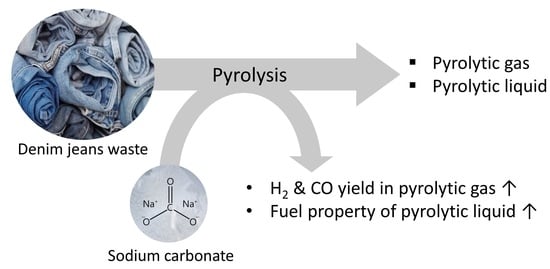
Pyrolysis of Denim Jeans Waste: Pyrolytic Product Modification by the Addition of Sodium Carbonate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. Pyrolysis Experiment and Product Analysis
3. Results and Discussion
3.1. Feedstock Characterization
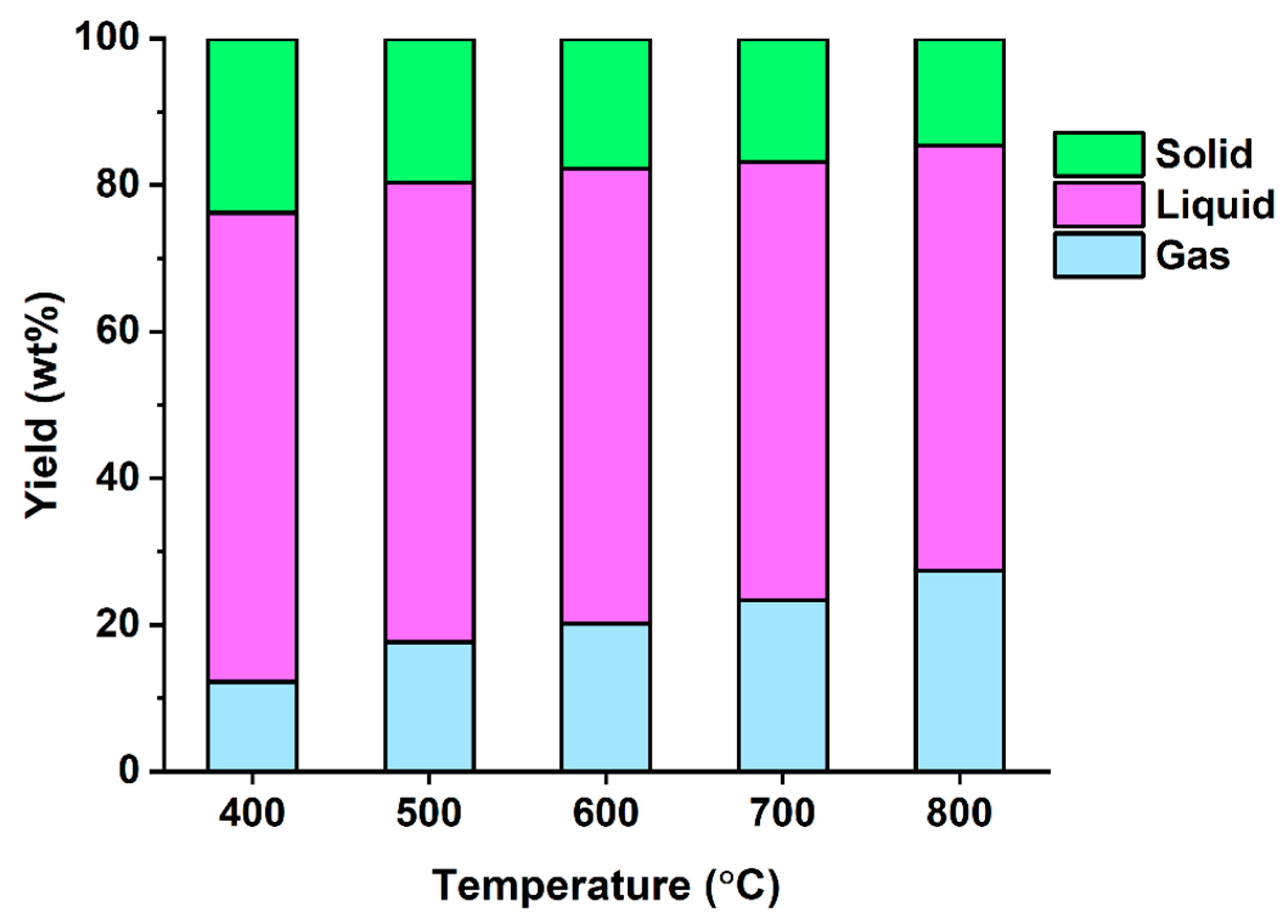
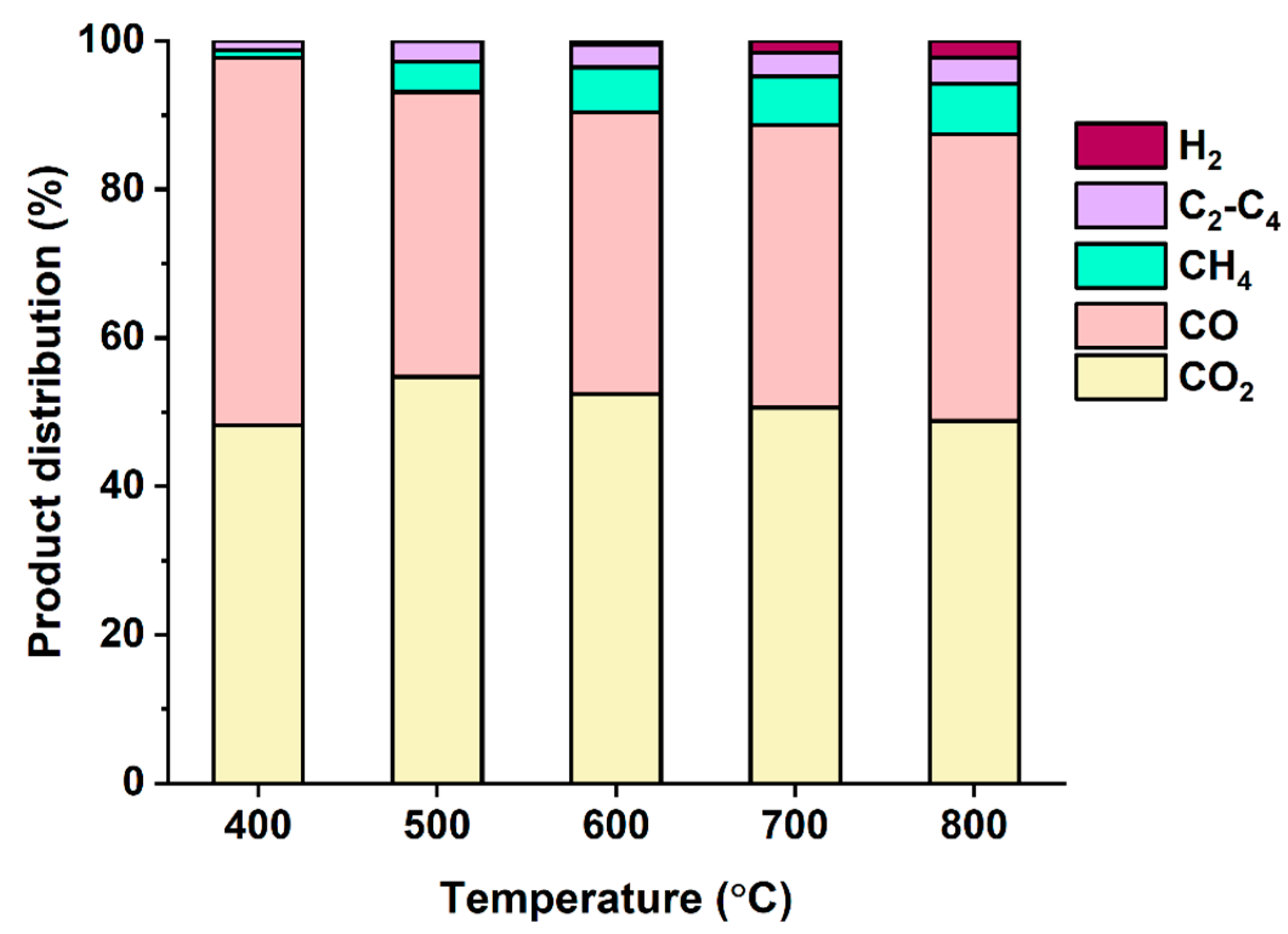
3.2. Pyrolysis of Denim Jeans Waste
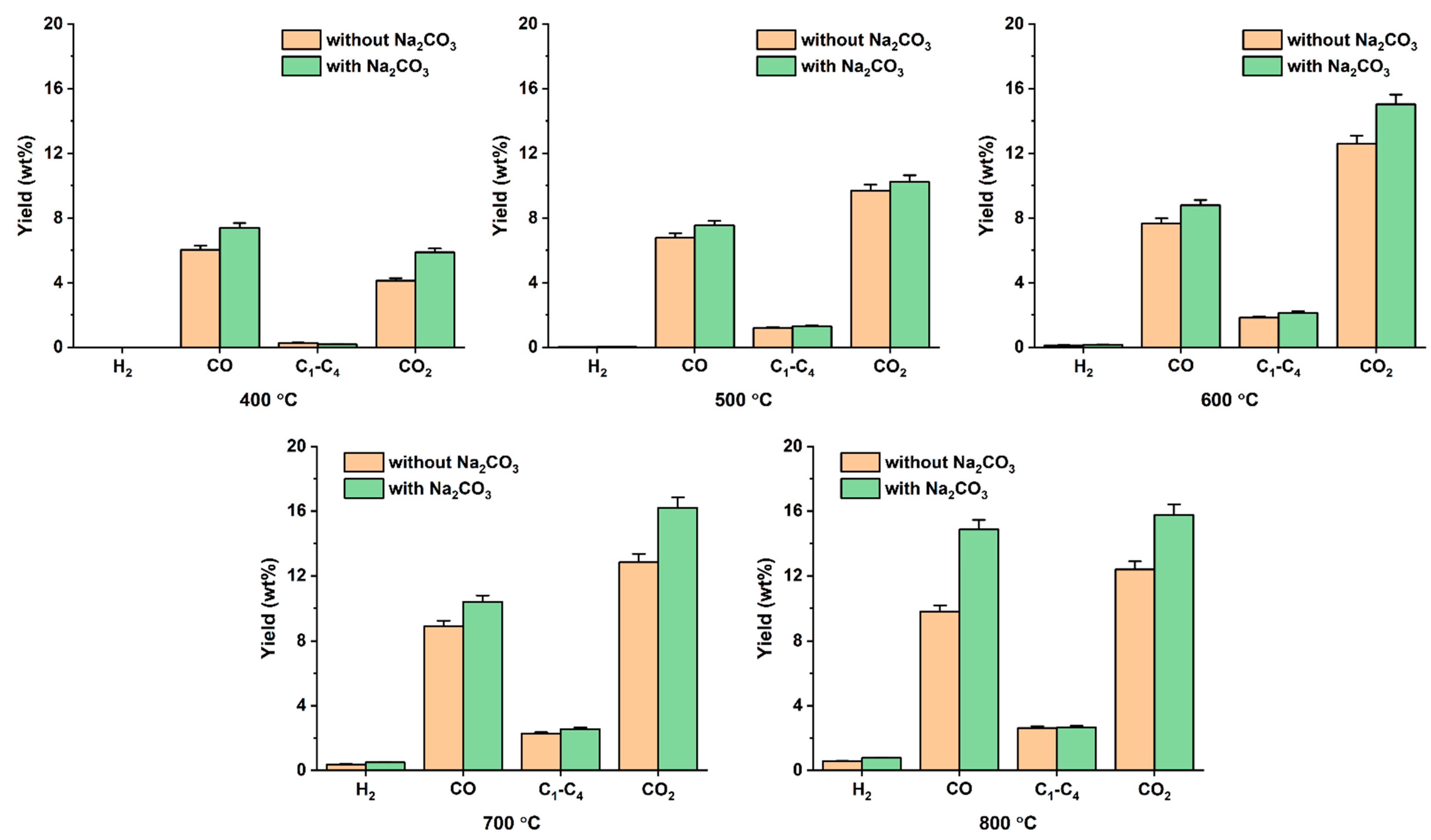
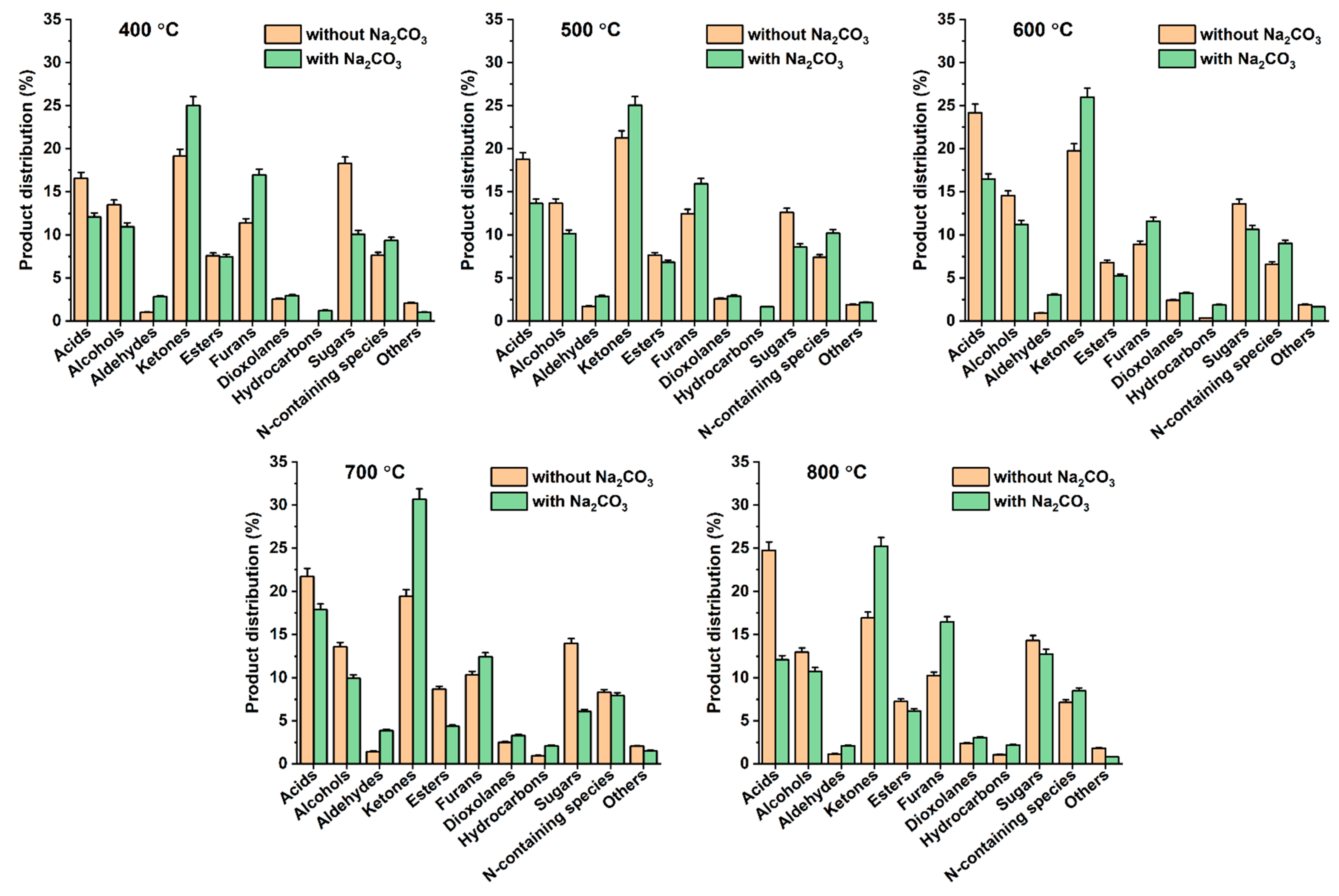
3.3. Effects of Na2CO3 Addition to Pyrolysis of Denim Jeans Waste
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- US EPA. Facts and Figures about Materials, Waste and Recycling—Textiles: Material-Specific Data; United States Environmental Protection Agency (US EPA): Washington, DC, USA, 2020.
- Dissanayake, D.G.K.; Weerasinghe, D.U.; Wijesinghe, K.A.P.; Kalpage, K.M.D.M.P. Developing a compression moulded thermal insulation panel using postindustrial textile waste. Waste Manag. 2018, 79, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Shukla, V.K.; Agarwal, P. Sustainable transformation in modest fashion through “RPET technology” and “Dry-Dye” process, using recycled PET plastic. Int. J. Recent Technol. Eng. 2019, 8, A1432058119. [Google Scholar] [CrossRef]
- EEA. Plastic in Textiles: Towards a Circular Economy for Synthetic Textiles in Europe; European Environment Agency (EEA): Copenhagen, Denmark, 2021. [Google Scholar]
- Lee, J.; Kwon, E.E.; Lam, S.S.; Chen, W.-H.; Rinklebe, J.; Park, Y.-K. Chemical recycling of plastic waste via thermocatalytic routes. J. Clean. Prod. 2021, 321, 128989. [Google Scholar] [CrossRef]
- Lee, N.; Joo, J.; Lin, K.-Y.A.; Lee, J. Thermochemical conversion of mulching film waste via pyrolysis with the addition of cattle excreta. J. Environ. Chem. Eng. 2021, 9, 106362. [Google Scholar] [CrossRef]
- Lee, J.; Lin, K.-Y.A.; Jung, S.; Kwon, E.E. Hybrid renewable energy systems involving thermochemical conversion process for waste-to-energy strategy. Chem. Eng. J. 2023, 452, 139218. [Google Scholar] [CrossRef]
- Park, C.; Lee, N.; Kim, J.; Lee, J. Co-pyrolysis of food waste and wood bark to produce hydrogen with minimizing pollutant emissions. Environ. Pollut. 2021, 270, 116045. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Park, Y.-K. Simultaneous upcycling of biodegradable plastic and sea shell wastes through thermocatalytic monomer recovery. ACS Sustain. Chem. Eng. 2022, 10, 13972–13979. [Google Scholar] [CrossRef]
- Kwon, D.; Yi, S.; Jung, S.; Kwon, E.E. Valorization of synthetic textile waste using CO2 as a raw material in the catalytic pyrolysis process. Environ. Pollut. 2021, 268, 115916. [Google Scholar] [CrossRef]
- Park, C.; Lin, K.-Y.A.; Kwon, E.E.; Lee, J.; Park, Y.-K. Energy recovery from banner waste through catalytic pyrolysis over cobalt oxide: Effects of catalyst configuration. Int. J. Energy Res. 2022, 46, 19051–19063. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.T.; Oh, L.S.; Kim, H.J.; Lee, J. Marine waste upcycling—Recovery of nylon monomers from fishing net waste using seashell waste-derived catalysts in a CO2-mediated thermocatalytic process. J. Mater. Chem. A 2022, 10, 20024–20034. [Google Scholar] [CrossRef]
- Ding, L.; Zhou, Z.; Guo, Q.; Huo, W.; Yu, G. Catalytic effects of Na2CO3 additive on coal pyrolysis and gasification. Fuel 2015, 142, 134–144. [Google Scholar] [CrossRef]
- Yu, D.; Jin, G.; Pang, Y.; Chen, Y.; Guo, S.; Shen, S. Gas characteristics of pine sawdust catalyzed pyrolysis by additives. J. Therm. Sci. 2021, 30, 333–342. [Google Scholar] [CrossRef]
- Zeng, K.; Yan, H.; Xia, H.; Zhang, L.; Zhang, Q. Catalytic pyrolysis of Eupatorium adenophorum by sodium salt. J. Mater. Cycles Waste Manag. 2021, 23, 1626–1635. [Google Scholar] [CrossRef]
- Lin, J.; Cheng, S. Catalytic pyrolysis of crofton weed: Comparison of their pyrolysis product and preliminary economic analysis. Environ. Prog. Sustain. Energy 2022, 41, e13742. [Google Scholar] [CrossRef]
- Sedef Uncu, A.; Cevza, C.; Banu, N.; Neslihan Sebla, Ö. Understanding denim recycling: A quantitative study with lifecycle assessment methodology. In Waste in Textile and Leather Sectors; Ayşegül, K., Ed.; IntechOpen: Rijeka, Croatia, 2020; Chapter 4. [Google Scholar]
- Kim, S.; Yang, W.; Lee, H.S.; Tsang, Y.F.; Lee, J. Effectiveness of CO2-mediated pyrolysis for the treatment of biodegradable plastics: A case study of polybutylene adipate terephthalate/polylactic acid mulch film. J. Clean. Prod. 2022, 372, 133763. [Google Scholar] [CrossRef]
- Yang, W.; Kim, K.-H.; Lee, J. Upcycling of decommissioned wind turbine blades through pyrolysis. J. Clean. Prod. 2022, 376, 134292. [Google Scholar] [CrossRef]
- Lee, N.; Lin, K.-Y.A.; Lee, J. Carbon dioxide-mediated thermochemical conversion of banner waste using cobalt oxide catalyst as a strategy for plastic waste treatment. Environ. Res. 2022, 213, 113560. [Google Scholar] [CrossRef]
- Valin, S.; Cances, J.; Castelli, P.; Thiery, S.; Dufour, A.; Boissonnet, G.; Spindler, B. Upgrading biomass pyrolysis gas by conversion of methane at high temperature: Experiments and modelling. Fuel 2009, 88, 834–842. [Google Scholar] [CrossRef]
- Chen, M.-q.; Wang, J.; Zhang, M.-x.; Chen, M.-g.; Zhu, X.-f.; Min, F.-f.; Tan, Z.-c. Catalytic effects of eight inorganic additives on pyrolysis of pine wood sawdust by microwave heating. J. Anal. Appl. Pyrolysis 2008, 82, 145–150. [Google Scholar] [CrossRef]
- Zhao, N.; Li, B.-X. The effect of sodium chloride on the pyrolysis of rice husk. Appl. Energy 2016, 178, 346–352. [Google Scholar] [CrossRef]
- Guo, D.-l.; Wu, S.-b.; Liu, B.; Yin, X.-l.; Yang, Q. Catalytic effects of NaOH and Na2CO3 additives on alkali lignin pyrolysis and gasification. Appl. Energy 2012, 95, 22–30. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, J.; Zhu, X. Corrosion properties of bio-oil and its emulsions with diesel. Chin. Sci. Bull. 2008, 53, 3726–3734. [Google Scholar] [CrossRef]

| Analysis | Composition | Content (wt%) |
|---|---|---|
| Ultimate analysis | C | 45.00 |
| H | 6.35 | |
| O | 32.16 | |
| N | 0.20 | |
| S | 1.20 | |
| Ash | 15.09 | |
| Total | 100 | |
| Proximate analysis | Volatile matter | 84.10 |
| Fixed carbon | 0.81 | |
| Ash | 15.09 | |
| Total | 100 |
| Component | 400 °C | 500 °C | 600 °C | 700 °C | 800 °C |
|---|---|---|---|---|---|
| Acids | 19.6 | 18.8 | 19.2 | 18.8 | 19.7 |
| Alcohols | 13.5 | 13.7 | 14.5 | 13.6 | 13.9 |
| Aldehydes | 1 | 1.7 | 1.5 | 1.4 | 1.1 |
| Ketones | 19.2 | 21.2 | 20.8 | 19.4 | 19.4 |
| Esters | 7.6 | 7.6 | 7.8 | 8.7 | 7.8 |
| Furans | 11.4 | 12.5 | 10.9 | 10.4 | 10.3 |
| Dioxolanes | 2.6 | 2.6 | 2.4 | 2.5 | 2.4 |
| Hydrocarbons | 0 | 0 | 0.4 | 0.9 | 1.1 |
| Sugars | 15.3 | 12.6 | 13.6 | 14 | 14.7 |
| N-containing species | 7.7 | 7.4 | 7 | 8.3 | 7.8 |
| Others | 2.1 | 1.9 | 1.9 | 2 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, J.; Choi, H.; Lin, K.-Y.A.; Lee, J. Pyrolysis of Denim Jeans Waste: Pyrolytic Product Modification by the Addition of Sodium Carbonate. Polymers 2022, 14, 5035. https://doi.org/10.3390/polym14225035
Joo J, Choi H, Lin K-YA, Lee J. Pyrolysis of Denim Jeans Waste: Pyrolytic Product Modification by the Addition of Sodium Carbonate. Polymers. 2022; 14(22):5035. https://doi.org/10.3390/polym14225035
Chicago/Turabian StyleJoo, Junghee, Heeyoung Choi, Kun-Yi Andrew Lin, and Jechan Lee. 2022. "Pyrolysis of Denim Jeans Waste: Pyrolytic Product Modification by the Addition of Sodium Carbonate" Polymers 14, no. 22: 5035. https://doi.org/10.3390/polym14225035