Development of Sustainable High Performance Epoxy Thermosets for Aerospace and Space Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermosetting Resins Manufacturing
2.3. Experimental Techniques
2.3.1. Differential Scanning Calorimetry (DSC)
2.3.2. Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.3.3. Thermoset’s Density
2.3.4. Dynamic Mechanical Analysis (DMA)
2.3.5. Shore Hardness Tests
2.3.6. Tensile Testing
2.3.7. Thermogravimetric Analysis (TGA)
2.3.8. Water Absorption (WA%)
2.3.9. Gel Content (GC)
3. Results and Discussions
3.1. Crosslinking Behavior of the Epoxy-Based Thermosetting Formulations
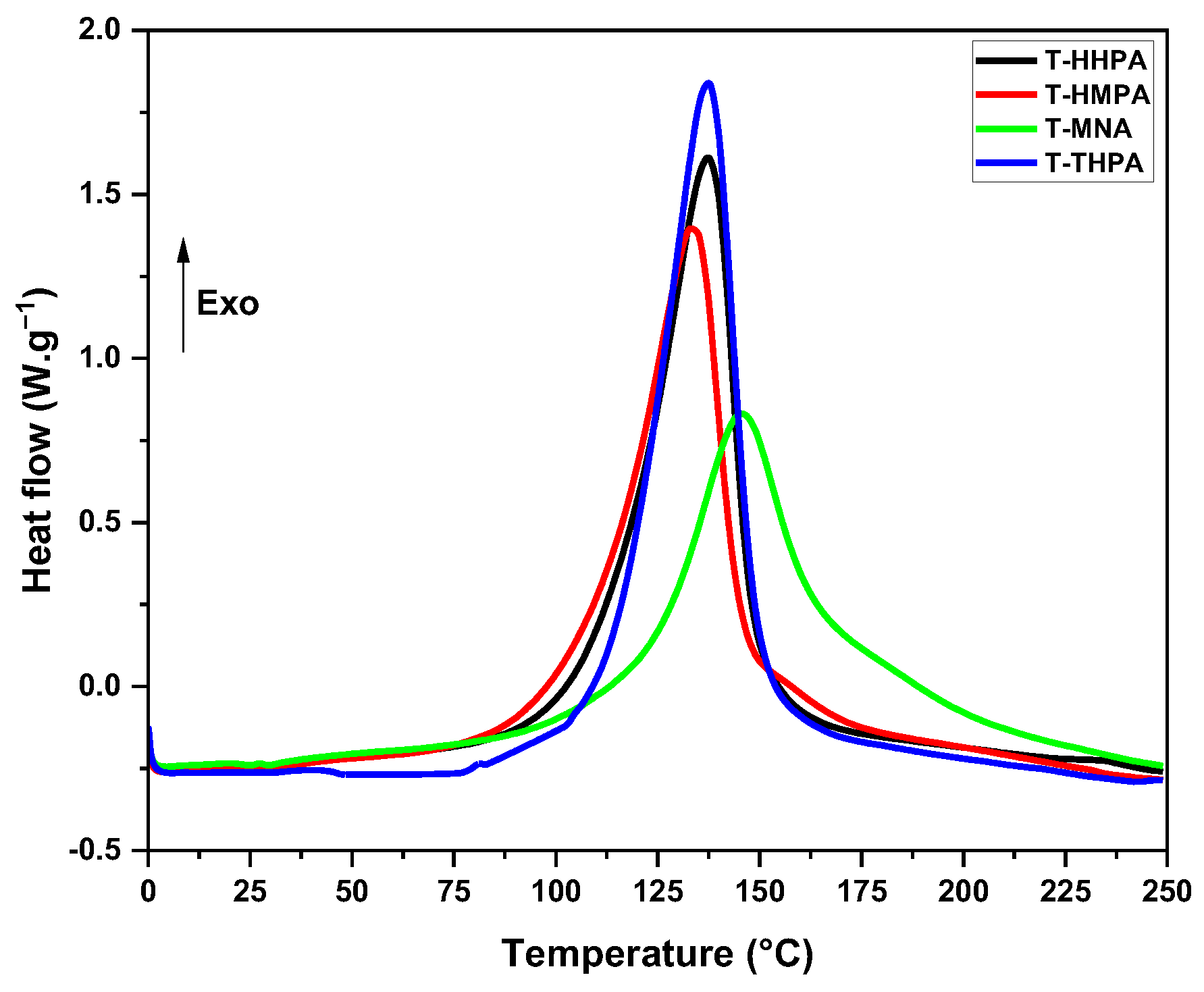
3.1.1. Differential Scanning Calorimetry (DSC) Investigation
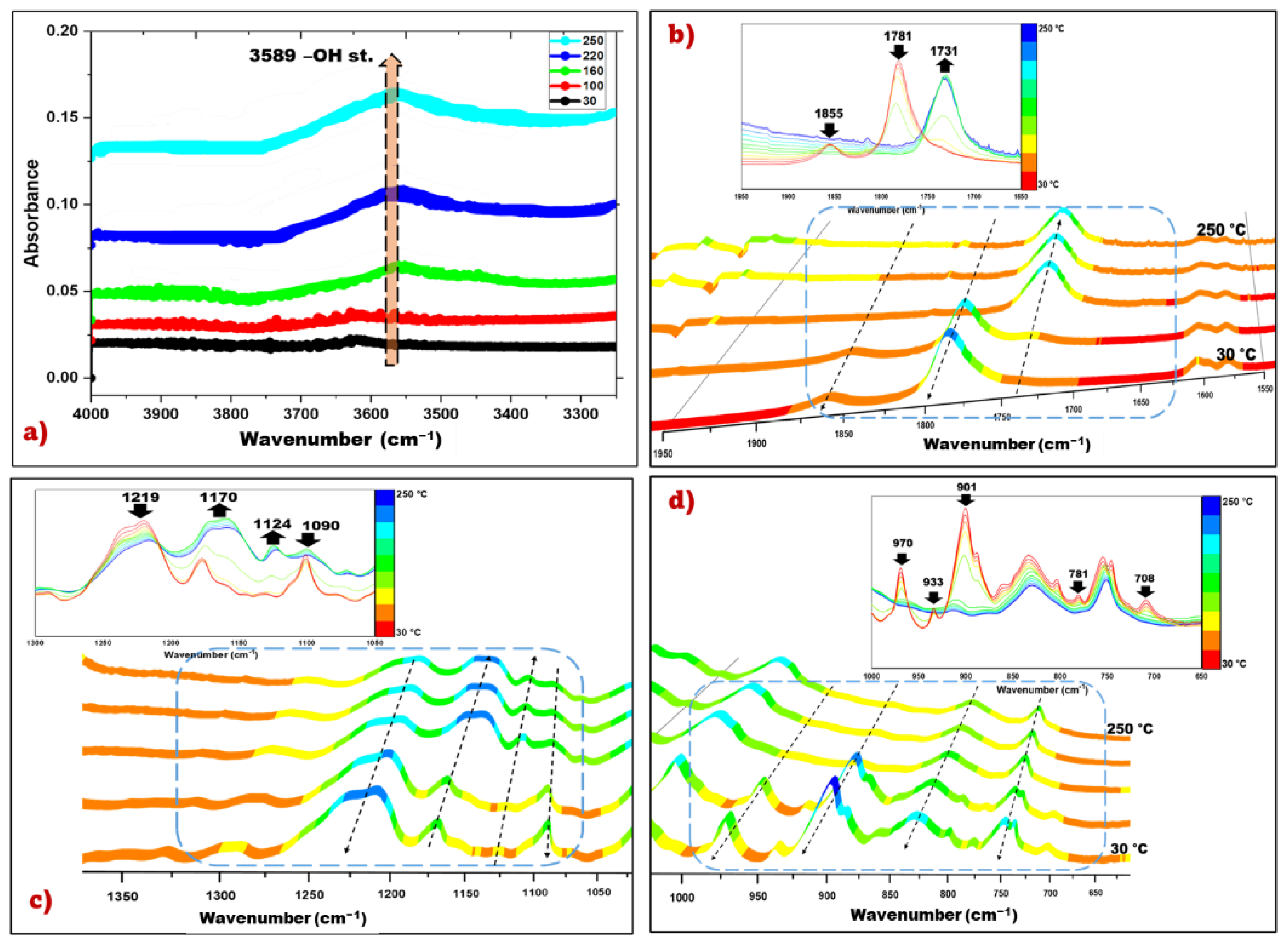
3.1.2. Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) Spectroscopy
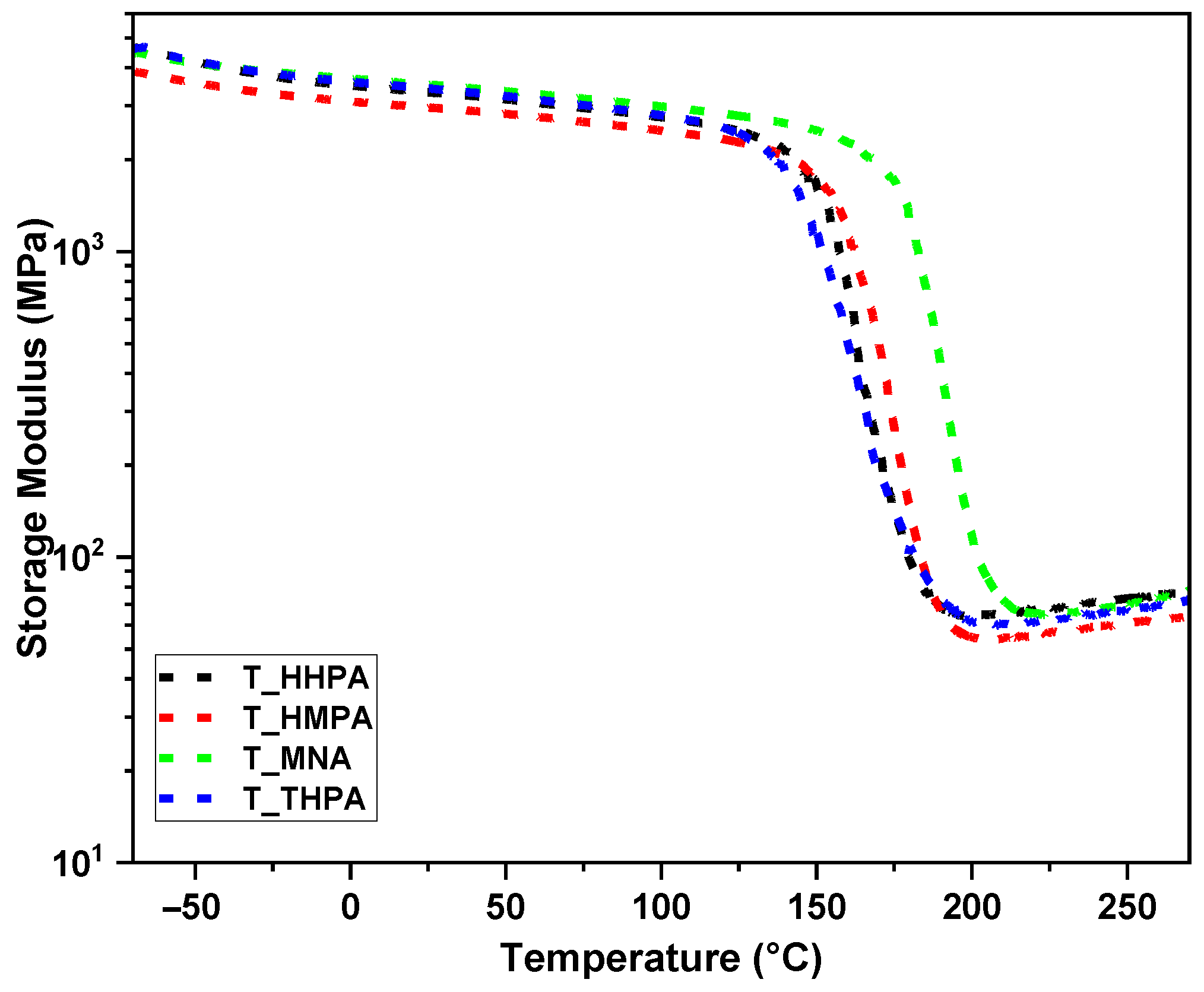
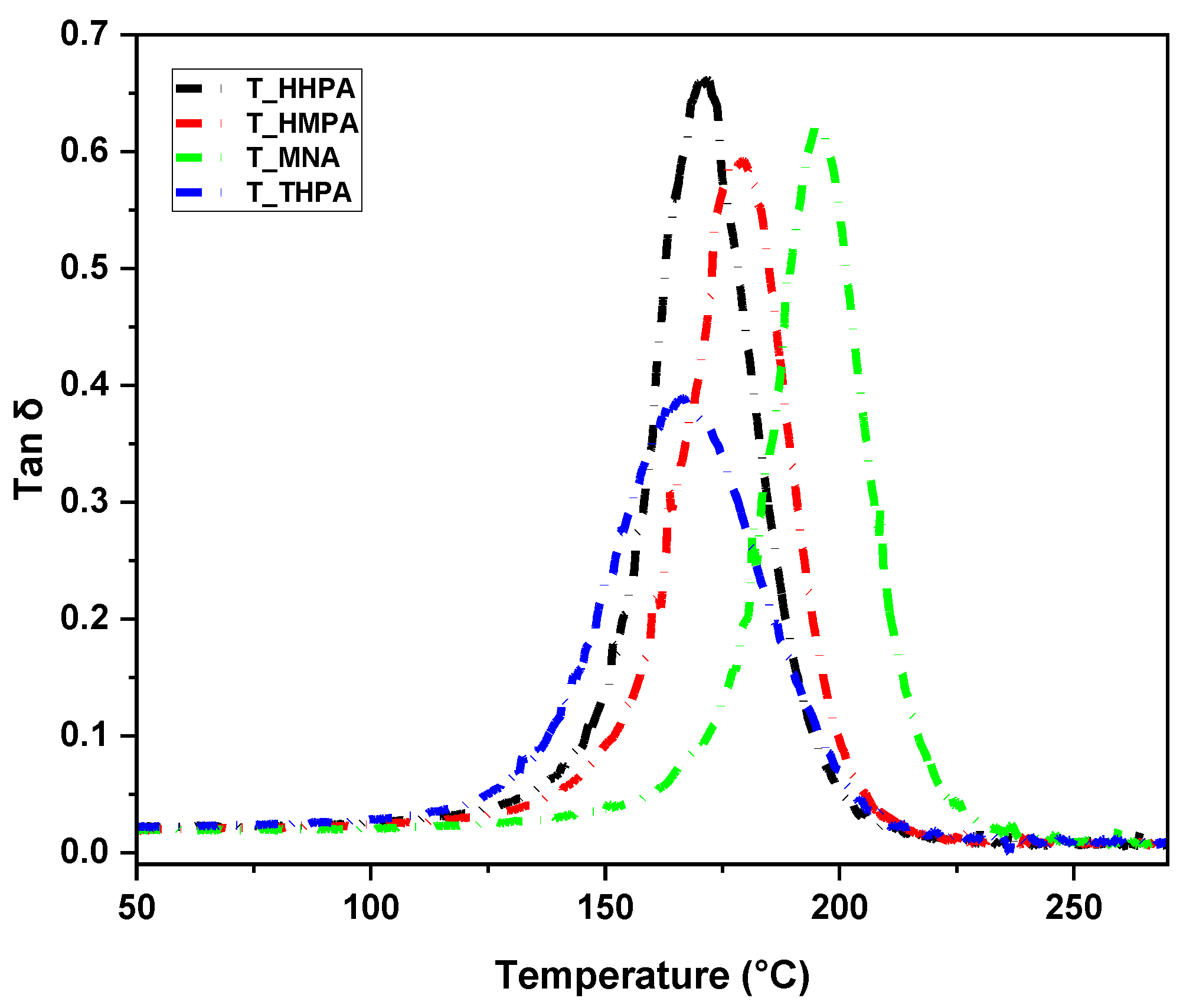
3.2. Thermomechanical Performances Investigation by DMA
3.3. Tensile Tests
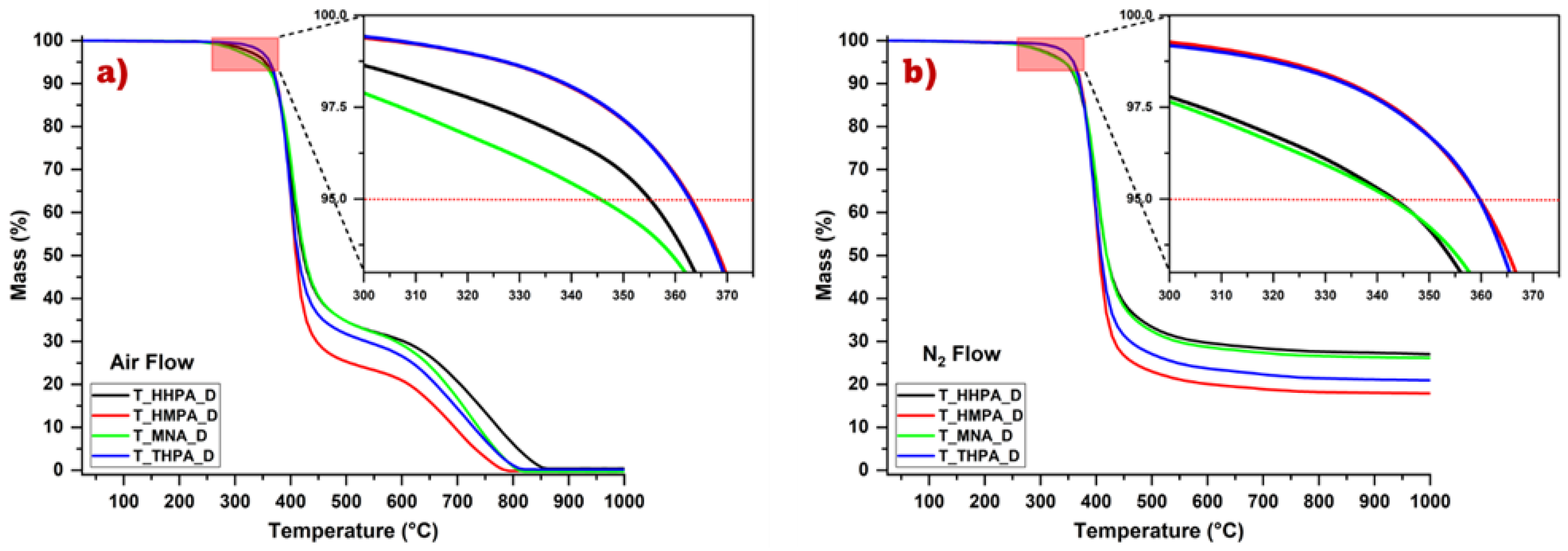
3.4. Thermal Stability of the Crosslinked Epoxy Resins
3.5. Moisture Behaviour of the Designed Epoxy Thermosets
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biron, M. Thermosets and Composites Material Selection, Applications, Manufacturing and Cost Analysis, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 8420041010110. [Google Scholar]
- Ramon, E.; Sguazzo, C.; Moreira, P. A Review of Recent Research on Bio-Based Epoxy Systems for Engineering Applications and Potentialities in the Aviation Sector. Aerospace 2018, 5, 110. [Google Scholar] [CrossRef] [Green Version]
- Ng, F.; Couture, G.; Philippe, C.; Boutevin, B.; Caillol, S. Bio-Based Aromatic Epoxy Monomers for Thermoset Materials. Molecules 2017, 22, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashouf Roudsari, G.; Mohanty, A.K.; Misra, M. Green Approaches To Engineer Tough Biobased Epoxies: A Review. ACS Sustain. Chem. Eng. 2017, 5, 9528–9541. [Google Scholar] [CrossRef]
- Wan, J.; Zhao, J.; Zhang, X.; Fan, H.; Zhang, J.; Hu, D.; Jin, P.; Wang, D.Y. Epoxy Thermosets and Materials Derived from Bio-Based Monomeric Phenols: Transformations and Performances. Prog. Polym. Sci. 2020, 108, 101287. [Google Scholar] [CrossRef]
- Pascault, J.-P.; Williams, R.J.J. Epoxy Polymers-New Materials and Innovations; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; ISBN 978-3-527-62871-1. [Google Scholar]
- Paolillo, S.; Bose, R.K.; Santana, M.H.; Grande, A.M. Intrinsic Self-Healing Epoxies in Polymer Matrix Composites (Pmcs) for Aerospace Applications. Polymers 2021, 13, 201. [Google Scholar] [CrossRef]
- Moreman, J.; Lee, O.; Trznadel, M.; David, A.; Kudoh, T.; Tyler, C.R. Acute Toxicity, Teratogenic, and Estrogenic Effects of Bisphenol A and Its Alternative Replacements Bisphenol S, Bisphenol F, and Bisphenol AF in Zebrafish Embryo-Larvae. Environ. Sci. Technol. 2017, 51, 12796–12805. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human Exposure to Bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Gundert-Remy, U.; Bodin, J.; Bosetti, C.; FitzGerald, R.; Hanberg, A.; Hass, U.; Hooijmans, C.; Rooney, A.A.; Rousselle, C.; van Loveren, H.; et al. Bisphenol A (BPA) Hazard Assessment Protocol. EFSA Support. Publ. 2017, 14, 1–58. [Google Scholar]
- Dinu, R.; Lafont, U.; Damiano, O.; Mija, A. High Glass Transition Materials from Sustainable Epoxy Resins with Potential Applications in the Aerospace and Space Sectors. ACS Appl. Polym. Mater. 2022, 4, 3636–3646. [Google Scholar] [CrossRef]
- Shieh, J.Y.; Ho, T.H.; Wang, C.S. Synthesis and Modification of a Trifunctional Epoxy Resin with Amino-Terminated Poly(dimethyl-siloxane)s for Semiconductor Encapsulation. Angew. Makromol. Chemie 1997, 245, 125–137. [Google Scholar] [CrossRef]
- Kodama, K.; Momota, M. Method of Synthesizing Polyphenol Compound and Positive Working Photoresist Composition Comprising Polyphenol Compound. U.S. Patent 5853949, 29 December 1998. [Google Scholar]
- Cornells, S.; De La Mare, H.E.; Bauer, R.S. Novel Polyglycidyl Amines. Patent 234609, 16 January 1987. [Google Scholar]
- Tess, R.W.H.; Calif, O. Process for Manufacture of Glycidyl Ethers of Polyhydric Phenols. U.S. Patent 2879259, 24 March 1959. [Google Scholar]
- Claire, W.E.S. Process for Preparing Epoxyalkyl Aryl Ethers. U.S. Patent 2892849, 30 June 1959. [Google Scholar]
- Reinking, N.H. Preparation of Monomeric Glycidyl Polyethers of Polyhydric Phenols. U.S. Patent 2943096, 28 June 1960. [Google Scholar]
- Lee, H.-J.; Lim, G.; Yang, E.; Kim, Y.-S.; Kwak, M.-G.; Kim, Y. Thermally Conductive Film Fabricated Using Perforated Graphite Sheet and UV-Curable Pressure-Sensitive Adhesive. Nanomaterials 2021, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M.; Hashem, T.; Gopalakrishnan, A.; Schäfer, A.I. Cyclodextrin Composite Nanofiber Membrane: Impact of the Crosslinker Type on Steroid Hormone Micropollutant Removal from Water. ACS Appl. Polym. Mater. 2021, 3, 2646–2656. [Google Scholar] [CrossRef]
- Agarwal, P.; Hefner, R.E.; Ge, S.; Tomlinson, I.; Rao, Y.; Dikic, T. Nanofiltration Membranes from Crosslinked Troger’s Base Polymers of Intrinsic Microporosity (PIMs). J. Memb. Sci. 2020, 595, 117501. [Google Scholar] [CrossRef]
- El Gazzani, S.; Nassiet, V.; Habas, J.P.; Freydier, C.; Hilleshein, A. High Temperature Epoxy Foam: Optimization of Process Parameters. Polymers 2016, 8, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, C.M.; Kim, J.G.; Kim, M.S.; Kim, K.M.; Kim, K.N. Development of a New Photocurable Composite Resin with Reduced Curing Shrinkage. Dent. Mater. 2002, 18, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, E.; Watson, D.A.; Lobo, R.F. Renewable Production of Phthalic Anhydride from Biomass-Derived Furan and Maleic Anhydride. Green Chem. 2014, 16, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Thiyagarajan, S.; Genuino, H.C.; Śliwa, M.; Van Der Waal, J.C.; De Jong, E.; Van Haveren, J.; Weckhuysen, B.M.; Bruijnincx, P.C.A.; Van Es, D.S. Substituted Phthalic Anhydrides from Biobased Furanics: A New Approach to Renewable Aromatics. ChemSusChem 2015, 8, 3052–3056. [Google Scholar] [CrossRef] [PubMed]
- Giarola, S.; Romain, C.; Williams, C.K.; Hallett, J.P.; Shah, N. Techno-Economic Assessment of the Production of Phthalic Anhydride from Corn Stover. Chem. Eng. Res. Des. 2016, 107, 181–194. [Google Scholar] [CrossRef] [Green Version]
- Galy, J.; Sabra, A.; Pascault, J.-P. Characterization of Epoxy Thermosetting Systems by Differential Scanning Calorimetry. Polym. Eng. Sci. 1986, 26, 1514–1523. [Google Scholar] [CrossRef]
- Leukel, J.; Burchard, W.; Krüger, R.P.; Much, H.; Schulz, G. Mechanism of the Anionic Copolymerization of Anhydride-Cured Epoxies—Analyzed by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF-MS). Macromol. Rapid Commun. 1996, 17, 359–366. [Google Scholar] [CrossRef]
- International ASTM D570-98(2018); Standard Test Method for Water Absorption of Plastics, Reapproved in 2018. ASTM: West Conshohocken, PA, USA, 2018.
- Tan, S.G.; Ahmad, Z.; Chow, W.S. Interpenetrating Polymer Network Structured Thermosets Prepared from Epoxidized Soybean Oil/Diglycidyl Ether of Bisphenol A. Polym. Int. 2014, 63, 273–279. [Google Scholar] [CrossRef]
- Ma, S.; Liu, X.; Fan, L.; Jiang, Y.; Cao, L.; Tang, Z.; Zhu, J. Synthesis and Properties of a Bio-Based Epoxy Resin with High Epoxy Value and Low Viscosity. ChemSusChem 2014, 7, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, I.A.; Andritsch, T.; Vaughan, A.S. On the Dielectric Behavior of Amine and Anhydride Cured Epoxy Resins Modified Using Multi-Terminal Epoxy Functional Network Modifier. Polymers 2019, 11, 1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, M.G.; Cabanelas, J.C.; Baselga, J. Applications of FTIR on Epoxy Resins—Identification, Monitoring the Curing Process, Phase Separation and Water Uptake. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophanides, T., Ed.; InTech: Rijeka, Croatia, 2012; Volume 16, p. 208. [Google Scholar]
- Duemichen, E.; Javdanitehran, M.; Erdmann, M.; Trappe, V.; Sturm, H.; Braun, U.; Ziegmann, G. Analyzing the Network Formation and Curing Kinetics of Epoxy Resins by in Situ Near-Infrared Measurements with Variable Heating Rates. Thermochim. Acta 2015, 616, 49–60. [Google Scholar] [CrossRef]
- Todorovic, A.; Resch-Fauster, K.; Mahendran, A.R.; Oreski, G.; Kern, W. Curing of Epoxidized Linseed Oil: Investigation of the Curing Reaction with Different Hardener Types. J. Appl. Polym. Sci. 2021, 138, 50239. [Google Scholar] [CrossRef]
- Zhao, W.; An, L.; Wang, S. Recyclable High-Performance Epoxy-Anhydride Resins with DMP-30 as the Catalyst of Transesterification Reactions. Polymers 2021, 13, 296. [Google Scholar] [CrossRef]
- Paramarta, A.; Webster, D.C. Bio-Based High Performance Epoxy-Anhydride Thermosets for Structural Composites: The Effect of Composition Variables. React. Funct. Polym. 2016, 105, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Mustata, F.; Tudorachi, N. Synthesis and Thermal Characterization of Some Hardeners for Epoxy Resins Based on Castor Oil and Cyclic Anhydrides. Ind. Crops Prod. 2021, 159, 113087. [Google Scholar] [CrossRef]
- Hill, L.W. Calculation of Crosslink Density in Short Chain Networks. Prog. Org. Coatings 1997, 31, 235–243. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953; ISBN 0801401348. [Google Scholar]
- Rios de Anda, A.; Sotta, P.; Modjinou, T.; Langlois, V.; Versace, D.; Renard, E. Multiscale Structural Characterization of Biobased Diallyl–Eugenol Polymer Networks. Macromolecules 2020, 53, 2187–2197. [Google Scholar] [CrossRef]
- Hübner, F.; Szpoganicz, E.; Demleitner, M.; Kuhnigk, J.; Altstädt, V.; Rios De Anda, A. Time Domain 1H NMR, Thermomechanical, and Rheology Multiscale Structural Characterization of Polydimethylsiloxane-Toughened Epoxy Nanocomposites for Liquid Composite Molding. ACS Appl. Polym. Mater. 2020, 2, 4779–4789. [Google Scholar] [CrossRef]
- Tobolsky, A.V. Properties and Structure of Polymers; Wiley: New York, NY, USA, 1960. [Google Scholar]
- Kalogeras, I.M.; Hagg Lobland, H.E. The Nature of the Glassy State: Structure and Glass Transitions. J. Mater. Educ. 2012, 34, 69–94. [Google Scholar]
- Fache, M.; Viola, A.; Auvergne, R.; Boutevin, B.; Caillol, S. Biobased Epoxy Thermosets from Vanillin-Derived Oligomers. Eur. Polym. J. 2015, 68, 526–535. [Google Scholar] [CrossRef]
- Campbell, F.C. Manufacturing Technology for Aerospace Structural Materials; Elsevier Ltd.: Oxford, UK, 2006; ISBN 9781856174954. [Google Scholar]
- Yi, X. Development of Multifunctional Composites for Aerospace Application. Multifunctionality of Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2015; pp. 367–418. [Google Scholar]
- Altuna, F.I.; Riccardi, C.C.; Marín Quintero, D.C.; Ruseckaite, R.A.; Stefani, P.M. Effect of an Anhydride Excess on the Curing Kinetics and Dynamic Mechanical Properties of Synthetic and Biogenic Epoxy Resins. Int. J. Polym. Sci. 2019, 2019, 5029153. [Google Scholar] [CrossRef]
- Chennapuram, M.; Yoshida, Y.; Endo, T. Curing Behavior and Properties of Epoxy Monomers with Ethylenediaminetetraacetic Dianhydride. J. Appl. Polym. Sci. 2022, 139, 51626. [Google Scholar] [CrossRef]
- Bajpai, A.; Davidson, J.R.; Robert, C. Studies on the Modification of Commercial Bisphenol-A-Based Epoxy Resin Using Different Multifunctional Epoxy Systems. Appl. Mech. 2021, 2, 419–430. [Google Scholar] [CrossRef]
- Brostow, W.; Hagg Lobland, H.E.; Narkis, M. Sliding Wear, Viscoelasticity, and Brittleness of Polymers. J. Mater. Res. 2006, 21, 2422–2428. [Google Scholar] [CrossRef] [Green Version]
- Brostow, W.; Hagg Lobland, H.E. Brittleness of Materials: Implications for Composites and a Relation to Impact Strength. J. Mater. Sci. 2010, 45, 242–250. [Google Scholar] [CrossRef]
- Brostow, W.; Hagg Lobland, H.E.; Narkis, M. The Concept of Materials Brittleness and Its Applications. Polym. Bull. 2011, 67, 1697–1707. [Google Scholar] [CrossRef]
- Engineering:Specific Strength. Available online: https://handwiki.org/wiki/Engineering:Specific_strength (accessed on 14 June 2022).
- Ma, S.; Kovash, C.S.; Webster, D.C. Effect of Solvents on the Curing and Properties of Fully Bio-Based Thermosets for Coatings. J. Coat. Technol. Res. 2017, 14, 367–375. [Google Scholar] [CrossRef]
- Heat Resistance Index and Classification of Plastic Profile Products. Available online: https://www.extrusionplastic.cn/news/plastic-profile-products.html (accessed on 26 July 2021).
- Pan, H.; Sun, G.; Zhao, T.; Wang, G. Thermal Properties of Epoxy Resins Crosslinked by an Aminated Lignin. Polym. Eng. Sci. 2015, 55, 924–932. [Google Scholar] [CrossRef]
- Vijayakumar, C.T.; Chitra, R.; Surender, R.; Pitchaimari, G.; Rajakumar, K. Development of Photodegradable Environment. Plast. Polym. Technol. 2013, 2, 22–37. [Google Scholar]
- Van Krevelen, D.W. Some Basic Aspects of Flame Resistance of Polymeric Materials. Polymer 1975, 16, 615–620. [Google Scholar] [CrossRef]
- Kumar, A.; T’Ien, J. Numerical Modeling of Limiting Oxygen Index Apparatus for Film Type Fuels. Int. J. Spray Combust. Dyn. 2012, 4, 299–322. [Google Scholar] [CrossRef]
- Capiel, G.; Uicich, J.; Fasce, D.; Montemartini, P.E. Diffusion and Hydrolysis Effects during Water Aging on an Epoxy-Anhydride System. Polym. Degrad. Stab. 2018, 153, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, P.; Ramírez, C.; Torres, A.; Abad, M.J.; Cano, J.; López, J.; López-Bueno, I.; Barral, L. Effect of Water Sorption on the Structure and Mechanical Properties of an Epoxy Resin System. J. Appl. Polym. Sci. 2001, 80, 71–80. [Google Scholar] [CrossRef]
- Pereira, A.A.C.; D’Almeida, J.R.M. Effect of the Hardener to Epoxy Monomer Ratio on the Water Absorption Behavior of the DGEBA/TETA Epoxy System. Polímeros 2016, 26, 30–37.
- Toscano, A.; Pitarresi, G.; Scafidi, M.; Di Filippo, M.; Spadaro, G.; Alessi, S. Water Diffusion and Swelling Stresses in Highly Crosslinked Epoxy Matrices. Polym. Degrad. Stab. 2016, 133, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Bouvet, G.; Dang, N.; Cohendoz, S.; Feaugas, X.; Mallarino, S.; Touzain, S. Impact of Polar Groups Concentration and Free Volume on Water Sorption in Model Epoxy Free Films and Coatings. Prog. Org. Coatings 2016, 96, 32–41. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: New York, NY, USA, 1979; ISBN 0198534116. [Google Scholar]
- Li, L.; Yu, Y.; Wu, Q.; Zhan, G.; Li, S. Effect of Chemical Structure on the Water Sorption of Amine-Cured Epoxy Resins. Corros. Sci. 2009, 51, 3000–3006. [Google Scholar] [CrossRef]
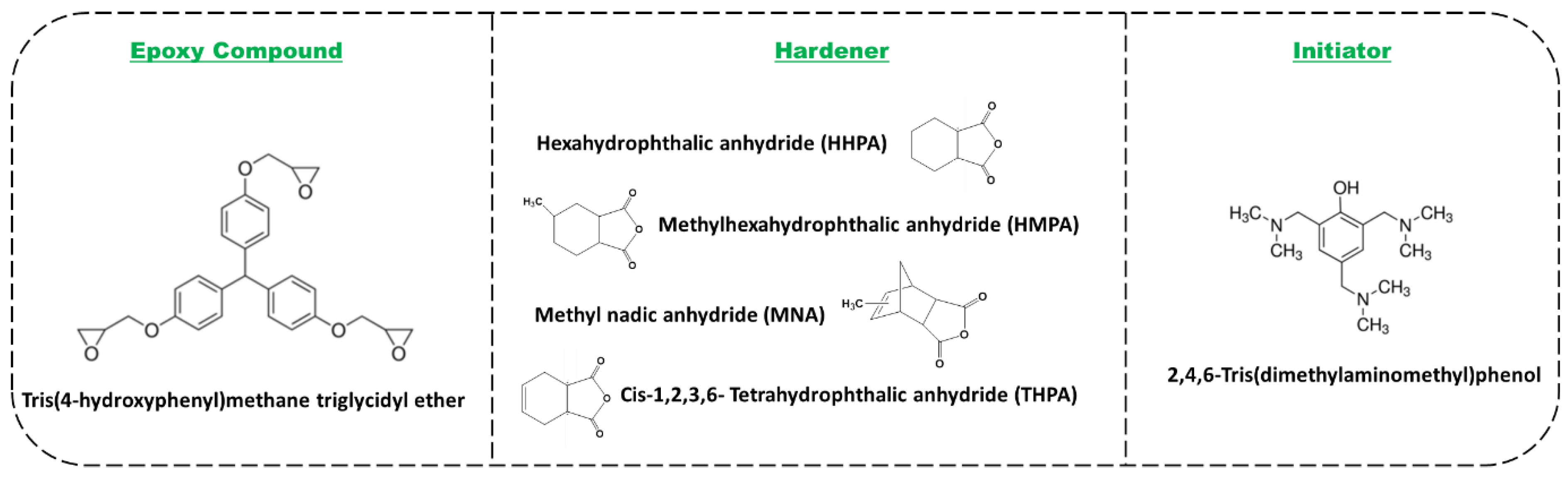

| Sample | Quantity (g) | |||||
|---|---|---|---|---|---|---|
| THPMTGE | D | HHPA | HMPA | MNA | THPA | |
| T-HHPA | 66 | 1 | 33 | - | - | - |
| T-HMPA | 64 | 1 | - | 35 | - | - |
| T-MNA | 63 | 1 | - | - | 36 | - |
| T-THPA | 66 | 1 | - | - | - | 33 |
| Sample | Tpeak (°C) | Tonset-Tend (°C) | ΔH (J·g−1) | GC (%) |
|---|---|---|---|---|
| T-HHPA | 138 | 60–180 | 341 | 99.91 |
| T-HMPA | 134 | 55–200 | 329 | 99.95 |
| T-THPA | 137 | 75–190 | 325 | 99.90 |
| T-MNA | 146 | 95–240 | 278 | 99.92 |
| Thermoset | Density (g/cm3) | Shore D | E′ at 25 °C (GPa) | E′ at 200 °C (GPa) | tan δ (°C) | Tg-DSC (°C) | ν (mmol∙cm−3) | Mc (g/mol) | Sample Aspect |
|---|---|---|---|---|---|---|---|---|---|
| T-HHPA | 1.13 | 88 | 3.3 | 0.08 | 171 | 179 | 5.70 | 204.43 |  |
| T-HMPA | 1.17 | 90 | 3 | 0.07 | 179 | 167 | 4.74 | 248.01 |  |
| T-THPA | 1.13 | 85 | 3.5 | 0.11 | 196 | 181 | 6.34 | 220.44 |  |
| T-MNA | 1.16 | 84 | 3.4 | 0.08 | 167 | 160 | 5.21 | 230.73 |  |
| Sample | Young’s Modulus (GPa) | Tensile Stress at Break (MPa) | Elongation at Break (%) | Specific Modulus E/ρ (106·m2/s2) | Specific Strength σ/ρ (kN·m/kg) | Specific Length σ/ρ·g (km) | B (%·Pa/1010) |
|---|---|---|---|---|---|---|---|
| T-HHPA | 1.25 ± 0.16 | 45 ± 11 | 4.8 ± 1.12 | 1.11 | 39.82 | 4.06 | 0.063 |
| T-HMPA | 1.25 ± 0.15 | 44 ± 11 | 5.1 ± 1.51 | 1.07 | 37.61 | 3.84 | 0.065 |
| T-THPA | 1.31 ± 0.18 | 41 ± 12 | 4.2 ± 1.42 | 1.16 | 36.28 | 3.70 | 0.068 |
| T-MNA | 1.29 ± 0.22 | 34 ± 27 | 3.9 ± 2.77 | 1.11 | 29.31 | 2.99 | 0.075 |
| Samples | T1% (°C) | T5% (°C) | T30% (°C) | Tdmax (°C) | Ts | Cy800 (%) | LOI (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Air | N2 | Air | N2 | Air | N2 | Air | N2 | Air | N2 | Air | N2 | Air | N2 | |
| T-HHPA | 291 | 261 | 350 | 345 | 399 | 395 | 399 | 399 | 186 | 184 | 6 | 28 | N/A | 28.7 |
| T-HMPA | 320 | 310 | 360 | 359 | 395 | 395 | 399 | 399 | 188 | 187 | 0 | 18 | N/A | 24.7 |
| T-MNA | 280 | 261 | 340 | 340 | 400 | 395 | 409 | 401 | 187 | 183 | 0.7 | 27 | N/A | 28.3 |
| T-THPA | 320 | 310 | 360 | 360 | 395 | 395 | 399 | 395 | 188 | 187 | 1 | 22 | N/A | 26.3 |
| Samples | Water Absorption (%) | |
|---|---|---|
| at 24 h | at Saturation | |
| T-HHPA | 0.48 | 0.90 |
| T-HMPA | 0.39 | 0.77 |
| T-THPA | 0.32 | 0.84 |
| T-MNA | 0.40 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, R.; Lafont, U.; Damiano, O.; Mija, A. Development of Sustainable High Performance Epoxy Thermosets for Aerospace and Space Applications. Polymers 2022, 14, 5473. https://doi.org/10.3390/polym14245473
Dinu R, Lafont U, Damiano O, Mija A. Development of Sustainable High Performance Epoxy Thermosets for Aerospace and Space Applications. Polymers. 2022; 14(24):5473. https://doi.org/10.3390/polym14245473
Chicago/Turabian StyleDinu, Roxana, Ugo Lafont, Olivier Damiano, and Alice Mija. 2022. "Development of Sustainable High Performance Epoxy Thermosets for Aerospace and Space Applications" Polymers 14, no. 24: 5473. https://doi.org/10.3390/polym14245473







