Bio-Inspired Muco-Adhesive Polymers for Drug Delivery Applications
Abstract
:1. Introduction
2. Muco-Adhesion Theories
2.1. Wetting Theory
2.2. Mechanical Inter-Locking Theory
2.3. Adsorption Theory
2.4. Electronic Transfer Theory
2.5. Fracture Theory
2.6. Diffusion Theory
3. Mechanisms of Muco-Adhesion
4. Development of Muco-Adhesives and Muco-Adhesive Drug Delivery Systems
4.1. Evaluating Muco-Adhesion
4.2. Delivery Sites and Routes for Muco-Adhesive Bio-Polymer Drug Delivery Systems
4.2.1. Buccal/Oral Cavity (Intra-Oral)
4.2.2. Ocular Cavity (Eye Conjunctiva)
4.2.3. Reproductive Lumen (Vaginal and Rectal)
4.2.4. Nasal Cavity
4.2.5. The GI Tract
5. Muco-Adhesive Bio-Polymers: From Basics to Applications
5.1. Characteristics of Bio-Polymers
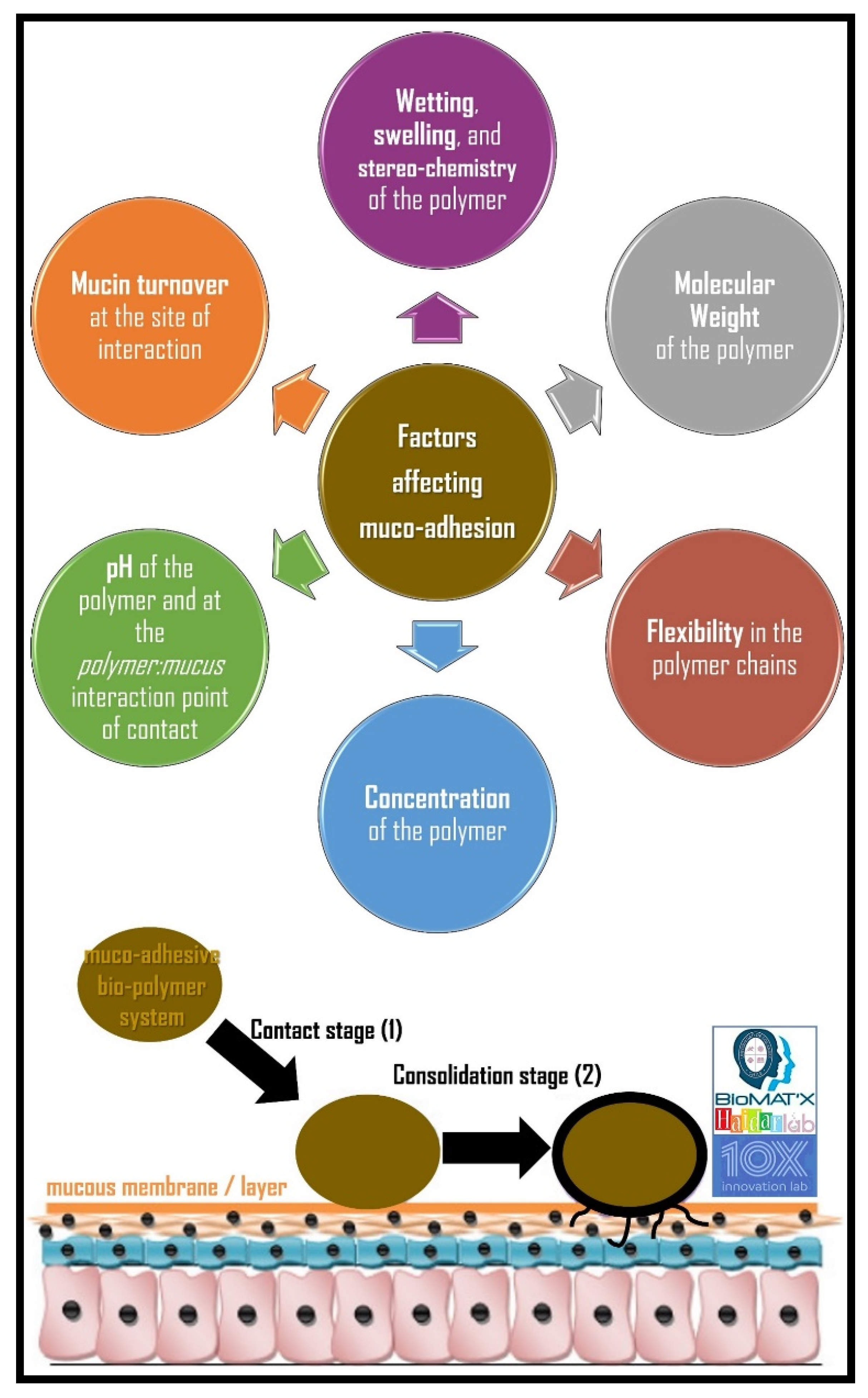
5.2. Factors Affecting Muco-Adhesion
5.2.1. Hydrophilicity
5.2.2. Molecular Weight
5.2.3. Cross-Linking and Swelling Factor
5.2.4. pH at the Muco-Adhesive Bio-Polymer-Substrate Interface
5.2.5. Concentration of the Active Bio-Polymer
5.2.6. Drug/Excipient Concentration
5.2.7. Mucin Turnover Rate
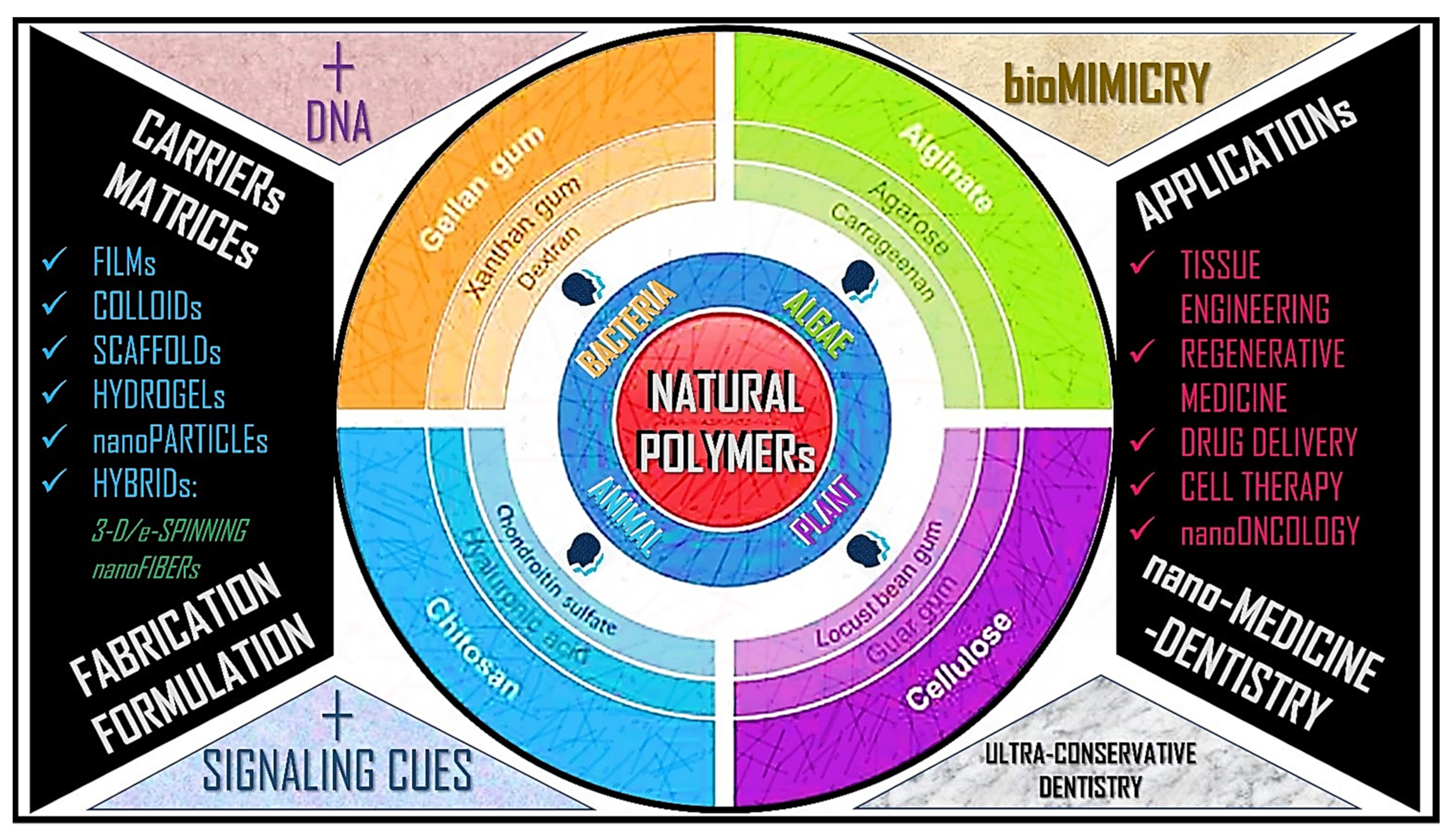
6. Bio-Inspired Polymers and Application in Drug Delivery
6.1. Chitosan
6.2. Mussel Adhesive Protein (MAP)
6.3. Alginate-PEGAc
6.4. Pectin-Sodium Carboxymethyl Cellulose System
6.5. Carbopol 934P
6.6. Spider Silk
6.7. Spider Silk 4RepCT Variants
6.8. Aggregate Silk Glue
6.9. Pyriform Silk
6.10. Silkworm
6.11. Sericin
6.12. Caddisfly Silk
6.13. TAPE
6.14. Edible Bird’s Nest
7. Current Developments
7.1. Chitin- and Chitosan-Based Systems
7.2. Alginates and Alginate-Based Systems
| Polymer | Source | Chemical Composition | Bio-Inspiration | Elastic Modulus | References |
|---|---|---|---|---|---|
| Aggregate Silk Glue | Natural | 64-mer (Gly-rich): Met-Gly-Tyr-Lys-Lys-Thr-Val-Gly-Lys-Asp-Gly-Gln-Ile-Val-Tyr-Thr-Met-Thr-Glu-Thr-Tyr-Gly-Gly-Ser-Gly-Gly-Asn-Gly-Gly-Asn-Gly-Gly-Asn-Gly-Gly-Pro-Gly-Gly-Asn-Gly-Gly-Asn-Gly-Gly-Pro-Ser-His-Gln-Thr-Pro-Gly-Gly-Gly-Ala-Pro-Gly-Met-Ser-Ser-Ser- Glu-Leu-Thr-Ala 36-mer (X1-Pro-Gly-X2-Gly, where X1 is Gln, Glu, or Arg and X2 is Ser or Asn): Gln-Pro-Gly-Asn-Gly-Gln-Pro-Gly-Ser-Gly-Gln-Pro-Gly-Ser-Gly-Glu-Pro-Gly-Ser-Gly-Gln-Pro-Gly-Ser-Gly-Gln-Pro-Gly-Tyr-Tyr-Arg-Pro-Gly-Gly-Lys-Gly 33-mer (Gly-Gly-X1/Asn-X2-Asn-X2-Asn, where X1 is Ala, Gly, Leu, or Ser and X2 is Val, Asp, Leu, Phe, or Met): Gly-Gly-Gln-Ser-Gly-Gly-Gly-Gly-Asn-Tyr-Asn-Val-Asn-Leu-Asn-Gly-Gly-Gly-His-Gly-Gly-His-Pro-Gly-Gly-Ser-Leu-Asn-Val-Asn-Ala-Asn-Gly | Araneoid orb-weaving spider silk glues | 0.1–0.4 mN | Opell et al.; Brooks et al.; Petrou et al.; Elices et al.; Elices et al.; Sahni et al.; Vasanthavada et al. [45,64,97,98,99,100] |
| Alginate | Natural | C12H20O12P2 | Alginate-thiol | ~6500 mN | Davidovich-Pinhas et al.; Zia et al.; PubChem [55,56,101] |
| Caddisfly Silk | Natural | O-phospho-ser cluster (Ser-X)n; X = Val, Leu, Ile, or Arg; n = 2–6 | Aquatic caddisworm | 32.7 ± 6.6 MPa (stress at fracture) | Lane et al.; Brooks; Wang et al.; Stewart et al. [45,80,81] |
| Carbopol 934-P | Synthetic | (C3H4O2)n | Carbomer | 0.3–13 Pa | Singla et al.; Bera et al.; Tamburic et al.; Takeuchi et al.; Blanco-Fuente et al.; NIH [58,60,61,62,102] |
| Chitosan + Derivatives | Natural | (C6H11NO4)n | Shellfish, insects, fungi | 32.4 ± 14.5 mN; 39 to 67 Pa | Felt et al., Dash et al.; Brooks; Lehr; Kim et al.; Sogias et al.; He et al.; Cho et al.; Zvarec et al.; Ways et al.; Shitrit et al.; Snyman et al.; Elgadir et al.; Kumar et al. [42,44,45,51,52,84,103,104,105,106,107] |
| Mussel Adhesive Protein (MAP) | Synthetic | [Ala-Lys-Pro-Ser-Tyr-Hyp-Hyp-Thr-Dopa-Lys]80 | DOPA, Mussel adhesive proteins from blue mussel (Mytilus edulis) | Uncertain | Ryu et al.; Schnurrer et al.; Lee et al.; Deacon et al.; Lim et al. [49,52,53,54,108] |
| Pectin-Sodium Carboxymethyl cellulose System | Natural | 1:1:2 ratio of carbopol, pectin, and sodium carboxymethylcellulose | Pectin and sodium carboxymethyl-cellulose | 23.2 ± 6.2 mN | Gupta et al. [57] |
| Pepsin-trypsin hydrolysates | Natural | Pentapeptides Pro-Phe-His-Pro-Tyr and Leu-Leu-Gly-Asp-Pro in f134–138 and f164–168 of cytochrome b, respectively | Swiftlet species Aerodramus fuciphagus of edible bird’s nest | 155 MPa | Ghassem et al.; Jessel et al.; Valles-Ayoub et al. [85,86,87] |
| Pyriform Silk | Natural/Synthetic | Two repetitive motifs: Gln-Gln-Ser-Ser-Van-Ala and Pro-X-Pro-X-Pro, where X is a variable amino acid residue | N. clavipes pyriform silk | 39.8 ± 8.9 mN | (Natural) Brooks; Wolff et al.; Blasingame et al.; Geurts et al. (Synthetic) Opell et al.; Brooks; Petrou et al.; Elices et al.; Peng et al. [45,64,65,66,67,68,97,109] |
| Sericin | Natural | [Ser-Ser-Thr-Gly-Ser-Ser-Ser-Asn-Thr-Asp-Ser-Asn-Ser-Asn-Ser-Val-Gly-Ser-Ser-Thr-Ser-Gly-Gly-Ser-Ser-Thr-Tyr-Gly-Tyr-Ser-Ser-Asn-Ser-Arg-Asp-Gly-Ser-Val]n | Silkworm-derived adhesive | 4.1 ± 2 N | Jiang et al.; Brooks; Freitas et al. [45,74,110] |
| Silkworm fibroin | Natural | Gly-Ala-Gly-Ala-Ser, Gly-Xn; X = Ala, Tyr, or Val | Silkworm-derived adhesive | 54 mN or 1466 Pa | Brooks; Jiang et al.; Yucel et al.; Serban et al.; Kundu et al.; Wei et al. [45,69,71,78,110,111] |
| TAPE | Natural | 1 g mL−1 in distilled water of tannic acid (C76H52O46) blended with 1 g mL−1 in distilled water of PEG (C2nH4n+2On+1) | Tannic acid in plants | Up to 1 kPa | Kim et al.; Shin et al. [83,84] |
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Brako, F.; Thorogate, R.; Mahalingam, S.; Raimi-Abraham, B.; Craig, D.Q.M.; Edirisinghe, M. Mucoadhesion of Progesterone-Loaded Drug Delivery Nanofiber Constructs. ACS Appl. Mater. Interfaces 2018, 10, 13381–13389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, R.F.; Shaikh, R.; Singh, R.R.T.; Garland, M.J.; Woolfson, A.D. Mucoadhesive drug delivery systems. J. Pharm. Bioallied Sci. 2011, 3, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Control. Release 2021, 341, 132–146. [Google Scholar] [CrossRef]
- Salamat-Miller, N.; Chittchang, M.; Johnston, T.P. The use of mucoadhesive polymers in buccal drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1666–1691. [Google Scholar] [CrossRef] [PubMed]
- Madhav, N.S.; Shakya, A.K.; Shakya, P.; Singh, K. Orotransmucosal drug delivery systems: A review. J. Control. Release 2009, 140, 2–11. [Google Scholar] [CrossRef]
- Xu, J.; Tam, M.; Samaei, S.; Lerouge, S.; Barralet, J.; Stevenson, M.M.; Cerruti, M. Mucoadhesive chitosan hydrogels as rectal drug delivery vessels to treat ulcerative colitis. Acta Biomater. 2017, 48, 247–257. [Google Scholar] [CrossRef]
- Pinnaratip, R.; Bhuiyan, M.S.A.; Meyers, K.; Rajachar, R.M.; Lee, B.P. Multifunctional Biomedical Adhesives. Adv. Healthc. Mater. 2019, 8, e1801568. [Google Scholar] [CrossRef]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef]
- Ahuja, A.; Khar, R.K.; Ali, J. Mucoadhesive Drug Delivery Systems. Drug Dev. Ind. Pharm. 1997, 23, 489–515. [Google Scholar] [CrossRef]
- Subramanian, P. Mucoadhesive Delivery System: A Smart Way to Improve Bioavailability of Nutraceuticals. Foods 2021, 10, 1362. [Google Scholar] [CrossRef]
- Mizrahi, B. Mucoadhesive Polymers for Delivery of Drugs to the Oral Cavity. Recent Patents Drug Deliv. Formul. 2008, 2, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Mahdi, S.; Kaur, J.; Iqbal, Z.; Talegaonkar, S.; Ahmad, F.J. Advances and potential applications of chitosan derivatives as mucoadhesive biomaterials in modern drug delivery. J. Pharm. Pharmacol. 2006, 58, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Semalty, M.; Semalty, A.; Kumar, G. Formulation and characterization of mucoadhesive buccal films of glipizide. Indian J. Pharm. Sci. 2008, 70, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Boddupalli, B.M.; Mohammed, Z.N.K.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381. [Google Scholar] [CrossRef] [Green Version]
- Krishnakumar, P. Wetting and Spreading Phenomena. 2010. Available online: http://guava.physics.uiuc.edu/~nigel/courses/563/Essays_2010/PDF/Krishnakumar.pdf (accessed on 4 April 2019).
- Edsman, K.; Hägerström, H. Pharmaceutical applications of mucoadhesion for the non-oral routes. J. Pharm. Pharmacol. 2005, 57, 3–22. [Google Scholar] [CrossRef]
- Young, T., III. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Shafrin, E.G.; Zisman, W.A. Constitutive relations in the wetting of low energy surfaces and the theory of the retraction method of preparing monolayers. J. Phys. Chem. 1960, 64, 519–524. [Google Scholar] [CrossRef]
- Packham, D.E. Theories of Fundamental Adhesion. In Handbook of Adhesion Technology; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–31. ISBN 9781315120942. [Google Scholar] [CrossRef]
- Kinloch, A.J. Adhesion and Adhesives: Science and Technology—Anthony J. Kinloch—Google Books; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Jiménez-Castellanos, M.R.; Zia, H.; Rhodes, C.T. Mucoadhesive Drug Delivery Systems. Drug Dev. Ind. Pharm. 1993, 19, 143–194. [Google Scholar] [CrossRef]
- Patel, D.; Smith, J.R.; Smith, A.W.; Grist, N.; Barnett, P.; Smart, J.D. An atomic force microscopy investigation of bioadhesive polymer adsorption onto human buccal cells. Int. J. Pharm. 2000, 200, 271–277. [Google Scholar] [CrossRef]
- Serra, L.; Doménech, J.; Peppas, N.A. Engineering design and molecular dynamics of mucoadhesive drug delivery systems as targeting agents. Eur. J. Pharm. Biopharm. 2009, 71, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Haidar, Z.S. Bio-Inspired/-Functional Colloidal Core-Shell Polymeric-Based NanoSystems: Technology Promise in Tissue Engineering, Bioimaging and NanoMedicine. Polymers 2010, 2, 323–352. [Google Scholar] [CrossRef] [Green Version]
- Mathiowitz, E.; Chickering, D.; Lehr, C.-M. (Eds.) Bioadhesive Drug Delivery Systems; CRC Press: Boca Raton, FL, USA, 1999; Volume 19992355. [Google Scholar] [CrossRef]
- Roy, S.; Pal, K.; Anis, A.; Pramanik, K.; Prabhakar, B. Polymers in Mucoadhesive Drug-Delivery Systems: A Brief Note Review Polymers in Mucoadhesive Drug-Delivery Systems: A Brief Note. Des. Monomers Polym. 2009, 12, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Das Neves, J.; Sarmento, B. (Eds.) Mucosal Delivery of Biopharmaceuticals; Springer US: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Bruschi, M.L.; Evangelista, R.C.; Gremião, M.P.D. Mucoadhesive drug delivery systems. Braz. J. Pharm. Sci. 2010, 46, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Khurana, S.; Madhav, N.V.S.; Tangri, P. Mucoadhesive Properties of the Mussel Adhesive Protein. Available online: www.ijpbs.net (accessed on 4 April 2019).
- Peppas, N.A.; Sahlin, J.J. Hydrogels as mucoadhesive and bioadhesive materials: A review. Biomaterials 1996, 17, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.O.; McConville, J.T. Manufacture and characterization of mucoadhesive buccal films. Eur. J. Pharm. Biopharm. 2011, 77, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Ajazuddin, D.K.; Tripathi, T.; Verma, J.; Maurya, S.P. Mechanism Responsible for Mucoadhesion of Mucoadhesive Drug Delivery System: A Review. Available online: www.ijabpt.com (accessed on 1 June 2019).
- Mahajan, P.; Kaur, A.; Aggarwal, G.; Harikumar, S.L. Mucoadhesive Drug Delivery System: A Review. 2013. Available online: http://www.ijddr.in (accessed on 1 June 2019).
- Hombach, J.; Bernkop-Schnürch, A. Mucoadhesive Drug Delivery Systems; Springer: Berlin/Heidelberg, Germany, 2010; pp. 251–266. [Google Scholar] [CrossRef]
- Lohani, A.; Chaudhary, G. Mucoadhesive microspheres: A novel approach to increase gastroretention. Chronicles Young-Sci. 2012, 3, 121. [Google Scholar] [CrossRef]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N.K. Mucoadhesion: A promising approach in drug delivery system. React. Funct. Polym. 2016, 100, 151–172. [Google Scholar] [CrossRef]
- Yadav, V.K.; Kumar, B. Mucoadhesive Polymers: Means of Improving the Mucoadhesive Properties of Drug Delivery System. 2010. Available online: https://www.researchgate.net/publication/222712279 (accessed on 18 May 2019).
- Russo, E.; Selmin, F.; Baldassari, S.; Gennari, C.G.M.; Caviglioli, G.; Cilurzo, F.; Minghetti, P.; Parodi, B. A focus on mucoadhesive polymers and their application in buccal dosage forms. J. Drug Deliv. Sci. Technol. 2016, 32, 113–125. [Google Scholar] [CrossRef]
- Patel, A.R.; Patel, D.A.; Chaudhry, S.V. Mucoadhesive buccal drug delivery system. J. Pharm. Life Sci. 2011. Available online: https://eds.s.ebscohost.com/eds/pdfviewer/pdfviewer?vid=0&sid=e0646e8b-5e52-49fd-90e5-53291c9397eb%40redis (accessed on 31 July 2022).
- Forooshani, P.K.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. Part A Polym. Chem. 2016, 55, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is Chitosan Mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Lehr, C.-M.; Bouwstra, J.A.; Schacht, E.H.; Junginger, H.E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm. 1992, 78, 43–48. Available online: https://biblio.ugent.be/publication/204037 (accessed on 2 June 2019). [CrossRef]
- He, P.; Davis, S.S.; Illum, L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int. J. Pharm. 1998, 166, 75–88. [Google Scholar] [CrossRef]
- Brooks, A.E. The Potential of Silk and Silk-Like Proteins as Natural Mucoadhesive Biopolymers for Controlled Drug Delivery. Front. Chem. 2015, 3, 65. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Soliman, G.M.; Barralet, J.; Cerruti, M. Mollusk Glue Inspired Mucoadhesives for Biomedical Applications. Langmuir 2012, 28, 14010–14017. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Zhong, W. Mussel-inspired hydrogel tissue adhesives for wound closure. RSC Adv. 2017, 7, 47380–47396. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.; Ryu, J.H.; Lee, H. Chitosan-catechol: A polymer with long-lasting mucoadhesive properties. Biomaterials 2015, 52, 161–170. [Google Scholar] [CrossRef]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef]
- Yamada, K.; Chen, T.; Kumar, G.; Vesnovsky, O.; Topoleski, L.D.T.; Payne, G.F. Chitosan Based Water-Resistant Adhesive. Analogy to Mussel Glue. Biomacromolecules 2000, 1, 252–258. [Google Scholar] [CrossRef]
- Zvarec, O.; Purushotham, S.; Masic, A.; Ramanujan, R.V.; Miserez, A. Catechol-Functionalized Chitosan/Iron Oxide Nanoparticle Composite Inspired by Mussel Thread Coating and Squid Beak Interfacial Chemistry. Langmuir 2013, 29, 10899–10906. [Google Scholar] [CrossRef] [PubMed]
- Schnurrer, J.; Lehr, C.-M. Intemational Journal of Pharmaceutics Notes Mucoadhesive Properties of the Mussel Adhesive Protein 1. Vol 141. 1996. Available online: https://pdf.sciencedirectassets.com/271189/1-s2.0-S0378517300X00348/1-s2.0-037851739604625X/main.pdf?x-amz-security-token=AgoJb3JpZ2luX2VjEJj%252F%252F%252F%252F%252F%252F%252F%252F%252F%252FwEaCXVzLWVhc3QtMSJIMEYCIQDHBzBH84W7ObRghjS1L%252BMPcC%252FPLgWKgtWseojDbCxYqQIhANqke5e%252 (accessed on 22 May 2019).
- Lee, B.P.; Dalsin, J.L.; Messersmith, P.B. Biomimetic Adhesive Polymers Based on Mussel Adhesive Proteins. In Biological Adhesives; Springer: Berlin/Heidelberg, Germany, 2006; pp. 257–278. [Google Scholar] [CrossRef]
- Deacon, M.P.; Davis, S.S.; Waite, J.H.; Harding, S.E. Structure and Mucoadhesion of Mussel Glue Protein in Dilute Solution. Biochemistry 1998, 37, 14108–14112. [Google Scholar] [CrossRef] [PubMed]
- Davidovich-Pinhas, M.; Bianco-Peled, H. Alginate–PEGAc: A new mucoadhesive polymer. Acta Biomater. 2011, 7, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.M.; Zia, F.; Zuber, M.; Rehman, S.; Ahmad, M.N. Alginate based polyurethanes: A review of recent advances and perspective. Int. J. Biol. Macromol. 2015, 79, 377–387. [Google Scholar] [CrossRef]
- Gupta, V.; Hwang, B.H.; Lee, J.; Anselmo, A.C.; Doshi, N.; Mitragotri, S. Mucoadhesive intestinal devices for oral delivery of salmon calcitonin. J. Control. Release 2013, 172, 753–762. [Google Scholar] [CrossRef]
- Bera, K.; Mazumder, B.; Khanam, J. Study of the Mucoadhesive Potential of Carbopol Polymer in the Preparation of Microbeads Containing the Antidiabetic Drug Glipizide. AAPS PharmSciTech 2015, 17, 743–756. [Google Scholar] [CrossRef]
- Tamburic, S.; Craig, D.Q.M. Rheological Evaluation of Polyacrylic Acid Hydrogels. Pharm. Pharmacol. Commun. 1995, 1, 107–109. [Google Scholar] [CrossRef]
- Tamburic, S.; Craig, D. An Investigation into the Rheological, Dielectric and Mucoadhesive Properties of Poly(Acrylic Acid) Gel Systems. Elsevier Sci. Pub. Co. 1995, 37, 59–68. Available online: https://www.infona.pl/resource/bwmeta1.element.elsevier-2231f5ea-1fbd-37db-95b6-77c5f4bd80e6 (accessed on 2 June 2019). [CrossRef]
- Blanco-Fuente, H.; Anguiano-Igea, S.; Otero-Espinar, F.J.; Blanco-Méndez, J. In-Vitro Bioadhesion of Carbopol Hydrogels. Vol 2. Elsevier/North Holland; 1996. Available online: https://www.infona.pl/resource/bwmeta1.element.elsevier-e8edf0b5-177e-395a-b237-6cac90cfe710 (accessed on 2 June 2019).
- Takeuchi, H.; Matsui, Y.; Yamamoto, H.; Kawashima, Y. Mucoadhesive properties of carbopol or chitosan-coated liposomes and their effectiveness in the oral administration of calcitonin to rats. J. Control. Release 2003, 86, 235–242. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12526820 (accessed on 2 June 2019). [CrossRef]
- Dodou, D.; Breedveld, P.; Wieringa, P.A. Mucoadhesives in the gastrointestinal tract: Revisiting the literature for novel applications. Eur. J. Pharm. Biopharm. 2005, 60, 1–16. [Google Scholar] [CrossRef]
- Petrou, G.; Jansson, R.; Högqvist, M.; Erlandsson, J.; Wågberg, L.; Hedhammar, M.; Crouzier, T. Genetically Engineered Mucoadhesive Spider Silk. Biomacromolecules 2018, 19, 3268–3279. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhang, Y.; Lu, L.; Shao, H.; Qin, K.; Hu, X.; Xia, X. Recombinant spider silk from aqueous solutions via a bio-inspired microfluidic chip. Sci. Rep. 2016, 6, 36473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blasingame, E.; Tuton-Blasingame, T.; Larkin, L.; Falick, A.M.; Zhao, L.; Fong, J.; Vaidyanathan, V.; Visperas, A.; Geurts, P.; Hu, X.; et al. Pyriform Spidroin 1, a Novel Member of the Silk Gene Family That Anchors Dragline Silk Fibers in Attachment Discs of the Black Widow Spider, Latrodectus hesperus. J. Biol. Chem. 2009, 284, 29097–29108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, J.O.; Grawe, I.; Wirth, M.; Karstedt, A.; Gorb, S.N. Spider’s super-glue: Thread anchors are composite adhesives with synergistic hierarchical organization. Soft Matter 2015, 11, 2394–2403. [Google Scholar] [CrossRef] [Green Version]
- Geurts, P.; Zhao, L.; Hsia, Y.; Gnesa, E.; Tang, S.; Jeffery, F.; La Mattina, C.; Franz, A.; Larkin, L.; Vierra, C. Synthetic Spider Silk Fibers Spun from Pyriform Spidroin 2, A Glue Silk Protein Discovered in Orb-Weaving Spider Attachment Discs. Biomacromolecules 2010, 11, 3495–3503. [Google Scholar] [CrossRef]
- Serban, M.A.; Panilaitis, B.; Kaplan, D.L. Silk fibroin and polyethylene glycol-based biocompatible tissue adhesives. J. Biomed. Mater. Res. Part A 2011, 98, 567–575. [Google Scholar] [CrossRef]
- Kundu, J.; Patra, C.; Kundu, S. Design, fabrication and characterization of silk fibroin-HPMC-PEG blended films as vehicle for transmucosal delivery. Mater. Sci. Eng. C 2008, 28, 1376–1380. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, Y.; Zhao, Y.; Luo, J.; Shao, H.; Hu, X. Bio-inspired capillary dry spinning of regenerated silk fibroin aqueous solution. Mater. Sci. Eng. C 2011, 31, 1602–1608. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Y.; Huang, Y.; Shao, H.; Hu, X. A bio-inspired microfluidic concentrator for regenerated silk fibroin solution. Sensors Actuators B Chem. 2011, 162, 435–440. [Google Scholar] [CrossRef]
- Kundu, B.; Kundu, S.C. Bio-inspired fabrication of fibroin cryogels from the muga silkworm Antheraea assamensis for liver tissue engineering. Biomed. Mater. 2013, 8, 055003. [Google Scholar] [CrossRef]
- Freitas, E.D.; Vidart, J.M.; Silva, E.A.; da Silva, M.G.; Vieira, M.G. Development of mucoadhesive sericin/alginate particles loaded with ibuprofen for sustained drug delivery. Particuology 2018, 41, 65–73. [Google Scholar] [CrossRef]
- Purwar, R.; Sharma, S.; Sahoo, P.; Srivastava, C.M. Flexible sericin/polyvinyl alcohol/clay blend films. Fibers Polym. 2015, 16, 761–768. [Google Scholar] [CrossRef]
- Kunz, R.I.; Brancalhão, R.M.C.; Ribeiro, L.D.F.C.; Natali, M.R.M. Silkworm Sericin: Properties and Biomedical Applications. BioMed Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Zhang, Y.; Zhang, J.; Huang, L.; Liu, J.; Li, Y.; Zhang, G.; Kundu, S.C.; Wang, L. Exploring natural silk protein sericin for regenerative medicine: An injectable, photoluminescent, cell-adhesive 3D hydrogel. Sci. Rep. 2014, 4, 7064. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Kundu, B.; Cai, Y.; Kundu, S.C.; Yao, J. Bio-inspired mineralization of hydroxyapatite in 3D silk fibroin hydrogel for bone tissue engineering. Colloids Surf. B Biointerfaces 2015, 134, 339–345. [Google Scholar] [CrossRef]
- Ashton, N.N.; Roe, D.R.; Weiss, R.B.; Cheatham, I.T.E.; Stewart, R.J. Self-Tensioning Aquatic Caddisfly Silk: Ca2+-Dependent Structure, Strength, and Load Cycle Hysteresis. Biomacromolecules 2013, 14, 3668–3681. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.D.; Kaur, S.; Weerasakare, G.M.; Stewart, R.J. Toughened hydrogels inspired by aquatic caddisworm silk. Soft Matter 2015, 11, 6981–6990. [Google Scholar] [CrossRef]
- Stewart, R.J.; Wang, C.S. Adaptation of Caddisfly Larval Silks to Aquatic Habitats by Phosphorylation of H-Fibroin Serines. Biomacromolecules 2010, 11, 969–974. [Google Scholar] [CrossRef]
- Wang, Y.J.; Sanai, K.; Nakagaki, M. A Novel Bioadhesive Protein of Silk Filaments Spun Underwater by Caddisfly Larvae. Adv. Mater. Res. 2009, 79–82, 1631–1634. [Google Scholar] [CrossRef]
- Kim, K.; Shin, M.; Koh, M.-Y.; Ryu, J.H.; Lee, M.S.; Hong, S.; Lee, H. TAPE: A Medical Adhesive Inspired by a Ubiquitous Compound in Plants. Adv. Funct. Mater. 2015, 25, 2402–2410. [Google Scholar] [CrossRef]
- Shin, M.; Kim, K.; Shim, W.; Yang, J.W.; Lee, H. Tannic Acid as a Degradable Mucoadhesive Compound. ACS Biomater. Sci. Eng. 2016, 2, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Ghassem, M.; Arihara, K.; Mohammadi, S.; Sani, N.A.; Babji, A.S. Identification of two novel antioxidant peptides from edible bird’s nest (Aerodramus fuciphagus) protein hydrolysates. Food Funct. 2017, 8, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Jessel, H.R.; Chen, S.; Osovski, S.; Efroni, S.; Rittel, D.; Bachelet, I. Design principles of biologically fabricated avian nests. Sci. Rep. 2019, 9, 4792. [Google Scholar] [CrossRef] [Green Version]
- Nutritional Supplement for Increasing Cellular Levels of N-Acetylneuraminate. Published Online 18 November 2015. Available online: https://patents.google.com/patent/US20190083516A1/en (accessed on 2 June 2019).
- Abruzzo, A.; Cerchiara, T.; Bigucci, F.; Gallucci, M.C.; Luppi, B. Mucoadhesive Buccal Tablets Based on Chitosan/Gelatin Microparticles for Delivery of Propranolol Hydrochloride. J. Pharm. Sci. 2015, 104, 4365–4372. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.W.; Lee, J.-S.; Ko, S.; Lee, H.G. Preparation and Characterization of Mucoadhesive Buccal Nanoparticles Using Chitosan and Dextran Sulfate. J. Agric. Food Chem. 2016, 64, 5384–5388. [Google Scholar] [CrossRef]
- Freag, M.S.; Saleh, W.M.; Abdallah, O.Y. Laminated chitosan-based composite sponges for transmucosal delivery of novel protamine-decorated tripterine phytosomes: Ex-vivo mucopenetration and in-vivo pharmacokinetic assessments. Carbohydr. Polym. 2018, 188, 108–120. [Google Scholar] [CrossRef]
- Garcia, M.T.J.; Freitas, C.D.P.; Graciano, T.B.; Coutinho, T.S.; Cressoni, C.B.; Pereira, S.A.D.L.; Shimano, M.M. Chitosan-based mucoadhesive gel for oral mucosal toluidine blue O delivery: The influence of a non-ionic surfactant. Photodiagnosis Photodyn. Ther. 2017, 20, 48–54. [Google Scholar] [CrossRef]
- Mai-Ngam, K.; Boonkitpattarakul, K.; Jaipaew, J.; Mai-Ngam, B. Evaluation of the Properties of Silk Fibroin Films from the Non-mulberry Silkworm Samia cynthia ricini for Biomaterial Design. J. Biomater. Sci. Polym. Ed. 2011, 22, 2001–2022. [Google Scholar] [CrossRef]
- Xu, J.; Strandman, S.; Zhu, J.X.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef]
- Shtenberg, Y.; Goldfeder, M.; Prinz, H.; Shainsky, J.; Ghantous, Y.; Abu El-Naaj, I.; Schroeder, A.; Bianco-Peled, H. Mucoadhesive alginate pastes with embedded liposomes for local oral drug delivery. Int. J. Biol. Macromol. 2018, 111, 62–69. [Google Scholar] [CrossRef]
- Kilicarslan, M.; Ilhan, M.; Inal, O.; Orhan, K. Preparation and evaluation of clindamycin phosphate loaded chitosan/alginate polyelectrolyte complex film as mucoadhesive drug delivery system for periodontal therapy. Eur. J. Pharm. Sci. 2018, 123, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Ghumman, S.A.; Noreen, S.; Muntaha, S.T. Linum usitatissimum seed mucilage-alginate mucoadhesive microspheres of metformin HCl: Fabrication, characterization and evaluation. Int. J. Biol. Macromol. 2020, 155, 358–368. [Google Scholar] [CrossRef]
- Opell, B.D.; Karinshak, S.E.; Sigler, M.A. Environmental response and adaptation of glycoprotein glue within the droplets of viscous prey capture threads from araneoid spider orb-webs. J. Exp. Biol. 2013, 216, 3023–3034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elices, M.; Guinea, G.V.; Plaza, G.R.; Karatzas, C.; Riekel, C.; Agulló-Rueda, F.; Daza, R.; Pérez-Rigueiro, J. Bioinspired Fibers Follow the Track of Natural Spider Silk. Macromolecules 2011, 44, 1166–1176. [Google Scholar] [CrossRef]
- Sahni, V.; Miyoshi, T.; Chen, K.; Jain, D.; Blamires, S.J.; Blackledge, T.A.; Dhinojwala, A. Direct Solvation of Glycoproteins by Salts in Spider Silk Glues Enhances Adhesion and Helps To Explain the Evolution of Modern Spider Orb Webs. Biomacromolecules 2014, 15, 1225–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasanthavada, K.; Hu, X.; Tuton-Blasingame, T.; Hsia, Y.; Sampath, S.; Pacheco, R.; Freeark, J.; Falick, A.M.; Tang, S.; Fong, J.; et al. Spider Glue Proteins Have Distinct Architectures Compared with Traditional Spidroin Family Members. J. Biol. Chem. 2012, 287, 35986–35999. [Google Scholar] [CrossRef]
- Calcium Alginate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/44630049 (accessed on 8 April 2020).
- Singla, A.K.; Chawla, M.; Singh, A. Potential Applications of Carbomer in Oral Mucoadhesive Controlled Drug Delivery System: A Review. Drug Dev. Ind. Pharm. 2000, 26, 913–924. [Google Scholar] [CrossRef]
- Shitrit, Y.; Bianco-Peled, H. Acrylated chitosan for mucoadhesive drug delivery systems. Int. J. Pharm. 2017, 517, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Felt-Baeyens, O.; Buri, P.; Gurny, R. Chitosan: A Unique Polysaccharide for Drug Delivery. Drug Dev. Ind. Pharm. 1998, 24, 979–993. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Cho, I.S.; Oh, H.M.; Cho, M.O.; Jang, B.S.; Cho, J.-K.; Park, K.H.; Kang, S.-W.; Huh, K.M. Synthesis and characterization of thiolated hexanoyl glycol chitosan as a mucoadhesive thermogelling polymer. Biomater. Res. 2018, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.; Choi, Y.S.; Kang, D.G.; Song, Y.H.; Cha, H.J. The adhesive properties of coacervated recombinant hybrid mussel adhesive proteins. Biomaterials 2010, 31, 3715–3722. [Google Scholar] [CrossRef] [PubMed]
- Elices, M.; Pérez-Rigueiro, J.; Plaza, G.; Guinea, G.V. Recovery in spider silk fibers. J. Appl. Polym. Sci. 2004, 92, 3537–3541. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, H.; Wang, C.; Wu, L.; Huang, J.; Guo, C. Tensile behavior and morphology of differently degummed silkworm (Bombyx mori) cocoon silk fibres. Mater. Lett. 2006, 60, 919–925. [Google Scholar] [CrossRef]
- Yucel, T.; Kojic, N.; Leisk, G.G.; Lo, T.J.; Kaplan, D.L. Non-equilibrium silk fibroin adhesives. J. Struct. Biol. 2010, 170, 406–412. [Google Scholar] [CrossRef]
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Protein release kinetics for core–shell hybrid nanoparticles based on the layer-by-layer assembly of alginate and chitosan on liposomes. Biomaterials 2008, 29, 1207–1215. [Google Scholar] [CrossRef]
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Biocompatibility and safety of a hybrid core–shell nanoparticulate OP-1 delivery system intramuscularly administered in rats. Biomaterials 2010, 31, 2746–2754. [Google Scholar] [CrossRef]
- Panotopoulos, G.P.; Aguayo, S.; Haidar, Z.S. Nonmotile Single-Cell Migration as a Random Walk in Nonuniformity: The “Extreme Dumping Limit” for Cell-to-Cell Communications. J. Heal. Eng. 2018, 2018, 9680713. [Google Scholar] [CrossRef] [Green Version]
- Panotopoulos, G.P.; Haidar, Z.S. Mathematical Modeling for Pharmaco-Kinetic and -Dynamic Predictions from Controlled Drug Release NanoSystems: A Comparative Parametric Study. Scientifica 2019, 2019, 9153876. [Google Scholar] [CrossRef] [PubMed]

| Theory | Summary | Equation(s) | Diagram | Label (s) |
|---|---|---|---|---|
| Wetting | The wetting theory is applicable to liquid bio-adhesives. It treats adhesion as an embedding process, whereby the lower the contact angle, the greater the adhesion. In this process, adhesive agents penetrate surface irregularities to spread through the surface. | γTA = γPT + γPAcos(θ) | 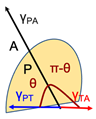 | T: Tissue; substrate surface; mucosal membrane P: Polymer; liquid; mucoadhesive material A: Air; vapor θ: Angle of contact angle between solid and liquid interface γTA: Interfacial tension between tissue and air γPT: Interfacial tension between polymer and tissue γPA: Interfacial tension between polymer and air |
| Mechanical Inter-locking or Keying | Mechanical inter-locking theory, or mechanical keying, proposes that the adhesion between a liquid and a rough or porous surface is due to the mechanical inter-locking as well as the increased surface rugosity. | OJS = C ∗ MIC ∗ ICC |  | OJS: Optimum joint strength C: Constant MIC: Mechanical interlocking component ICC: Interfacial chemical component A: Air T: Tissue |
| Adsorption | The adsorption theory states that adhesion is caused by molecular bonding between the mucus membrane and muco-adhesive device. | N/A | 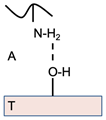 | T: Tissue A: Air |
| Electronic | The electronic transfer theory proposes that electron transfer between the mucus membrane and the muco-adhesive substrate results in attractive layers within a double layer of electrical charges, at the interface. | N/A |  | T: Tissue A: Air |
| Fracture | A commonly used theory in mechanical measuring muco-adhesion, the fracture theory, calculates the force necessary to detach two surfaces after adhesion is established. | Sm = Fm/A0σ = ((E ∗ ε)/L)0.5ε = Wr + WiE = [σ/ε]ε→ 0 = [[F/A0]/[∆l/l0]]∆l→0 | 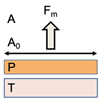 | Sm: Adhesive strength/detachment force Fm: Maximal force for detachment A0: Surface area encompassed by muco-adhesive system T: Tissue A: Air P: Polymer muco-adhesive σ: Fracture strength E: Young’s modulus for elasticity ε: Fracture energy L: Critical crack length Wr: Reversible adhesion work Wi: Irreversible adhesion work ∆l: Change in thickness l0: original thickness |
| Diffusion | A semi-permanent adhesive bond is formed via the deep inter-penetration and entanglement of the polymer and mucin chains, with the adhesive force being proportional to the degree of penetration. | l = (t ∗ Db)½ | 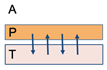 | I: Interpenetration depth t: Contact Time Db: Diffusion co-efficient of mucoadhesive T: Tissue P: Polymer muco-adhesive A: Air |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jawadi, Z.; Yang, C.; Haidar, Z.S.; Santa Maria, P.L.; Massa, S. Bio-Inspired Muco-Adhesive Polymers for Drug Delivery Applications. Polymers 2022, 14, 5459. https://doi.org/10.3390/polym14245459
Jawadi Z, Yang C, Haidar ZS, Santa Maria PL, Massa S. Bio-Inspired Muco-Adhesive Polymers for Drug Delivery Applications. Polymers. 2022; 14(24):5459. https://doi.org/10.3390/polym14245459
Chicago/Turabian StyleJawadi, Zina, Christine Yang, Ziyad S. Haidar, Peter L. Santa Maria, and Solange Massa. 2022. "Bio-Inspired Muco-Adhesive Polymers for Drug Delivery Applications" Polymers 14, no. 24: 5459. https://doi.org/10.3390/polym14245459