Maltol-Incorporated Acetylated Cassava Starch Films for Shelf-Life-Extension Packaging of Bakery Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Film Preparation
2.2. Scanning Electron Microscopy (SEM)
2.3. X-ray Diffraction (XRD)
2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5. Dynamic Mechanical Thermal Analysis (DMTA)
2.6. Thermal Degradation Behavior of the Films
2.7. Mechanical Properties
2.8. Water Vapor Permeability
2.9. Oxygen Permeability
2.10. Application as Active Packaging for Bakery Products
2.11. Statistical Analysis
3. Results and Discussion
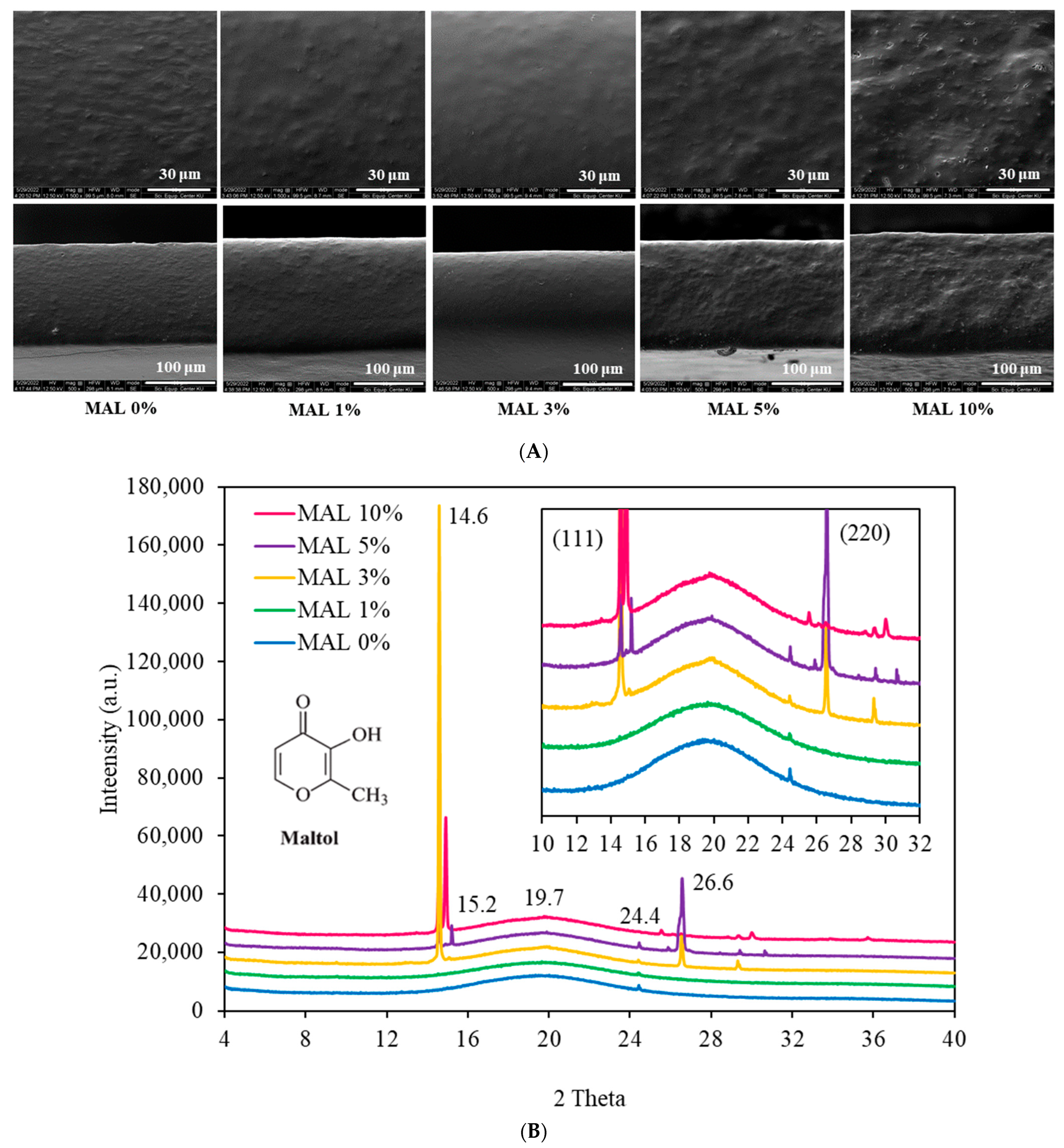
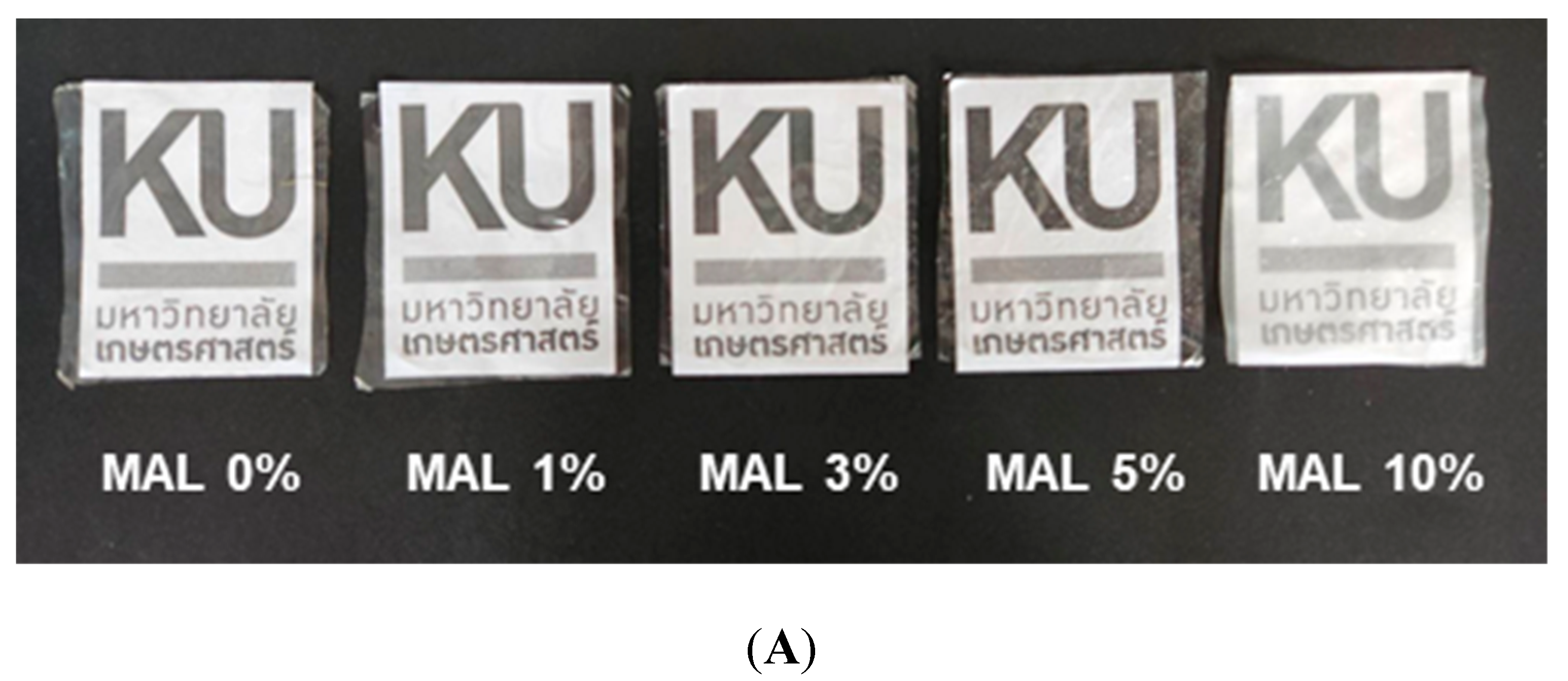
3.1. Microstructures
3.2. X-ray Diffraction
3.3. FTIR
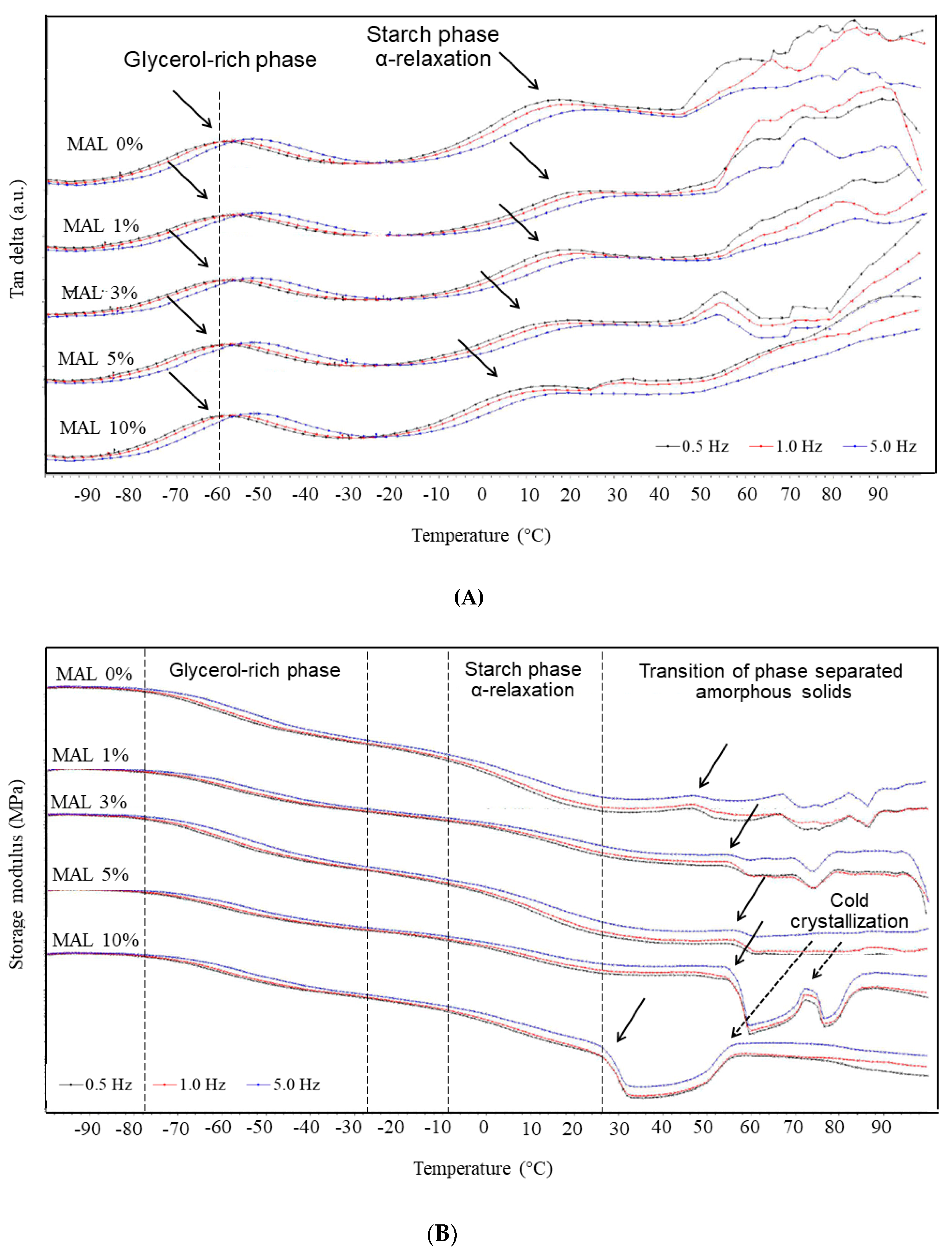
3.4. DMTA
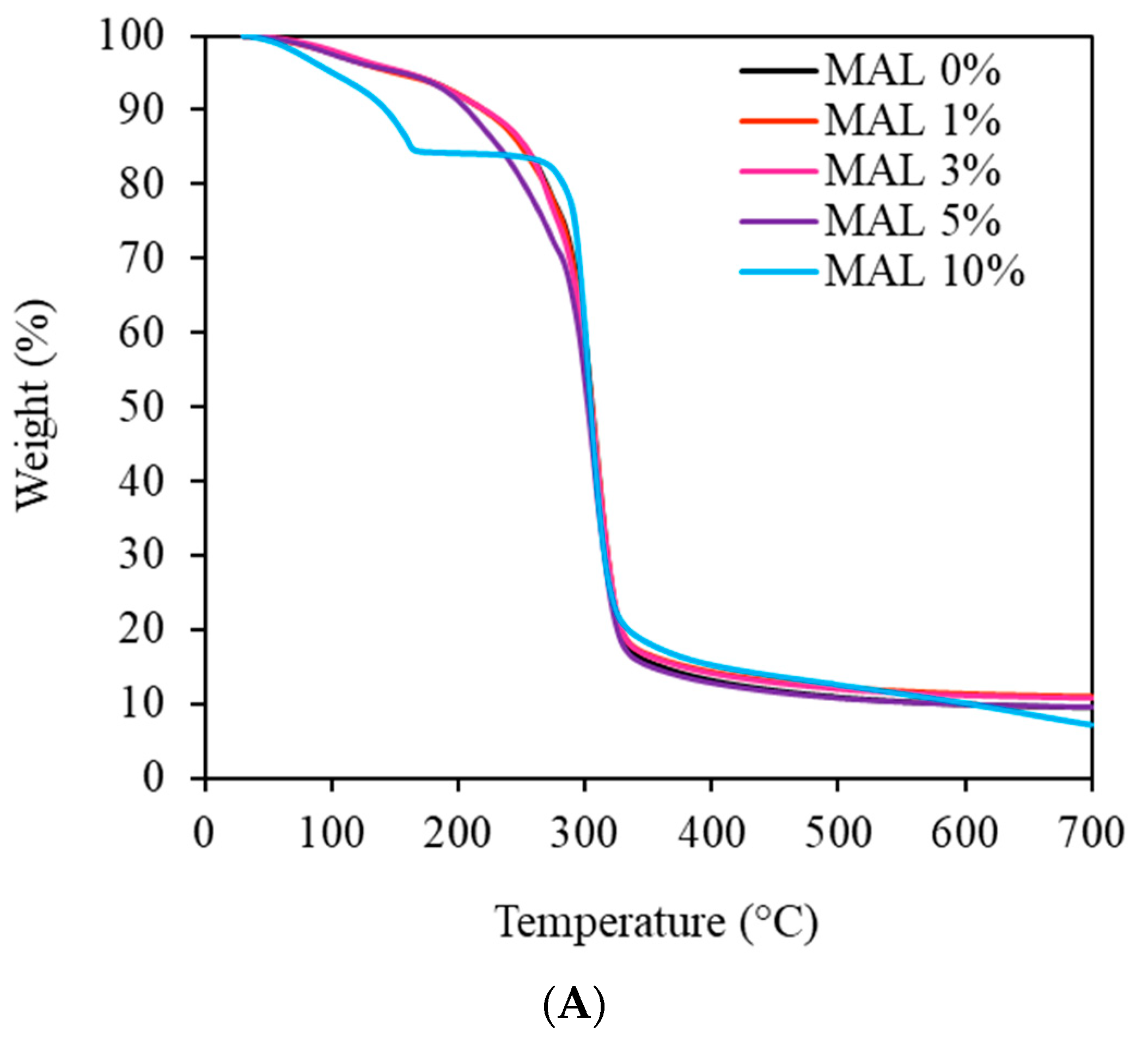
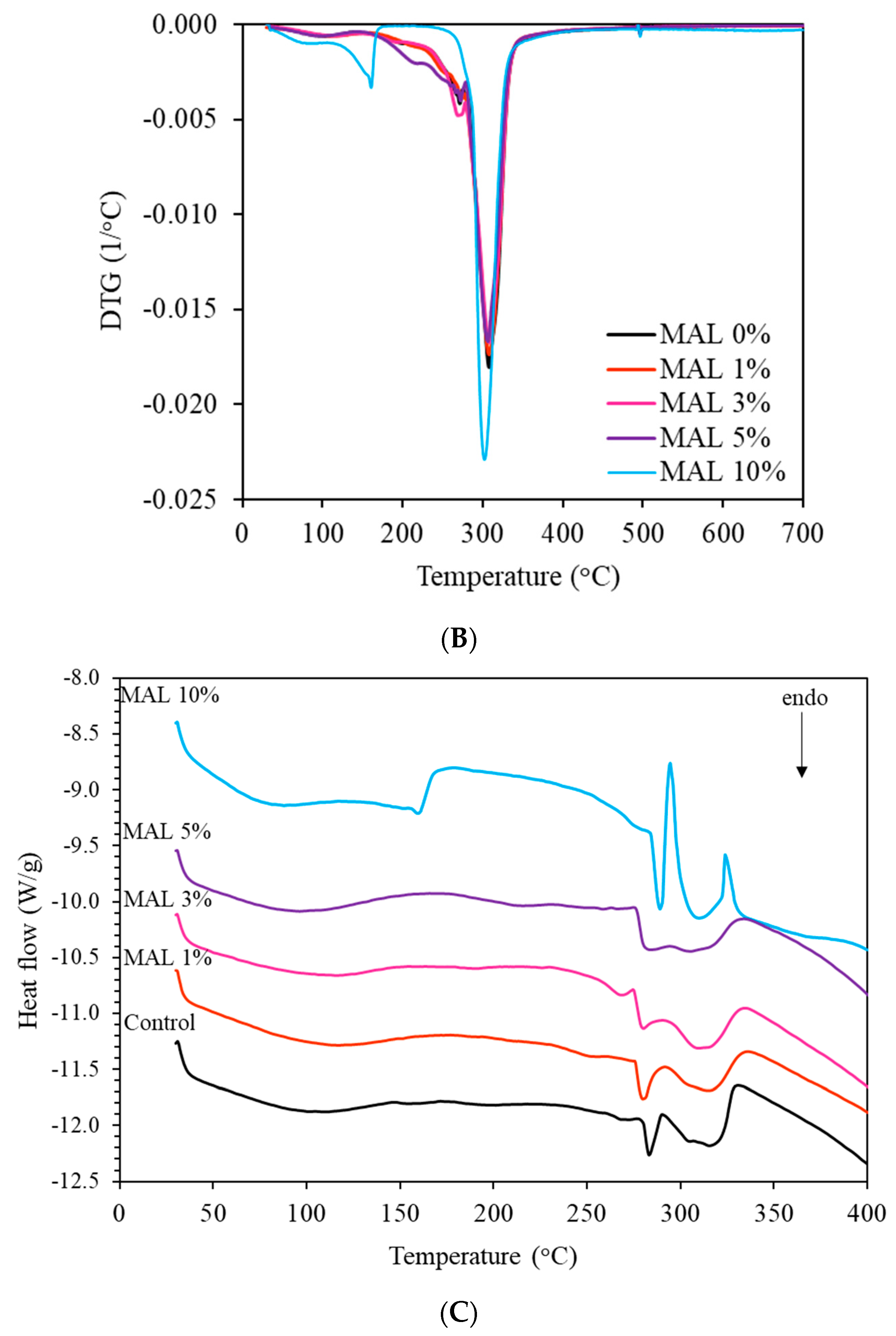
3.5. Thermal Degradation Behavior of the Films
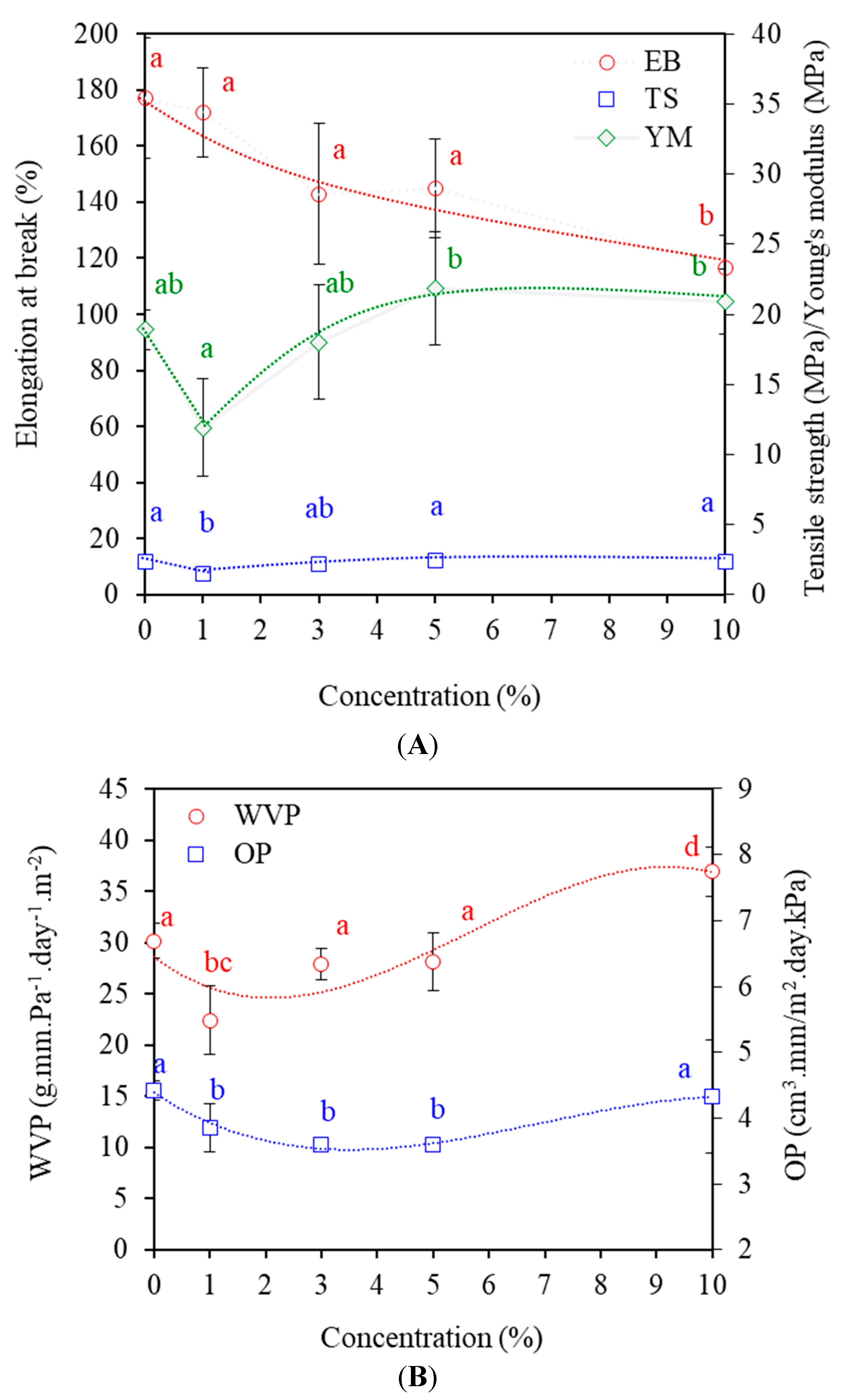
3.6. Mechanical Properties
3.7. Barrier Properties
3.8. Application as Active Packaging for Bakery Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bertuzzi, M.A.; Vidaurre, E.C.; Armada, M.; Gottifredi, J.C. Water vapor permeability of edible starch based films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- Jariyasakoolroj, P.; Tashiro, K.; Chinsirikul, W.; Kerddonfag, N.; Chirachanchai, S. Microstructural analyses of biaxially oriented polylactide/modified thermoplastic starch film with drastic improvement in toughness. Macromol. Mater. Eng. 2019, 304, 1900340. [Google Scholar] [CrossRef]
- Shaikh, M.; Haider, S.; Ali, T.M.; Hasnain, A. Physical, thermal, mechanical and barrier properties of pearl millet starch films as affected by levels of acetylation and hydroxypropylation. Int. J. Biol. Macromol. 2019, 124, 209–219. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Ashogbon, A.O.; Kumar, M. Recent advances in thermoplastic starches for food packaging: A review. Food Packag. Shelf Life 2021, 30, 100743. [Google Scholar] [CrossRef]
- Derungs, I.; Rico, M.; López, J.; Barral, L.; Montero, B.; Bouza, R. Influence of the hydrophilicity of montmorillonite on structure and properties of thermoplastic wheat starch/montmorillonite bionanocomposites. Polym. Adv. Technol. 2021, 32, 4479–4489. [Google Scholar] [CrossRef]
- Teixeira, S.C.; Silva, R.R.A.; de Oliveira, T.V.; Stringheta, P.C.; Pinto, M.R.M.R.; Soares, N.D.F.F. Glycerol and triethyl citrate plasticizer effects on molecular, thermal, mechanical, and barrier properties of cellulose acetate films. Food Biosci. 2021, 42, 101202. [Google Scholar] [CrossRef]
- Singh, G.P.; Bangar, S.P.; Yang, T.; Trif, M.; Kumar, V.; Kumar, D. Effect on the Properties of Edible Starch-Based Films by the Incorporation of Additives: A Review. Polymers 2022, 14, 1987. [Google Scholar] [CrossRef]
- Yao, S.; Wang, B.J.; Weng, Y.M. Preparation and characterization of mung bean starch edible films using citric acid as cross-linking agent. Food Packag. Shelf Life 2022, 32, 100845. [Google Scholar] [CrossRef]
- Sukudom, N.; Jariyasakoolroj, P.; Jarupan, L.; Tansin, K. Mechanical, thermal, and biodegradation behaviors of poly (vinyl alcohol) biocomposite with reinforcement of oil palm frond fiber. J. Mater. Cycles Waste Manag. 2019, 21, 125–133. [Google Scholar] [CrossRef]
- Jariyasakoolroj, P.; Rojanaton, N.; Jarupan, L. Crystallization behavior of plasticized poly (lactide) film by poly (l-lactic acid)-poly (ethylene glycol)-poly (l-lactic acid) triblock copolymer. Polym. Bull. 2020, 77, 2309–2323. [Google Scholar] [CrossRef]
- Klinmalai, P.; Srisa, A.; Laorenza, Y.; Katekhong, W.; Harnkarnsujarit, N. Antifungal and plasticization effects of carvacrol in biodegradable poly (lactic acid) and poly (butylene adipate terephthalate) blend films for bakery packaging. LWT 2021, 152, 112356. [Google Scholar] [CrossRef]
- Jariyasakoolroj, P.; Chirachanchai, S. In Situ Chemical Modification of Thermoplastic Starch with Poly (L-lactide) and Poly (butylene succinate) for an Effectively Miscible Ternary Blend. Polymers 2022, 14, 825. [Google Scholar] [CrossRef]
- Promsorn, J.; Harnkarnsujarit, N. Pyrogallol loaded thermoplastic cassava starch based films as bio-based oxygen scavengers. Ind. Crops Prod. 2022, 186, 115226. [Google Scholar] [CrossRef]
- Famá, L.; Flores, S.K.; Gerschenson, L.; Goyanes, S. Physical characterization of cassava starch biofilms with special reference to dynamic mechanical properties at low temperatures. Carbohydr. Polym. 2006, 66, 8–15. [Google Scholar] [CrossRef]
- Batpho, K.; Boonsupthip, W.; Rachtanapun, C. Antimicrobial activity of collagen casing impregnated with nisin against foodborne microorganisms associated with ready-to-eat sausage. Food Control. 2017, 73, 1342–1352. [Google Scholar] [CrossRef]
- Qian, M.; Liu, D.; Zhang, X.; Yin, Z.; Ismail, B.B.; Ye, X.; Guo, M. A review of active packaging in bakery products: Applications and future trends. Trends Food Sci. Technol. 2021, 114, 459–471. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Blown film extrusion of PBAT/TPS/ZnO nanocomposites for shelf-life extension of meat packaging. Colloids Surf. B Biointerfaces 2022, 214, 112472. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Hadidi, M.; Forough, M.; Nafchi, A.M.; Mousavi Khaneghah, A. The control of fungi and mycotoxins by food active packaging: A review. Crit. Rev. Food Sci. Nutr. 2022, 1–19. [Google Scholar] [CrossRef]
- Naqvi, S.S.; Anwer, H.; Siddiqui, A.; Zohra, R.R.; Ali, S.A.; Shah, M.R.; Hashim, S. Novel synthesis of maltol capped copper nanoparticles and their synergistic antibacterial activity with antibiotics. Plasmonics 2021, 16, 1915–1928. [Google Scholar] [CrossRef]
- Ziklo, N.; Bibi, M.; Salama, P. The antimicrobial mode of action of Maltol and its synergistic efficacy with selected cationic surfactants. Cosmetics 2021, 8, 86. [Google Scholar] [CrossRef]
- Morassi, L.L.; Bernardi, A.O.; Amaral, A.L.; Chaves, R.D.; Santos, J.L.; Copetti, M.V.; Sant’Ana, A.S. Fungi in cake production chain: Occurrence and evaluation of growth potential in different cake formulations during storage. Food Res. Int. 2018, 106, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Saud, R.; Pokhrel, S.; Yadav, P.N. Synthesis, characterization and antimicrobial activity of maltol functionalized chitosan derivatives. J. Macromol. Sci. 2019, 56, 375–383. [Google Scholar] [CrossRef]
- Burgess, J.; Fawcett, J.; Russell, D.R.; Hider, R.C.; Hossain, M.B.; Stoner, C.R.; Van der Helm, D. Two polymorphic forms of 3-hydroxy-2-methyl-4H-pyran-4-one (maltol). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1996, 52, 2917–2920. [Google Scholar] [CrossRef]
- Romero-Bastida, C.A.; Tapia-Blácido, D.R.; Méndez-Montealvo, G.; Bello-Pérez, L.A.; Velázquez, G.; Alvarez-Ramirez, J. Effect of amylose content and nanoclay incorporation order in physicochemical properties of starch/montmorillonite composites. Carbohydr. Polym. 2016, 152, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.H.; Kociok-Köhn, G.; Molloy, K.C. Copper and zinc complexes of kojic acid and related ligands. Transit. Met. Chem. 2015, 40, 459–470. [Google Scholar] [CrossRef]
- Behera, B.; Das, P.K. Blue-and red-shifting hydrogen bonding: A gas phase FTIR and Ab initio study of RR′ CO··DCCl3 and RR′ S··DCCl3 complexes. J. Phys. Chem. A 2018, 122, 4481–4489. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pan, H.; Jia, S.; Wang, Z.; Tian, H.; Han, L.; Zhang, H. In-situ reaction compatibilization modification of poly (butylene succinate-co-terephthalate)/polylactide acid blend films by multifunctional epoxy compound. Int. J. Biol. Macromol. 2022, 213, 934–943. [Google Scholar] [CrossRef]
- Forssell, P.M.; Mikkilä, J.M.; Moates, G.K.; Parker, R. Phase and glass transition behaviour of concentrated barley starch-glycerol-water mixtures, a model for thermoplastic starch. Carbohydr. Polym. 1997, 34, 275–282. [Google Scholar] [CrossRef]
- Wadaugsorn, K.; Panrong, T.; Wongphan, P.; Harnkarnsujarit, N. Plasticized hydroxypropyl cassava starch blended PBAT for improved clarity blown films: Morphology and properties. Ind. Crops Prod. 2022, 176, 114311. [Google Scholar] [CrossRef]
- Babaee, M.; Garavand, F.; Rehman, A.; Jafarazadeh, S.; Amini, E.; Cacciotti, I. Biodegradability, physical, mechanical and antimicrobial attributes of starch nanocomposites containing chitosan nanoparticles. Int. J. Biol. Macromol. 2022, 195, 49–58. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Harnkarnsujarit, N. Migration, aggregations and thermal degradation behaviors of TiO2 and ZnO incorporated PBAT/TPS nanocomposite blown films. Food Packag. Shelf Life 2022, 33, 100901. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, W.; Zhao, X.; Liu, Y.; Gao, W. Study on the morphology, crystalline structure and thermal properties of yellow ginger starch acetates with different degrees of substitution. Thermochim. Acta 2009, 495, 57–62. [Google Scholar] [CrossRef]
- Daniel, C.; Zhovner, D.; Guerra, G. Thermal stability of nanoporous crystalline and amorphous phases of poly (2, 6-dimethyl-1, 4-phenylene) oxide. Macromolecules 2013, 46, 449–454. [Google Scholar] [CrossRef]
- Kishi, M.; Nagatsuka, K.; Toda, T. Effect of membrane hydrophobicity and thickness on energy-efficient dissolved oxygen removal from algal culture. Front. Bioeng. Biotechnol. 2020, 8, 978. [Google Scholar] [CrossRef]
- Caicedo, C.; Díaz-Cruz, C.A.; Jiménez-Regalado, E.J.; Aguirre-Loredo, R.Y. Effect of Plasticizer Content on Mechanical and Water Vapor Permeability of Maize Starch/PVOH/Chitosan Composite Films. Materials 2022, 15, 1274. [Google Scholar] [CrossRef]
- Fongin, S.; Granados, A.E.A.; Harnkarnsujarit, N.; Hagura, Y.; Kawai, K. Effects of maltodextrin and pulp on the water sorption, glass transition, and caking properties of freeze-dried mango powder. J. Food Eng. 2019, 247, 95–103. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Promhuad, K.; Bumbudsanpharoke, N.; Wadaugsorn, K.; Sonchaeng, U.; Harnkarnsujarit, N. Maltol-Incorporated Acetylated Cassava Starch Films for Shelf-Life-Extension Packaging of Bakery Products. Polymers 2022, 14, 5342. https://doi.org/10.3390/polym14245342
Promhuad K, Bumbudsanpharoke N, Wadaugsorn K, Sonchaeng U, Harnkarnsujarit N. Maltol-Incorporated Acetylated Cassava Starch Films for Shelf-Life-Extension Packaging of Bakery Products. Polymers. 2022; 14(24):5342. https://doi.org/10.3390/polym14245342
Chicago/Turabian StylePromhuad, Khwanchat, Nattinee Bumbudsanpharoke, Kiattichai Wadaugsorn, Uruchaya Sonchaeng, and Nathdanai Harnkarnsujarit. 2022. "Maltol-Incorporated Acetylated Cassava Starch Films for Shelf-Life-Extension Packaging of Bakery Products" Polymers 14, no. 24: 5342. https://doi.org/10.3390/polym14245342
APA StylePromhuad, K., Bumbudsanpharoke, N., Wadaugsorn, K., Sonchaeng, U., & Harnkarnsujarit, N. (2022). Maltol-Incorporated Acetylated Cassava Starch Films for Shelf-Life-Extension Packaging of Bakery Products. Polymers, 14(24), 5342. https://doi.org/10.3390/polym14245342