The Influence of Organofunctional Substituents of Spherosilicates on the Functional Properties of PLA/TiO2 Composites Used in 3D Printing (FDM/FFF)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
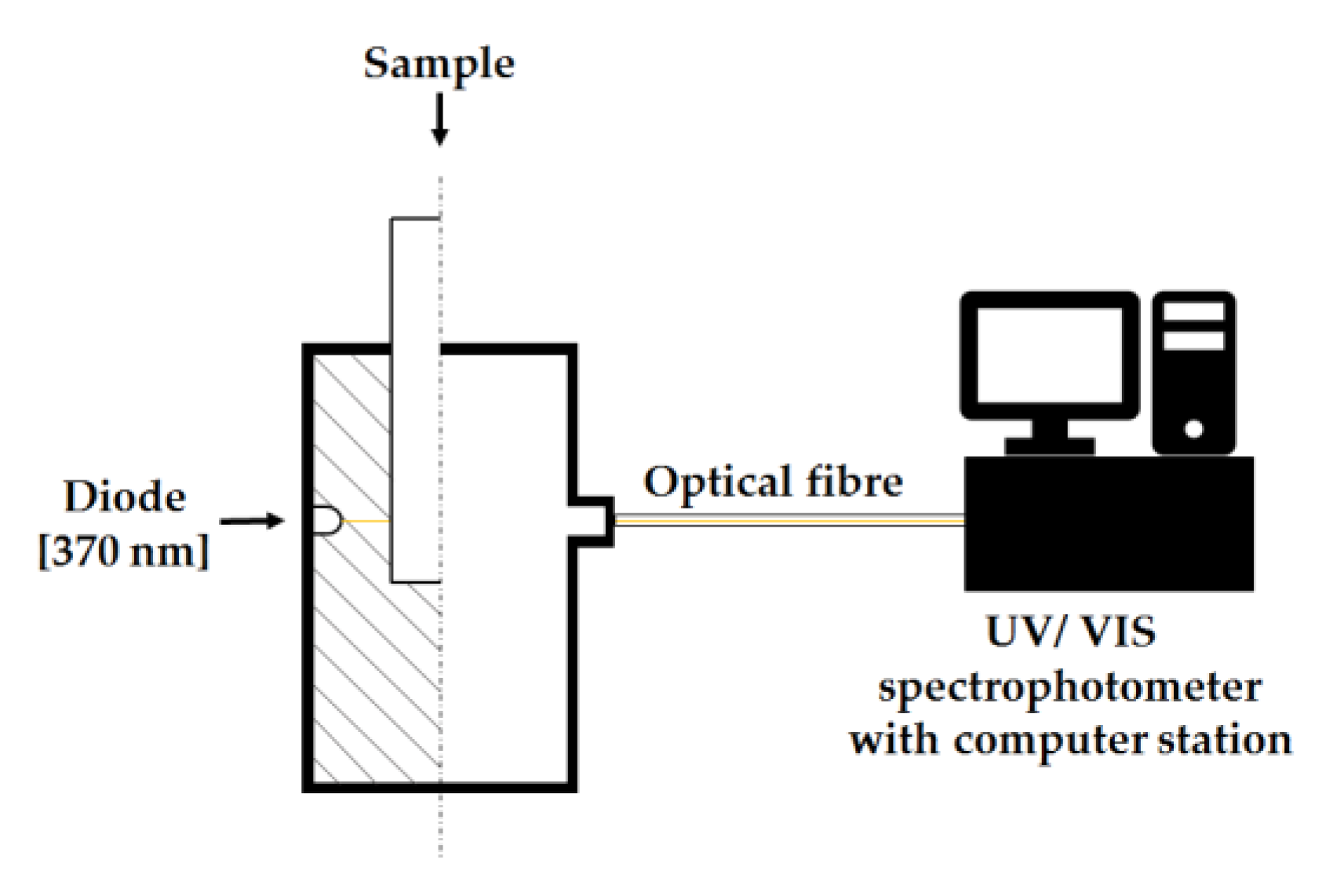
2.2. Analyses
2.3. The Procedure for Synthesis of Octaspherosilicate Derivatives
2.4. The Procedure of Mixing TiO2 with the Modifier
2.5. Fabrication of Filaments
2.5.1. Preparation of Granulates
2.5.2. Extrusion of Filaments
2.6. 3D Printing (FDM)
3. Results and Discussion
3.1. Chemical Characterization of Modifiers
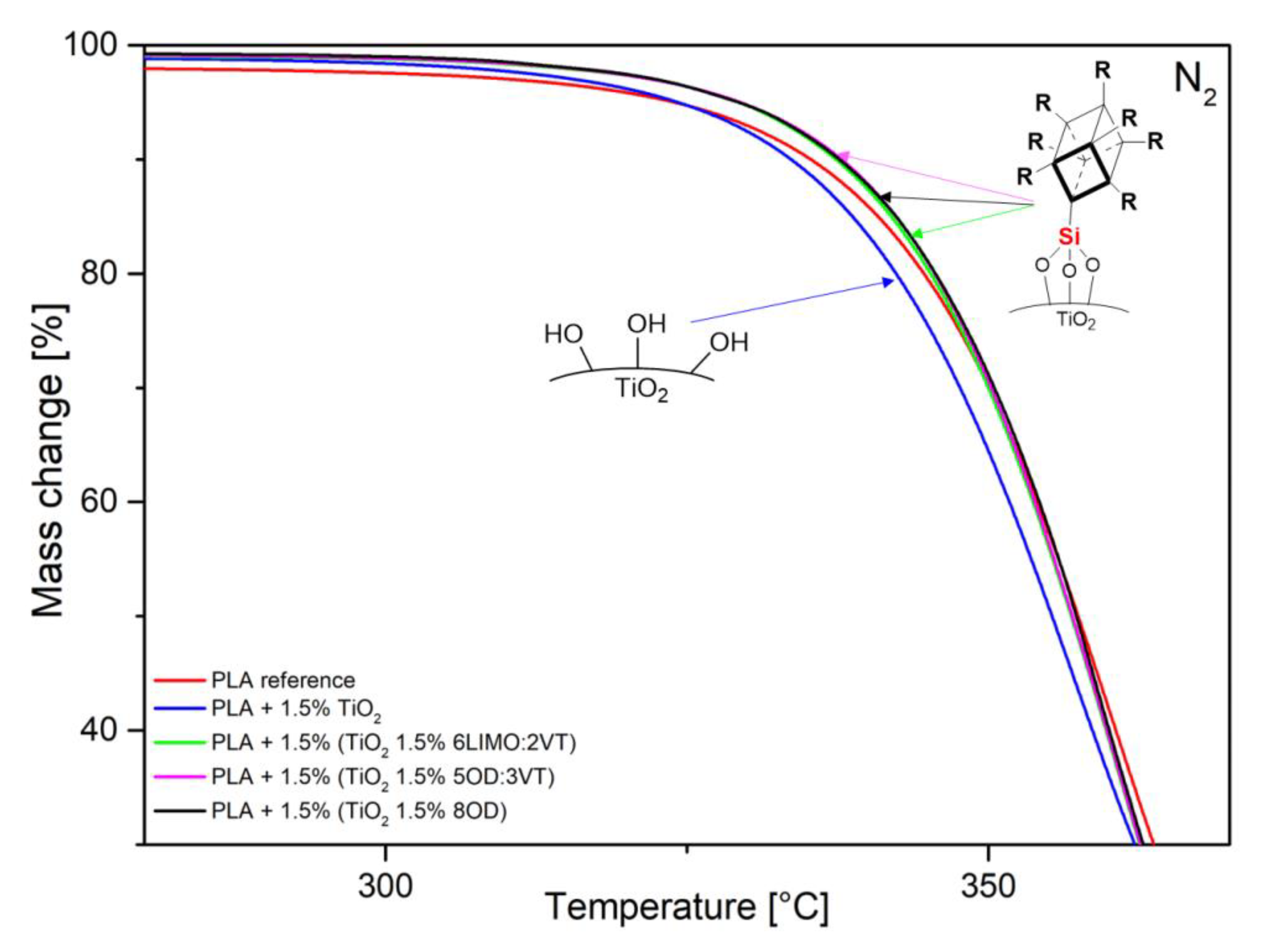
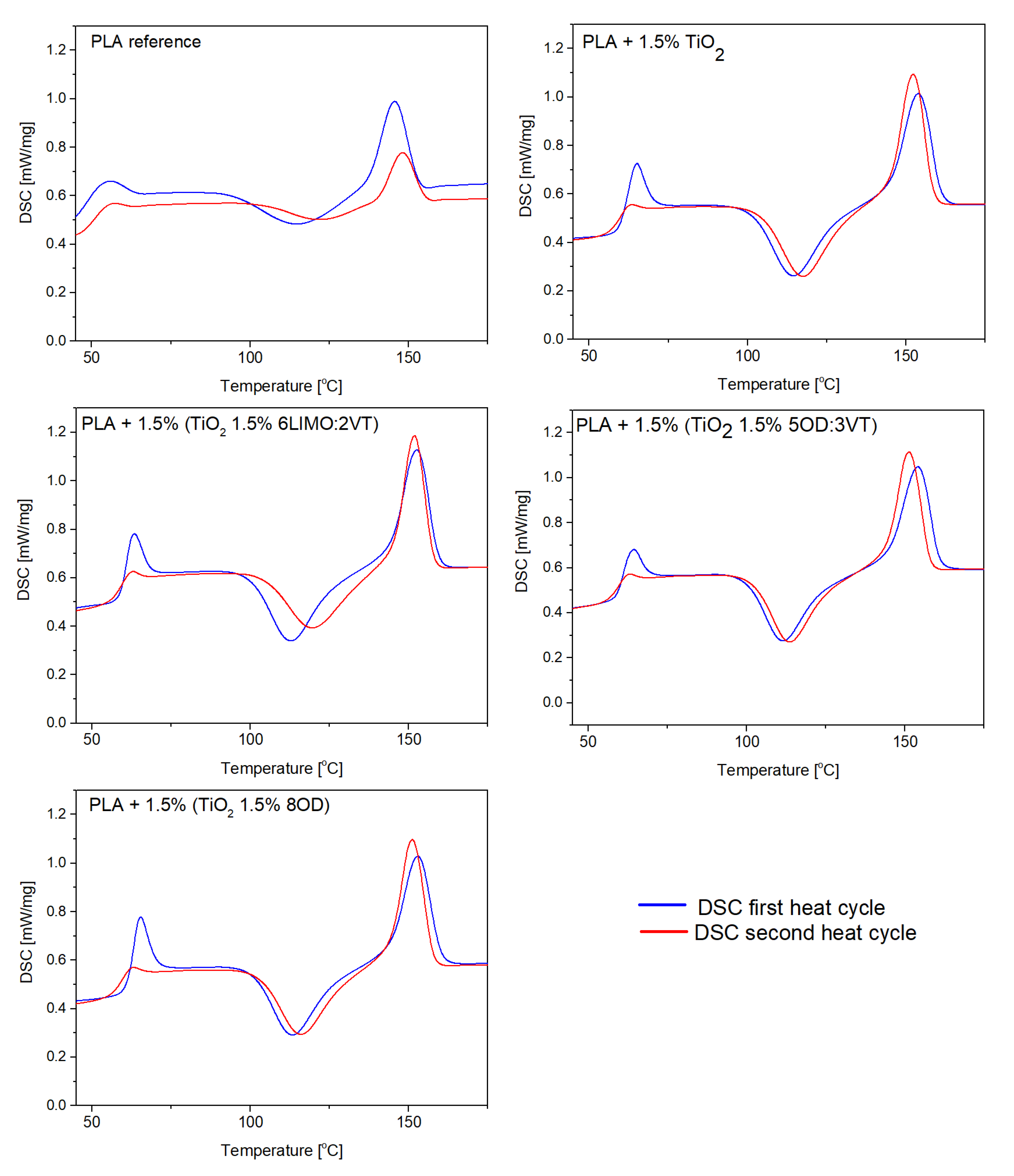
3.2. Thermal Analysis (TGA, DSC)
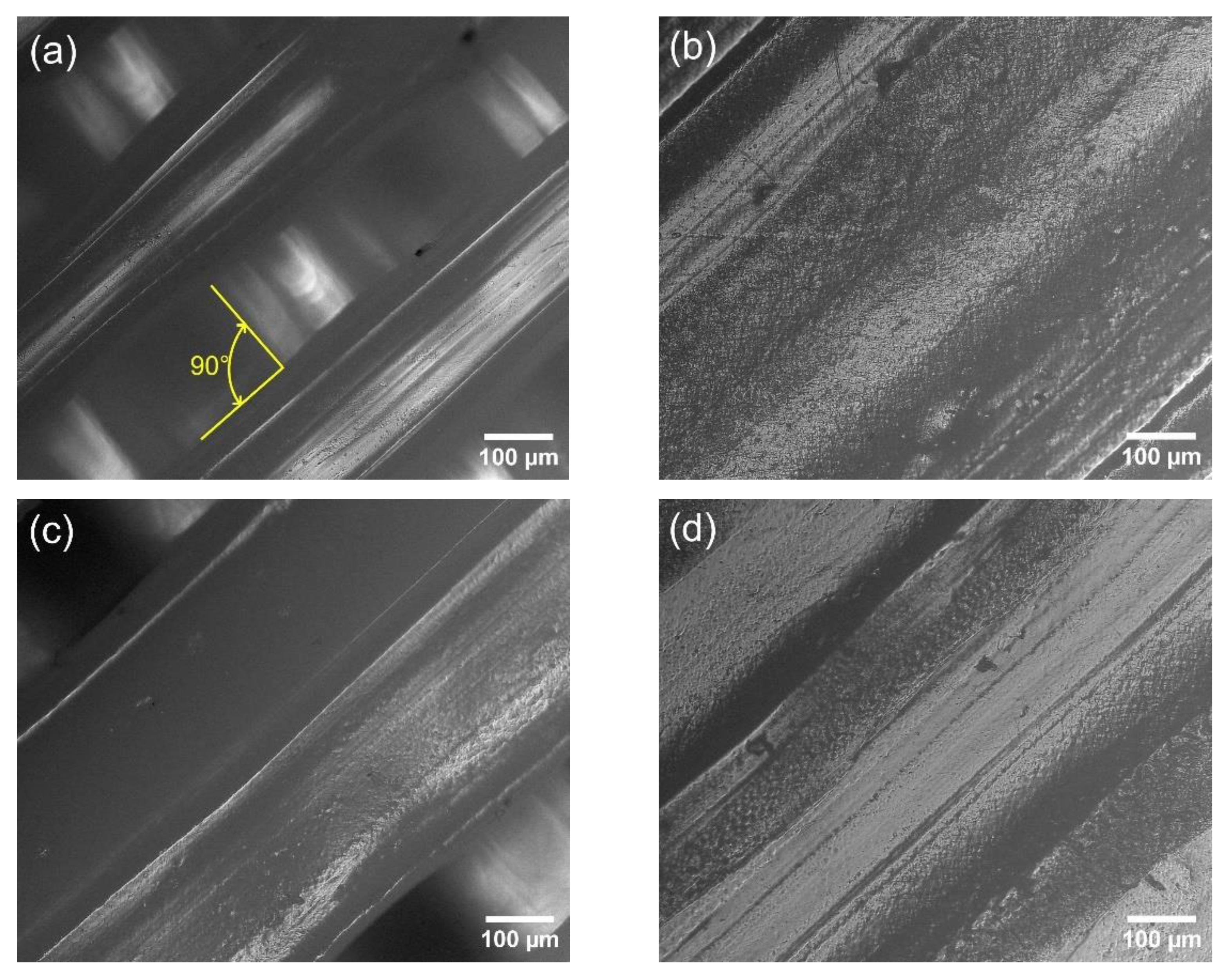
3.3. Images of the PLA/TiO2 Composites Surface
3.4. Water Contact Angle Analysis (WCA)
3.4.1. WCA of Modified TiO2
3.4.2. WCA of PLA/TiO2/Organosilicon Compound Composites
3.5. Mass Flow Rate (MFR)
3.6. Measurement of the Relative Opacity
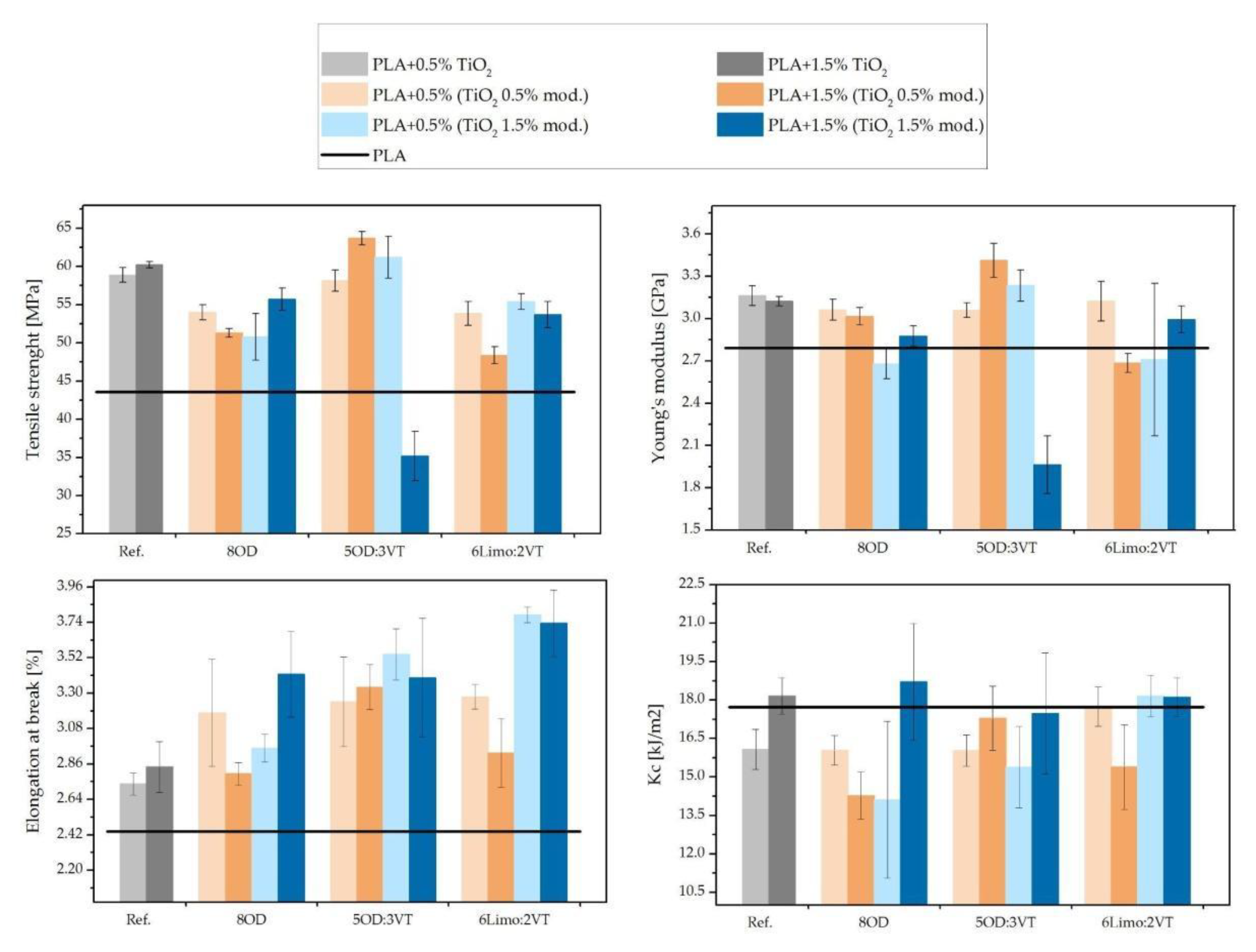
3.7. Tensile Strength and Impact Strength Tests
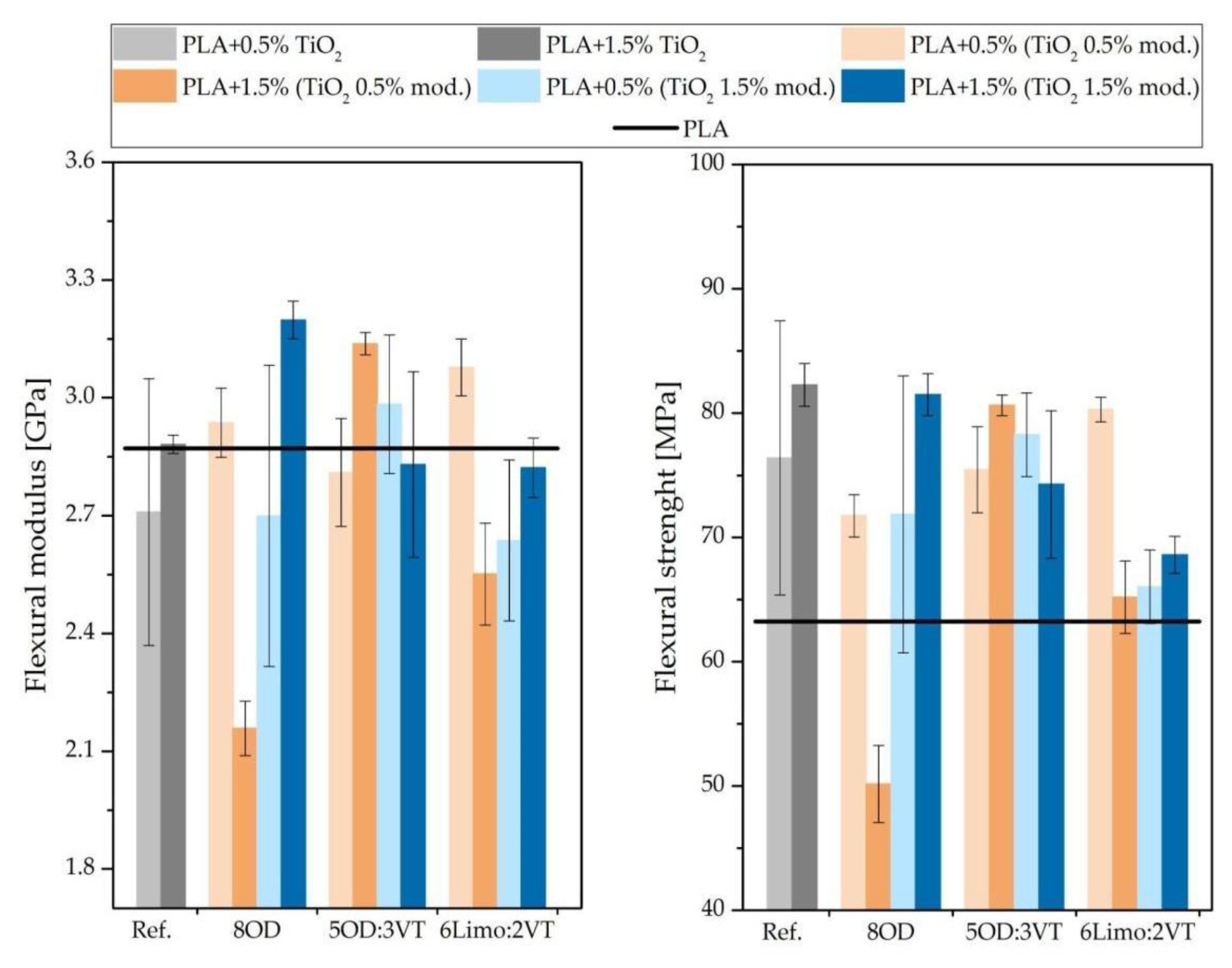
3.8. Flexural Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olam, M.; Tosun, N. Assessment of 3D Printings Produced in Fused Deposition Modeling Printer Using Polylactic Acid/TiO2/Hydroxyapatite Composite Filaments. J. Mater. Eng. Perform. 2022, 31, 4554–4565. [Google Scholar] [CrossRef]
- Cardoso, P.; Oliveira, M.; Oliveira, M.; Thire, R.M.d.M. 3D Printed Parts of Polylactic Acid Reinforced with Carbon Black and Alumina Nanofillers for Tribological Applications. Macromol. Symp. 2020, 394, 2000155. [Google Scholar] [CrossRef]
- McQueen, A.; Ballentine, M.; Lauren, R.; Laber, C.; Das, A.; Bortner, M.; Kennedy, A. Photocatalytic Degradation of Polycyclic Aromatic Hydrocarbons in Water by 3D Printed TiO2 Composites. ACS ES&T Water 2022, 2, 137–147. [Google Scholar]
- Torres, J.; Cole, M.; Owji, A.; DeMastry, Z.; Gordon, A. An Approach for Mechanical Property Optimization of Fused Deposition Modeling with Polylactic Acid via Design of Experiments. Rapid Prototyp. J. 2016, 22, 387–404. [Google Scholar] [CrossRef]
- Ertane, E.G.; Dorner-Reisel, A.; Baran, O.; Welzel, T.; Matner, V.; Svoboda, S. Processing and Wear Behaviour of 3D Printed PLA Reinforced with Biogenic Carbon. Adv. Tribol. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Borkowski, G.; Martyła, A.; Dobrosielska, M.; Marciniak, P.; Gabriel, E.; Głowacka, J.; Jałbrzykowski, M.; Pakuła, D.; Przekop, R. Carbonate Lake Sediments in the Plastics Processing-Preliminary Polylactide Composite Case Study: Mechanical and Structural Properties. Materials 2022, 15, 6106. [Google Scholar] [CrossRef]
- Loyo, C.; Moreno-Serna, V.; Fuentes, J.; Amigo, N.; Sepúlveda, F.A.; Ortiz, J.; Rivas, L.; Ulloa, M.; Benavente, R.; Zapata, P. PLA/CaO nanocomposites with antimicrobial and photodegradation properties. Polym. Degrad. Stab. 2022, 197, 1–14. [Google Scholar] [CrossRef]
- Fonseca, C.; Ochoa, A.; Ulloa, M.; Alvarez, E.; Canales, D.; Zapata, P. Poly(lactic acid)/TiO2 nanocomposites as alternative biocidal and antifungal materials. Mater. Sci. Eng. C. 2015, 57, 314–320. [Google Scholar] [CrossRef]
- Widiastuti, I. Polylactide nanocomposites for packaging materials: A review. In Proceedings of the 6th Nanoscience And Nanotechnology Symposium, American Institute of Physics Inc., Surakarta, Indonesia, 4–5 November 2015. [Google Scholar]
- Gálvez, J.; Aguirre, J.C.; Salazar, M.H.; Mondragón, B.V.; Wagner, E.; Caicedo, C. Effect of extrusion screw speed and plasticizer proportions on the rheological, thermal, mechanical, morphological and superficial properties of PLA. Polymers 2020, 12, 2020. [Google Scholar] [CrossRef]
- Murarriu, J.; Dubois, P. PLA compositers: Fromproductiontion to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Salahuddin, N.; Abdelwahab, M.; Gaber, M.; Elneana, S. Synthesis and design of norfloxacin drug delivery system based on PLA/TiO2 nanocomposites: Antibacterial and antitumor activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 2020. [Google Scholar] [CrossRef] [PubMed]
- Milovanović, S.; Marković, D.; Pantić, M.; Pavlović, S.; Knapczyk-Korczak, J.; Stachewicz, U.; Novak, Z. Development of advanced floating poly(lactic acid)-based materials for colored wastewater treatment. J. Supercrit Fluids 2021, 177, 105328. [Google Scholar] [CrossRef]
- Su, S.; Huang, Y.; Qu, S.; Liu, W.; Liu, R.; Li, L. Microdiamond/PLA composites with enhanced thermal conductivity. Diam. Relat. Mater. 2018, 81, 161–167. [Google Scholar] [CrossRef]
- Cifuentes, S.; Frutos, E.; Benavente, R.; Loren, V.; Gonzalez-Carrasco, J. Assessment of mechanical behavior of PLA composites reinforced with Mg micro-particles through depth-sensing indentations analysis. J. Mech. Behav. Biomed. Mater. 2017, 65, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Brounstein, Z.; Yeager, C.; Labouriau, A. Development of Antimicrobial PLA Composites for Fused Filament Fabrication. Polymers 2021, 13, 580. [Google Scholar] [CrossRef]
- Bayer, I. Thermomechanical Properties of Polylactic Acid-Graphene Composites: A State-of-the-Art Review for Biomedical. Materials 2017, 10, 748. [Google Scholar] [CrossRef] [Green Version]
- Grande, R.; Pessan, L.; Carvalho, A. Ternary melt blends of poly(lactic acid)/poly(vinyl alcohol)-chitosan. Ind. Crops Prod. 2015, 72, 159–165. [Google Scholar] [CrossRef]
- Li, H.-Z.; Chen, S.-C.; Wang, Y.-Z. Thermoplastic PVA/PLA Blends with Improved Processability and Hydrophobicity. Ind. Eng. Chem. Res. 2014, 53, 17355–17361. [Google Scholar] [CrossRef]
- Mallick, S.; Ahmad, Z.; Touati, F.; Bhadra, J.; Shakoor, R.; Al-Thani, N. PLA-TiO2 nanocomposites: Thermal, morphological, structural, and humidity sensing properties. Ceram. Int. 2018, 44, 16507–16513. [Google Scholar] [CrossRef]
- Ponnamma, D.; Cabibihan, J.; Rajan, M.; Pethaiah, S.; Deshmukh, K.; Gogoi, J.; Pasha, S.; Ahamed, M.; Krishnegowda, J. Synthesis, optimization and applications of ZnO/polymer nanocomposites. Mater. Sci. Eng. C 2019, 98, 1210–1240. [Google Scholar]
- González, E.S.; Olmos, D.; Lorente, M.; Vélaz, I.; González-Benito, J. Preparation and characterization of polymer composite materials based on PLA/TiO2. Polymers 2018, 10, 1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Wang, L.; Zou, Y.; Sheng, X.; Chang, L.; Yang, D. Electrochemically deposited Cu2O on TiO2 nanorod arrays for photovoltaic application. Electrochem. Solid-State Lett. 2021, 15, H34. [Google Scholar] [CrossRef]
- Brząkalski, D.; Przekop, R.; Sztorch, B.; Frydrych, M.; Pakuła, D.; Jałbrzykowski, M.; Markiewicz, G.; Marciniec, B. Why POSS-Type Compounds Should Be Considered Nanomodifiers, Not Nanofillers-A Polypropylene Blends Case Study. Polymers 2021, 13, 2124. [Google Scholar] [CrossRef] [PubMed]
- Zeybek, Y.; Kaynak, C. Behaviour of PLA/POSS nanocomposites: Effects of filler content, functional group and copolymer compatibilization, Polym. Polym. Compos. 2021, 29, 485–500. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, S.; Liu, W.; Shi, H.; Liang, L.; Liu, C.; Pi, K.; Zhang, W.; Zeng, J. Design on the corrosion protection of eco-friendly and multifunctional polyhedral oligomeric silsesquioxane functionalized graphene oxide reinforced waterborne polyurethane, Colloids Surf. A Physicochem. Eng. Asp. 2020, 640, 127718. [Google Scholar] [CrossRef]
- Kodal, M.; Wis, A.; Ozkoc, G. The mechanical, thermal and morphological properties of γ-irradiated PLA/TAIC and PLA/OvPOSS, Radiat. Phys. Chem. 2018, 153, 214–225. [Google Scholar]
- Baldi, F.; Bignotti, F.; Fina, A.; Tabuani, D.; Riccò, T. Mechanical characterization of polyhedral oligomeric silsesquioxane/polypropylene blends. J. Appl. Polym. Sci. 2007, 105, 935–943. [Google Scholar] [CrossRef]
- Sirin, H.; Kodal, M.; Ozkoc, G. The influence of POSS type on the properties of PLA. Polym. Compos. 2016, 37, 1497–1506. [Google Scholar] [CrossRef]
- Turan, D.; Sirin, H.; Ozkoc, G. Effects of POSS particles on the mechanical, thermal, and morphological properties of PLA and plasticised PLA. J. Appl. Polym. Sci. 2011, 121, 1067–1075. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, J. Thermal ablation mechanism of polyimide reinforced with POSS under atomic oxygen bombardment. Appl. Surf. Sci. 2021, 567, 150578. [Google Scholar] [CrossRef]
- Batibay, G.; Gunkara, O.; Ocal, N.; Arsu, N. Thioxanthone attached polyhedral oligomeric silsesquioxane (POSS) nano-photoinitiator for preparation of PMMA hybrid networks in air atmosphere. Prog. Org. Coat. 2019, 149, 105935. [Google Scholar] [CrossRef]
- Zhu, Y.; Ramadani, E.; Egap, E. Thiol ligand capped quantum dot as an efficient and oxygen tolerance photoinitiator for aqueous phase radical polymerization and 3D printing under visible light. Polym. Chem. 2021, 12, 5106–5116. [Google Scholar] [CrossRef]
- Filho, N.; de Aquino, H.; Pires, G.; Caetano, G. Relationship between the dielectric and mechanical properties and the ratio of epoxy resin to hardener of the hybrid thermosetting polymers. J. Braz. Chem. Soc. 2020, 17, 533–541. [Google Scholar] [CrossRef]
- Brząkalski, D.; Przekop, R.E.; Frydrych, M.; Pakuła, D.; Dobrosielska, M.; Sztorch, B.; Marciniec, B. Where ppm Quantities of Silsesquioxanes Make a Difference—Silanes and Cage Siloxanes as TiO2 Dispersants and Stabilizers for Pigmented Epoxy Resins. Materials 2022, 15, 494. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, Z.; Wei, M.; Lu, T.; Nong, D.; Zhao, J.; Gao, X.; Teng, L. Catalytic effect of nanosized ZnO and TiO2 on thermal degradation of poly(lactic acid) and isoconversional kinetic analysis. Thermochim. Acta 2019, 672, 14–24. [Google Scholar] [CrossRef]







| No | Code | Organosilicon Compound | Amount of Organosilicon Compound [%] | Amount of TiO2 [%] |
|---|---|---|---|---|
| 1 | PLA | - | - | - |
| 2 | PLA + 0.5% TiO2 | - | - | 0.5 |
| 3 | PLA + 1.5% TiO2 | - | - | 1.5 |
| 4 | PLA + 0.5% (TiO2 0.5% 8OD) | SS:8OD | 0.5 | 0.5 |
| 5 | PLA + 0.5% (TiO2 1.5% 8OD) | SS:8OD | 1.5 | 0.5 |
| 6 | PLA + 1.5% (TiO2 0.5% 8OD) | SS:8OD | 0.5 | 1.5 |
| 7 | PLA + 1.5% (TiO2 1.5% 8OD) | SS:8OD | 1.5 | 1.5 |
| 8 | PLA + 0.5% (TiO2 0.5% 5OD:3VT) | SS:5OD:3VTMOS | 0.5 | 0.5 |
| 9 | PLA + 0.5% (TiO2 1.5% 5OD:3VT) | SS:5OD:3VTMOS | 1.5 | 0.5 |
| 10 | PLA + 1.5% (TiO2 0.5% 5OD:3VT) | SS:5OD:3VTMOS | 0.5 | 1.5 |
| 11 | PLA + 1.5% (TiO2 1.5% 5OD:3VT) | SS:5OD:3VTMOS | 1.5 | 1.5 |
| 12 | PLA + 0.5% (TiO2 0.5% 6Limo:2VT) | SS:6LIMO:2VTMOS | 0.5 | 0.5 |
| 13 | PLA + 0.5% (TiO2 1.5% 6Limo:2VT) | SS:6LIMO:2VTMOS | 1.5 | 0.5 |
| 14 | PLA + 1.5% (TiO2 0.5% 6Limo:2VT) | SS:6LIMO:2VTMOS | 0.5 | 1.5 |
| 15 | PLA + 1.5% (TiO2 1.5% 6Limo:2VT) | SS:6LIMO:2VTMOS | 1.5 | 1.5 |
| Properties | Values |
|---|---|
| Layer height | 0.2 mm |
| First layer height | 0.2 mm |
| Number of shells | 2 |
| Top and bottom layers number | 3 |
| Nozzle diameter | 0.4 mm |
| Infill density | 100% |
| First layer speed | 20 mm/s |
| Printing speed | 60 mm/s |
| Bed temp. | 60 °C |
| Extruder temp. | 210 °C |
| Code | Onset Temperature [°C] |
|---|---|
| PLA reference | 342.6 |
| PLA + 1.5% TiO2 | 338.7 |
| PLA + 1.5% (TiO2 1.5% 6LIMO:2VT) | 343.0 |
| PLA + 1.5% (TiO2 1.5% 5OD:3VT) | 343.0 |
| PLA + 1.5% (TiO2 1.5% 8OD) | 343.7 |
| Code | Heat Cycle | Glass Transition (Tg) [°C] | Crystallization (Tc) [°C] | Melting (Tm) [°C] |
|---|---|---|---|---|
| PLA | first | 62.0 | 124.5 | 154.5 |
| second | 62.9 | 127.6 | 153.9 | |
| PLA + 1.5% TiO2 | first | 64.3 | 114.5 | 154.0 |
| second | 63.1 | 117.3 | 152.3 | |
| PLA + 1.5% (TiO2 1.5% 5OD:3VT) | first | 63.6 | 111.4 | 151.1 |
| second | 62.5 | 113.6 | 154.7 | |
| PLA + 1.5% (TiO2 1.5% 6LIMO:2VT) | first | 63.2 | 112.5 | 152.5 |
| second | 62.3 | 119.3 | 151.8 | |
| PLA + 1.5% (TiO2 1.5% 8OD) | first | 65.1 | 113.3 | 152.8 |
| second | 62.7 | 115.7 | 151.1 |
| Sample Name | Contact Angle [°] |
|---|---|
| TiO2 | 0 |
| TiO2 0.5% 5OD:3VT | 0 |
| TiO2 1.5% 5OD:3VT | 141.4 ± 3.4 |
| TiO2 0.5% 6LIMO:2VT | 0 |
| TiO2 1.5% 6LIMO:2VT | 138.9 ± 2.9 |
| TiO2 0.5% 8OD | 0 |
| TiO2 1.5% 8OD | 142.8 ± 3.5 |
| Code | Contact Angle [°] |
|---|---|
| PLA | 71.3 ± 3.1 |
| PLA + 1.5% TiO2 | 66.8 ± 5.5 |
| PLA + 1.5% (TiO2 0.5% 5OD:3VT) | 71.0 ± 1.7 |
| PLA + 1.5% (TiO2 1.5% 5OD:3VT) | 82.5 ± 1.1 |
| PLA + 1.5% (TiO2 0.5% 6LIMO:2VT) | 71.6 ± 1.0 |
| PLA + 1.5% (TiO2 1.5% 6LIMO:2VT) | 76.6 ± 3.8 |
| PLA + 1.5% (TiO2 0.5% 8OD) | 70.8 ± 1.6 |
| PLA + 1.5% (TiO2 1.5% 8OD) | 77.4 ± 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sztorch, B.; Pakuła, D.; Kustosz, M.; Romanczuk-Ruszuk, E.; Gabriel, E.; Przekop, R.E. The Influence of Organofunctional Substituents of Spherosilicates on the Functional Properties of PLA/TiO2 Composites Used in 3D Printing (FDM/FFF). Polymers 2022, 14, 5493. https://doi.org/10.3390/polym14245493
Sztorch B, Pakuła D, Kustosz M, Romanczuk-Ruszuk E, Gabriel E, Przekop RE. The Influence of Organofunctional Substituents of Spherosilicates on the Functional Properties of PLA/TiO2 Composites Used in 3D Printing (FDM/FFF). Polymers. 2022; 14(24):5493. https://doi.org/10.3390/polym14245493
Chicago/Turabian StyleSztorch, Bogna, Daria Pakuła, Magdalena Kustosz, Eliza Romanczuk-Ruszuk, Ewa Gabriel, and Robert E. Przekop. 2022. "The Influence of Organofunctional Substituents of Spherosilicates on the Functional Properties of PLA/TiO2 Composites Used in 3D Printing (FDM/FFF)" Polymers 14, no. 24: 5493. https://doi.org/10.3390/polym14245493
APA StyleSztorch, B., Pakuła, D., Kustosz, M., Romanczuk-Ruszuk, E., Gabriel, E., & Przekop, R. E. (2022). The Influence of Organofunctional Substituents of Spherosilicates on the Functional Properties of PLA/TiO2 Composites Used in 3D Printing (FDM/FFF). Polymers, 14(24), 5493. https://doi.org/10.3390/polym14245493









