Fabrication of Inorganic Coatings Incorporated with Functionalized Graphene Oxide Nanosheets for Improving Fire Retardancy of Wooden Substrates
Abstract
1. Introduction
2. Experimental Section
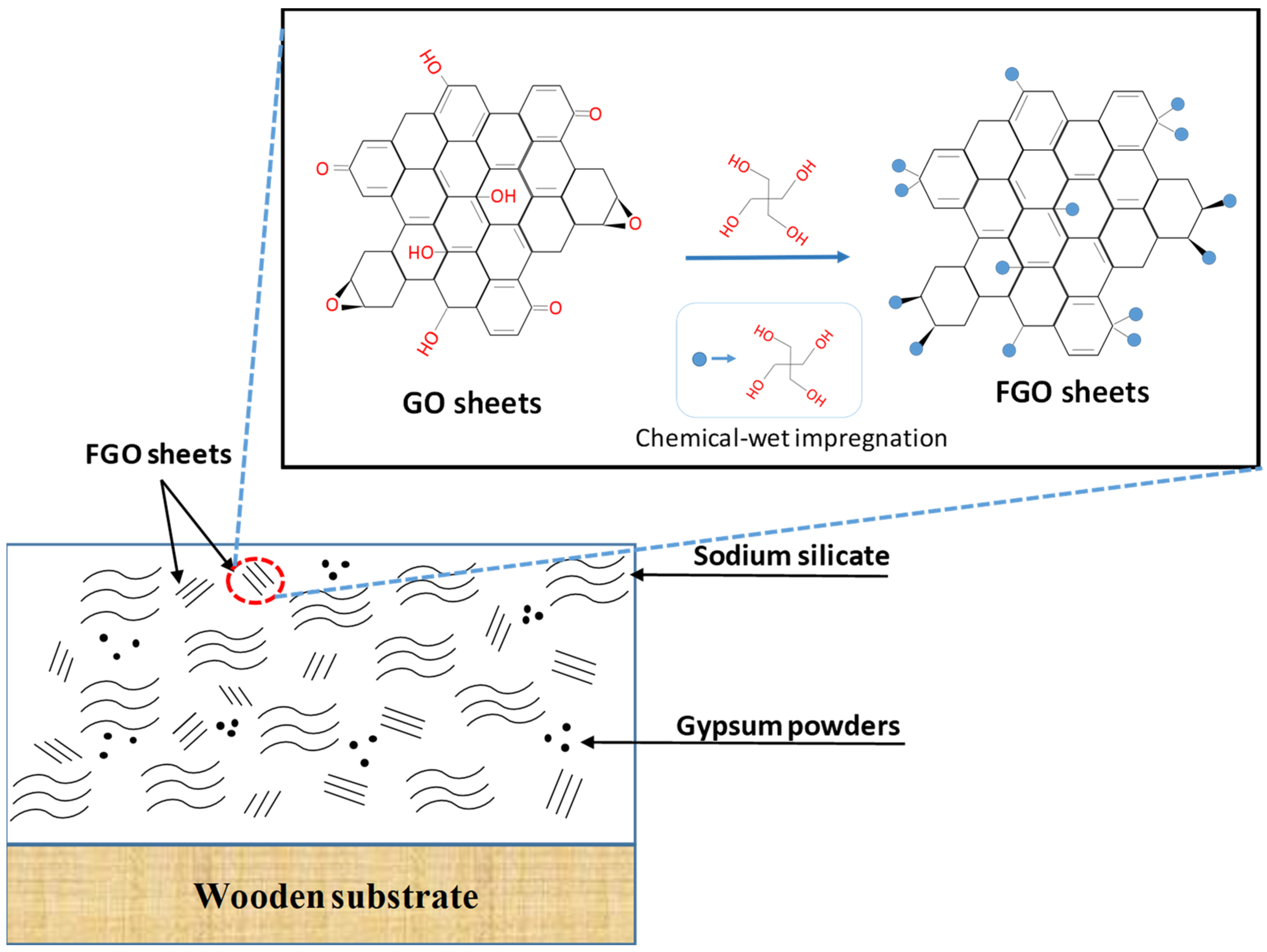
2.1. Synthesis of FGO Sheets
2.2. Fireproof Performance of FGO-Filled Coatings
2.3. Materials’ Characterization
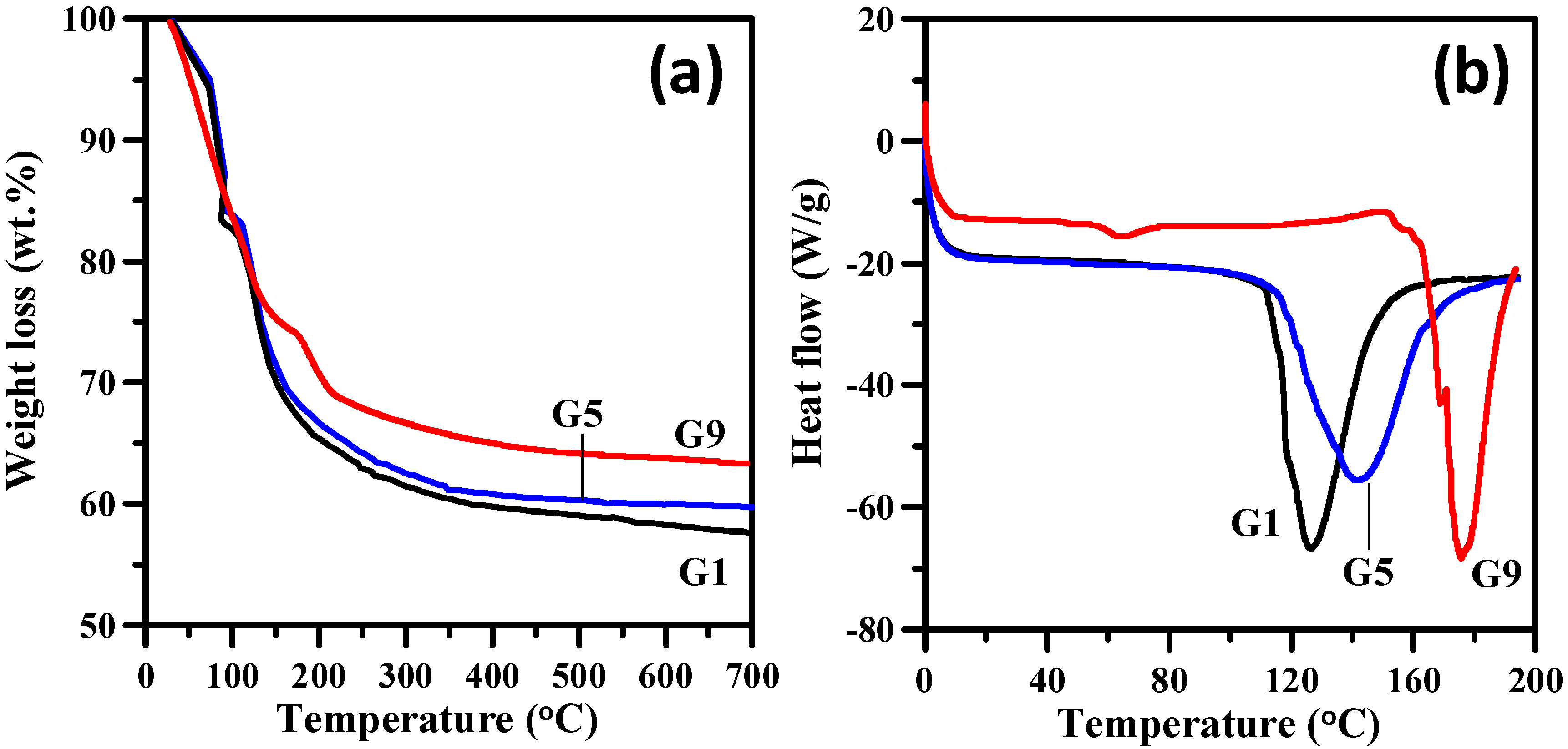
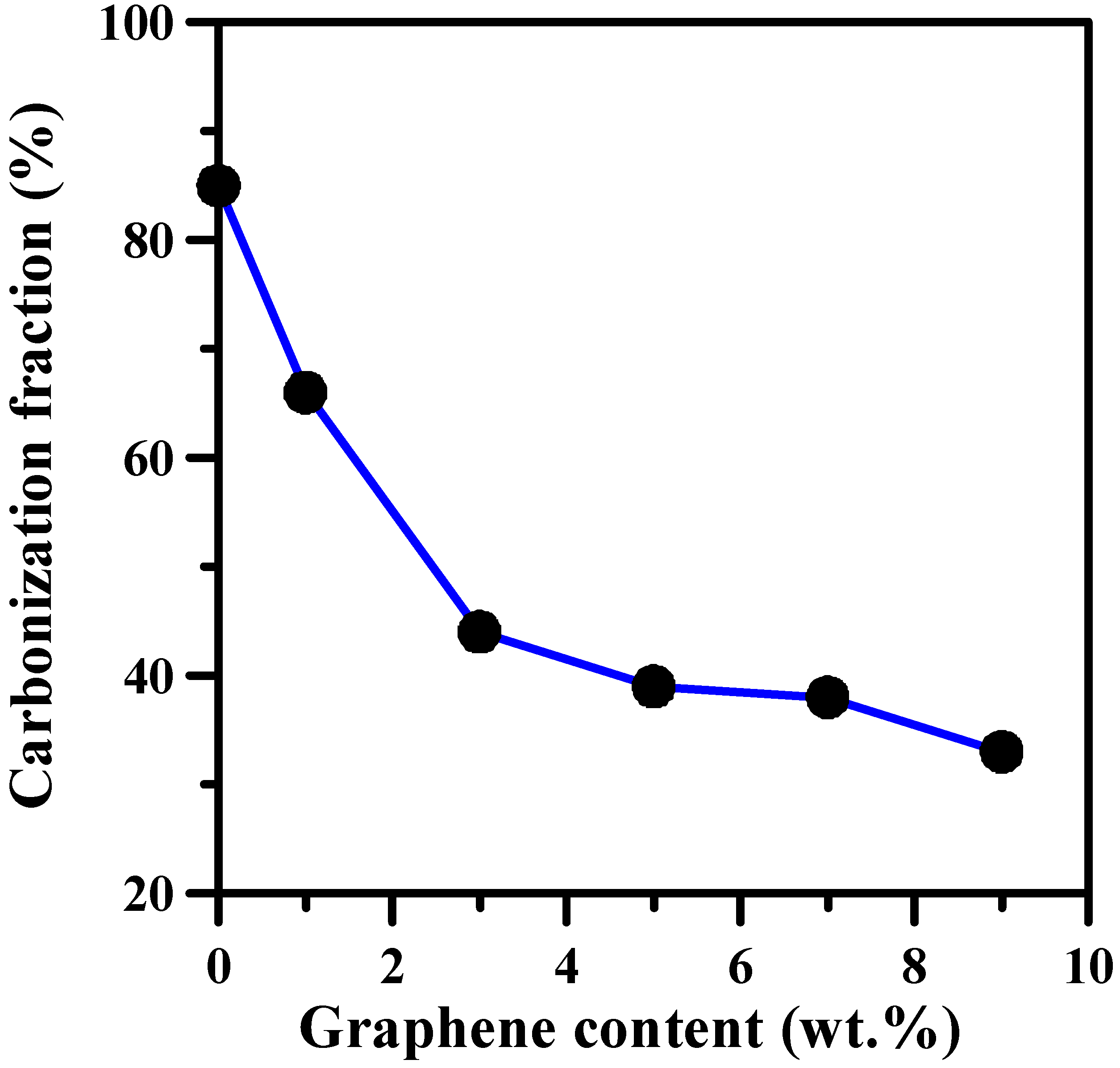
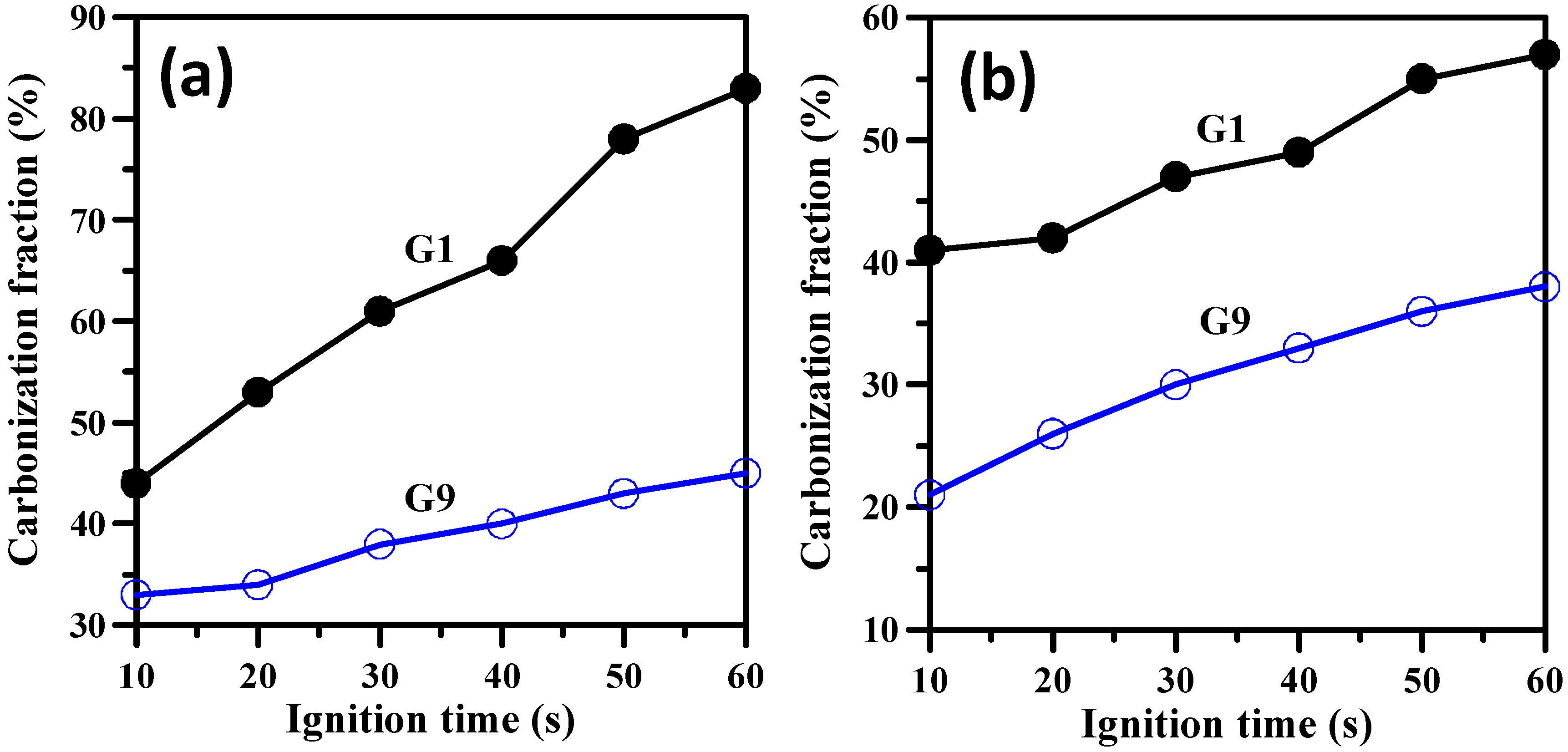
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puri, R.G.; Khanna, A.S. Intumescent coatings: A review on recent progress. J. Coat. Technol. Res. 2017, 14, 1–20. [Google Scholar] [CrossRef]
- Saxena, N.K.; Gupta, D.R. Development and evaluation of fire retardant Coatings. Fire Technol. 1990, 26, 329–341. [Google Scholar] [CrossRef]
- Pan, H.; Wang, W.; Pan, Y.; Song, L.; Hu, Y.; Liew, K.M. Formation of self-extinguishing flame retardant biobased coating on cotton fabrics via Layer-by-Layer assembly of chitin derivatives. Carbohydr. Polym. 2015, 115, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Tong, B.; Du, T.; Zhang, X.; Meng, Y.; Liu, X.; Tian, X. Unique nanobrick wall nanocoating for flame-retardant cotton fabric via layer-by-layer assembly technique. Cellulose 2016, 23, 3341–3354. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Li, Y.; Sun, J. Intumescent flame-retardant and self-healing superhydrophobic coatings on cotton fabric. ACS Nano 2015, 9, 4070–4076. [Google Scholar] [CrossRef]
- Shin, Y.J.; Shin, M.J.; Shin, J.S. Flame retardant properties of cyclotriphosphazene derivatives for ABS. Polym. Polym. Compos. 2018, 26, 309–314. [Google Scholar] [CrossRef]
- Wirasaputra, A.; Yao, X.; Zhu, Y.; Liu, S.; Yuan, Y.; Zhao, J.; Fu, Y. Flame-retarded epoxy resins with a curing agent of DOPO-triazine based anhydride. Macromol. Mater. Eng. 2016, 301, 982–991. [Google Scholar] [CrossRef]
- Wei, H.; Zhu, Z.; Sun, H.; Peng, M.; Liang, W.; Li, A. Graphene and poly(ionic liquid) modified polyurethane sponges with enhanced flame-retardant properties. J. Appl. Polym. Sci. 2017, 134, 45477. [Google Scholar] [CrossRef]
- Dai, K.; Sun, S.; Xu, W.; Song, Y.; Deng, Z.; Qian, X. Covalently-functionalized graphene oxide via introduction of bifunctional phosphorus containing molecules as an effective flame retardant for polystyrene. RSC Adv. 2018, 8, 24993–25000. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wan, J.T.; Wang, D.Y. Carbon family materials for flame retardant polymeric materials. Prog. Polym. Sci. 2017, 69, 22–46. [Google Scholar] [CrossRef]
- Chavali, K.S.; Pethsangave, D.; Patankar, K.; Khose, R.V.; Wadekar, P.; Maiti, S.; Adivarekar, R.V.; Some, S. Graphene-based intumescent flame retardant on cotton fabric. J. Mater. Sci. 2020, 55, 14197–14210. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Drzal, L.T. A novel approach to create a highly ordered monolayer film of graphene nanosheets at the liquid−liquid interface. Nano Lett. 2009, 9, 167–172. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Gu, J.L.; Chen, Y.C.; Tzou, D.Y. Pulse microwave synthesis of palladium catalysts on graphene electrodes for proton exchange membrane fuel cells. Electrochim. Acta 2013, 98, 39–47. [Google Scholar] [CrossRef]
- Gu, S.; Hsieh, C.T.; Mallick, B.C.; Gandomi, Y.A. Infrared-assisted synthesis of highly amidized graphene quantum dots as metal-free electrochemical catalysts. Electrochim. Acta 2013, 360, 137009. [Google Scholar] [CrossRef]
- Daugherty, M.C.; Gu, S.; Aaron, D.S.; Mallick, B.C.; Gandomi, Y.A.; Hsieh, C.T. Decorating sulfur and nitrogen co-doped graphene quantum dots on graphite felt as high-performance electrodes for vanadium redox flow batteries. J. Power Sources 2020, 477, 228709. [Google Scholar] [CrossRef]
- Paek, S.M.; Yoo, E.; Honma, I. Enhanced cyclic performance and lithium storage capacity of SnO2/graphene nanoporous electrodes with three-dimensionally delaminated flexible structure. Nano Lett. 2009, 9, 72–75. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Lee, W.Y.; Lee, C.E.; Teng, H. Electrochemical capacitors fabricated with tin oxide/graphene oxide nanocomposites. J. Phys. Chem. C 2014, 118, 15146–15153. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Le, M.T.; Huang, S.C. Thermal and mechanical behavior of hybrid polymer nanocomposite reinforced with graphene nanoplatelets. Materials 2015, 8, 5526–5536. [Google Scholar] [CrossRef] [PubMed]
- Daimatsu, K.; Sugimoto, H.; Kato, Y.; Nakanishi, E.; Inomata, K.; Amekawa, Y.; Kazuki, T. Preparation and physical properties of flame retardant acrylic resin containing nanosized aluminum hydroxide. Polym. Degrad. Stab. 2007, 92, 1433–1438. [Google Scholar] [CrossRef]
- Some, S.; Kim, Y.; Yoon, Y.; Yoo, H.; Lee, S.; Park, Y.; Lee, H. High-quality reduced graphene oxide by a dual function chemical reduction and healing process. Sci. Rep. 2013, 3, 1929. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, L.; Yang, H.; Xing, W.; Kandol, B.; Hu, Y. Simultaneous reduction and surface functionalization of graphene oxide with POSS for reducing fire hazards in epoxy composites. J. Mater. Chem. 2012, 22, 22037–22043. [Google Scholar] [CrossRef]
- Wang, Z.; Ni, L.; Gu, Z.; Cui, F.; Jiang, J.; Chen, L. The influences of graphene and carbon nanotubes on properties of waterborne intumescent fire resistive coating. Powder Technol. 2021, 385, 572–579. [Google Scholar]
- Wang, C.; Tang, G.; Zhao, J.; Han, Y. Effect of flaky graphite with different particle sizes on flame resistance of intumescent flame retardant coating. Results Mater. 2020, 5, 100061. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J. Effect of graphite on the flame resistance of silica fume-based geopolymeric coatings. Mater. Chem. Phys. 2020, 239, 122088. [Google Scholar] [CrossRef]
- Esmailpour, A.; Majidi, R.; Taghiyari, H.R.; Ganjkhani, M.; Armaki, S.M.M.; Papadopoulos, A.N. Improving fire retardancy of beech wood by graphene. Polymers 2020, 12, 303. [Google Scholar] [CrossRef]
- Cai, W.; Wang, B.-B.; Wang, X.; Zhu, Y.-L.; Li, Z.-X.; Xu, Z.-M.; Song, L.; Hu, W.-Z.; Hu, Y. Recent progress in two-dimensional nanomaterials following graphene for improving fire safety of polymer (nano) composites. Chin. J. Polym. Sci. 2021, 39, 935–956. [Google Scholar] [CrossRef]
- Huang, G.; Han, D.; Jin, Y.; Song, P.; Yan, Q.; Gao, C. Fabrication of nitrogen-doped graphene decorated with organophosphorus and lanthanum towards high-performance polymer nanocomposites. ACS Appl. Nano Mater. 2020, 1, 3204–3213. [Google Scholar] [CrossRef]
- Fang, F.; Ran, S.; Fang, Z.; Song, P.; Wang, H. Improved flame resistance and thermo-mechanical properties of epoxy resin nanocomposites from functionalized graphene oxide via self-assembly in water. Compos. B. Eng. 2019, 165, 406–416. [Google Scholar] [CrossRef]
- Gu, S.; Hsieh, C.T.; Mallick, B.C.; Gandomi, Y.A.; Chang, J.K.; Li, J.; Liaw, P.K. Amino-functionalization on graphene oxide sheets using an atomic layer amidation technique. J. Mater. Chem. 2020, 8, 700–705. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Hsu, S.M.; Lin, J.Y.; Teng, H. Electrochemical capacitors based on graphene oxide sheets using different aqueous electrolytes. J. Phys. Chem. C 2011, 115, 12367–12374. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Lin, C.Y.; Chen, Y.F.; Lin, J.S.; Teng, H. Silver nanorods attached to graphene sheets as anode materials for lithium-ion batteries. Carbon 2013, 62, 109–116. [Google Scholar] [CrossRef]
- Yew, M.C.; Sulong, N.H.R.; Yew, M.K.; Amalina, M.A.; Johan, M.R. Eggshells: A novel bio-filler for intumescent flame-retardant coatings. Prog. Org. Coat. 2015, 81, 116–124. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Hsu, S.M.; Lin, J.Y. Fabrication of graphene-based electrochemical capacitors. Jpn. J. Appl. Phys. 2012, 51, 01AH06. [Google Scholar] [CrossRef]
- Ma, H.Y.; Tong, L.F.; Xu, Z.B.; Fang, Z.P. Functionalizing carbon nanotubes by grafting on intumescent flame retardant: Nanocomposite synthesis, morphology, rheology, and flammability. Adv. Funct. Mater. 2008, 18, 414–421. [Google Scholar] [CrossRef]
- Feng, Y.; He, C.; Wen, Y.; Ye, Y.; Zhou, X.; Xie, X.; Mai, Y.W. Superior flame retardancy and smoke suppression of epoxy-based composites with phosphorus/nitrogen co-doped grapheme. J. Hazard. Mater. 2018, 346, 140–151. [Google Scholar] [CrossRef]
- Lowden, L.A.; Hull, T.R. Flammability behaviour of wood and a review of the methods for its reduction. Fire Sci. Rev. 2013, 2, 4. [Google Scholar] [CrossRef]
- Yang, H.C.; Tsai, T.P.; Hsieh, C.T. Enhancement on fireproof performance of construction coatings using calcium sulfate whiskers prepared from wastewater. Chem. Pap. 2017, 71, 1343–1350. [Google Scholar] [CrossRef]
- Corrias, M.; Caussat, B.; Ayral, A.; Durand, J.; Kihn, Y.; Kalck, P.; Serp, P. Carbon nanotubes produced by fluidized bed catalytic CVD: Approach of the process. Chem. Eng. Sci. 2003, 58, 4475–4482. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Hung, W.-M.; Chen, W.-Y.; Lin, J.-Y. Microwave-assisted polyol synthesis of Pt-Zn electrocatalysts on carbon nanotube electrodes for methanol oxidation. Inter. J. Hydrogen Energy 2011, 36, 2765–2772. [Google Scholar] [CrossRef]
- Jiang, J.X.; Li, J.Z.; Gao, Q. Effect of flame retardant treatment on dimensional stability and thermal degradation of wood. Constr Build Mater. 2015, 75, 74–81. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Du, F.; Douglas, J.F.; Winey, K.I.; Jr, R.H.H.; Shields, J.R. Nanoparticle networks reduce the flammability of polymer nanocomposites. Nat. Mater. 2005, 4, 928–933. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, B.; Wang, F.; Zhao, Y.; Liu, C.; Chen, J.; Shen, C. Thermal degradation mechanism and kinetics of polycarbonate/silica nanocomposites. Polym. Degrad. Stab. 2014, 107, 129–138. [Google Scholar] [CrossRef]
- de Hoyos-Martínez, P.L.; Issaoui, H.; Herrera, R.; Labidi, J.; Bouhtoury, F. C-E.. Wood fireproofing coatings based on biobased phenolic resins. ACS Sustain. Chem. Eng. 2021, 9, 1729–1740. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Q.; Zhang, X.; Pan, H.; Lu, Y. Graphene oxide-filled multilayer coating to improve flame-retardant and smoke suppression properties of flexible polyurethane foam. J. Mater. Sci. 2016, 51, 10361–10374. [Google Scholar] [CrossRef]
- Yuan, B.; Sun, Y.; Chen, X.; Shi, Y.; Dai, H.; He, S. Poorly-/well-dispersed graphene: Abnormal influence on flammability and fire behavior of intumescent flame retardant. Compos. Part A Appl. Sci. Manuf. 2018, 109, 345–354. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasi, T.-P.; Hsieh, C.-T.; Yang, H.-C.; Huang, H.-Y.; Wu, M.-W.; Ashraf Gandomi, Y. Fabrication of Inorganic Coatings Incorporated with Functionalized Graphene Oxide Nanosheets for Improving Fire Retardancy of Wooden Substrates. Polymers 2022, 14, 5542. https://doi.org/10.3390/polym14245542
Tasi T-P, Hsieh C-T, Yang H-C, Huang H-Y, Wu M-W, Ashraf Gandomi Y. Fabrication of Inorganic Coatings Incorporated with Functionalized Graphene Oxide Nanosheets for Improving Fire Retardancy of Wooden Substrates. Polymers. 2022; 14(24):5542. https://doi.org/10.3390/polym14245542
Chicago/Turabian StyleTasi, Tsung-Pin, Chien-Te Hsieh, Hsi-Chi Yang, Heng-Yu Huang, Min-Wei Wu, and Yasser Ashraf Gandomi. 2022. "Fabrication of Inorganic Coatings Incorporated with Functionalized Graphene Oxide Nanosheets for Improving Fire Retardancy of Wooden Substrates" Polymers 14, no. 24: 5542. https://doi.org/10.3390/polym14245542
APA StyleTasi, T.-P., Hsieh, C.-T., Yang, H.-C., Huang, H.-Y., Wu, M.-W., & Ashraf Gandomi, Y. (2022). Fabrication of Inorganic Coatings Incorporated with Functionalized Graphene Oxide Nanosheets for Improving Fire Retardancy of Wooden Substrates. Polymers, 14(24), 5542. https://doi.org/10.3390/polym14245542









