Efficient Cationization of Cotton for Salt-Free Dyeing by Adjusting Fiber Crystallinity through Alcohol-Water-NaOH Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment Process of Cotton Fibers
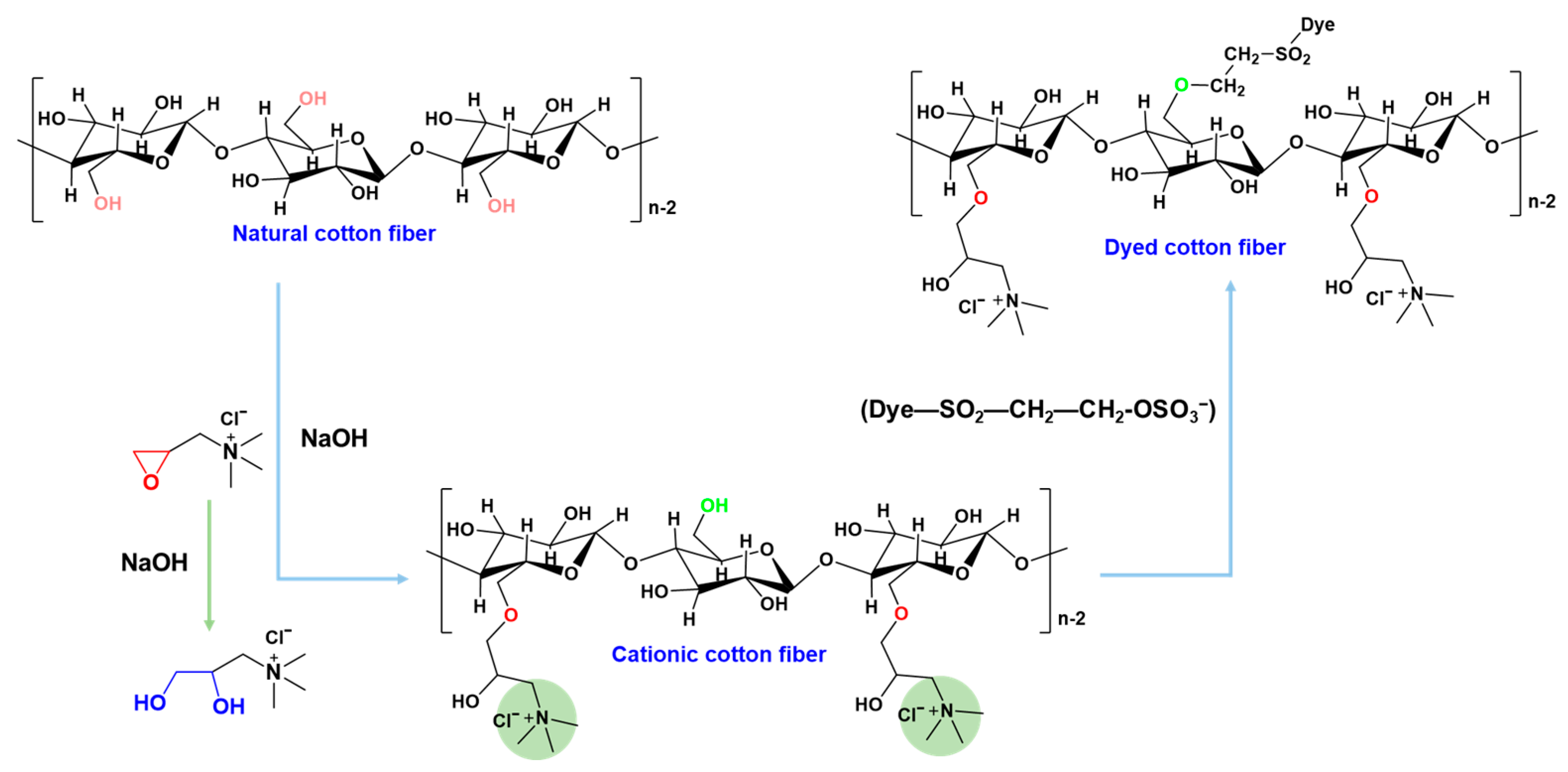
2.3. Preparation of Cationic Fibers
2.4. Dyeing Procedures
2.5. Characterization
2.6. Measurements
3. Results and Discussion
3.1. Exploration of Pretreatment Process
3.2. XRD Analysis of Cotton Fibers
3.3. FT-IR Analysis of Cotton Fibers
3.4. SEM Analysis of Surface Morphology of Cotton Fibers
3.5. Water Contact Angle Analysis of Cotton Fibers
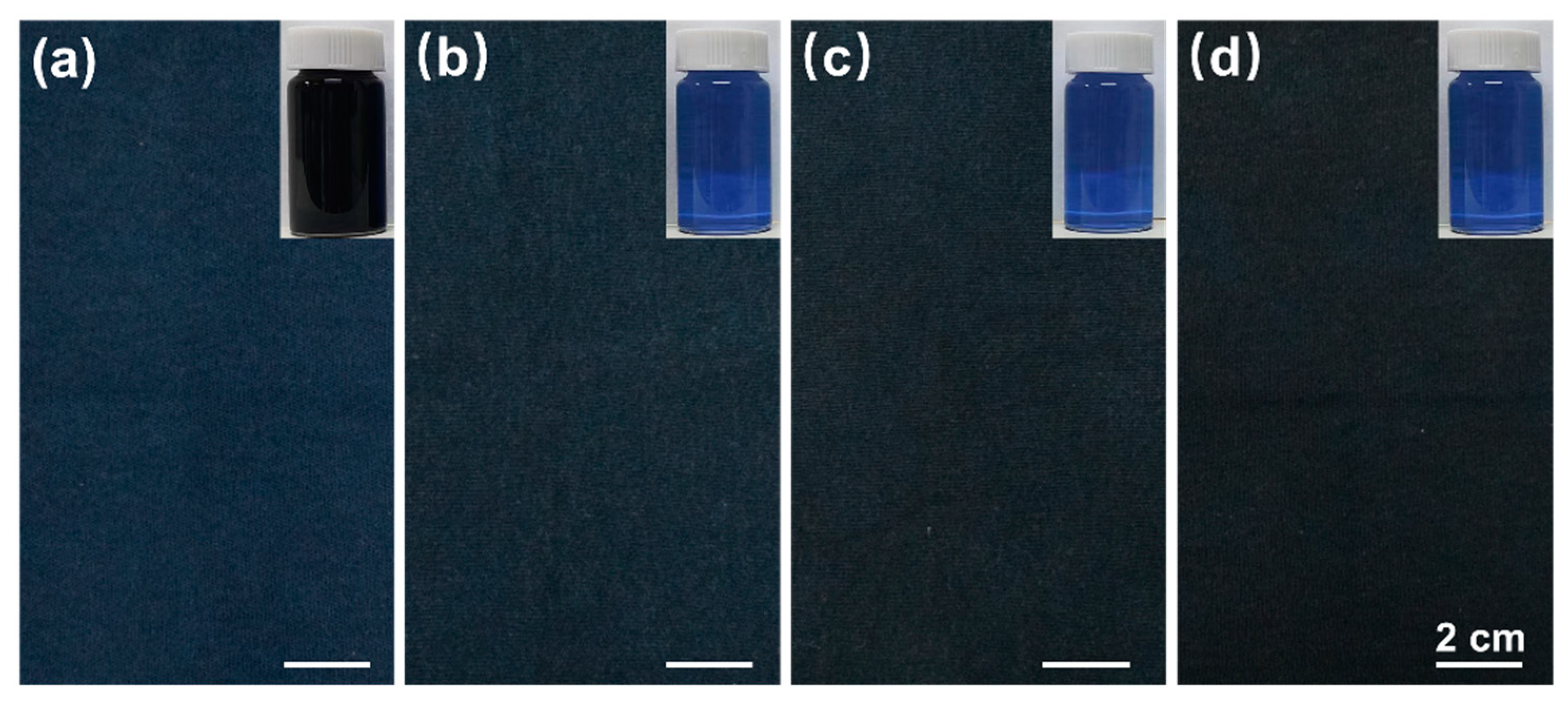
3.6. Comparison of the Performance of Different Dyed Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fang, L.; Zhang, X.; Sun, D. Chemical modification of cotton fabrics for improving utilization of reactive dyes. Carbohydr. Polym. 2013, 91, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Shu, D.; Fang, K.; Liu, X.; Cai, Y.; Zhang, X.; Zhang, J. Cleaner coloration of cotton fabric with reactive dyes using a pad-batch-steam dyeing process. J. Clean. Prod. 2018, 196, 935–942. [Google Scholar] [CrossRef]
- Ma, W.; Yan, S.; Meng, M.; Zhang, S. Preparation of betaine-modified cationic cellulose and its application in the treatment of reactive dye wastewater. J. Appl. Polym. Sci. 2014, 131, 40522. [Google Scholar] [CrossRef]
- Mu, B.; Liu, L.; Li, W.; Yang, Y. High sorption of reactive dyes onto cotton controlled by chemical potential gradient for reduction of dyeing effluents. J. Environ. Manag. 2019, 239, 271–278. [Google Scholar] [CrossRef]
- Irfan, M.; Zhang, H.; Syed, U.; Hou, A. Low liquor dyeing of cotton fabric with reactive dye by an eco-friendly technique. J. Clean. Prod. 2018, 197, 1480–1487. [Google Scholar] [CrossRef]
- Dong, W.; Zhou, M.; Li, Y.; Zhai, S.; Jin, K.; Fan, Z.; Zhao, H.; Zou, W.; Cai, Z. Low-salt dyeing of cotton fabric grafted with pH-responsive cationic polymer of polyelectrolyte 2-(N,N-dimethylamino)ethyl methacrylate. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124573. [Google Scholar] [CrossRef]
- Abkenar, S.S.; Malek, R.M.A.; Mazaheri, F. Salt-free dyeing isotherms of cotton fabric grafted with PPI dendrimers. Cellulose 2015, 22, 897–910. [Google Scholar] [CrossRef]
- Wang, L.; Ma, W.; Zhang, S.; Teng, X.; Yang, J. Preparation of cationic cotton with two-bath pad-bake process and its application in salt-free dyeing. Carbohydr. Polym. 2009, 78, 602–608. [Google Scholar] [CrossRef]
- Zhai, S.; Li, Y.; Dong, W.; Zhao, H.; Ma, K.; Zhang, H.; Wang, H.; Zhao, Y.; Li, X.; Cai, Z. Cationic cotton modified by 3-chloro-2-hydroxypropyl trimethyl ammonium chloride for salt-free dyeing with high levelling performance. Cellulose 2021, 29, 633–646. [Google Scholar] [CrossRef]
- Fu, S.; Farrell, M.J.; Hauser, P.J.; Hinks, D.; Jasper, W.J.; Ankeny, M.A. Real-time dyebath monitoring of reactive dyeing on cationized cotton for levelness control: Part 2—Effects of leveling agents and dye dosing. Cellulose 2017, 24, 3061–3071. [Google Scholar] [CrossRef]
- Niu, T.; Wang, X.; Wu, C.; Sun, D.; Zhang, X.; Chen, Z.; Fang, L. Chemical modification of cotton fabrics by a bifunctional cationic polymer for salt-Free reactive dyeing. ACS Omega 2020, 5, 15409–15416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, S.; Qian, W.; He, J.; Dong, X. Reactive dyeing of cationized cotton fabric: The effect of cationization level. ACS Sustain. Chem. Eng. 2021, 9, 12355–12364. [Google Scholar] [CrossRef]
- Hauser, P.J.; Tabba, A.H. Improving the environmental and economic aspects of cotton dyeing using a cationised cotton. Color. Technol. 2001, 117, 282–288. [Google Scholar] [CrossRef]
- Ramamoorthy, A.; Helmy, H.M.; Rajbhandari, R.; Hauser, P.; El-Shafei, A. Plasma induced graft polymerization of cationic and fluorocarbon monomers into cotton: Enhanced dyeability and photostability. Ind. Eng. Chem. Res. 2016, 55, 8501–8508. [Google Scholar] [CrossRef]
- Farrell, M.J.; Ormond, R.B.; Gabler, W.J. Quantitative analysis of trimethyl amine in cotton fabrics cationized with 3-chloro-2-hydroxypropyltrimethylammonium chloride. Cellulose 2015, 22, 3435–3439. [Google Scholar] [CrossRef]
- De Vries, T.S.; Davies, D.R.; Miller, M.C.; Cynecki, W.A. Kinetics of the cationization of cotton. Ind. Eng. Chem. Res. 2014, 53, 9686–9694. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.; Zhang, X.; Shi, Y. Solvent effect on carboxymethylation of cellulose. J. Appl. Polym. Sci. 1993, 49, 741–746. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, W.; Zhang, C.; Navik, R.; Ding, X.; Mia, M.S.; Pervez, M.N.; Mondal, M.I.H.; Lin, L.; Cai, Y. Post-treatment of reactive dyed cotton fabrics by caustic mercerization and liquid ammonia treatment. Cellulose 2021, 28, 7435–7453. [Google Scholar] [CrossRef]
- Zhang, F.; Pang, Z.; Dong, C.; Liu, Z. Preparing cationic cotton linter cellulose with high substitution degree by ultrasonic treatment. Carbohydr. Polym. 2015, 132, 214–220. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, K.; Fang, K.; Shi, F.; Pan, Y.; Sun, F.; Wang, D.; Xie, R.; Chen, W. Insights into coloration enhancement of mercerized cotton fabric on reactive dye digital inkjet printing. RSC Adv. 2022, 12, 10386–10394. [Google Scholar] [CrossRef]
- Sawada, D.; Hanson, L.; Wada, M.; Nishiyama, Y.; Langan, P. The initial structure of cellulose during ammonia pretreatment. Cellulose 2014, 21, 1117–1126. [Google Scholar] [CrossRef]
- Shang, X.; Wang, Q.; Jiang, Z.; Ma, H. Influence of liquid ammonia on the structure of wool fiber. J. Mol. Liq. 2022, 367, 120426. [Google Scholar] [CrossRef]
- Thao Ho, T.T.; Zimmermann, T.; Caseri, W.R.; Smith, P. Liquid ammonia treatment of (cationic) nanofibrillated cellulose/vermiculite composites. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 638–648. [Google Scholar] [CrossRef]
- Lee, I.Y.; Kim, S.D.; Hwang, C.S.; Kim, S.R.; Park, S.W. Effects of liquid ammonia treatment on the physical properties of knit fabric. In IOP Conference Series: Materials Science and Engineering; IOP: Atlanta, GA, USA, 2016; Volume 141, p. 012019. [Google Scholar]
- Yokota, H. The mechanism of cellulose alkalization in the isopropyl alcohol–water–sodium hydroxide–cellulose system. J. Appl. Polym. Sci. 1985, 30, 263–277. [Google Scholar] [CrossRef]
- Mansikkamäki, P.; Lahtinen, M.; Rissanen, K. Structural changes of cellulose crystallites induced by mercerisation in different solvent systems; determined by powder X-ray Diffraction method. Cellulose 2005, 12, 233–242. [Google Scholar] [CrossRef]
- Olaru, N.; Olaru, L.; Stoleriu, A.; Ţi˘mpu, D. Carboxymethylcellulose synthesis in organic media containing ethanol and/or acetone. J. Appl. Polym. Sci. 1998, 67, 481–486. [Google Scholar] [CrossRef]
- Stigsson, V.; Kloow, G.; Germgård, U. The influence of the solvent system used during manufacturing of CMC. Cellulose 2006, 13, 705–712. [Google Scholar] [CrossRef]
- Im, W.; Lee, S.; Abhari, A.R.; Youn, H.J.; Lee, H.L. Optimization of carboxymethylation reaction as a pretreatment for production of cellulose nanofibrils. Cellulose 2018, 25, 3873–3883. [Google Scholar] [CrossRef]
- Aguado, R.; Lourenço, A.F.; Ferreira, P.J.T.; Moral, A.; Tijero, A. The relevance of the pretreatment on the chemical modification of cellulosic fibers. Cellulose 2019, 26, 5925–5936. [Google Scholar] [CrossRef]
- Fu, S.; Hinks, D.; Hauser, P.; Ankeny, M. High efficiency ultra-deep dyeing of cotton via mercerization and cationization. Cellulose 2013, 20, 3101–3110. [Google Scholar] [CrossRef]
- Fu, S.; Farrell, M.; Hauser, P.; Ankeny, M. Influence of liquor ratio and amount of dyestuff in producing ultradeep black dyeing using mercerised and cationised cotton. Color. Technol. 2016, 132, 232–237. [Google Scholar] [CrossRef]
- Ambjörnsson, H.A.; Schenzel, K.; Germgård, U. Carboxymethyl cellulose produced at different mercerization conditions and characterized by NIR FT Raman Spectroscopy in combination with multivariate analytical methods. BioResources 2013, 8, 1918–1932. [Google Scholar] [CrossRef]
- Ling, Z.; Chen, S.; Zhang, X.; Xu, F. Exploring crystalline-structural variations of cellulose during alkaline pretreatment for enhanced enzymatic hydrolysis. Bioresour. Technol. 2017, 224, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Mannu, A.; Panzeri, W.; Theeuwen, C.H.J.; Mele, A. An integrated approach to optimizing cellulose mercerization. Polymers 2020, 12, 1559. [Google Scholar] [CrossRef]
- Nagarajan, S.; Skillen, N.C.; Irvine, J.T.S.; Lawton, L.A.; Robertson, P.K.J. Cellulose II as bioethanol feedstock and its advantages over native cellulose. Renew. Sust. Energ. Rev. 2017, 77, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Nomura, S.; Kugo, Y.; Erata, T. 13C NMR and XRD studies on the enhancement of cellulose II crystallinity with low concentration NaOH post-treatments. Cellulose 2020, 27, 3553–3563. [Google Scholar] [CrossRef]
- Široký, J.; Blackburn, R.S.; Bechtold, T.; Taylor, J.; White, P. Attenuated total reflectance Fourier-transform Infrared spectroscopy analysis of crystallinity changes in lyocell following continuous treatment with sodium hydroxide. Cellulose 2010, 17, 103–115. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Manian, A.P.; Braun, D.E.; Široká, B.; Bechtold, T. Distinguishing liquid ammonia from sodium hydroxide mercerization in cotton textiles. Cellulose 2022, 29, 4183–4202. [Google Scholar] [CrossRef]
- Tarbuk, A.; Grancaric, A.M.; Leskovac, M. Novel cotton cellulose by cationisation during the mercerisation process—Part 1: Chemical and morphological changes. Cellulose 2014, 21, 2167–2179. [Google Scholar] [CrossRef]
- Zaman, M.W.; Han, J.; Zhang, X. Evaluating wettability of geotextiles with contact angles. Geotext. Geomembr. 2022, 50, 825–833. [Google Scholar] [CrossRef]
- Patiño, A.; Canal, C.; Rodríguez, C.; Caballero, G.; Navarro, A.; Canal, J.M. Surface and bulk cotton fibre modifications: Plasma and cationization. Influence on dyeing with reactive dye. Cellulose 2011, 18, 1073–1083. [Google Scholar] [CrossRef]
- Ma, W.; Shen, K.; Xiang, N.; Zhang, S. Combinative scouring, bleaching, and cationization pretreatment of greige knitted cotton fabrics for facilely achieving salt-free reactive dyeing. Molecules 2017, 22, 2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arivithamani, N.; Giri Dev, V.R. Characterization and comparison of salt-free reactive dyed cationized cotton hosiery fabrics with that of conventional dyed cotton fabrics. J. Clean. Prod. 2018, 183, 579–589. [Google Scholar] [CrossRef]
- Acharya, S.; Abidi, N.; Rajbhandari, R.; Meulewaeter, F. Chemical cationization of cotton fabric for improved dye uptake. Cellulose 2014, 21, 4693–4706. [Google Scholar] [CrossRef]
- Arivithamani, N.; Giri Dev, V.R. Salt-free reactive dyeing of cotton hosiery fabrics by exhaust application of cationic agent. Carbohydr. Polym. 2016, 152, 1–11. [Google Scholar] [CrossRef]








| Samples | Crystallinity |
|---|---|
| NC | 72.3% |
| PC(EtOH-70) | 61.2% |
| PC(NPA-60) | 54.6% |
| PC(IPA-60) | 54.2% |
| PC(TBA-60) | 54.9% |
| Samples | GTA Dosage (g/L) | E% | F% | K/S | Rub Fastness | Wash Fastness | |||
|---|---|---|---|---|---|---|---|---|---|
| Dry | Wet | Color Change | Staining on Cotton | Staining on Wool | |||||
| NC | × | 79.8% | 72.9% | 35.5 | 4–5 | 3–4 | 4–5 | 4–5 | 4–5 |
| NCC | 72.5 | 95.1% | 94.8% | 38.4 | 5 | 4 | 4–5 | 4–5 | 4–5 |
| PCC(EtOH) | 35 | 95.9% | 95.3% | 38.7 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 |
| PCC(NPA) | 30 | 96.5% | 96.0% | 39.3 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 |
| PCC(IPA) | 30 | 98.0% | 97.8% | 39.3 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 |
| PCC(TBA) | 30 | 96.6% | 96.1% | 39.3 | 4–5 | 4–5 | 4 | 4–5 | 4–5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, A.; Ma, W.; Yang, Z.; Zhang, S. Efficient Cationization of Cotton for Salt-Free Dyeing by Adjusting Fiber Crystallinity through Alcohol-Water-NaOH Pretreatment. Polymers 2022, 14, 5546. https://doi.org/10.3390/polym14245546
Wu A, Ma W, Yang Z, Zhang S. Efficient Cationization of Cotton for Salt-Free Dyeing by Adjusting Fiber Crystallinity through Alcohol-Water-NaOH Pretreatment. Polymers. 2022; 14(24):5546. https://doi.org/10.3390/polym14245546
Chicago/Turabian StyleWu, Aini, Wei Ma, Zhiyu Yang, and Shufen Zhang. 2022. "Efficient Cationization of Cotton for Salt-Free Dyeing by Adjusting Fiber Crystallinity through Alcohol-Water-NaOH Pretreatment" Polymers 14, no. 24: 5546. https://doi.org/10.3390/polym14245546
APA StyleWu, A., Ma, W., Yang, Z., & Zhang, S. (2022). Efficient Cationization of Cotton for Salt-Free Dyeing by Adjusting Fiber Crystallinity through Alcohol-Water-NaOH Pretreatment. Polymers, 14(24), 5546. https://doi.org/10.3390/polym14245546






