Influence of Stabilization Additive on Rheological, Thermal and Mechanical Properties of Recycled Polypropylene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Methods
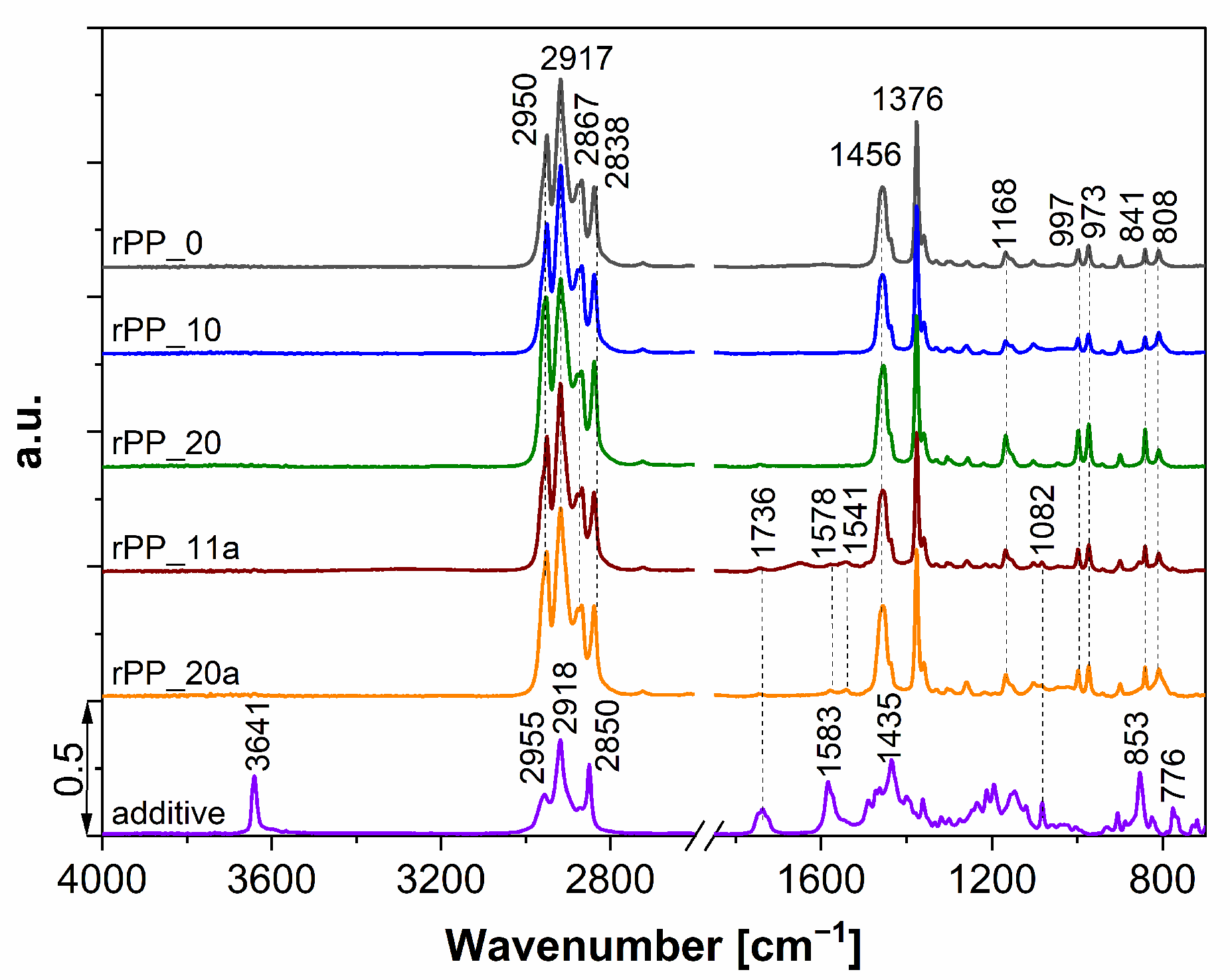
2.2.1. ATR-IR Characterization
2.2.2. Polarized Optical Microscopy
2.2.3. Thermal Properties
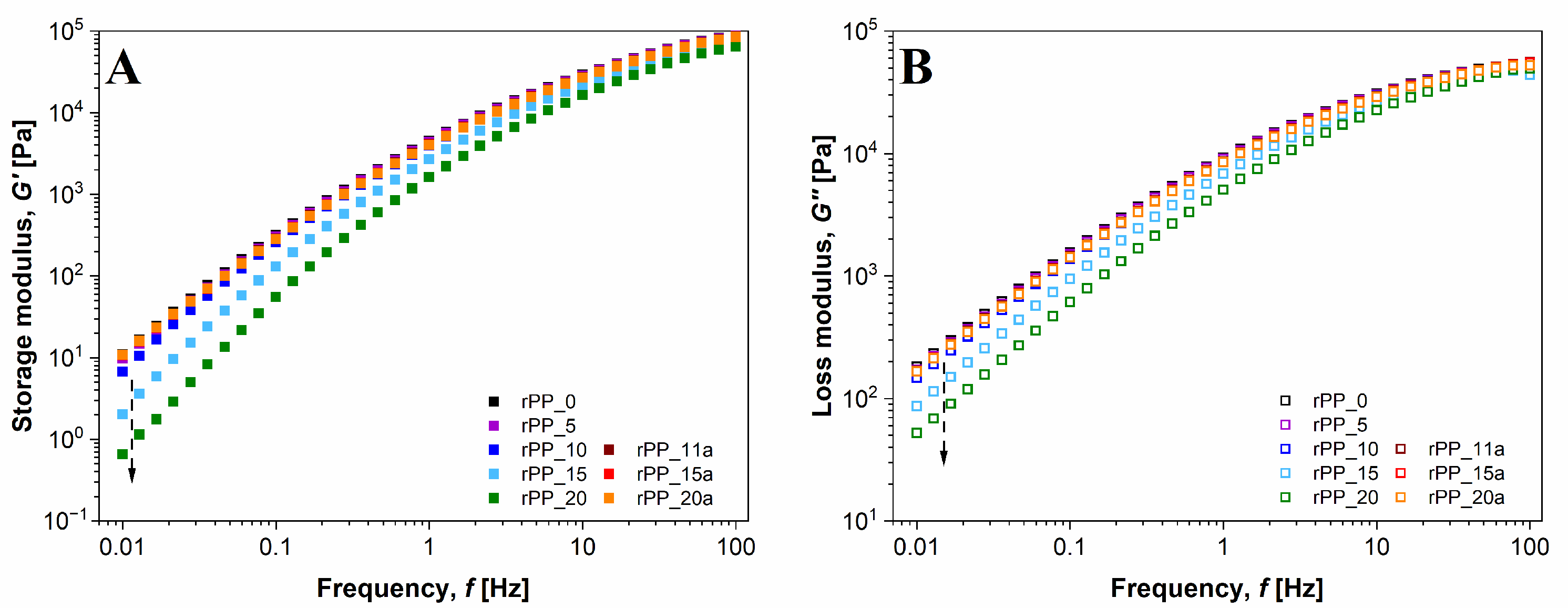
2.2.4. Rheological Properties
2.2.5. Mechanical Characterization
Nanoindentation
Time-Dependent Measurements
Dynamic Mechanical Thermal Analysis (DMTA)
3. Results and Discussion
3.1. ATR-IR Characterization
3.2. Polarized Optical Microscopy
3.3. Thermal Properties
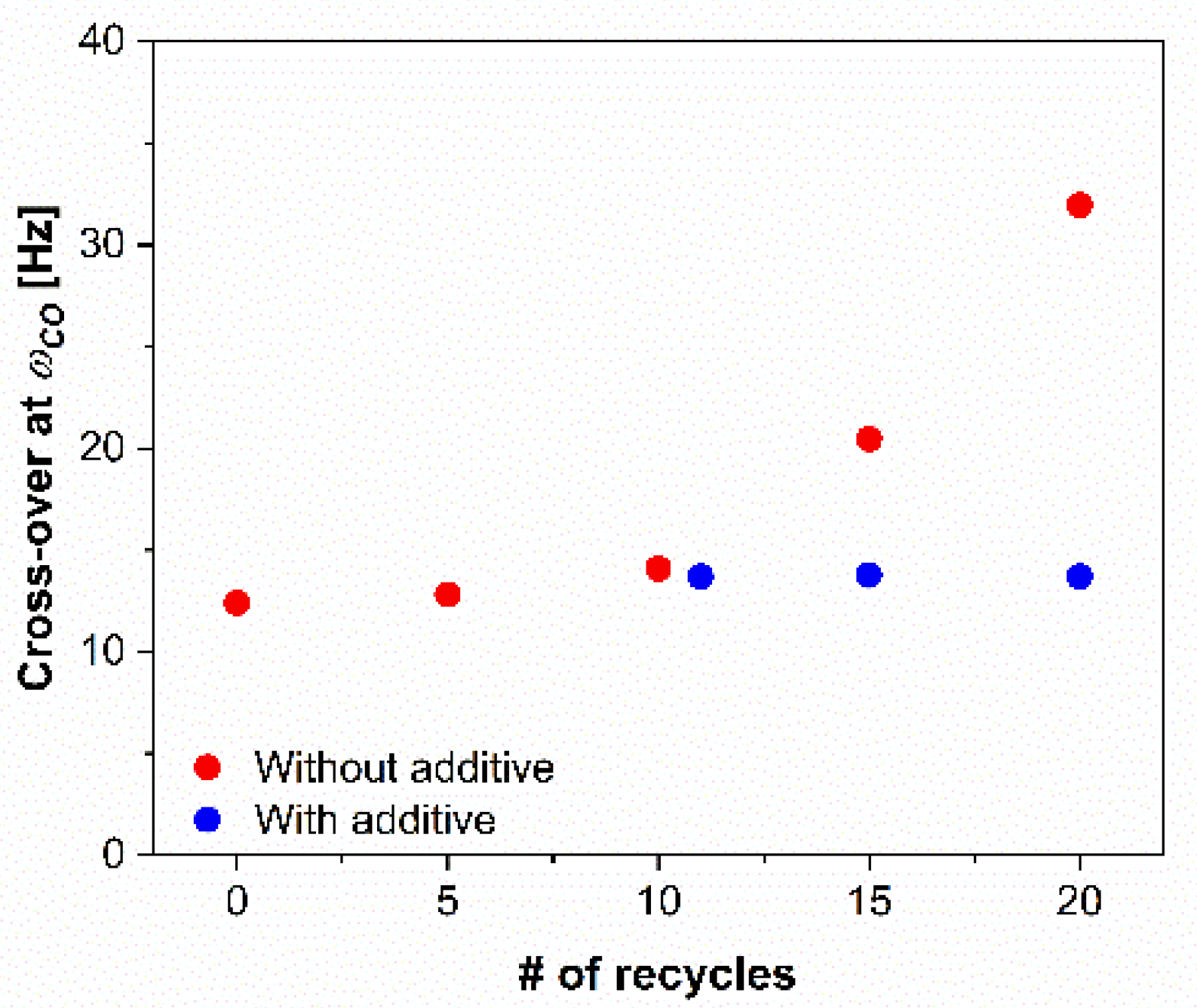
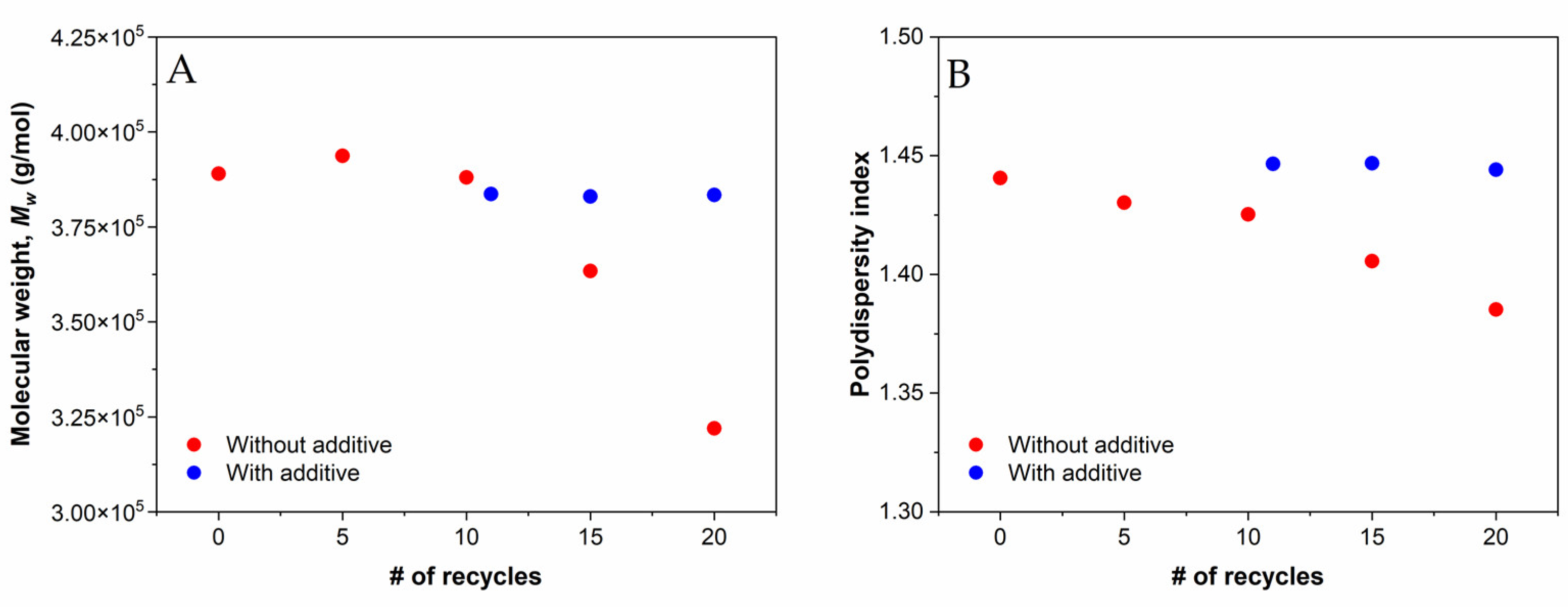
3.4. Rheological Properties
3.5. Mechanical Properties
3.5.1. Nanoindentation
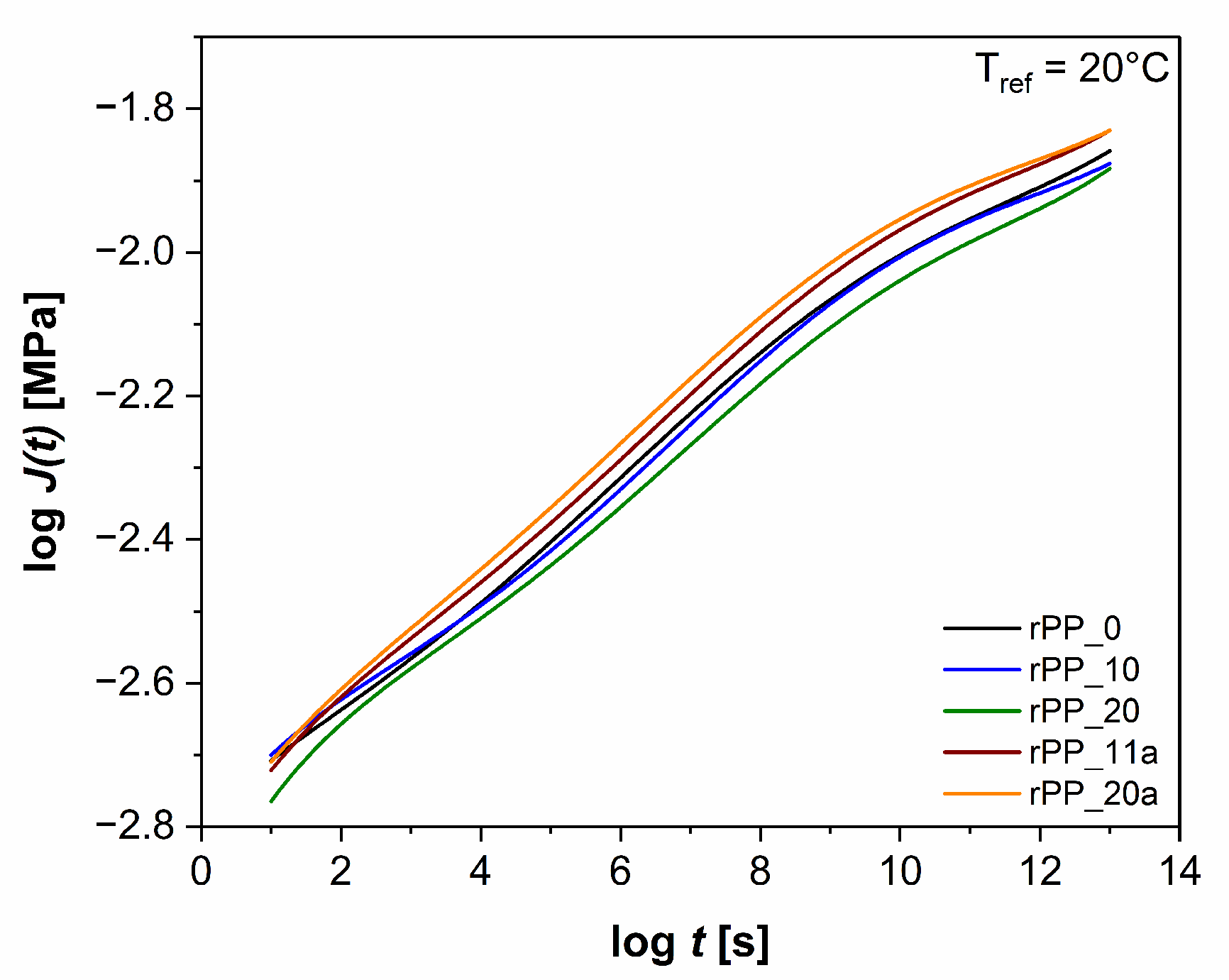
3.5.2. Time-Dependent Behavior
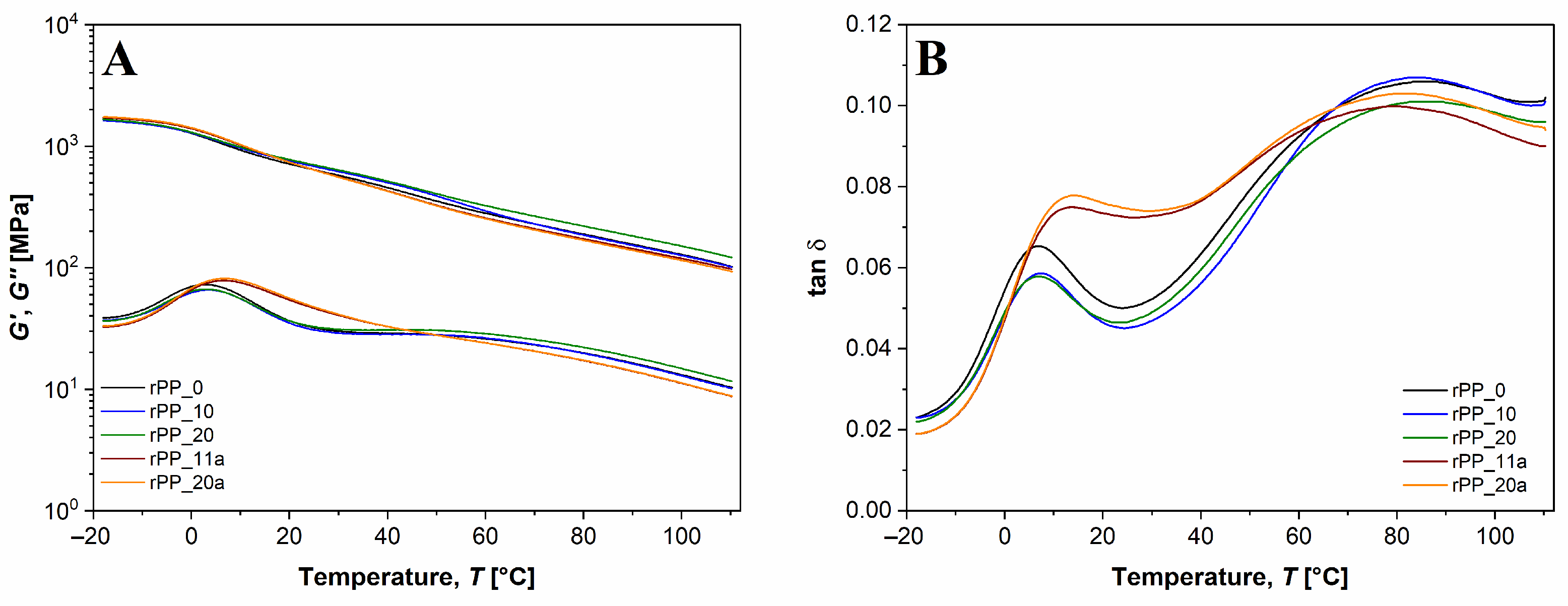
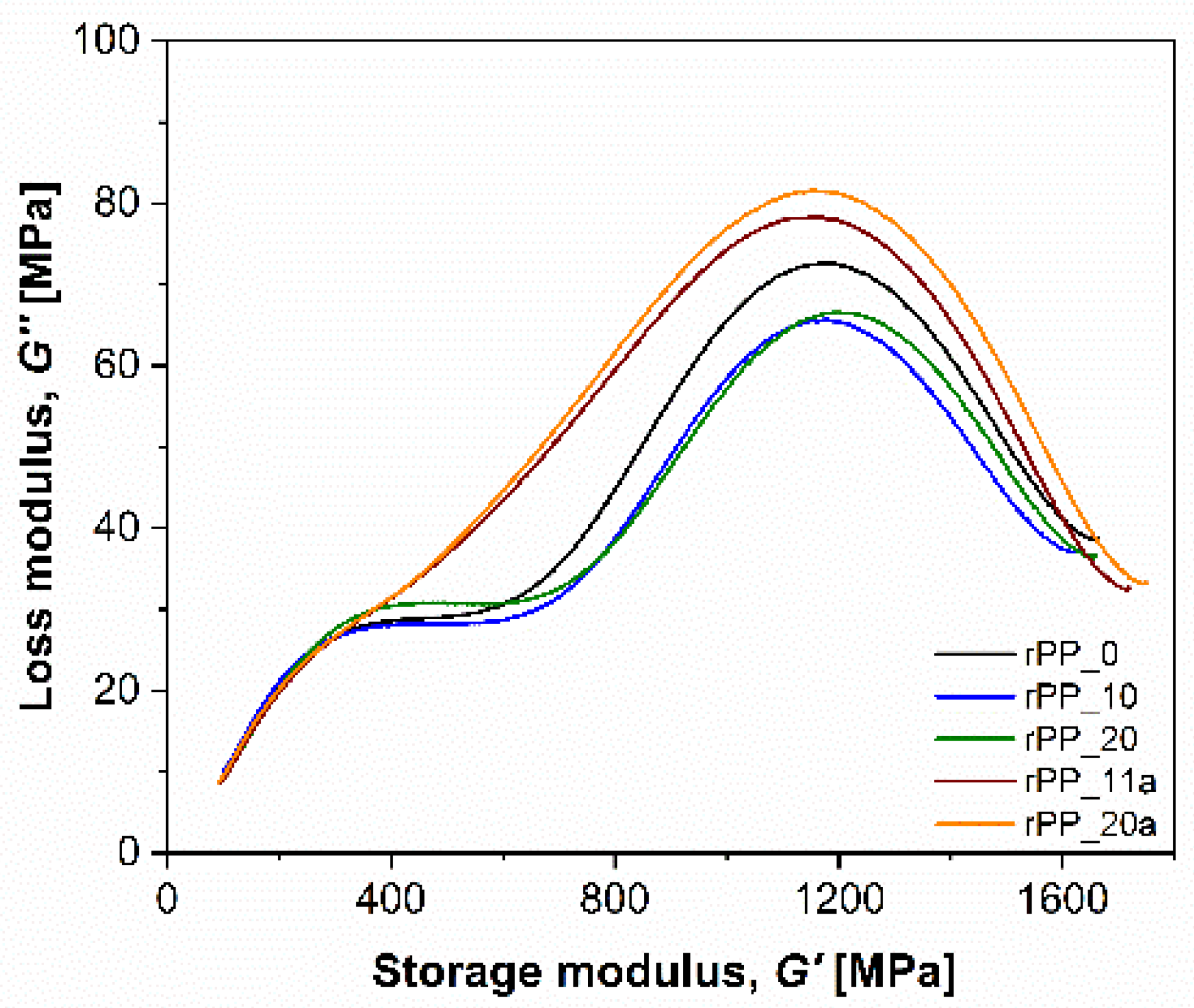
3.5.3. Dynamic Mechanical Thermal Analysis (DMTA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roland, G.; Jambeck, J.R.; Lavender, L.K. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Ajorloo, M.; Ghodrat, M.; Kang, W.-H. Incorporation of Recycled Polypropylene and Fly Ash in Polypropylene-Based Composites for Automotive Applications. J. Polym. Environ. 2021, 29, 1298–1309. [Google Scholar] [CrossRef]
- Khalaj, M.-J.; Ahmadi, H.; Lesankhosh, R.; Khalaj, G. Study of physical and mechanical properties of polypropylene nanocomposites for food packaging application: Nano-clay modified with iron nanoparticles. Trends Food Sci. Technol. 2016, 51, 41–48. [Google Scholar] [CrossRef]
- Aizenshtein, E. Polypropylene fibres and yarns in the current state of development. Fibre Chem. 2008, 40, 399–405. [Google Scholar] [CrossRef]
- Karian, H. Handbook of Polypropylene and Polypropylene Composites; Marcel Dekker: New York, NY, USA, 2003; ISBN 0824740645/9780824740641. [Google Scholar]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Peterson, J.D.; Vyazovkin, S.; Wight, C.A. Kinetics of the Thermal and Thermo-Oxidative Degradation of Polystyrene, Polyethylene and Poly(propylene). Macromol. Chem. Phys. 2001, 202, 775–784. [Google Scholar] [CrossRef]
- Esmizadeh, E.; Tzoganakis, C.; Mekonnen, T.H. Degradation Behavior of Polypropylene during Reprocessing and Its Biocomposites: Thermal and Oxidative Degradation Kinetics. Polymers 2020, 12, 1627. [Google Scholar] [CrossRef]
- Martinez Jothar, L.; Montes-Zavala, I.; Rivera-García, N.; Díaz-Ceja, Y.; Pérez, E.; Waldo-Mendoza, M. Thermal degradation of polypropylene reprocessed in a co-rotating twin-screw extruder: Kinetic model and relationship between Melt Flow Index and Molecular weight. Rev. Mex. Ing. Quím. 2021, 20, 1079–1091. [Google Scholar] [CrossRef]
- Da Costa, H.M.; Ramos, V.D.; de Oliveira, M.G. Degradation of polypropylene (PP) during multiple extrusions: Thermal analysis, mechanical properties and analysis of variance. Polym. Test. 2007, 26, 676–684. [Google Scholar] [CrossRef]
- Spicker, C.; Rudolph, N.; Kühnert, I.; Aumnate, C. The use of rheological behavior to monitor the processing and service life properties of recycled polypropylene. Food Packag. Shelf Life 2019, 19, 174–183. [Google Scholar] [CrossRef]
- Stocchi, A.; Pettarin, V.; Izer, A.; Bárány, T.; Czigány, T.; Bernal, C. Fracture Behavior of Recyclable All-Polypropylene Composites Composed of α- and β-Modifications. J. Thermoplast. Compos. Mater. 2011, 24, 805–818. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Franco, J.M. Influence of polymer reprocessing cycles on the microstructure and rheological behavior of polypropylene/mineral oil oleogels. Polym. Test. 2015, 45, 12–19. [Google Scholar] [CrossRef]
- González-González, V.A.; Neira-Velázquez, G.; Angulo-Sánchez, J.L. Polypropylene chain scissions and molecular weight changes in multiple extrusion. Polym. Degrad. Stab. 1998, 60, 33–42. [Google Scholar] [CrossRef]
- Altay, L.; Sarikanat, M.; Sağlam, M.; Uysalman, T.; Seki, Y. The effect of various mineral fillers on thermal, mechanical, and rheological properties of polypropylene. Res. Eng. Struct. Mater. 2021, 7, 361–373. [Google Scholar] [CrossRef]
- Caicedo, C.; Vázquez-Arce, A.; Ossa, O.H.; De La Cruz, H.; Maciel-Cerda, A. Physicomechanical behavior of composites of polypropylene, and mineral fillers with different process cycles. DYNA 2018, 85, 260–268. [Google Scholar] [CrossRef]
- Techawinyutham, L.; Sumrith, N.; Srisuk, R.; Techawinyutham, W.; Siengchin, S.; Mavinkere Rangappa, S. Thermo-mechanical, rheological and morphology properties of polypropylene composites: Residual CaCO3 as a sustainable by-product. Polym. Compos. 2021, 42, 4643–4659. [Google Scholar] [CrossRef]
- Saw, L.T.; Uy Lan, D.N.; Rahim, N.A.A.; Mohd Kahar, A.W.; Viet, C.X. Processing degradation of polypropylene-ethylene copolymer-kaolin composites by a twin-screw extruder. Polym. Degrad. Stab. 2015, 111, 32–37. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef]
- Pfaendner, R.; Herbst, H.; Hoffmann, K. Innovative concept for the upgrading of recyclates by restabilization and repair molecules. Macromol. Symp. 1998, 135, 97–111. [Google Scholar] [CrossRef]
- Ton-That, M.; Denault, J. Development of Composites Based on Natural Fibers; The Institute of Textile Science: Ottawa, ON, Canada, 2007. [Google Scholar]
- Barbeș, L.; Radulescu, C.; Stihi, C. ATR–FTIR spectrometry characterisation of polymeric materials. Rom. Rep. Phys. 2014, 66, 765–777. [Google Scholar]
- Karger-Kocsis, J. Polypropylene Structure, Blends and Composites: Volume 3 Composites; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 9401105235. [Google Scholar]
- Li, X.; Bhushan, B. A review of nanoindentation continuous stiffness measurement technique and its applications. Mater. Charact. 2002, 48, 11–36. [Google Scholar] [CrossRef]
- Oseli, A.; Prodan, T.; Susič, E.; Slemenik Perše, L. The effect of short fiber orientation on long term shear behavior of 40% glass fiber reinforced polyphenylene sulfide. Polym. Test. 2020, 81, 106262. [Google Scholar] [CrossRef]
- Lacoste, J.; Vaillant, D.; Carlsson, D.J. Gamma-, photo-, and thermally-initiated oxidation of isotactic polypropylene. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 715–722. [Google Scholar] [CrossRef]
- Strömberg, E.; Karlsson, S. The Design of a Test Protocol to Model the Degradation of Polyolefins During Recycling and Service Life. J. Appl. Polym. Sci. 2009, 112, 1835–1844. [Google Scholar] [CrossRef]
- Andreassen, E. Infrared and Raman spectroscopy of polypropylene. In Polypropylene; Karger-Kocsis, J., Ed.; Springer: Dordrecht, The Netherlands, 1999; pp. 320–328. ISBN 978-94-011-4421-6. [Google Scholar]
- Zieba-Palus, J. The usefulness of infrared spectroscopy in examinations of adhesive tapes for forensic purposes. Forensic Sci. Criminol. 2017, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zdiri, K.; Elamri, A.; Hamdaoui, M.; Harzallah, O.; Khenoussi, N.; Brendlé, J. Elaboration and Characterization of Recycled PP/Clay Nanocomposites. J. Mater. Environ. Sci. 2018, 9, 2370–2378. [Google Scholar]
- Hamad, K.; Kaseem, M.; Deri, F. Recycling of waste from polymer materials: An overview of the recent works. Polym. Degrad. Stab. 2013, 98, 2801–2812. [Google Scholar] [CrossRef]
- Da Costa, H.M.; Ramos, V.D.; Rocha, M.C.G. Rheological properties of polypropylene during multiple extrusion. Polym. Test. 2005, 24, 86–93. [Google Scholar] [CrossRef]
- Schmiederer, D.; Gardocki, A.; Kühnert, I.; Schmachtenberg, E. Local thermo-oxidative degradation in injection molding. Polym. Eng. Sci. 2008, 48, 717–722. [Google Scholar] [CrossRef]
- Khak, M.; Ramazani, A.S.A. Rheological measurement of molecular weight distribution of polymers. e-Polymers 2013, 13, 1. [Google Scholar] [CrossRef]
- Bayazian, H.; Schoeppner, V. Investigation of molecular weight distributions during extrusion process of polypropylene by rheometry experiment. AIP Conf. Proc. 2019, 2065, 30044. [Google Scholar] [CrossRef]
- Jubinville, D.; Esmizadeh, E.; Tzoganakis, C.; Mekonnen, T. Thermo-mechanical recycling of polypropylene for the facile and scalable fabrication of highly loaded wood plastic composites. Compos. Part B Eng. 2021, 219, 108873. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Flores, A.; Ania, F.; Baltá-Calleja, F.J. From the glassy state to ordered polymer structures: A microhardness study. Polymer 2009, 50, 729–746. [Google Scholar] [CrossRef] [Green Version]
- Zdiri, K.; Elamri, A.; Hamdaoui, M.; Harzallah, O.; Khenoussi, N.; Brendlé, J. Reinforcement of recycled PP polymers by nanoparticles incorporation. Green Chem. Lett. Rev. 2018, 11, 296–311. [Google Scholar] [CrossRef] [Green Version]
- Bourmaud, A.; Le Duigou, A.; Baley, C. What is the technical and environmental interest in reusing a recycled polypropylene–hemp fibre composite? Polym. Degrad. Stab. 2011, 96, 1732–1739. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Properties of Polymers; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Saba, N.; Jawaid, M.; Alothman, O.Y.; Paridah, M.T. A review on dynamic mechanical properties of natural fibre reinforced polymer composites. Constr. Build. Mater. 2016, 106, 149–159. [Google Scholar] [CrossRef]
- Mazidi, M.M.; Razavi Aghjeh, M.K.; Khonakdar, H.A.; Reuter, U. Structure–property relationships in super-toughened polypropylene-based ternary blends of core–shell morphology. RSC Adv. 2016, 6, 1508–1526. [Google Scholar] [CrossRef]
- Hidalgo-Salazar, M.; Luna Vera, F.; Correa, J. Biocomposites from Colombian sugarcane bagasse with polypropylene: Mechanical, thermal and viscoelastic properties. In Characterizations of Some Composite Materials; Books on Demand: Dorderstedt, Germany, 2019; ISBN 978-1-78984-912-7. [Google Scholar]
- Hoyos, M.; Tiemblo, P.; Gómez-Elvira, J.M. The role of microstructure, molar mass and morphology on local relaxations in isotactic polypropylene. The α relaxation. Polymer 2007, 48, 183–194. [Google Scholar] [CrossRef]
- Gitsas, A.; Floudas, G. Pressure Dependence of the Glass Transition in Atactic and Isotactic Polypropylene. Macromolecules 2008, 41, 9423–9429. [Google Scholar] [CrossRef]
- Gaska, K.; Manika, G.C.; Gkourmpis, T.; Tranchida, D.; Gitsas, A.; Kádár, R. Mechanical Behavior of Melt-Mixed 3D Hierarchical Graphene/Polypropylene Nanocomposites. Polymers 2020, 12, 1309. [Google Scholar] [CrossRef] [PubMed]





| Sample | Abbreviation |
|---|---|
| Virgin PP | rPP_0 |
| 5× reprocessed PP | rPP_5 |
| 10× reprocessed PP | rPP_10 |
| 15× reprocessed PP | rPP_15 |
| 20× reprocessed PP | rPP_20 |
| 11× reprocessed PP with additive | rPP_11a |
| 15× reprocessed PP with additive | rPP_15a |
| 20× reprocessed PP with additive | rPP_20a |
| Sample | Abbreviation |
|---|---|
| Melt temperature (Tm) | 190 °C |
| Waiting time | 3 min |
| Mold temperature | 50 °C |
| Holding pressure | 500 bar |
| Injection time | 10 s |
| Post-pressure | 100 bar |
| Post-processing time | 10 s |
| Melt temperature (Tm) | 190 °C |
| 1st Heating | Cooling | 2nd Heating | |||||
|---|---|---|---|---|---|---|---|
| # of Recycles | Hm (J/g) | Tm (°C) | Hc (J/g) | Tc (°C) | Hm (J/g) | Tm (°C) | Xc (%) |
| rPP_0 | 99.0 | 166.0 | 100.9 | 112.9 | 105.7 | 162.0 | 51.1 |
| rPP_5 | 99.5 | 166.1 | 101.8 | 120.2 | 106.4 | 163.3 | 51.4 |
| rPP_10 | 99.7 | 165.7 | 103.1 | 120.4 | 108.2 | 163.5 | 52.3 |
| rPP_15 | 98.8 | 166.1 | 103.6 | 121.2 | 108.5 | 163.0 | 52.4 |
| rPP_20 | 94.0 | 165.5 | 102.3 | 121.5 | 106.7 | 162.5 | 51.3 |
| rPP_11a | 91.0 | 165.1 | 95.5 | 120.1 | 99.6 | 162.9 | 48.1 |
| rPP_15a | 91.2 | 165.2 | 96.8 | 120.6 | 101.1 | 163.3 | 48.8 |
| rPP_20a | 91.3 | 165.3 | 97.1 | 120.9 | 101.7 | 163.3 | 49.1 |
| Sample | Tg (°C) 1 | Tg (°C) 2 | α Relaxation Peak (°C) |
|---|---|---|---|
| rPP_0 | 6.3 | 2.5 | 86.7 |
| rPP_10 | 7.5 | 3.2 | 84.5 |
| rPP_20 | 6.7 | 3.0 | 86.7 |
| rPP_11a | 13.5 | 6.2 | 81.0 |
| rPP_20a | 13.5 | 6.7 | 82.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihelčič, M.; Oseli, A.; Huskić, M.; Slemenik Perše, L. Influence of Stabilization Additive on Rheological, Thermal and Mechanical Properties of Recycled Polypropylene. Polymers 2022, 14, 5438. https://doi.org/10.3390/polym14245438
Mihelčič M, Oseli A, Huskić M, Slemenik Perše L. Influence of Stabilization Additive on Rheological, Thermal and Mechanical Properties of Recycled Polypropylene. Polymers. 2022; 14(24):5438. https://doi.org/10.3390/polym14245438
Chicago/Turabian StyleMihelčič, Mohor, Alen Oseli, Miroslav Huskić, and Lidija Slemenik Perše. 2022. "Influence of Stabilization Additive on Rheological, Thermal and Mechanical Properties of Recycled Polypropylene" Polymers 14, no. 24: 5438. https://doi.org/10.3390/polym14245438
APA StyleMihelčič, M., Oseli, A., Huskić, M., & Slemenik Perše, L. (2022). Influence of Stabilization Additive on Rheological, Thermal and Mechanical Properties of Recycled Polypropylene. Polymers, 14(24), 5438. https://doi.org/10.3390/polym14245438







