Molecular Dynamics Simulation of the Interfacial Shear Properties between Thermoplastic Polyurethane and Functionalized Graphene Sheet
Abstract
1. Introduction
2. Materials and Methods
2.1. PCFF Forcefield
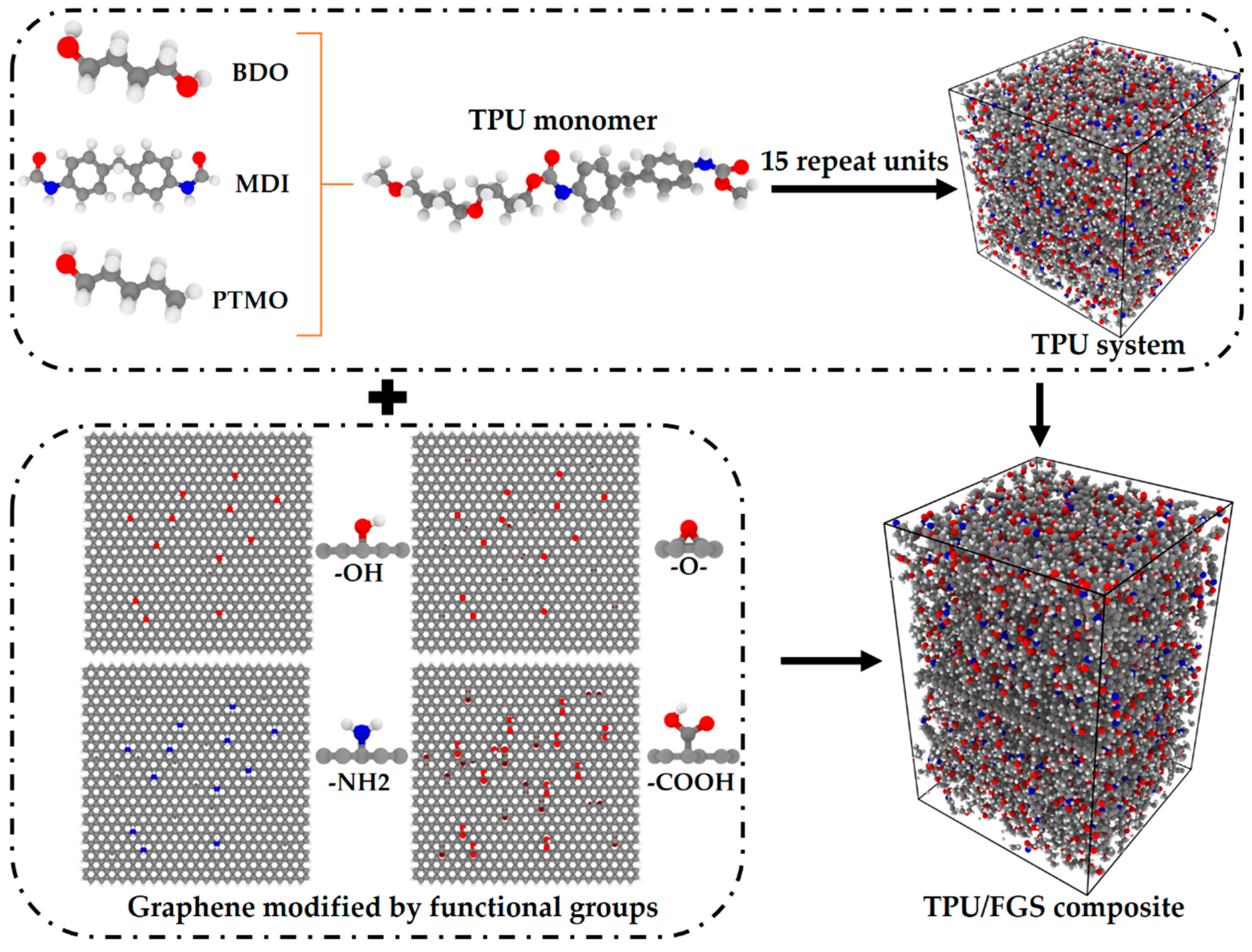
2.2. Model Preparation
2.3. Simulation Details
3. Results and Discussion
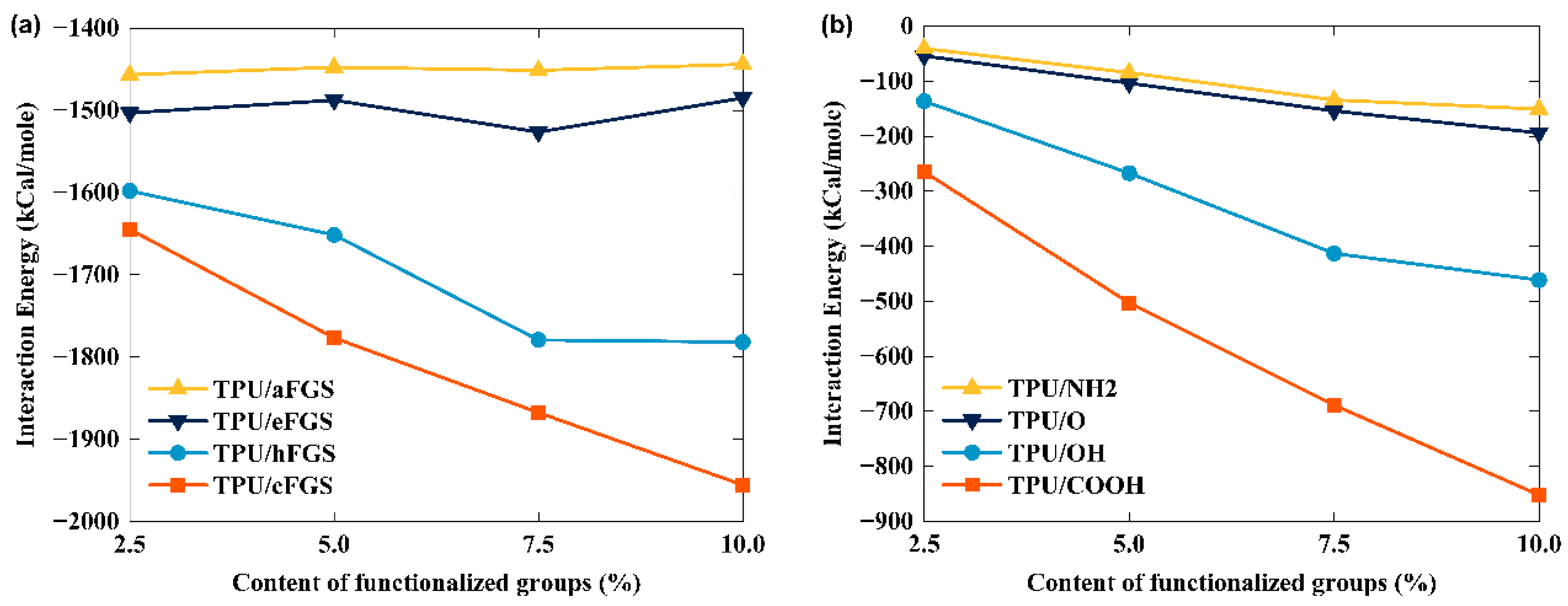
3.1. Interfacial Energy between TPU and the FGS
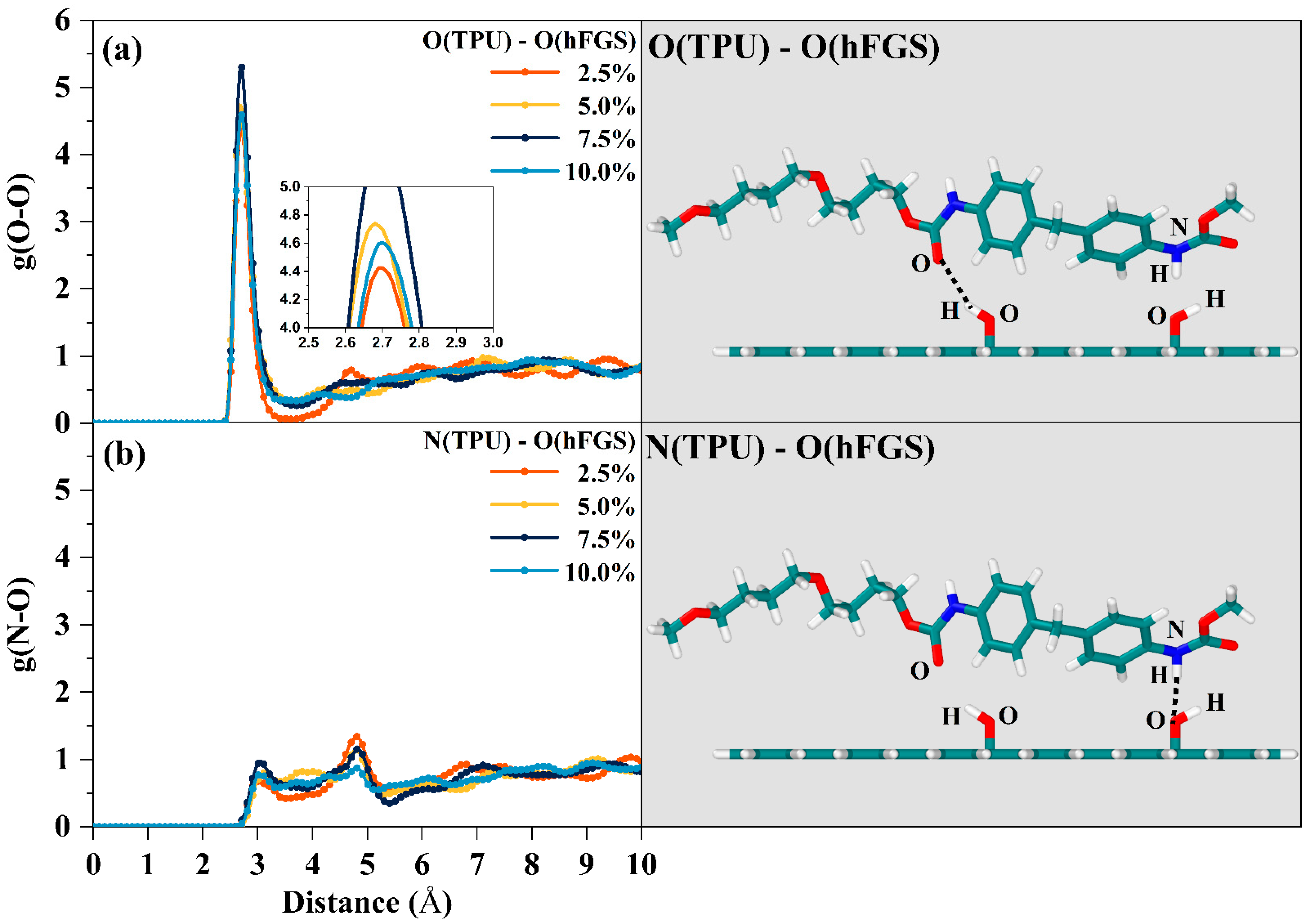
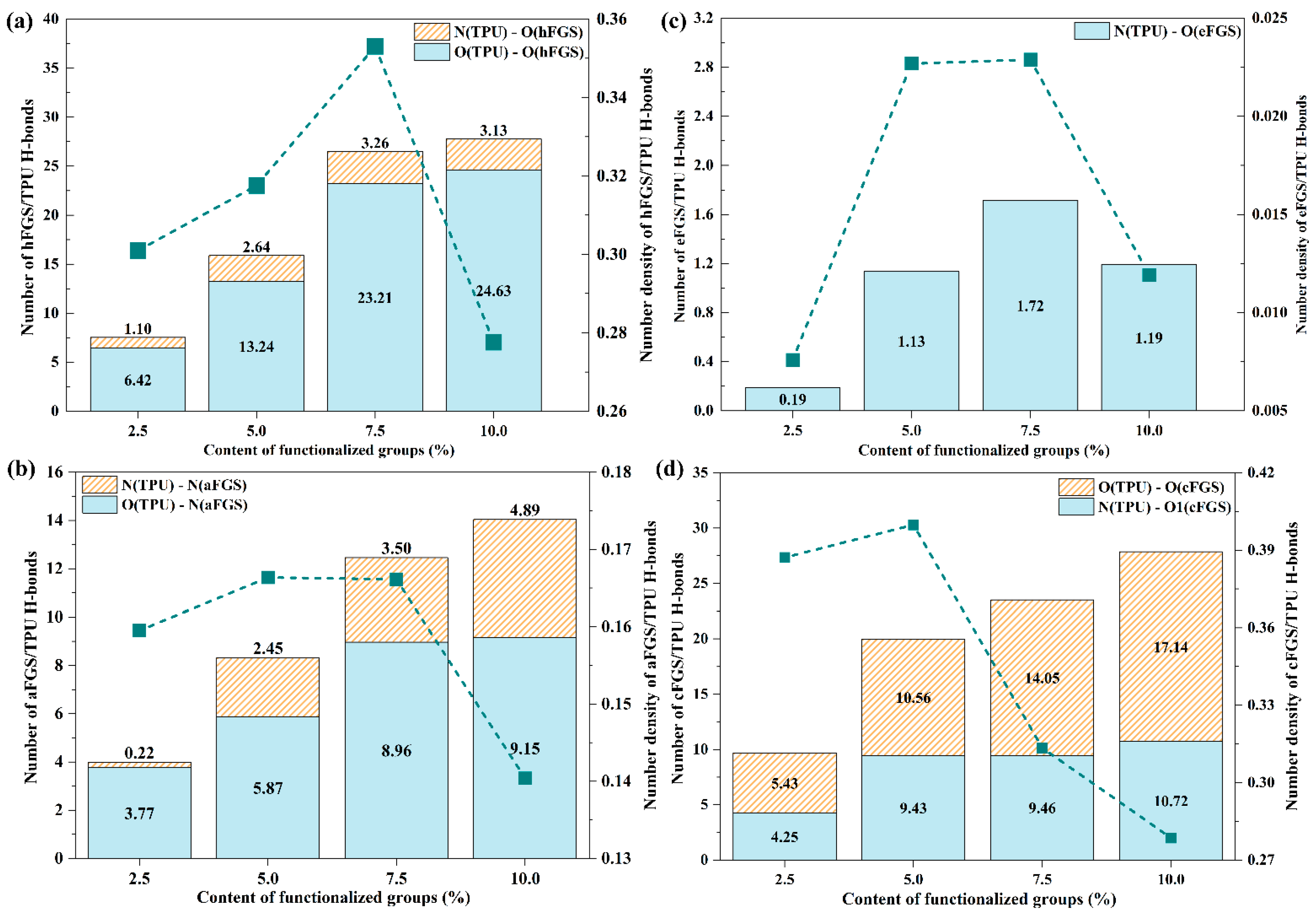
3.2. Hydrogen Bonding
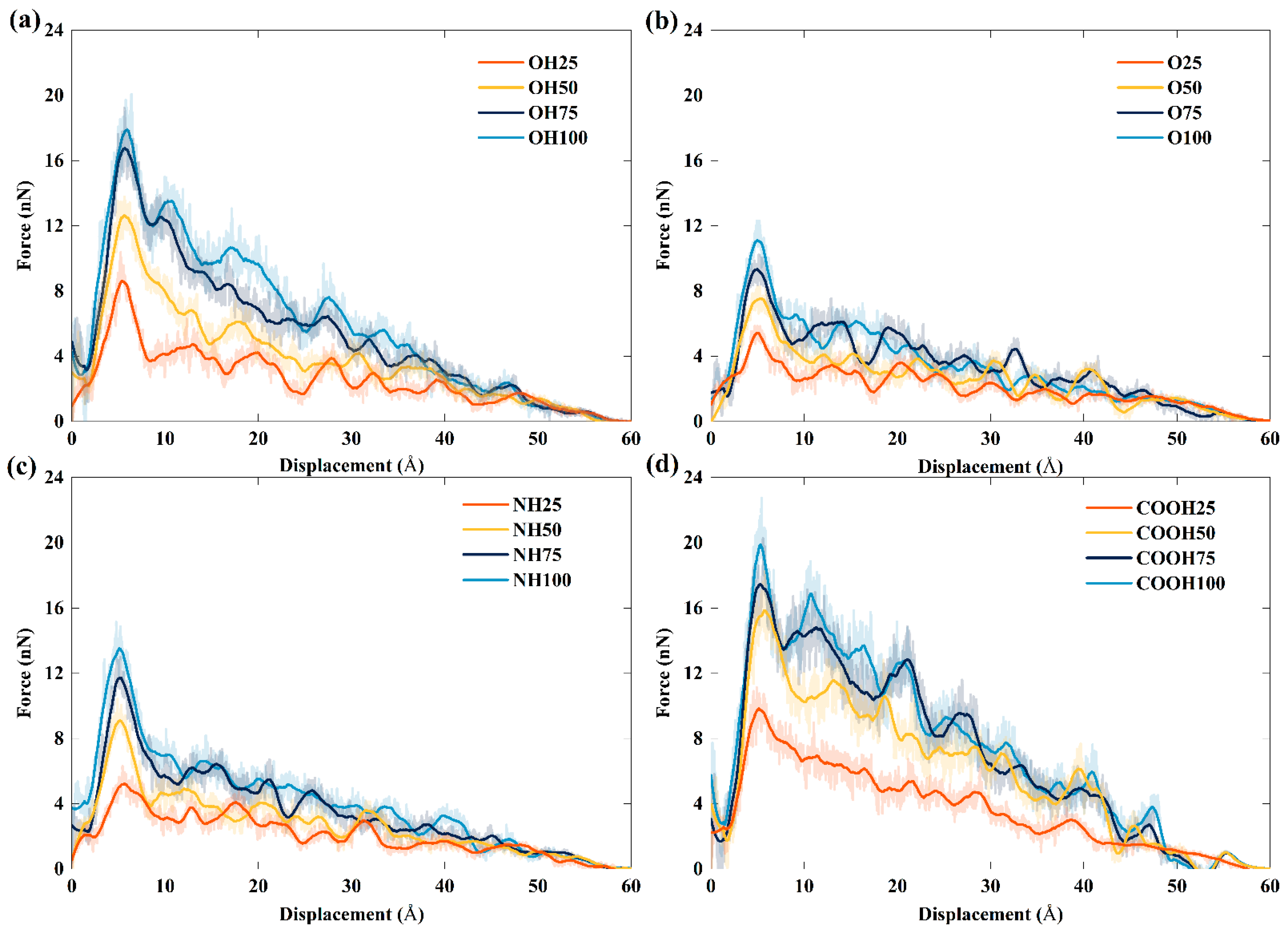
3.3. Pull-Out Simulation
4. Conclusions
- The interfacial shear resistance of TPU/hFGS and TPU/cFGS models is higher than that of TPU/aFGS and TPU/eFGS models, which is due to the stronger electrostatic interaction and H-bond interactions between TPU and functional groups with higher polarity, such as the –COOH or the –OH group. In addition, during the process of pulling out, the electrostatic interaction and H-bond interactions dominate the traction-separation behavior of the FGS compared to the mechanical interlocking effect caused by the size of functional groups.
- According to the RDF analysis, the TPU matrix has a larger possibility to form H-bonds with the –OH, –NH2, and –COOH groups than with the –O– group. Except for the inability to form hydrogen bonds between the O atoms of the –NH group of the TPU matrix and the –OH group of the cFGS due to the limitation of contact sites between the TPU matrix and the –COOH group, all the former three functional groups mentioned can form two types of H-bonds with the TPU matrix through nonbonded interactions between their polar atoms. This also leads to the reduced probability of forming H-bonds between the TPU matrix and the cFGS, which is reflected in the electrostatic energy, and the total amount of H-bonds between TPU and the cFGS are slightly higher than those between TPU and the hFGS, while the number of H-bonds formed between the –OH group of the cFGS and the TPU matrix is lower than that between the –OH group of the hFGS and the TPU matrix.
- With the increase in the functional group content, the hydrogen bond formation between TPU and the FGS tends to a saturation state.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Russo, P.; Acierno, D.; Capezzuto, F.; Buonocore, G.G.; Di Maio, L.; Lavorgna, M. Thermoplastic Polyurethane/Graphene Nanocomposites: The Effect of Graphene Oxide on Physical Properties. AIP Conf. Proc. 2015, 1695, 1–6. [Google Scholar] [CrossRef]
- Njoroge, J.L.; Cagin, T. Atomistic Simulation of Graphene-Polyurethane Nanocomposite for Use in Ballistic Applications. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2013. [Google Scholar]
- Pokharel, P.; Lee, S.H.; Lee, D.S. Thermal, Mechanical, and Electrical Properties of Graphene Nanoplatelet/Graphene Oxide/Polyurethane Hybrid Nanocomposite. J. Nanosci. Nanotechnol. 2015, 15, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Maji, P.K.; Guchhait, P.K.; Bhowmick, A.K. Effect of Nanoclays on Physico-Mechanical Properties and Adhesion of Polyester-Based Polyurethane Nanocomposites: Structure-Property Correlations. J. Mater. Sci. 2009, 44, 5861–5871. [Google Scholar] [CrossRef]
- Lee, S.I.; Hahn, Y.B.; Nahm, K.S.; Lee, Y.S. Synthesis of Polyether-Based Polyurethane-Silica Nanocomposites with High Elongation Property. Polym. Adv. Technol. 2005, 16, 328–331. [Google Scholar] [CrossRef]
- Rueda, L.; Saralegui, A.; Fernández D’Arlas, B.; Zhou, Q.; Berglund, L.A.; Corcuera, M.A.; Mondragon, I.; Eceiza, A. Cellulose Nanocrystals/Polyurethane Nanocomposites. Study from the Viewpoint of Microphase Separated Structure. Carbohydr. Polym. 2013, 92, 751–757. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.; Dixit, A.R. A Review on the Mechanical and Thermal Properties of Graphene and Graphene-Based Polymer Nanocomposites: Understanding of Modelling and MD Simulation. Mol. Simul. 2020, 46, 136–154. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Xu, C.; Zhang, M.; Shang, X. Preparation of Graphene/Poly(Vinyl Alcohol) Nanocomposites with Enhanced Mechanical Properties and Water Resistance. Polym. Int. 2011, 60, 816–822. [Google Scholar] [CrossRef]
- Yasmin, A.; Luo, J.J.; Daniel, I.M. Processing of Expanded Graphite Reinforced Polymer Nanocomposites. Compos. Sci. Technol. 2006, 66, 1182–1189. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Yang, H.; Xing, W.; Lu, H. In Situ Polymerization of Graphene Nanosheets and Polyurethane with Enhanced Mechanical and Thermal Properties. J. Mater. Chem. 2011, 21, 4222–4227. [Google Scholar] [CrossRef]
- Khatir, N.M.; Ahmadi, A.; Taghizade, N.; Motevali khameneh, S.; Faghihnasiri, M. Electronic Transport Properties of Nanoribbons of Graphene and ψ-Graphene-Based Lactate Nanobiosensor. Superlattices Microstruct. 2020, 145, 106603. [Google Scholar] [CrossRef]
- Khatir, N.M.; Fatoorehchi, H.; Ahmadi, A.; Khoshnoodfar, A.; Faghihnasiri, M. Investigating the Adsorption of the Thyroid Stimulating Hormones Molecules on Graphene Sheets by the Density Functional Theory for Possible Nano-Biosensor Applications. J. Chem. Pet. Eng. 2021, 55, 385–392. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Zhang, Y.; Liao, Y.; Zhou, H. The Effect of Defects on the Interfacial Mechanical Properties of Graphene/Epoxy Composites. RSC Adv. 2017, 7, 46101–46108. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, G.; Shang, L. Effects of Temperature and Hard Segment Content on the Interfacial Mechanical Properties of Graphene/Thermoplastic Polyurethane Composites: A Molecular Dynamics Study. Comput. Mater. Sci. 2022, 213, 111635. [Google Scholar] [CrossRef]
- Sahraei, A.A.; Mokarizadeh, A.H.; Foroutan, M.; George, D.; Rodrigue, D.; Baniassadi, M. Atomistic Simulation of Interfacial Properties and Damage Mechanism in Graphene Nanoplatelet/Epoxy Composites. Comput. Mater. Sci. 2020, 184, 109888. [Google Scholar] [CrossRef]
- Sahraei, A.A.; Mokarizadeh, A.H.; George, D.; Rodrigue, D.; Baniassadi, M.; Foroutan, M. Insights into Interphase Thickness Characterization for Graphene/Epoxy Nanocomposites: A Molecular Dynamics Simulation. Phys. Chem. Chem. Phys. 2019, 21, 19890–19903. [Google Scholar] [CrossRef]
- Liu, F.; Hu, N.; Zhang, J.; Atobe, S.; Weng, S.; Ning, H.; Liu, Y.; Wu, L.; Zhao, Y.; Mo, F.; et al. The Interfacial Mechanical Properties of Functionalized Graphene-Polymer Nanocomposites. RSC Adv. 2016, 6, 66658–66664. [Google Scholar] [CrossRef]
- Mo, M.; Zhao, W.; Chen, Z.; Yu, Q.; Zeng, Z.; Wu, X.; Xue, Q. Excellent Tribological and Anti-Corrosion Performance of Polyurethane Composite Coatings Reinforced with Functionalized Graphene and Graphene Oxide Nanosheets. RSC Adv. 2015, 5, 56486–56497. [Google Scholar] [CrossRef]
- Pokharel, P.; Pant, B.; Pokhrel, K.; Pant, H.R.; Lim, J.G.; Lee, D.S.; Kim, H.Y.; Choi, S. Effects of Functional Groups on the Graphene Sheet for Improving the Thermomechanical Properties of Polyurethane Nanocomposites. Compos. Part B Eng. 2015, 78, 192–201. [Google Scholar] [CrossRef]
- Skountzos, E.N.; Anastassiou, A.; Mavrantzas, V.G.; Theodorou, D.N. Determination of the Mechanical Properties of a Poly(Methyl Methacrylate) Nanocomposite with Functionalized Graphene Sheets through Detailed Atomistic Simulations. Macromolecules 2014, 47, 8072–8088. [Google Scholar] [CrossRef]
- Pishehvarz, G.; Erfan-Niya, H.; Zaminpayma, E. The Role of Hydrogen Bonding in Interaction Energy at the Interface of Conductive Polymers and Modified Graphene-Based Nanosheets: A Reactive Molecular Dynamics Study. Comput. Mater. Sci. 2018, 155, 499–523. [Google Scholar] [CrossRef]
- Yang, S.; Shin, H.; Cho, M. Contribution of Oxygen Functional Groups in Graphene to the Mechanical and Interfacial Behaviour of Nanocomposites: Molecular Dynamics and Micromechanics Study. Int. J. Mech. Sci. 2021, 189, 105972. [Google Scholar] [CrossRef]
- Bian, C.; Yan, N.; Zhu, G.W.; Qu, H.J.; Guan, Y.W.; Li, H.Y.; Luan, T.; Gong, J.L. Interfacial Interaction Mechanism of Graphene/Phenolic Resin Composites: A Molecular Dynamics Study. J. Phys. Conf. Ser. 2021, 1765, 012005. [Google Scholar] [CrossRef]
- Prasitnok, K.; In-noi, O. Functionalized Graphenes as Nanofillers for Polylactide: Molecular Dynamics Simulation Study. Polym. Compos. 2020, 41, 294–305. [Google Scholar] [CrossRef]
- Melro, L.S.; Pyrz, R.; Jensen, L.R. A Molecular Dynamics Study on the Interaction between Epoxy and Functionalized Graphene Sheets. IOP Conf. Ser. Mater. Sci. Eng. 2016, 139, 012036. [Google Scholar] [CrossRef]
- Park, C.; Yun, G.J. Characterization of Interfacial Properties of Graphene-Reinforced Polymer Nanocomposites by Molecular Dynamics-Shear Deformation Model. J. Appl. Mech. Trans. ASME 2018, 85, 091007. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, G.; Wang, W.; Zhu, S.; Guo, L.; Zhang, Z.; Li, P. Effects of Different Functional Groups in Graphene Nanofiber on the Mechanical Property of Polyvinyl Alcohol Composites by the Molecular Dynamic Simulations. J. Mol. Liq. 2019, 277, 261–268. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Sun, H.; Mumby, S.J.; Maple, J.R.; Hagler, A.T. An ab initio cff93 all-atom force field for polycarbonates. J. Am. Chem. Soc. 1994, 116, 2978–2987. [Google Scholar] [CrossRef]
- Awasthi, A.P.; Lagoudas, D.C.; Hammerand, D.C. Modeling of Graphene-Polymer Interfacial Mechanical Behavior Using Molecular Dynamics. Model. Simul. Mater. Sci. Eng. 2009, 17, 015002. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, J.; Zhu, H.; Xu, Y.; Wang, M.; Wang, B.; Yang, C. Molecular Dynamics Simulation of the Interface Properties of Continuous Carbon Fiber/ Polyimide Composites. Appl. Surf. Sci. 2021, 563, 150370. [Google Scholar] [CrossRef]
- Nikkhah, S.J.; Moghbeli, M.R.; Hashemianzadeh, S.M. Investigation of the Interface between Polyethylene and Functionalized Graphene: A Computer Simulation Study. Curr. Appl. Phys. 2015, 15, 1188–1199. [Google Scholar] [CrossRef]
- Eastwood, J.W.; Hockney, R.W. Shaping the Force Law in Two-Dimensional Particle-Mesh Models. J. Comput. Phys. 1974, 16, 342–359. [Google Scholar] [CrossRef]
- Dewapriya, M.A.N.; Miller, R.E. Molecular Dynamics Study of the Mechanical Behaviour of Ultrathin Polymer–Metal Multilayers under Extreme Dynamic Conditions. Comput. Mater. Sci. 2020, 184, 109951. [Google Scholar] [CrossRef]
- Talapatra, A.; Datta, D. Atomistic Investigation of the Interfacial Mechanical Characteristics of Graphene Reinforced Thermoplastic Polyurethane Composite. Compos. Interfaces 2021, 28, 395–427. [Google Scholar] [CrossRef]
- Zhou, R.; Gao, W.; Xia, L.; Wu, H.; Guo, S. The Study of Damping Property and Mechanism of Thermoplastic Polyurethane/Phenolic Resin through a Combined Experiment and Molecular Dynamics Simulation. J. Mater. Sci. 2018, 53, 9350–9362. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Hou, D.; Zhang, J. Tuning Interfacial Structure and Mechanical Properties of Graphene Oxide Sheets/Polymer Nanocomposites by Controlling Functional Groups of Polymer. Appl. Surf. Sci. 2020, 504, 144152. [Google Scholar] [CrossRef]
- Karatasos, K.; Kritikos, G. Characterization of a Graphene Oxide/Poly(Acrylic Acid) Nanocomposite by Means of Molecular Dynamics Simulations. RSC Adv. 2016, 6, 109267–109277. [Google Scholar] [CrossRef]
- Morita, M.; Oya, Y.; Kato, N.; Mori, K.; Koyanagi, J. Effect of Electrostatic Interactions on the Interfacial Energy between Thermoplastic Polymers and Graphene Oxide: A Molecular Dynamics Study. Polymers 2022, 14, 2579. [Google Scholar] [CrossRef]
- He, Y.; Xie, D.; Zhang, X. The Structure, Microphase-Separated Morphology, and Property of Polyurethanes and Polyureas. J. Mater. Sci. 2014, 49, 7339–7352. [Google Scholar] [CrossRef]
- Hasheminejad, K.; Montazeri, A. Enhanced Interfacial Characteristics in PLA/Graphene Composites through Numerically-Designed Interface Treatment. Appl. Surf. Sci. 2020, 502, 144150. [Google Scholar] [CrossRef]
- Lv, C.; Xue, Q.; Xia, D.; Ma, M.; Xie, J.; Chen, H. Effect of Chemisorption on the Interfacial Bonding Characteristics of Graphene-Polymer Composites. J. Phys. Chem. C 2010, 114, 6588–6594. [Google Scholar] [CrossRef]
- Xiaolin, J.; Min, X.; Minhui, W.; Yuanhao, M.; Wencong, Z.; Yanan, Z.; Haoxiang, R.; Xun, L. Preparation and Molecular Dynamics Study of Polyurethane Damping Elastomer Containing Dynamic Disulfide Bond and Multiple Hydrogen Bond. Eur. Polym. J. 2022, 162, 110893. [Google Scholar] [CrossRef]
- Ren, Z.; Ma, D.; Yang, X. H-Bond and Conformations of Donors and Acceptors in Model Polyether Based Polyurethanes. Polymer 2003, 44, 6419–6425. [Google Scholar] [CrossRef]
- Ren, Z.; Zeng, X.; Yang, X.; Ma, D.; Hsu, S.L. Molecular Modeling of the H-Bonds in Polyurethane with Multiple Donors and Acceptors. Polymer 2005, 46, 12337–12347. [Google Scholar] [CrossRef]
- Bera, M.; Maji, P.K. Effect of Structural Disparity of Graphene-Based Materials on Thermo-Mechanical and Surface Properties of Thermoplastic Polyurethane Nanocomposites. Polymer 2017, 119, 118–133. [Google Scholar] [CrossRef]
- Zhang, X.; Nguyen, H.; Daly, M.; Nguyen, S.B.T.; Espinosa, H.D. Nanoscale Toughening of Ultrathin Graphene Oxide-Polymer Composites: Mechanochemical Insights into Hydrogen-Bonding/van Der Waals Interactions, Polymer Chain Alignment, and Steric Parameters. Nanoscale 2019, 11, 12305–12316. [Google Scholar] [CrossRef]
- Choi, J.Y.; Zhang, X.; Nguyen, H.T.; Roenbeck, M.R.; Mao, L.; Soler-Crespo, R.; Nguyen, S.B.T.; Espinosa, H.D. Atomistic Mechanisms of Adhesion and Shear Strength in Graphene Oxide-Polymer Interfaces. J. Mech. Phys. Solids 2021, 156, 104578. [Google Scholar] [CrossRef]
- Jin, Y.; Duan, F.; Mu, X. Functionalization Enhancement on Interfacial Shear Strength between Graphene and Polyethylene. Appl. Surf. Sci. 2016, 387, 1100–1109. [Google Scholar] [CrossRef]
- Nie, F.; Jian, W.; Lau, D. An Atomistic Study on the Thermomechanical Properties of Graphene and Functionalized Graphene Sheets Modified Asphalt. Carbon N. Y. 2021, 182, 615–627. [Google Scholar] [CrossRef]
- Wang, P.; Qiao, G.; Hou, D.; Jin, Z.; Wang, M.; Zhang, J.; Sun, G. Functionalization Enhancement Interfacial Bonding Strength between Graphene Sheets and Calcium Silicate Hydrate: Insights from Molecular Dynamics Simulation. Constr. Build. Mater. 2020, 261, 120500. [Google Scholar] [CrossRef]
- Cook, R.F.; Thouless, M.D.; Clarke, D.R.; Kroll, M.C. Stick-Slip during Fibre Pull-Out. Scr. Metall. 1989, 23, 1725–1730. [Google Scholar] [CrossRef]
- Xia, W.; Song, J.; Meng, Z.; Shao, C.; Keten, S. Designing Multi-Layer Graphene-Based Assemblies for Enhanced Toughness in Nacre-Inspired Nanocomposites. Mol. Syst. Des. Eng. 2016, 1, 40–47. [Google Scholar] [CrossRef]
- Karimi, M.R.; Abrinia, K.; Hamdia, K.M.; Hashemianzadeh, S.M.; Baniassadi, M. Effects of Functional Group Type and Coverage on the Interfacial Strength and Load Transfer of Graphene-Polyethylene Nanocomposites: A Molecular Dynamics Simulation. Appl. Phys. A Mater. Sci. Process. 2022, 128, 341. [Google Scholar] [CrossRef]
- Yao, X.; Shamsaei, E.; Sagoe-Crentsil, K.; Duan, W. The Interaction of Graphene Oxide with Cement Mortar: Implications on Reinforcing Mechanisms. J. Mater. Sci. 2022, 57, 3405–3415. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zou, G.; Liu, J. Molecular Dynamics Simulation of the Interfacial Shear Properties between Thermoplastic Polyurethane and Functionalized Graphene Sheet. Polymers 2022, 14, 5032. https://doi.org/10.3390/polym14225032
Wang Y, Zou G, Liu J. Molecular Dynamics Simulation of the Interfacial Shear Properties between Thermoplastic Polyurethane and Functionalized Graphene Sheet. Polymers. 2022; 14(22):5032. https://doi.org/10.3390/polym14225032
Chicago/Turabian StyleWang, Yuyang, Guangping Zou, and Junpeng Liu. 2022. "Molecular Dynamics Simulation of the Interfacial Shear Properties between Thermoplastic Polyurethane and Functionalized Graphene Sheet" Polymers 14, no. 22: 5032. https://doi.org/10.3390/polym14225032
APA StyleWang, Y., Zou, G., & Liu, J. (2022). Molecular Dynamics Simulation of the Interfacial Shear Properties between Thermoplastic Polyurethane and Functionalized Graphene Sheet. Polymers, 14(22), 5032. https://doi.org/10.3390/polym14225032





