Biofunctional Hyaluronic Acid/κ-Carrageenan Injectable Hydrogels for Improved Drug Delivery and Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
Development of Thermoreversible Hydrogel
2.3. In Vitro Characterization of Hydrogel
2.3.1. 1H NMR and FTIR
2.3.2. Thermogravimetric Analysis (TGA–DSC)
2.3.3. Scanning Electron Microscopy (SEM)
2.4. In Vitro Studies of Injectable Hydrogel
2.4.1. Gelation Time and Temperature
2.4.2. Sol–Gel Phase Transition (Tsol–gel)
2.4.3. Optical Transmittance and Temperature-Induced Change
2.4.4. Rheological Measurement
2.4.5. Equilibrium Swelling Ratio
2.4.6. In Vitro Drug Loading
2.4.7. In Vitro Drug Release and Release Kinetics
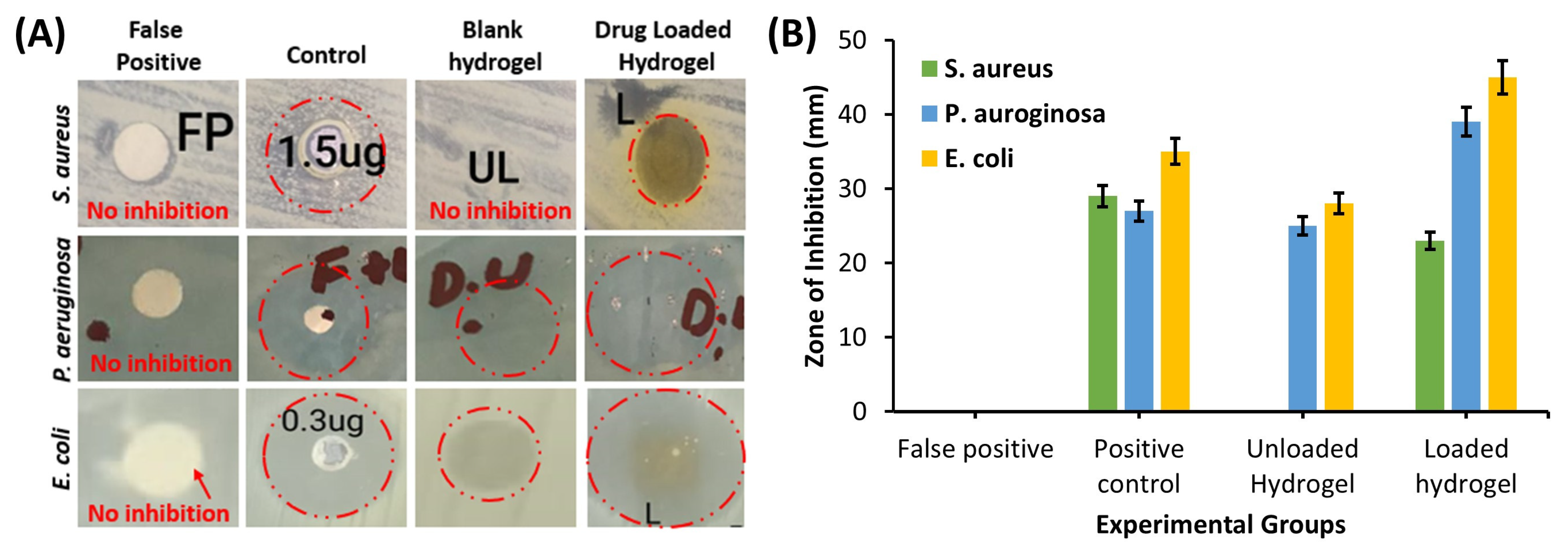
2.4.8. Antibacterial Activity
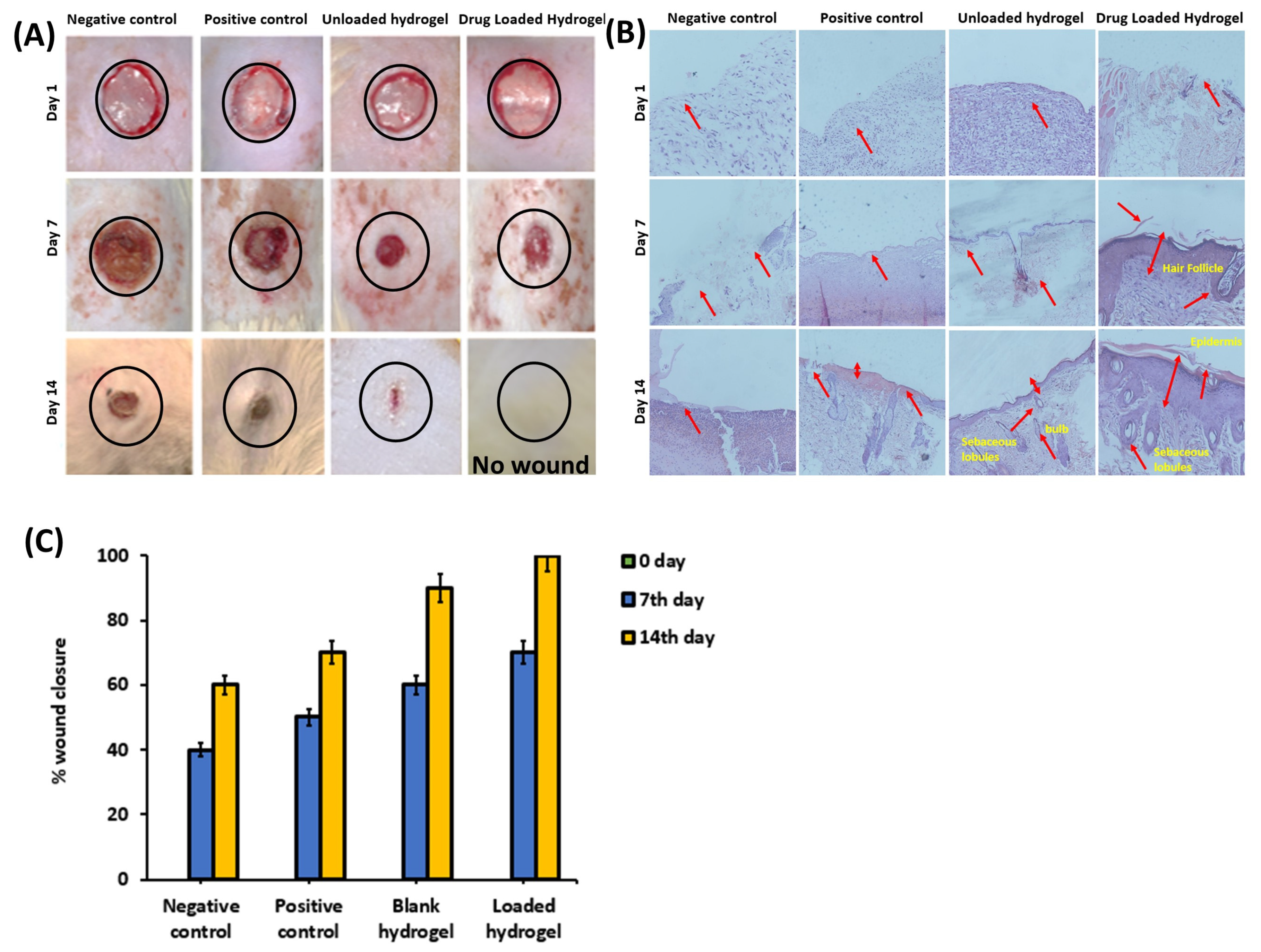
2.5. In Vivo Wound-Healing Analysis
2.6. Wound-Contraction Analysis and Histological Evaluation
3. Results
3.1. In Vitro Characterization of Hydrogel
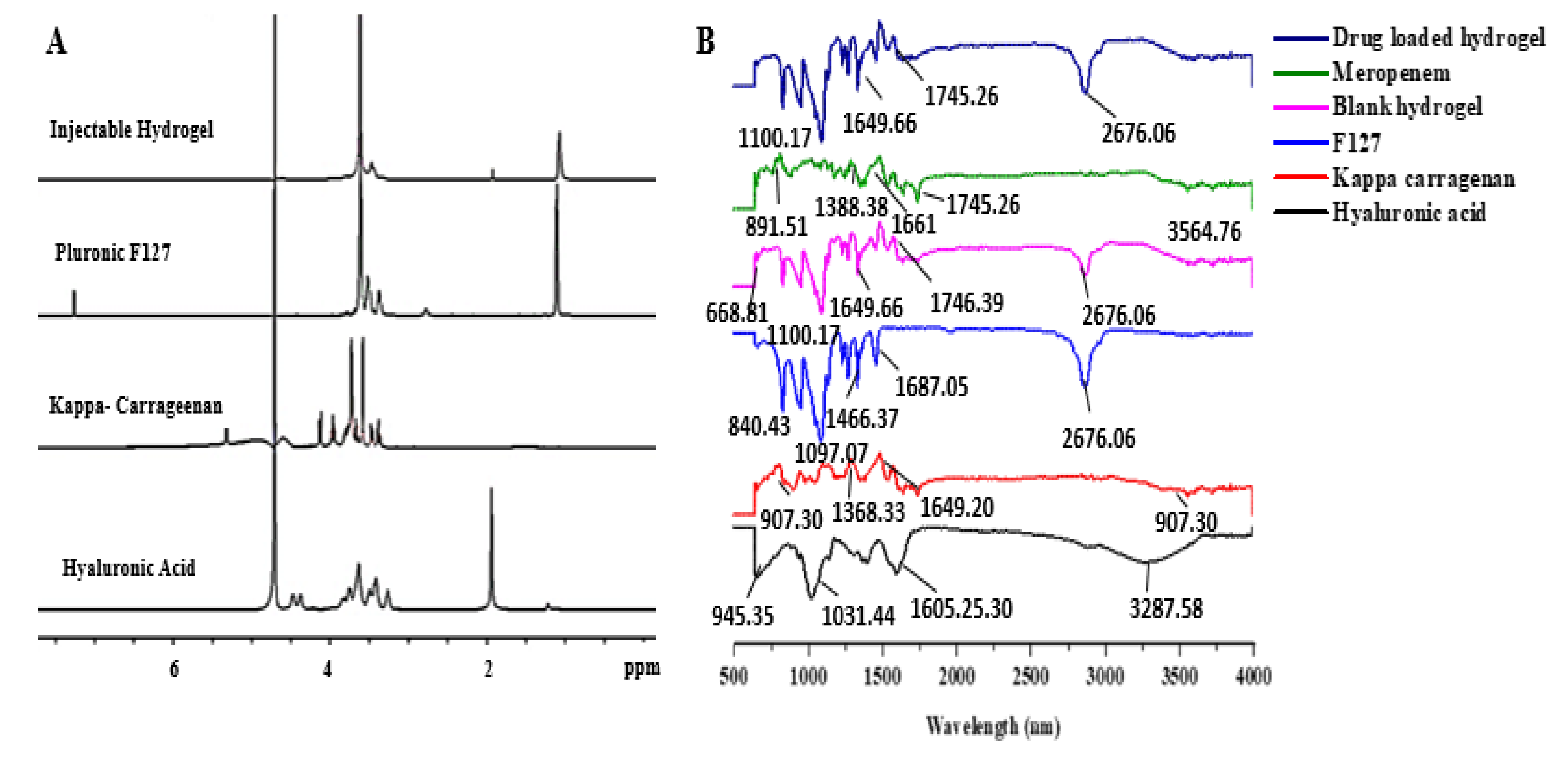
3.1.1. 1H NMR
3.1.2. FTIR
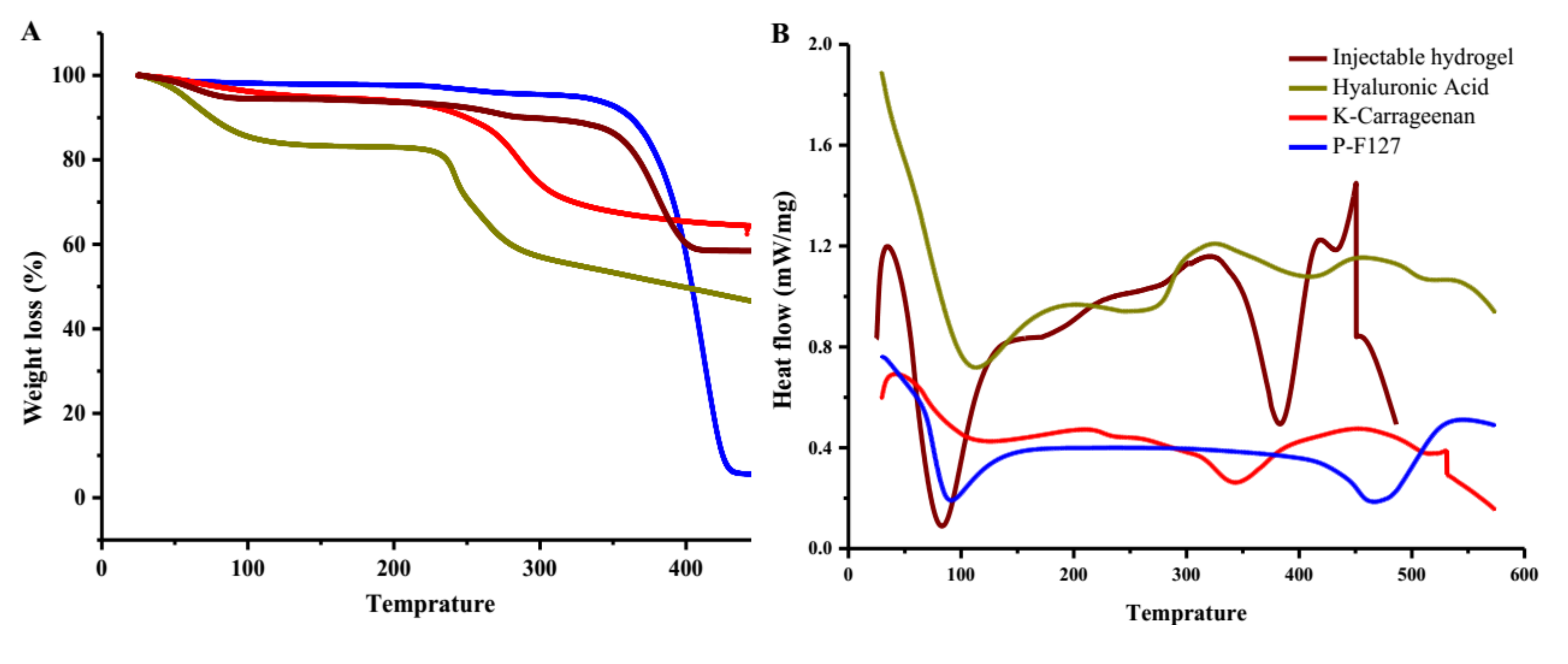
3.1.3. Thermogravimetric and Differential Scanning Calorimetry Thermographs
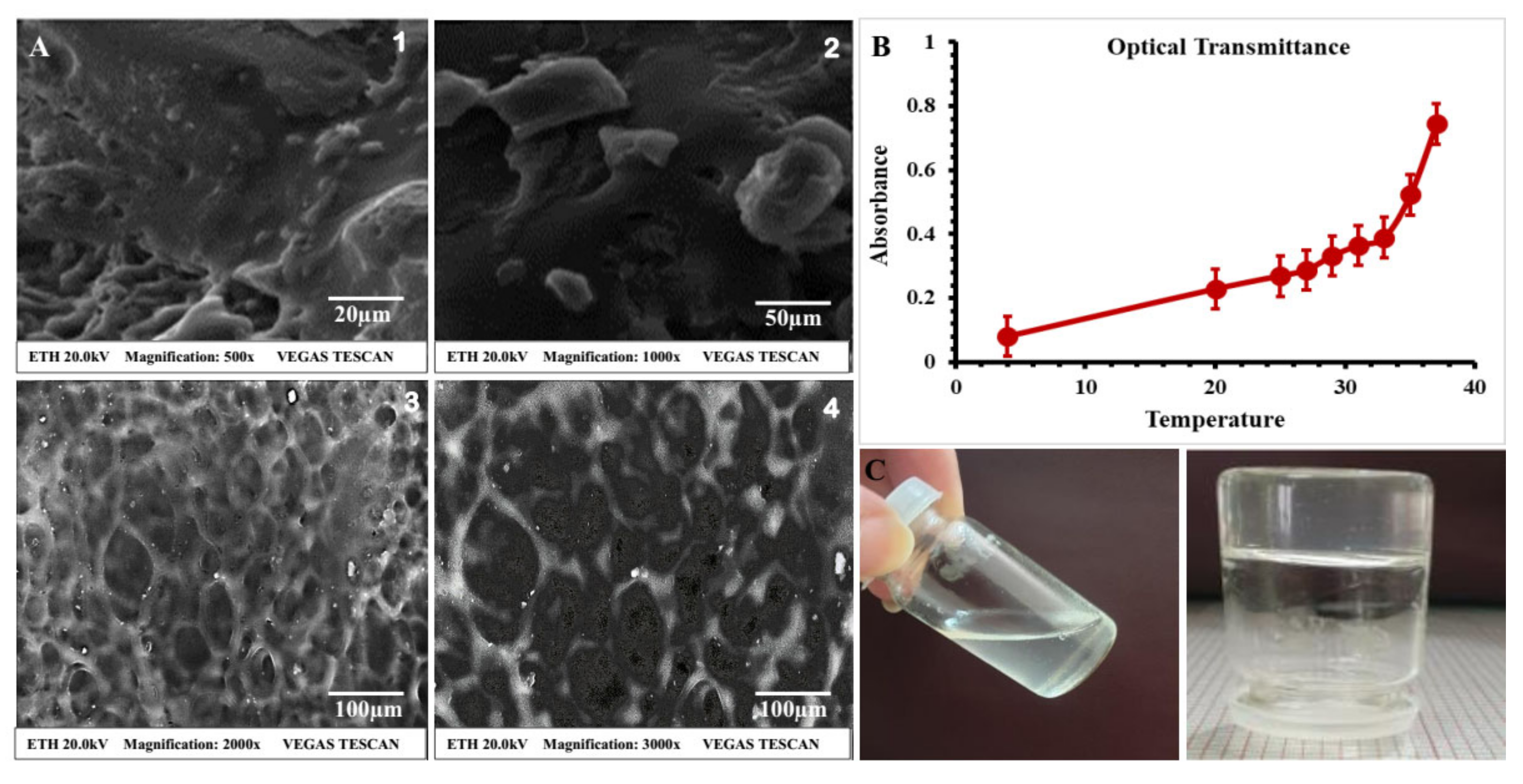
3.1.4. Scanning Electron Microscopy (SEM)
3.2. In Vitro Studies
3.2.1. Physical Appearance and Clarity of Thermosensitive Hydrogel
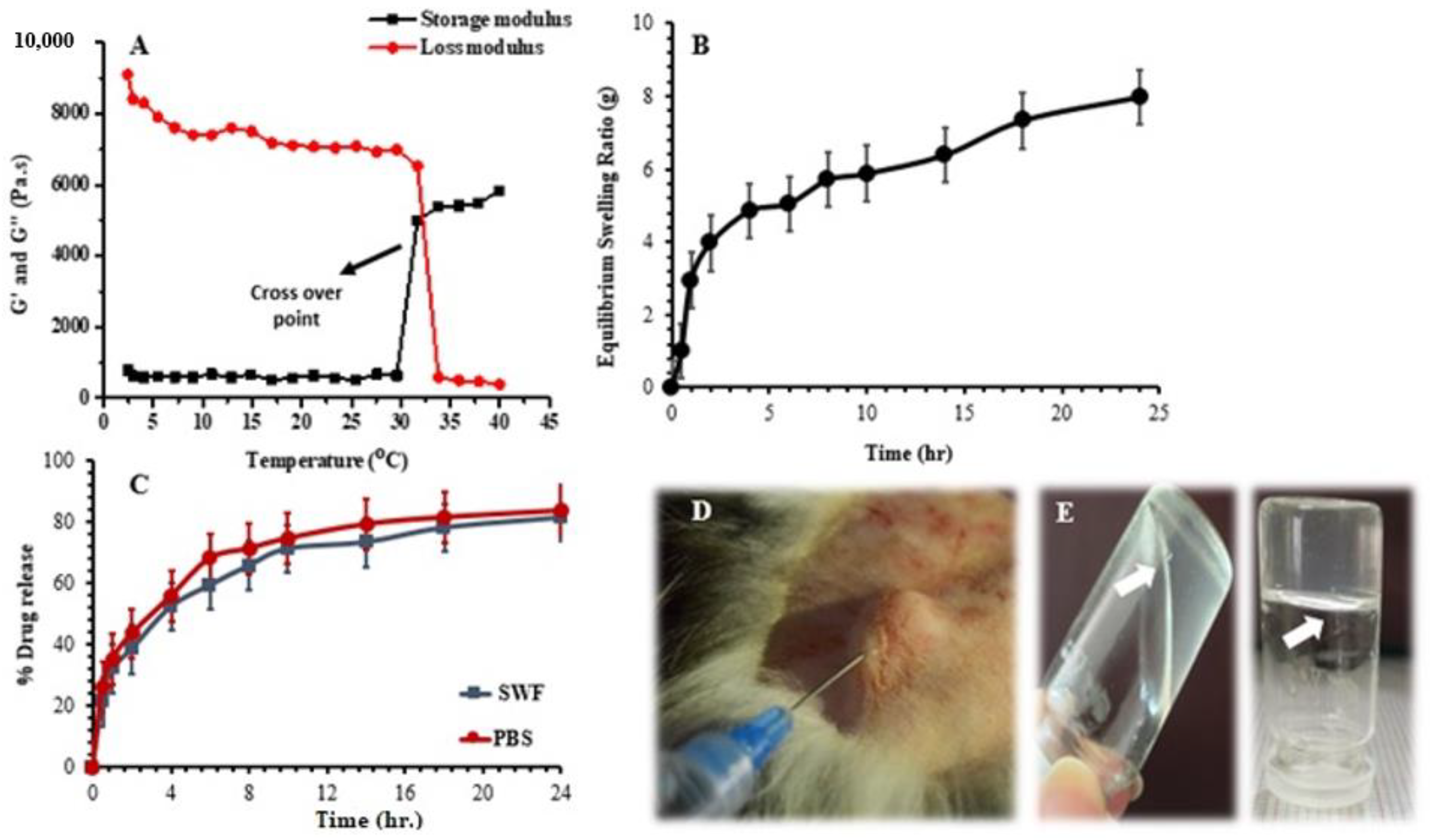
3.2.2. Gelation Time and Temperature
3.2.3. Sol–Gel Phase Transition Analysis (Tsol–gel)
3.2.4. Rheological Study
3.2.5. Optical Transmittance and Temperature-Induced Change
3.2.6. Equilibrium Swelling Ratio (ESR)
3.2.7. In Vitro Drug-Release Studies
3.2.8. Drug-Release Kinetics
3.2.9. Antibacterial Activity
3.3. In Vivo Wound-Healing Analysis
3.3.1. Animal Studies
3.3.2. Histological Examination
4. Discussion
4.1. In Vitro Characterization of Hydrogel
4.1.1. 1H NMR
4.1.2. FTIR
4.1.3. Thermogravimetric and Differential Scanning Calorimetry Thermographs
4.1.4. Scanning Electron Microscopy (SEM)
4.2. In Vitro Studies
4.2.1. Physical Appearance and Clarity of Thermosensitive Hydrogel
4.2.2. Gelation Time and Temperature
4.2.3. Sol–Gel Phase Transition Analysis (Tsol–gel)
4.2.4. Rheological Study
4.2.5. Optical Transmittance and Temperature-Induced Change
4.2.6. Equilibrium Swelling Ratio (ESR)
4.2.7. In Vitro Drug-Release Studies
4.2.8. Drug-Release Kinetics
4.2.9. Antibacterial Activity
4.3. In Vivo Wound-Healing Analysis
4.3.1. Animal Studies
4.3.2. Histological Examination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayaramudu, T.; Varaprasad, K.; Reddy, K.K.; Pyarasani, R.D.; Akbari-Fakhrabadi, A.; Amalraj, J. Alginate-based composite materials for wound dressing application: A mini review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, G.S.; Cooper, D.M.; Knighton, D.R.; Margolis, D.J.; Percoraro, R.E.; Rodeheaver, G. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen. 1994, 2, 165–170. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Karadsheh, M.; Nelson, J.; Rechner, B.; Wilcox, R. Application of a Skin Adhesive to Maintain Seal in Negative Pressure Wound Therapy: Demonstration of a New Technique. Wounds 2017, 29, E106–E110. [Google Scholar]
- Qin, Y.; Hu, H.; Luo, A. The conversion of calcium alginate fibers into alginic acid fibers and sodium alginate fibers. J. Appl. Polym. Sci. 2006, 101, 4216–4221. [Google Scholar] [CrossRef]
- Crane, M.J.; Henry, W.L., Jr.; Tran, H.L.; Albina, J.E.; Jamieson, A.M. Assessment of Acute Wound Healing using the Dorsal Subcutaneous Polyvinyl Alcohol Sponge Implantation and Excisional Tail Skin Wound Models. J. Vis. Exp. 2020, 157, 32281981. [Google Scholar] [CrossRef] [PubMed]
- James, G.A.; Swogger, E.; Wolcott, R.; Pulcini, E.; Secor, D.; Sestrich, P. Biofilms in chronic wounds. Adv. Wound Care 2008, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.A.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Control Release 2022, 341, 132–146. [Google Scholar] [CrossRef]
- Ahmad, U.; Sohail, M.; Ahmad, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Shah, S.A. Chitosan based thermosensitive injectable hydrogels for controlled delivery of loxoprofen: Development, characterization and in-vivo evaluation. Int. J. Biol. Macromol. 2019, 129, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Sohail, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Mahmood, A.; Mahmood, A. pH-responsive CAP-co-poly (methacrylic acid)-based hydrogel as an efficient platform for controlled gastrointestinal delivery: Fabrication, characterization, in vitro and in vivo toxicity evaluation. Drug Deliv. Transl. Res. 2019, 9, 555–577. [Google Scholar] [CrossRef] [PubMed]
- Zia, M.A.; Sohail, M.; Minhas, M.U.; Sarfraz, R.M.; Khan, S.; de Matas, M.; Kousar, M. HEMA based pH-sensitive semi IPN microgels for oral delivery; a rationale approach for ketoprofen. Drug Dev. Ind. Pharm. 2020, 46, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B.J.B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; West, B.; McLemore, R.; Pauken, C.; Vernon, B.L.J.B. In-situ injectable physically and chemically gelling NIPAAm-based copolymer system for embolization. Biomacromolecules 2006, 7, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef]
- Teotia, A.; Sami, H.; Kumar, A. Thermo-responsive polymers: Structure and design of smart materials. In Switchable and Responsive Surfaces and Materials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–43. [Google Scholar]
- Pitto-Barry, A.; Barry, N.P. Pluronic® block-copolymers in medicine: From chemical and biological versatility to rationalisation and clinical advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef]
- Hassan, A.; Abdel-Mohsen, A.; Elhadidy, H. Adsorption of arsenic by activated carbon, calcium alginate and their composite beads. Int. J. Biol. Macromol. 2014, 68, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, K.; Zhang, X.; Zhu, Z.; Yan, S.; Sun, T. Comparison of two hyaluronic acid formulations for safety and efficacy (chase) study of knee osteoarthritis: A multicenter, randomized, double-blind, 26 week non-inferiority trial comparing durolane® to artz®. Osteoarthr. Cartil. 2014, 22, S397. [Google Scholar] [CrossRef]
- Park, S.-N.; Lee, H.J.; Lee, K.H.; Suh, H.J.B. Biological characterization of EDC-crosslinked collagen–hyaluronic acid matrix in dermal tissue restoration. Biomaterials. 2003, 24, 1631–1641. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B., Jr.; Carvalho, I.J.C.P. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Pereira, R.C.; Scaranari, M.; Castagnola, P.; Grandizio, M.; Azevedo, H.S.; Reis, R. Novel injectable gel (system) as a vehicle for human articular chondrocytes in cartilage tissue regeneration. J. Tissue Eng. Regen. Med. 2009, 3, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.G.; Caridade, S.G.; Mano, J.F.; Reis, R.L.; Gomes, M.E.J.J.; Medicine, R. Chondrogenic potential of injectable κ-carrageenan hydrogel with encapsulated adipose stem cells for cartilage tissue-engineering applications. J. Tissue Eng. Regen. Med. 2015, 9, 550–563. [Google Scholar] [CrossRef]
- Mouton, J.W.; van den Anker, J.N.J.C.p. Meropenem clinical pharmacokinetics. Clin. Pharmacokinet. 1995, 28, 275–286. [Google Scholar] [CrossRef]
- Tanaka, S.; Matsui, H.; Kasai, M.; Kunishiro, K.; Kakeya, N.; Shirahase, H. Novel prodrugs of meropenem with two lipophilic promoieties: Synthesis and pharmacokinetics. J. Antibiot. 2011, 64, 233. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, A.M.; Meissner, A.; Harding, R.A.; Wong, C.A.; Aldrich, C.C.; Remmel, R.P.J.B. Synthesis, pH-dependent, and plasma stability of meropenem prodrugs for potential use against drug-resistant tuberculosis. Bioorg. Med. Chem. 2013, 21, 5605–5617. [Google Scholar] [CrossRef]
- Yang, J.-A.; Yeom, J.; Hwang, B.W.; Hoffman, A.S.; Hahn, S.K. In situ-forming injectable hydrogels for regenerative medicine. Prog. Polym. Sci. 2014, 39, 1973–1986. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Park, W.; Park, H.; Lee, D.-K.; Na, K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr. Polym. 2017, 156, 403–408. [Google Scholar] [CrossRef]
- Yeh, M.-Y.; Zhao, J.-Y.; Hsieh, Y.-R.; Lin, J.-H.; Chen, F.-Y.; Chakravarthy, R.D.; Hung, S.-C.J.R.A. Reverse thermo-responsive hydrogels prepared from Pluronic F127 and gelatin composite materials. RSC Adv. 2017, 7, 21252–21257. [Google Scholar] [CrossRef]
- Tan, H.; Ramirez, C.M.; Miljkovic, N.; Li, H.; Rubin, J.P.; Marra, K.G.J.B. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials 2009, 30, 6844–6853. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; Van de Velde, F.; Ribeiro-Claro, P.J. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef]
- Khaliq, T.; Sohail, M.; Minhas, M.U.; Shah, S.A.; Jabeen, N.; Khan, S.; Rashid, H. Self-crosslinked chitosan/κ-carrageenan-based biomimetic membranes to combat diabetic burn wound infections. Int. J. Biol. Macromol. 2021, 197, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Sohail, M.; Khan, S.A.; Kousar, M. Improved drug delivery and accelerated diabetic wound healing by chondroitin sulfate grafted alginate-based thermoreversible hydrogels. Mater. Sci. Eng. C 2021, 126, 112169. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Sohail, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Mahmood, A.; Abbasi, M. Curcumin-laden hyaluronic acid-co-Pullulan-based biomaterials as a potential platform to synergistically enhance the diabetic wound repair. International J. Biol. Macromol. 2021, 185, 350–368. [Google Scholar] [CrossRef]
- Sun Han Chang, R.; Lee, J.C.-W.; Pedron, S.; Harley, B.A.; Rogers, S. A Rheological analysis of the gelation kinetics of an enzyme cross-linked PEG hydrogel. Biomacromolecules 2019, 20, 2198–2206. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E. and P. Rupérez, FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Balqis, A.I.; Khaizura, M.N.; Russly, A.; Hanani, Z.N. Effects of plasticizers on the physicochemical properties of kappa-carrageenan films extracted from Eucheuma cottonii. Int. J. Biol. Macromol. 2017, 103, 721–732. [Google Scholar] [CrossRef]
- Kusumaningrum, I.K.; Wijaya, A.R.; Marfuah, S.; Fadilah, M. Optimation of Alkoxide formed step on Carboxymethyl Kappa Carrageenan synthesis. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bogor, Indonesia, 25 October 2018. [Google Scholar]
- Al-Sibani, M.; Al-Harrasi, A.; Rhh, N. Characterization of Linear and Chemically Cross-linked Hyaluronic acid using Various Analytical Techniques Including FTIR, ESI-MS, H1 NMR, and SEM. J. Biochem. Anal. Stud. 2018, 3, 2576–5833. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Grabska, S.; Kaczmarek, B.; Michalska, M. The miscibility of collagen/hyaluronic acid/chitosan blends investigated in dilute solutions and solids. J. Mol. Liq. 2016, 220, 726–730. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kiarostami, K.; Khatir, N.M.; Rezania, S.; Muhamad, I.I.; Hosseini, F. Effect of zinc content on structural, functional, morphological, and thermal properties of kappa-carrageenan/NaCMC nanocomposites. Polym. Test. 2021, 93, 106922. [Google Scholar] [CrossRef]
- Branca, C.; Khouzami, K.; Wanderlingh, U.; D’Angelo, G. Effect of intercalated chitosan/clay nanostructures on concentrated pluronic F127 solution: A FTIR-ATR, DSC and rheological study. J. Colloid Interface Sci. 2018, 517, 221–229. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Y.; Peng, S.; Chen, X.; Zou, L.; Liu, W.; Liu, C. Liposomes consisting of pluronic F127 and phospholipid: Effect of matrix on morphology, stability and curcumin delivery. J. Dispers. Sci. Technol. 2019, 41, 207–213. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Grabska, S.; Kaczmarek, B. Surface and thermal properties of collagen/hyaluronic acid blends containing chitosan. Int. J. Biol. Macromol. 2016, 92, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, K.; Alina, S.; Grabska, S.; Michalska, M. Characterisation of chitosan/hyaluronic acid blend films modified by collagen. Prog. Chem. Appl. Chitin Deriv. 2017, 22, 125–134. [Google Scholar] [CrossRef]
- Layek, R.K.; Uddin, M.E.; Kim, N.H.; Lau, A.K.T.; Lee, J.H. Noncovalent functionalization of reduced graphene oxide with pluronic F127 and its nanocomposites with gum Arabic. Compos. Part B Eng. 2017, 128, 155–163. [Google Scholar] [CrossRef]
- Yap, L.-S.; Yang, M.-C. Evaluation of hydrogel composing of Pluronic F127 and carboxymethyl hexanoyl chitosan as injectable scaffold for tissue engineering applications. Colloids Surf. B Biointerfaces 2016, 146, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.; Ahmad, M.; Minhas, M.U.; Barkat, K.; Khalid, M.F. pH-responsive smart gels of block copolymer [pluronic F127-co-poly (acrylic acid)] for controlled delivery of Ivabradine hydrochloride: Its toxicological evaluation. J. Polym. Res. 2019, 26, 212. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Reddy, K.K.; Pyarasani, R.D.; Akbari-Fakhrabadi, A.; Amalraj, J. Chitosan-pluronic based Cu nanocomposite hydrogels for prototype antimicrobial applications. Int. J. Biol. Macromol. 2020, 143, 825–832. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Kulkarni, A.R.; Aminabhavi, T.M. Blend microspheres of poly (3-hydroxybutyrate) and cellulose acetate phthalate for colon delivery of 5-fluorouracil. Ind. Eng. Chem. Res. 2011, 50, 10414–10423. [Google Scholar] [CrossRef]
- Wahid, F.; Zhou, Y.-N.; Wang, H.-S.; Wan, T.; Zhong, C.; Chu, L.-Q. Injectable self-healing carboxymethyl chitosan-zinc supramolecular hydrogels and their antibacterial activity. Int. J. Biol. Macromol. 2018, 114, 1233–1239. [Google Scholar] [CrossRef]
- Ojha, J.; Nanda, R.; Dorai, K. NMR investigation of the thermogelling properties, anomalous diffusion, and structural changes in a Pluronic F127 triblock copolymer in the presence of gold nanoparticles. Colloid Polym. Sci. 2020, 298, 1–15. [Google Scholar] [CrossRef]
- Picheth, G.F.; Da Silva, L.C.; Giglio, L.P.; Plivelic, T.S.; de Oliveira, M.G. S-nitrosothiol-terminated Pluronic F127: Influence of microstructure on nitric oxide release. J. Colloid Interface Sci. 2020, 576, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.; Zhang, Z.; Lian, H.; Chen, X.; Xiao, C.; Zhuang, X.; Chen, X. Injectable electroactive hydrogels based on Pluronic® F127 and tetraaniline copolymer. Eur. Polym. J. 2017, 88, 67–74. [Google Scholar] [CrossRef]
- Wende, F.J.; Xue, Y.; Nestor, G.; Öhrlund, Å.; Sandström, C. Relaxation and diffusion of water protons in BDDE cross-linked hyaluronic acid hydrogels investigated by NMR spectroscopy—Comparison with physicochemical properties. Carbohydr. Polym. 2020, 248, 116768. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Du, L.; Matsukawa, S. NMR study on the network structure of a mixed gel of kappa and iota carrageenans. Carbohydr. Polym. 2016, 150, 57–64. [Google Scholar] [CrossRef]
- Yang, D.; Yang, H. The temperature dependent extraction of polysaccharides from eucheuma and the rheological synergistic effect in their mixtures with kappa carrageenan. LWT 2020, 129, 109515. [Google Scholar] [CrossRef]
- Chirumamilla, S.K.; Padasala, U.D.; Aravally, H.; Vuppalapati, L.; Cherukuri, S. Solubility and Dissolution Enhancement of Meropenem by Nano Suspension Approach. J. Young Pharm. 2017, 9, 429–435. [Google Scholar] [CrossRef]
- Mangal, S.; Park, H.; Zeng, L.; Heidi, H.Y.; Lin, Y.-W.; Velkov, T.; Zhou, Q.T. Composite particle formulations of colistin and meropenem with improved in-vitro bacterial killing and aerosolization for inhalation. Int. J. Pharm. 2018, 548, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Liew, J.W.Y.; Loh, K.S.; Ahmad, A.; Lim, K.L.; Wan Daud, W.R. Synthesis and characterization of modified κ-carrageenan for enhanced proton conductivity as polymer electrolyte membrane. PLoS ONE 2017, 12, e0185313. [Google Scholar] [CrossRef]
- Du, L.; Brenner, T.; Xie, J.; Matsukawa, S. A study on phase separation behavior in kappa/iota carrageenan mixtures by micro DSC, rheological measurements and simulating water and cations migration between phases. Food Hydrocoll. 2016, 55, 81–88. [Google Scholar] [CrossRef]
- Schieber, J.; Lazar, R.; Bohacs, K.; Klimentidis, R.; Dumitrescu, M.; Ottmann, J. An SEM study of porosity in the Eagle Ford Shale of Texas—Pore types and porosity distribution in a depositional and sequence-stratigraphic context. In The Eagle Ford Shale: A Renaissance in U.S. Oil Production; American Association of Petroleum Geologists: Tulsa, OK, USA, 2016; pp. 167–186. [Google Scholar]
- Klaver, J.; Desbois, G.; Littke, R.; Urai, J.L. BIB-SEM pore characterization of mature and post mature Posidonia Shale samples from the Hils area, Germany. Int. J. Coal Geol. 2016, 158, 78–89. [Google Scholar] [CrossRef]
- Yang, M.; Wang, L.; Xia, Y. Ammonium persulphate induced synthesis of polymethyl methacrylate grafted sodium alginate composite films with high strength for food packaging. Int. J. Biol. Macromol. 2019, 124, 1238–1245. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, S.-H.; Mao, S.-H.; Tsai, M.-J.; Chou, P.-Y.; Liao, C.-H.; Chen, J.-P. Injectable thermosensitive hydrogel containing hyaluronic acid and chitosan as a barrier for prevention of postoperative peritoneal adhesion. Carbohydr. Polym. 2017, 173, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Ohi, H.; Taya, M. Gelatin/Hyaluronic Acid Content in Hydrogels Obtained through Blue Light-Induced Gelation Affects Hydrogel Properties and Adipose Stem Cell Behaviors. Biomolecules 2019, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Tytgat, L.; Vagenende, M.; Declercq, H.; Martins, J.; Thienpont, H.; Ottevaere, H.; Van Vlierberghe, S. Synergistic effect of κ-carrageenan and gelatin blends towards adipose tissue engineering. Carbohydr. Polym. 2018, 189, 1–9. [Google Scholar] [CrossRef]
- Cidade, M.; Ramos, D.; Santos, J.; Carrelo, H.; Calero, N.; Borges, J. Injectable Hydrogels Based on Pluronic/Water Systems Filled with Alginate Microparticles for Biomedical Applications. Materials 2019, 12, 1083. [Google Scholar] [CrossRef] [PubMed]
- Quah, S.P.; Smith, A.J.; Preston, A.N.; Laughlin, S.T.; Bhatia, S.R. Large-area alginate/PEO-PPO-PEO hydrogels with thermoreversible rheology at physiological temperatures. Polymer 2018, 135, 171–177. [Google Scholar] [CrossRef]
- Demeter, M.; Virgolici, M.; Vancea, C.; Scarisoreanu, A.; Kaya, M.G.A.; Meltzer, V. Network structure studies on γ–irradiated collagen–PVP superabsorbent hydrogels. Radiat. Phys. Chem. 2017, 131, 51–59. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C 2020, 107, 110195. [Google Scholar] [CrossRef]
- Yokota, S.; Tagawa, S.; Kondo, T. Facile surface modification of amphiphilic cellulose nanofibrils prepared by aqueous counter collision. Carbohydr. Polym. 2020, 255, 117342. [Google Scholar] [CrossRef] [PubMed]
- Dehghan-Baniani, D.; Chen, Y.; Wang, D.; Bagheri, R.; Solouk, A.; Wu, H. Injectable in situ forming kartogenin-loaded chitosan hydrogel with tunable rheological properties for cartilage tissue engineering. Colloids Surf. B Biointerfaces 2020, 192, 111059. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Teixeira, L.M.; Dijkstra, P.J.; Karperien, M.; Van Blitterswijk, C.; Zhong, Z.; Feijen, J. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Teixeira, L.M.; Krouwels, A.; Dijkstra, P.J.; Van Blitterswijk, C.; Karperien, M.; Feijen, J. Synthesis and characterization of hyaluronic acid–poly (ethylene glycol) hydrogels via Michael addition: An injectable biomaterial for cartilage repair. Acta Biomater. 2010, 6, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, X.; Li, J.; Hao, X.; Lin, J.; Cheng, D.; Lu, Y.J.P. Thermosensitive behavior and antibacterial activity of cotton fabric modified with a chitosan-poly (N-isopropylacrylamide) interpenetrating polymer network hydrogel. Polymers 2016, 8, 110. [Google Scholar] [CrossRef]
- Kim, W.K.; Choi, J.H.; Shin, M.E.; Kim, J.W.; Kim, P.Y.; Kim, N.; Khang, G. Evaluation of cartilage regeneration of chondrocyte encapsulated gellan gum-based hyaluronic acid blended hydrogel. Int. J. Biol. Macromol. 2019, 141, 51–59. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kharaziha, M.; Kermanpur, A.; Mokhtari, H. Sprayable and injectable visible-light Kappa-carrageenan hydrogel for in-situ soft tissue engineering. Int. J. Biol. Macromol. 2019, 138, 590–601. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, P.; Jin, Y.; Ren, F. Injectable, strongly compressible hyaluronic acid hydrogels via incorporation of Pluronic F127 diacrylate nanomicelles. Mater. Lett. 2019, 243, 112–115. [Google Scholar] [CrossRef]
- Chen, M.; Li, C.; Nie, F.; Liu, X.; Pipinos, I.I.; Li, X. Synthesis and characterization of a hyaluronic acid-based hydrogel with antioxidative and thermosensitive properties. RSC Adv. 2020, 10, 33851–33860. [Google Scholar] [CrossRef]
- Nascimento, M.; Franco, M.; Yokaichyia, F.; de Paula, E.; Lombello, C.; de Araujo, D. Hyaluronic acid in Pluronic F-127/F-108 hydrogels for postoperative pain in arthroplasties: Influence on physico-chemical properties and structural requirements for sustained drug-release. Int. J. Biol. Macromol. 2018, 111, 1245–1254. [Google Scholar] [CrossRef]
- Azizi, S.; Mohamad, R.; Rahim, R.A.; Mohammadinejad, R.; Ariff, A.B. Hydrogel beads bio-nanocomposite based on Kappa-Carrageenan and green synthesized silver nanoparticles for biomedical applications. Int. J. Biol. Macromol. 2017, 104, 423–431. [Google Scholar] [CrossRef]
- Selvakumaran, S.; Muhamad, I.I.; Abd Razak, S.I. Evaluation of kappa carrageenan as potential carrier for floating drug delivery system: Effect of pore forming agents. Carbohydr. Polym. 2016, 135, 207–214. [Google Scholar] [CrossRef]
- Meshkini, A.; Oveisi, H. Methotrexate-F127 conjugated mesoporous zinc hydroxyapatite as an efficient drug delivery system for overcoming chemotherapy resistance in osteosarcoma cells. Colloids Surf. B Biointerfaces 2017, 158, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Mdlovu, N.V.; Mavuso, F.A.; Lin, K.-S.; Chang, T.-W.; Chen, Y.; Wang, S.S.-S.; Lin, Y.-S. Iron oxide-pluronic F127 polymer nanocomposites as carriers for a doxorubicin drug delivery system. Colloids Surf. A Physicochem. Eng. Asp. 2019, 562, 361–369. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
| Formulation | HA (%w/w) | κ-C (%w/w) | F127 (%w/w) | Meropenem | Gel Temp | Gel Time |
|---|---|---|---|---|---|---|
| (% w/w) | (°C) | (s) | ||||
| HC-1 | 3 | 0.2 | 21 | 1 | 36 | 13 |
| HC-2 | 4 | 0.2 | 21 | 1 | 35 | 10 |
| HC-3 | 5 | 0.2 | 21 | 1 | 34 | 8 |
| HC-4 | 4 | 0.1 | 21 | 1 | 33 | 9 |
| HC-5 | 4 | 0.2 | 21 | 1 | 35 | 10 |
| HC-6 | 4 | 0.3 | 21 | 1 | 37 | 12 |
| HC-7 | 4 | 0.2 | 21 | 1 | 36 | 15 |
| HC-8 | 4 | 0.2 | 19 | 1 | 33 | 10 |
| HC-9 | 4 | 0.2 | 21 | 1 | 31 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ijaz, U.; Sohail, M.; Usman Minhas, M.; Khan, S.; Hussain, Z.; Kazi, M.; Ahmed Shah, S.; Mahmood, A.; Maniruzzaman, M. Biofunctional Hyaluronic Acid/κ-Carrageenan Injectable Hydrogels for Improved Drug Delivery and Wound Healing. Polymers 2022, 14, 376. https://doi.org/10.3390/polym14030376
Ijaz U, Sohail M, Usman Minhas M, Khan S, Hussain Z, Kazi M, Ahmed Shah S, Mahmood A, Maniruzzaman M. Biofunctional Hyaluronic Acid/κ-Carrageenan Injectable Hydrogels for Improved Drug Delivery and Wound Healing. Polymers. 2022; 14(3):376. https://doi.org/10.3390/polym14030376
Chicago/Turabian StyleIjaz, Uzma, Muhammad Sohail, Muhammad Usman Minhas, Shahzeb Khan, Zahid Hussain, Mohsin Kazi, Syed Ahmed Shah, Arshad Mahmood, and Mohammed Maniruzzaman. 2022. "Biofunctional Hyaluronic Acid/κ-Carrageenan Injectable Hydrogels for Improved Drug Delivery and Wound Healing" Polymers 14, no. 3: 376. https://doi.org/10.3390/polym14030376
APA StyleIjaz, U., Sohail, M., Usman Minhas, M., Khan, S., Hussain, Z., Kazi, M., Ahmed Shah, S., Mahmood, A., & Maniruzzaman, M. (2022). Biofunctional Hyaluronic Acid/κ-Carrageenan Injectable Hydrogels for Improved Drug Delivery and Wound Healing. Polymers, 14(3), 376. https://doi.org/10.3390/polym14030376










