Electrospun 5-Chloro-7-iodo-8-hydroxyquinoline (Clioquinol)-Containing Poly(3-hydroxybutyrate)/Polyvinylpyrrolidone Antifungal Materials Prospective as Active Dressings against Esca
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of the Mats
2.2.1. Fabrication of PVP,CQinPHB Mats by Electrospinning
2.2.2. Fabrication of PVP,CQonPHB Mats by Electrospinning in Conjunction with Electrospraying
2.3. Characterization of the Mats
2.4. In Vitro CQ Release from the Fibrous Mats
2.5. In Vitro Antifungal Assay
3. Results and Discussion
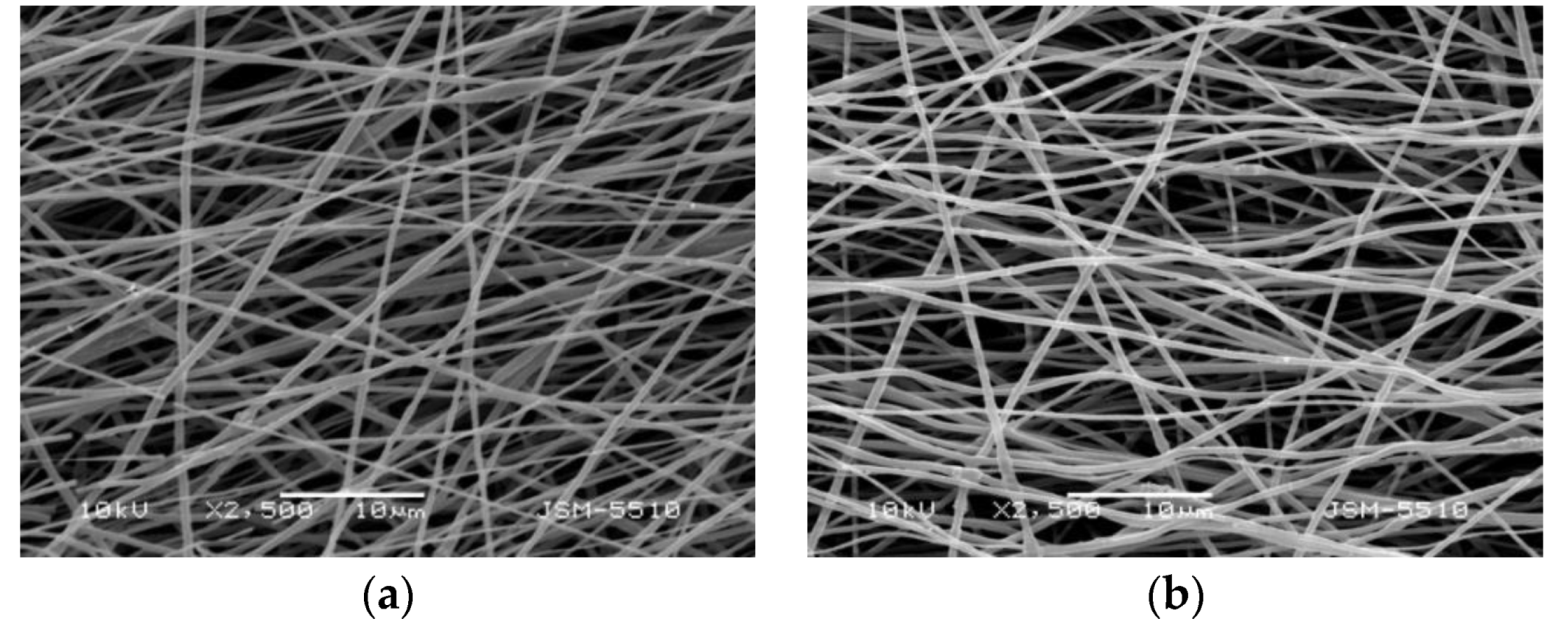
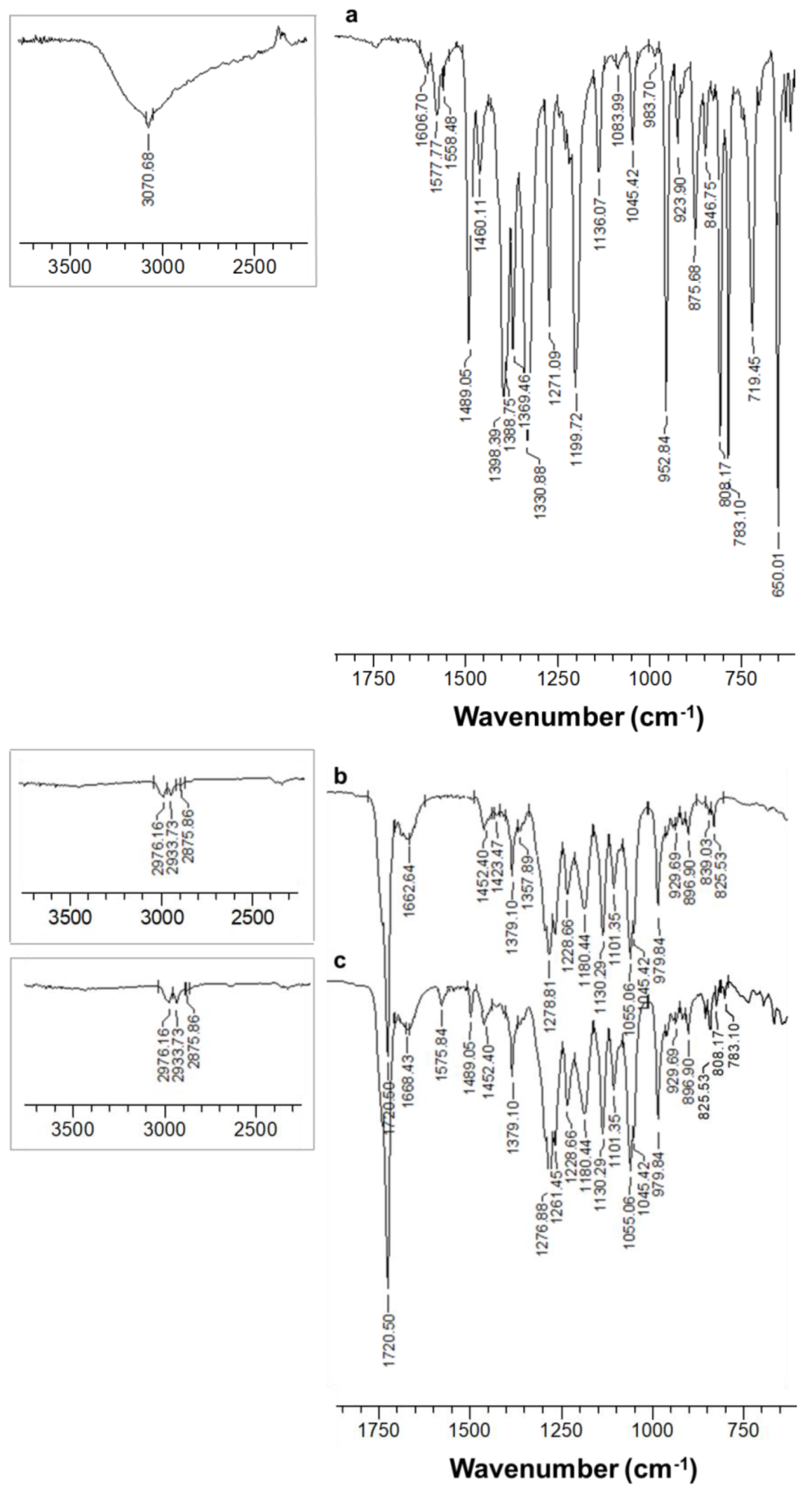
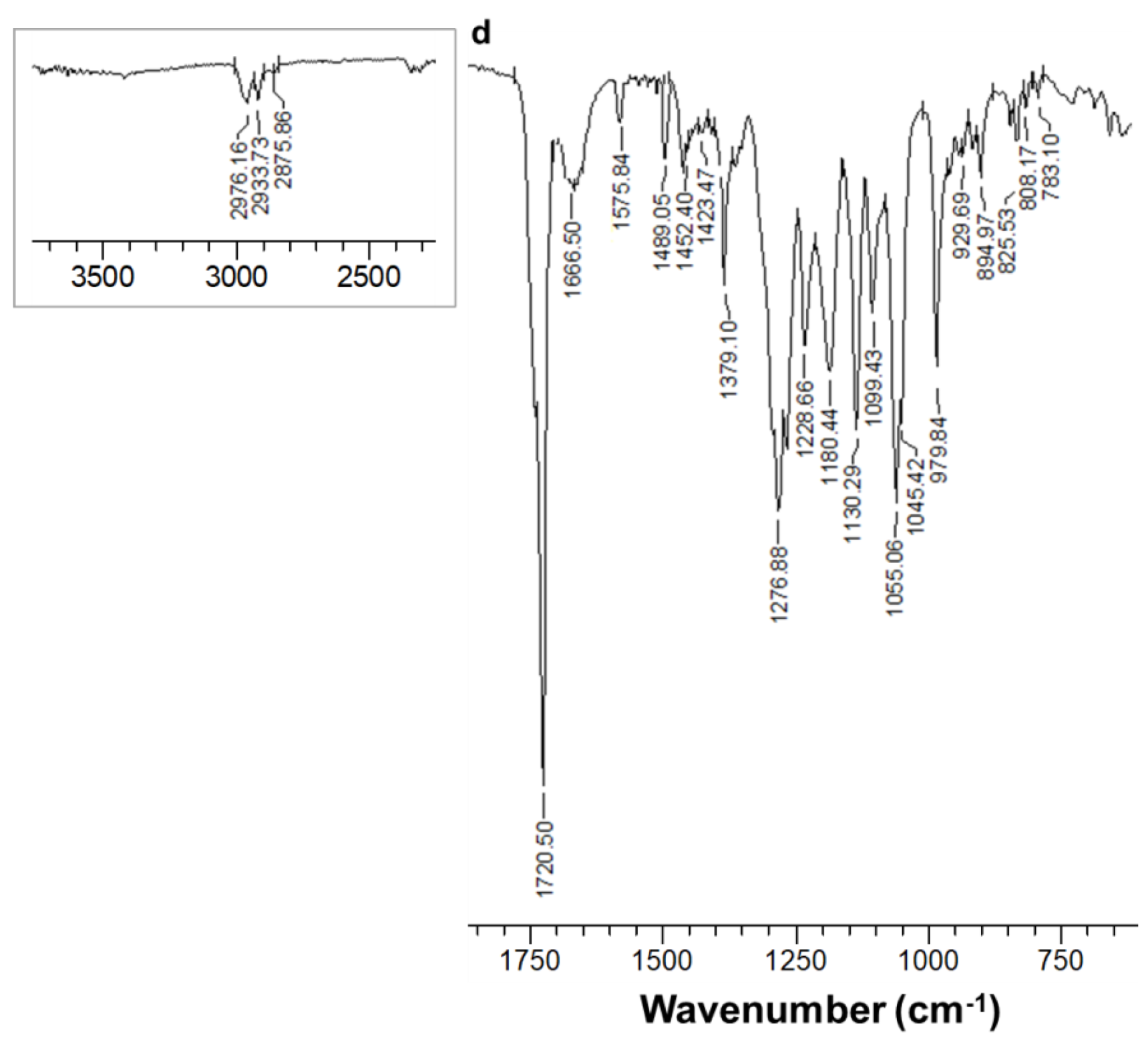
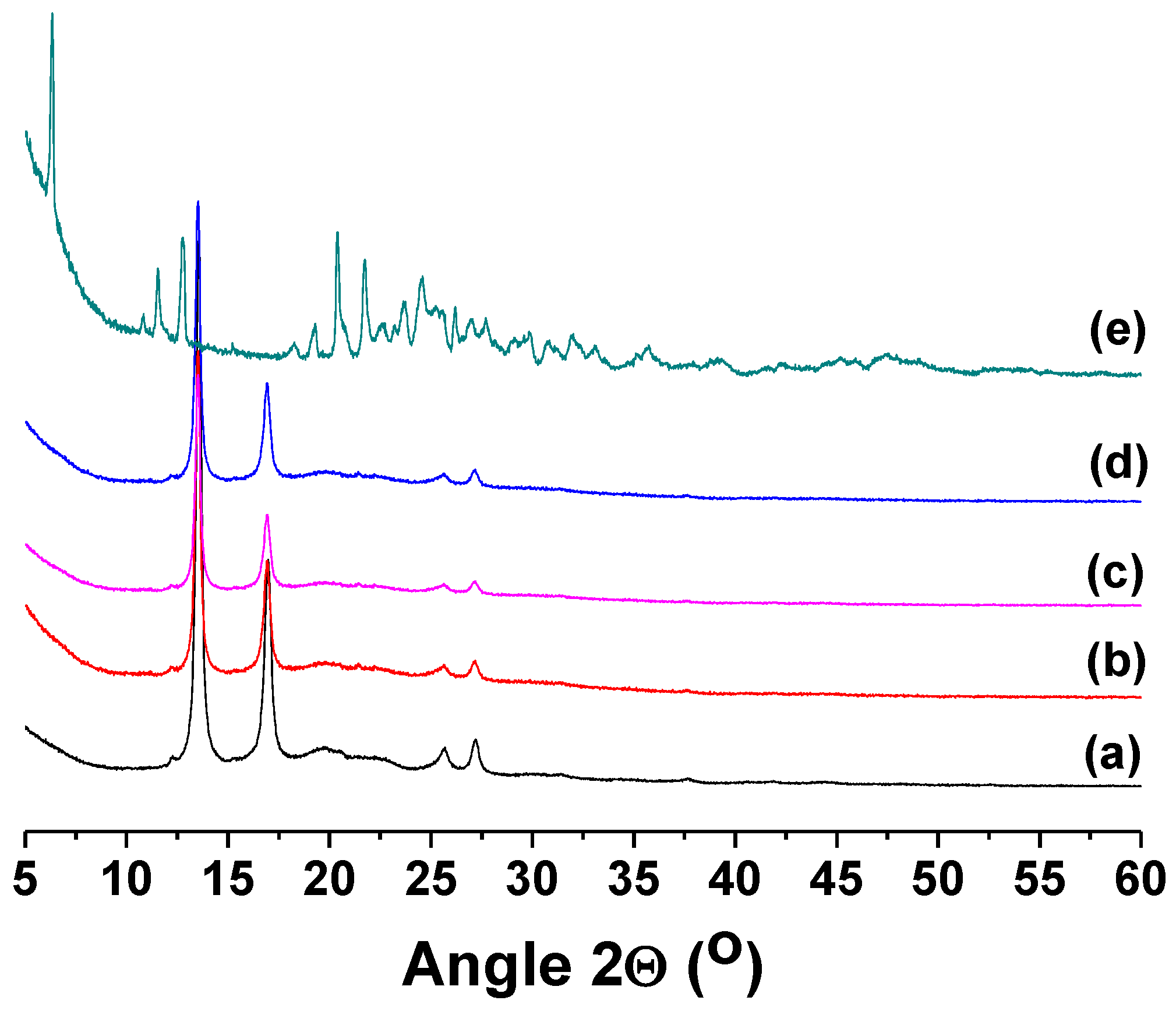
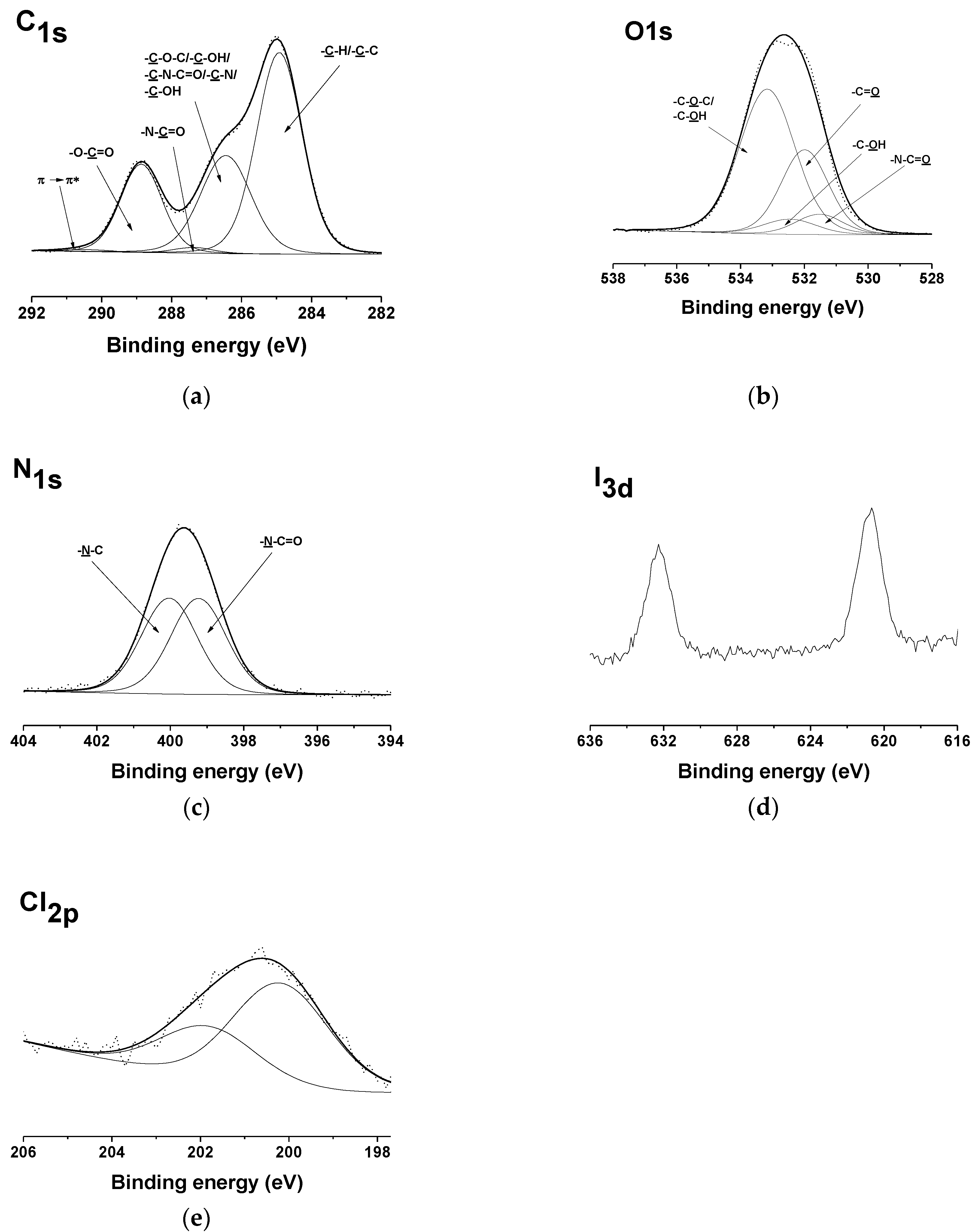
3.1. Fabrication and Characterization of Fibrous Materials
3.2. In Vitro CQ Release Studies
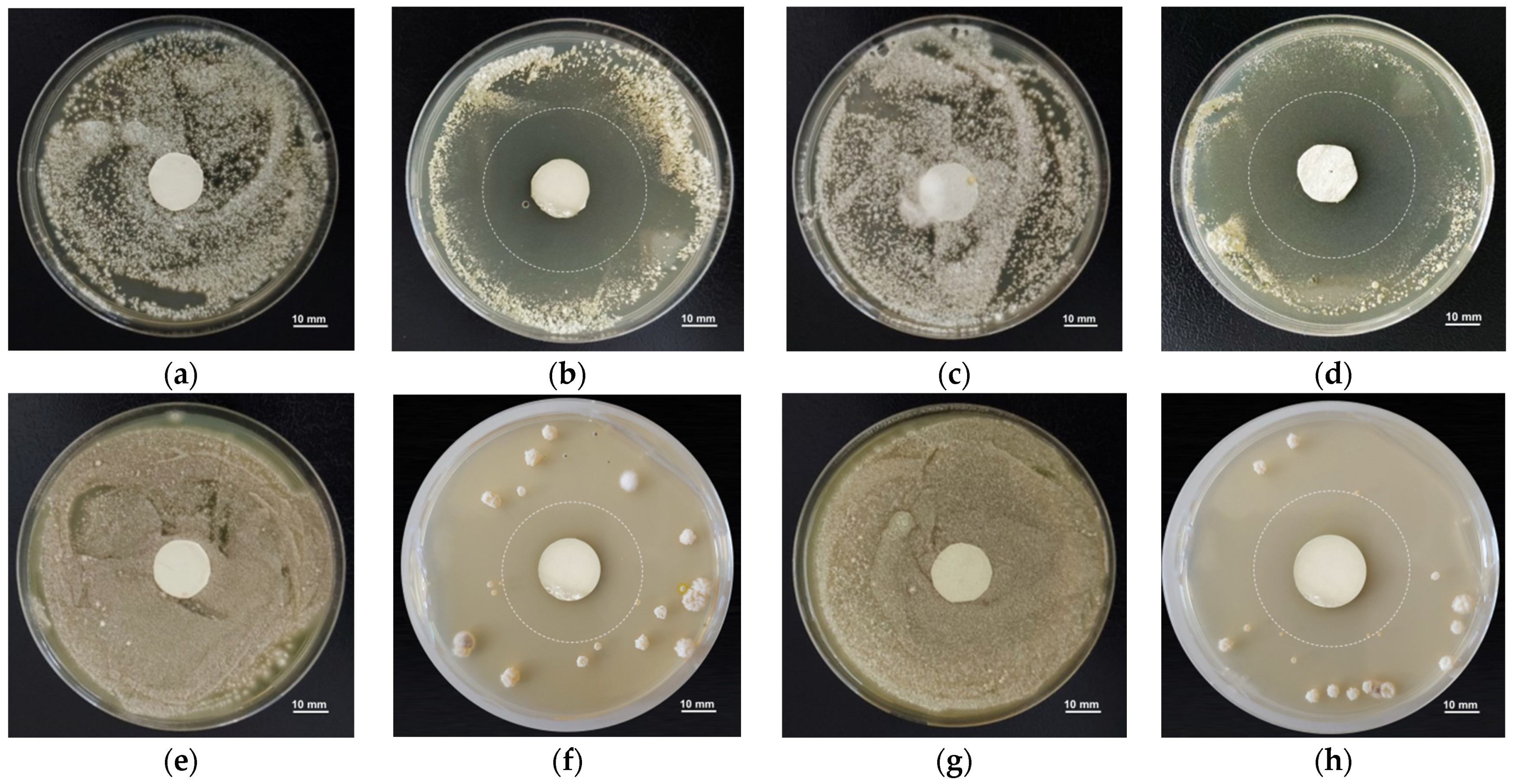
3.3. Antifungal Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mugnai:, L.; Graniti, A.; Surico, G. Esca (black measles) and brown wood-streaking: Two old and elusive diseases of grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surico, G. Towards a redefi nition of the diseases within the esca complex of grapevine. Phytopathol. Mediterr. 2009, 48, 5–10. [Google Scholar]
- Hrycan, J.; Hart, M.; Bowen, P.; Forge, T.; Úrbez-Torres, J.R. Grapevine trunk disease fungi: Their roles as latent pathogens and stress factors that favour disease development and symptom expression. Phytopathol. Mediterr. 2020, 59, 395–424. [Google Scholar]
- Crous, P.W.; Gams, W.; Wingfield, M.J.; Van Wyk, P.S. Phaeoacremonium gen. nov. associated with wilt and decline diseases of woody hosts and human infections. Mycologia 1996, 88, 786–796. [Google Scholar] [CrossRef] [Green Version]
- Crous, P.; Grams, W. Phaeoemoniella chlamydospora gen. et comb. nov., a causal organism of Petri grapevine decline and esca. Phytopathol. Mediterr. 2000, 39, 112–118. [Google Scholar]
- Fuente, M.; Fontaine, F.; Gramaje, D.; Armengol, J.; Smart, R.; Nagy, Z.; Borgo, M.; Rego, C.; Corio-Costet, M. Grapevine Trunk Diseases. A Review, 1st ed.; OIV Publications: Paris, France, 2016. [Google Scholar]
- Ciancio, A.; Mukerji, K. (Eds.) Integrated Management of Diseases Caused by Fungi, Phytoplasma and Bacteria; Springer: Berlin, Germany, 2008. [Google Scholar]
- Gramaje, D.; Úrbez-Torres, J.; Sosnowski, M. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Gao, Q.; Zhang, X. Developments of advanced electrospinning techniques: A critical review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.-G. Electrospun medicated nanofibers for wound healing: Review. Membranes 2021, 11, 770. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mills, D.; Urbanska, A.; Saeb, M.; Venugopal, J.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineerin applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Meraz-Dávila, S.; Pérez-García, C.E.; Feregrino-Perez, A.A. Challenges and advantages of electrospun nanofibers in agriculture: A review. Mater. Res. Express 2021, 8, 042001. [Google Scholar] [CrossRef]
- Sett, S.; Lee, M.; Weith, M.; Pourdeyhim, B.; Yarin, A. Biodegradable and biocompatible soy protein/polymer/adhesive sticky nano-textured interfacial membranes for prevention of esca fungi invasion, into pruning cuts and wounds of vines. J. Mater. Chem. B 2015, 3, 2147–2162. [Google Scholar] [CrossRef]
- Buchholz, V.; Molnar, M.; Wang, H.; Reich, S.; Agarwal, S.; Fischer, M.; Greiner, A. Protection of vine plants against Esca disease by breathable electrospun antifungal nonwovens. Macromol. Biosci. 2016, 16, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Spasova, M.; Stoilova, O.; Manolova, N.; Rashkov, I.; Naydenov, M. Electrospun eco-friendly materials based on poly(3-hydroxybutyrate) (PHB) and TiO2 with antifungal activity prospective for esca treatment. Polymers 2020, 12, 1384. [Google Scholar] [CrossRef]
- Oliveri, V.; Vecchio, G. 8-Hydroxyquinolines in medicinal chemistry: A structural perspective. Eur. J. Med. Chem. 2016, 120, 252–274. [Google Scholar] [CrossRef] [PubMed]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. 8-Hydroxyquinolines: A review of their metal chelating properties and medicinalapplications. Drug Des. Dev. Ther. 2013, 7, 1157–1178. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Luxami, V.; Paul, K. Insights of 8-hydroxyquinolines: A novel target in medicinal chemistry. Bioorg. Chem. 2021, 108, 104633. [Google Scholar] [CrossRef]
- Spasova, M.; Manolova, N.; Rashkov, I.; Naydenov, M. Electrospun 5-chloro-8-hydroxyquinoline-loaded cellulose acetate/polyethylene glycol antifungal membranes against esca. Polymers 2019, 11, 1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nachev, N.; Spasova, M.; Tsekova, P.; Manolova, N.; Rashkov, I.; Naydenov, M. Electrospun polymer-fungicide nanocomposites for grapevine protection. Polymers 2021, 13, 3673. [Google Scholar] [CrossRef] [PubMed]
- Alsterholm, M.; Karami, N.; Faergemann, J. Antimicrobial activity of topical skin pharmaceuticals—An in vitro study. Acta Derm. Venereol. 2010, 90, 239–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, Z.; Ran, X.; Dai, Y.; Ran, Y. Clioquinol, an alternative antimicrobial agent against common pathogenic microbe. J. Mycol. Med. 2018, 28, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Pippi, B.; Reginatto, P.; Da Rosa Monte Machado, G.; Bergamo, V.Z.; Lana, D.F.D.; Teixeira, M.L.; Franco, L.L.; Alves, R.J.; Andrade, S.F.; Fuentefria, A.M. Evaluation of 8-hydroxyquinoline derivatives as hits for antifungal drug design. Med. Mycol. 2017, 55, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.B.; Zou, J.; Zheng, Y.; Naslund, M.J.; Costello, L.C. Zinc ionophore (clioquinol) inhibition of human ZIP1-deficient prostate tumor growth in the mouse ectopic xenograft model: A zinc approach for the efficacious treatment of prostate cancer. Int. J. Cancer Clin. Res. 2016, 3, 037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Raymick, J.; Sarkar, S.; Lahiri, D.K.; Ray, B.; Holtzman, D.; Dumas, M.; Schmued, L.C. Efficacy and toxicity of clioquinol treatment and A-beta42 inoculation in the APP/PSI mouse model of Alzheimer’s disease. Curr. Alzheimer Res. 2013, 10, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, D.I.; Hare, D.J.; Billings, J.L.; Sedjahtera, A.; Nurjono, M.; Arthofer, E.; George, S.; Culvenor, J.G.; Bush, A.I.; Adlard, P.A. Clioquinol improves cognitive, motor function, and microanatomy of the alphasynuclein hA53T transgenic mice. ACS Chem. Neurosci. 2016, 7, 119–129. [Google Scholar] [CrossRef]
- Huntington Study Group Reach2HD Investigators. Safety, tolerability, and efficacy of PBT2 in Huntington’s disease: A phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2015, 14, 39–47. [Google Scholar] [CrossRef]
- Poirier, Y.; Dennis, D.E.; Nawrath, C.; Somerville, C. Progress toward biologically produced biodegradable thermoplastics. Adv. Mater. 1993, 5, 30–37. [Google Scholar] [CrossRef]
- Kumagai, Y.; Doi, Y. Enzymatic degradation and morphologies of binary blends of microbial poly(3-hydroxybutyrate) with poly(e-caprolactone) poly(1,4-butylene adipate) and poly(vinyl acetate). Polym. Degrad. Stab. 1992, 36, 241–248. [Google Scholar] [CrossRef]
- Carswell-Pomerantz, T.; Hill, D.J.T.; O’Donnell, J.H.; Pomery, P.J. An electro spin resonance study of the radiation chemistry of poly(hydroxybutyrate). Radiat. Phys. Chem. 1995, 45, 737–744. [Google Scholar] [CrossRef]
- Kurakula, M.; Koteswara Rao, G.S.N. Moving polyvinyl pyrrolidone electrospun nanofibers and bioprinted scaffolds toward multidisciplinary biomedical applications. Eur. Polym. J. 2020, 136, 109919. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. The use of poly(N-vinyl pyrrolidone) in the delivery of drugs: A review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wei, Z.; Zhao, H.; Liu, T.; Dong, A.; Zhang, J. Electrospinning of ibuprofen-loaded composite nanofibers for improving the performances of transdermal patches. J. Nanosci. Nanotechnol. 2013, 13, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Bruni, G.; Maggi, L.; Tammaro, L.; Lorenzo, R.D.; Friuli, V.; D’Aniello, S.; Maietta, M.; Berbenni, V.; Milanese, C.; Girella, A.; et al. Electrospun fibers as potential carrier systems for enhanced drug release of perphenazine. Int. J. Pharm. 2016, 511, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Vigh, T.; Démuth, B.; Balogh, A.; Galata, D.L.; Van Assche, I.; Mackie, C.; Vialpando, M.; Van Hove, B.; Psathas, P.; Borbás, E.; et al. Oral bioavailability enhancement of flubendazole by developing nanofibrous solid dosage forms. Drug Dev. Ind. Pharm. 2017, 43, 1126–1133. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA. 1997–2018. Available online: https://imagej.nih.gov/ij/ (accessed on 3 January 2016).
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: Poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Falcón-Piñeiro, A.; Remesal, E.; Noguera, M.; Ariza, J.J.; Guillamón, E.; Baños, A.; Navas-Cortes, J.A. Antifungal activity of propyl-propane-thiosulfinate (PTS) and propyl-propane-thiosulfonate (PTSO) from Allium cepa against Verticillium dahliae: In vitro and in planta assays. J. Fungi 2021, 7, 736. [Google Scholar] [CrossRef]
- Kovalcika, A.; Meixner, K.; Mihalic, M.; Zeilinger, W.; Fritz, I.; Fuchs, W.; Kucharczyk, P.; Stelzer, F.; Drosg, B. Characterization of polyhydroxyalkanoates produced by Synechocystis salina from digestate supernatant. Int. J. Biol. Macromol. 2017, 102, 497–504. [Google Scholar] [CrossRef]
- Porter, M.; Yu, J. Monitoring the in situ crystallization of native biopolyestergranules in Ralstonia eutropha via infrared spectroscopy. J. Microbiol. Methods 2011, 87, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Rahma, A.; Munir, M.M.; Khairurrijal, K.; Prasetyo, A.; Suendo, V.; Rachmawati, H. Intermolecular interactions and the release pattern of electrospun curcumin-polyvinyl(pyrrolidone) fiber. Biol. Pharm. Bull. 2016, 39, 163–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignatova, M.; Manolova, N.; Rashkov, I.; Markova, N. Antibacterial and antioxidant electrospun materials from poly(3-hydroxybutyrate) and polyvinylpyrrolidone containing caffeic acid phenethyl ester—“In” and “on” strategies for enhanced solubility. Int. J. Pharm. 2018, 545, 342–356. [Google Scholar] [CrossRef]
- Wagner, C.C.; Calvo, S.; Torre, M.H.; Baran, E.J. Vibrational spectra of clioquinol and its Cu(II) complex. J. Raman Spectrosc. 2007, 38, 373–376. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Development of flexible materials based on plasticized electrospun PLA–PHB blends: Structural, thermal, mechanical and disintegration properties. Eur. Polym. J. 2015, 73, 433–446. [Google Scholar] [CrossRef]
- Illangakoon, U.E.; Gill, H.; Shearman, G.C.; Parhizkar, M.; Mahalingam, S.; Chatterton, N.P.; Williams, G.R. Fast dissolving paracetamol/caffeine nanofibers prepared by electrospinning. Int. J. Pharm. 2014, 477, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, D.B.; Clark, D.T.; Baker, A.D.; Thompson, M. X-ray photoelectron spectra of uranium (VI) complexes of 8-hydroxyquinoline. J. Chem. Soc. D Chem. Commun. 1971, 24, 1600–1601. [Google Scholar] [CrossRef]
- Saadeh, H.A.; Sweidan, K.A.; Mubarak, M.S. Recent advances in the synthesis and biological activity of 8-hydroxyquinolines. Molecules 2020, 25, 4321. [Google Scholar] [CrossRef] [PubMed]
- Pippi, B.; Lopes, W.; Reginatto, P.; Silva, F.K.; Joaquim, A.R.; Alves, R.J.; Silveira, G.P.; Vainstein, M.H.; Andrade, S.F.; Fuentefria, A.M. New insights into the mechanism of antifungal action of 8-hydroxyquinolines. Saudi Pharm. J. 2018, 27, 41–48. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignatova, M.; Nachev, N.; Spasova, M.; Manolova, N.; Rashkov, I.; Naydenov, M. Electrospun 5-Chloro-7-iodo-8-hydroxyquinoline (Clioquinol)-Containing Poly(3-hydroxybutyrate)/Polyvinylpyrrolidone Antifungal Materials Prospective as Active Dressings against Esca. Polymers 2022, 14, 367. https://doi.org/10.3390/polym14030367
Ignatova M, Nachev N, Spasova M, Manolova N, Rashkov I, Naydenov M. Electrospun 5-Chloro-7-iodo-8-hydroxyquinoline (Clioquinol)-Containing Poly(3-hydroxybutyrate)/Polyvinylpyrrolidone Antifungal Materials Prospective as Active Dressings against Esca. Polymers. 2022; 14(3):367. https://doi.org/10.3390/polym14030367
Chicago/Turabian StyleIgnatova, Milena, Nasko Nachev, Mariya Spasova, Nevena Manolova, Iliya Rashkov, and Mladen Naydenov. 2022. "Electrospun 5-Chloro-7-iodo-8-hydroxyquinoline (Clioquinol)-Containing Poly(3-hydroxybutyrate)/Polyvinylpyrrolidone Antifungal Materials Prospective as Active Dressings against Esca" Polymers 14, no. 3: 367. https://doi.org/10.3390/polym14030367
APA StyleIgnatova, M., Nachev, N., Spasova, M., Manolova, N., Rashkov, I., & Naydenov, M. (2022). Electrospun 5-Chloro-7-iodo-8-hydroxyquinoline (Clioquinol)-Containing Poly(3-hydroxybutyrate)/Polyvinylpyrrolidone Antifungal Materials Prospective as Active Dressings against Esca. Polymers, 14(3), 367. https://doi.org/10.3390/polym14030367








