Wavelength-Tunable and Water-Stable Cesium–Lead-Based All-Bromide Nanocrystal–Polymer Composite Films Using Ultraviolet-Curable Prepolymer as an Anti-Solvent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Precursors and Prepolymer
2.3. Synthesis of IPeNCs and Its Composite Films
2.4. Characterization and LED Color Measurements
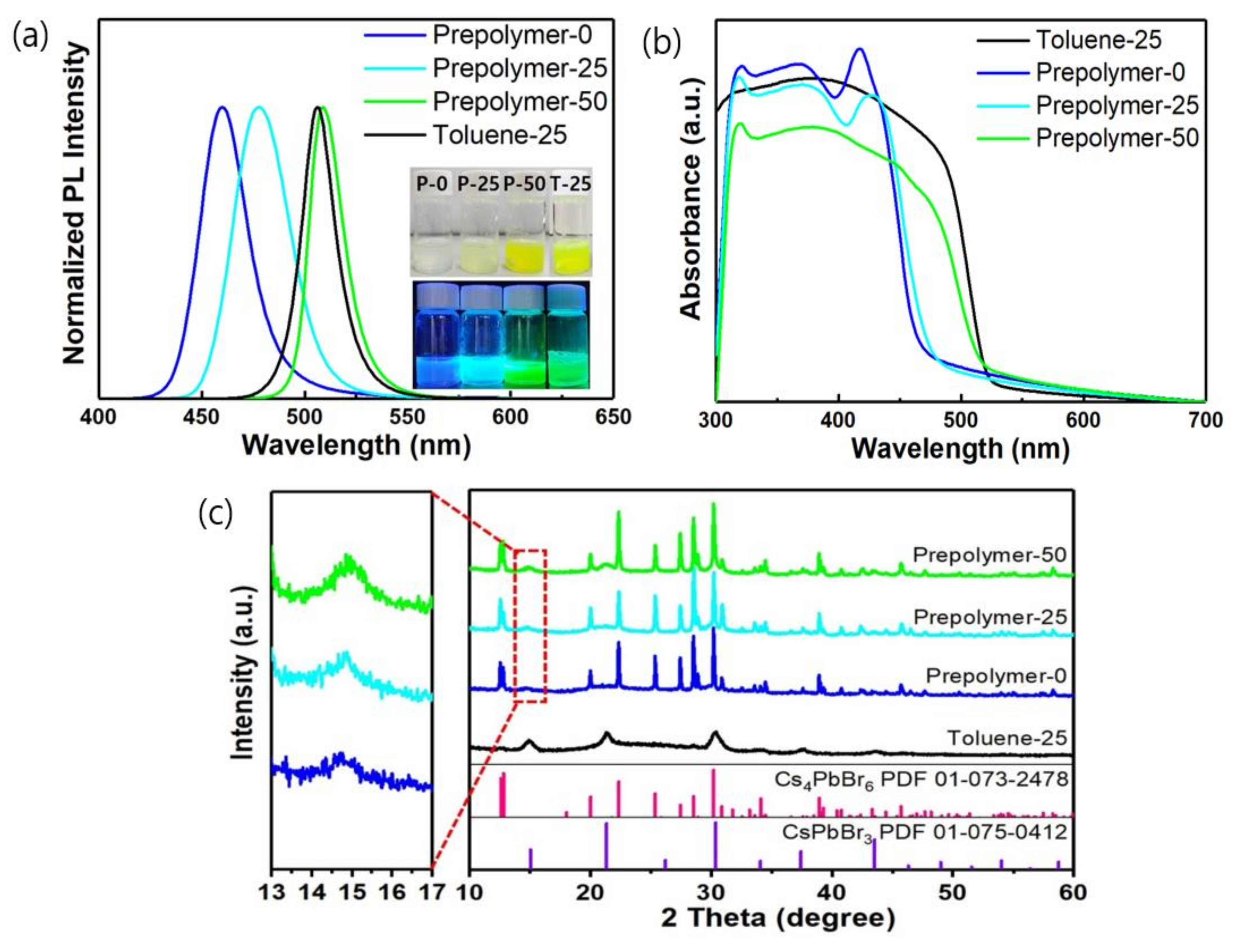
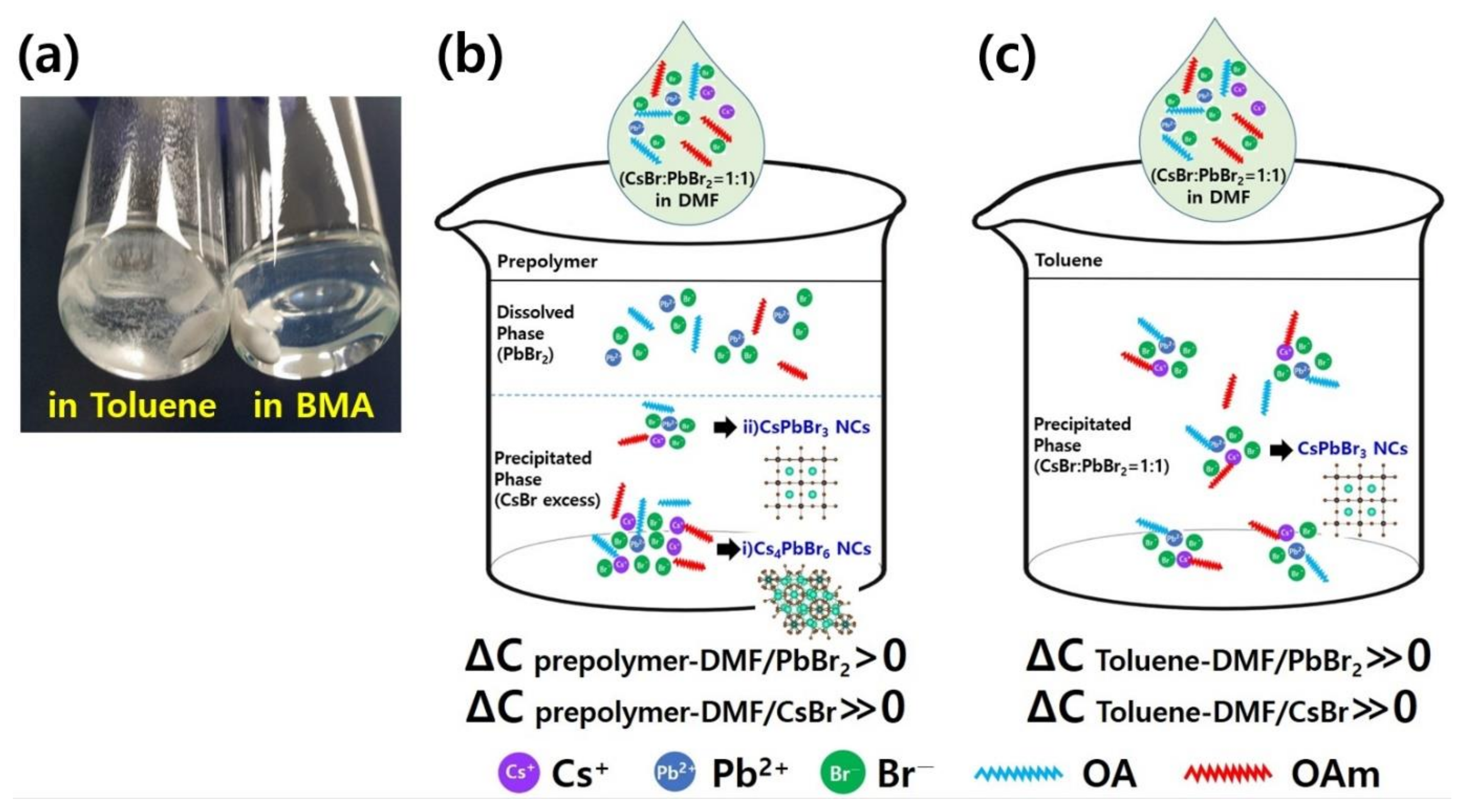
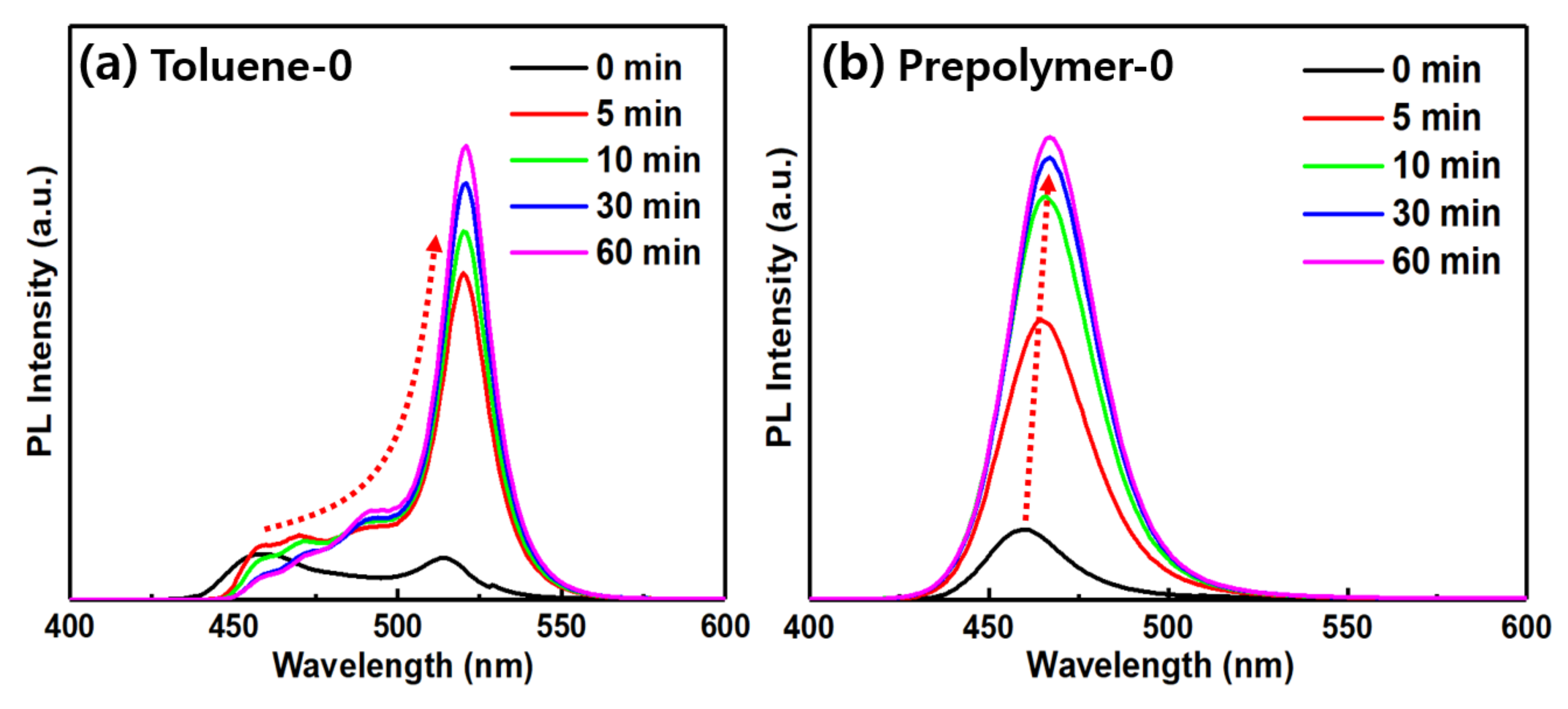
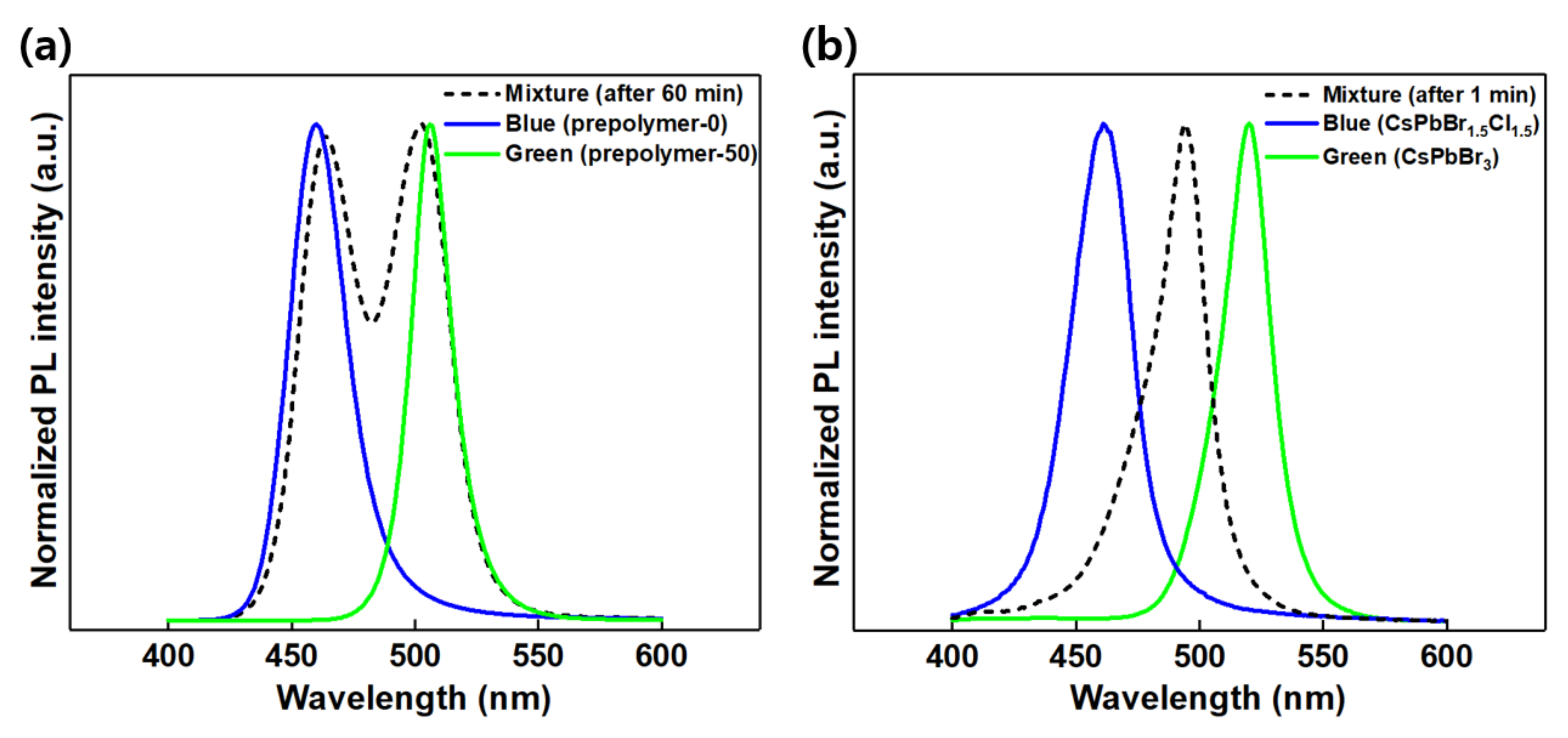
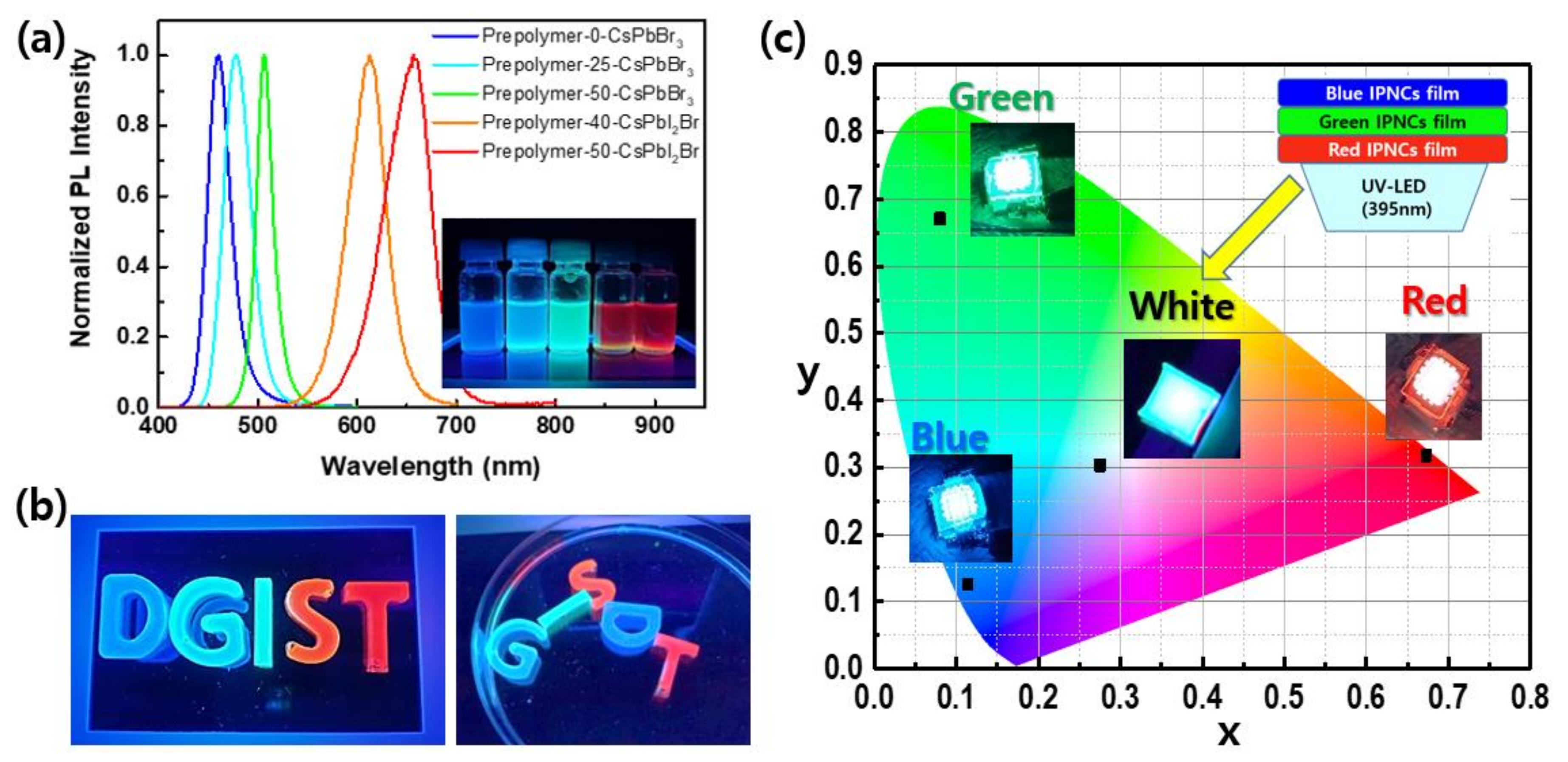
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, H.; Bodnarchuk, M.I.; Kershaw, S.V.; Kovalenko, M.V.; Rogach, A.L. Lead halide perovskite nanocrystals in the research spotlight: Stability and defect tolerance. ACS Energy Lett. 2017, 2, 2071–2083. [Google Scholar] [CrossRef] [Green Version]
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 quantum dots for lighting and displays: Room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, X.; Sun, X.; Zhang, X. Deep blue emission of all-bromide-based cesium lead perovskite nanocrystals. J. Phys. Chem. C 2020, 124, 1617–1622. [Google Scholar] [CrossRef]
- Peng, X.; Yan, C.; Chun, F.; Li, W.; Fu, X.; Yang, W. A review of low-dimensional metal halide perovskites for blue light emitting diodes. J. Alloys Compd. 2021, 883, 160727. [Google Scholar] [CrossRef]
- Yang, D.; Huo, D. Cation doping and strain engineering of CsPbBr3-based perovskite light emitting diodes. J. Mater. Chem. C 2020, 8, 6640–6653. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, G.; Yin, Y.; Miao, J.; Li, K.; Wang, C.; Xu, X.; Shen, C.; Meng, H. Aluminum-doped cesium lead bromide perovskite nanocrystals with stable blue photoluminescence used for display backlight. Adv. Sci. 2017, 4, 1700335. [Google Scholar] [CrossRef]
- Bi, C.; Wang, S.; Li, Q.; Kershaw, S.V.; Tian, J.; Rogach, A.L. Thermally stable copper(II)-doped cesium lead halide perovskite quantum dots with strong blue emission. J. Phys. Chem. Lett. 2019, 10, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Tsiwah, E.A.; Li, T.; Hu, J.; Li, Z.; Ding, Y.; Deng, Z.; Chen, W.; Xu, L.; Gao, P.; et al. Trivalent ion mediated abnormal growth of all inorganic perovskite nanocrystals and their divergent emission properties. Nanoscale 2019, 11, 7903–7912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Hu, Y.; Pei, Y.; Wang, S.; Shi, Z.; Colvin, V.L.; Wang, S.; Zhang, Y.; Yu, W.W. Strong blue emission from Sb3+-doped super small CsPbBr3 nanocrystals. J. Phys. Chem. Lett. 2019, 10, 1750–1756. [Google Scholar] [CrossRef]
- Xie, Y.; Peng, B.; Bravic, I.; Yu, Y.; Dong, Y.; Liang, R.; Ou, Q.; Monserrat, B.; Zhang, S. Highly efficient blue-emitting CsPbBr3 perovskite nanocrystals through neodymium doping. Adv. Sci. 2020, 7, 2001698. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Hu, Z.; Liu, Z.; Wu, Z.; Ono, L.K.; Qi, Y. Engineering green-to-blue emitting CsPbBr3 quantum-dot films with efficient ligand passivation. ACS Energy Lett. 2019, 4, 2731–2738. [Google Scholar] [CrossRef]
- Yang, H.; Feng, Y.; Tu, Z.; Su, K.; Fan, X.; Liu, B.; Shi, Z.; Zhang, Y.; Zhao, C.; Zhang, B. Blue emitting CsPbBr3 perovskite quantum dot inks obtained from sustained release tablets. Nano Res. 2019, 12, 3129–3134. [Google Scholar] [CrossRef]
- Xu, X.; He, H.; Fang, Z.; Lou, H.; Lin, C.; Chen, L.; Ye, Z. Ultrasonication-assisted ambient-air synthesis of monodispersed blue-emitting CsPbBr3 quantum dots for white light emission. ACS Appl. Nano Mater. 2019, 2, 6874–6879. [Google Scholar] [CrossRef]
- Tomanova, K.; Cuba, V.; Brik, M.G.; Mihokova, E.; Turtos, R.M.; Lecoq, P.; Auffray, E.; Nikl, M. On the structure, synthesis, and characterization of ultrafast blue-emitting CsPbBr3 nanoplatelets. APL Mater. 2019, 7, 011104. [Google Scholar] [CrossRef] [Green Version]
- Shamsi, J.; Kubicki, D.; Anaya, M.; Liu, Y.; Ji, K.; Frohna, K.; Gray, C.P.; Friend, R.H.; Stranks, S.D. Stable hexylphosphonate-capped blue-emitting quantum-confined CsPbBr3 nanoplatelets. ACS Energy Lett. 2020, 5, 1900–1907. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, B.; Wang, Z.; Hung, T.F.; Susha, A.S.; Zhong, H.; Rogach, A.L. Water resistant CsPbX3 nanocrystals coated with polyhedral oligomeric silsesquioxane and their use as solid state luminophores in all-perovskite white light-emitting devices. Chem. Sci. 2016, 7, 5699–5703. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.C.; Lin, S.Y.; Tang, A.C.; Singh, B.P.; Tong, H.C.; Chen, C.Y.; Lee, Y.C.; Tsai, T.L.; Liu, R.S. Mesoporous silica particles integrated with all-inorganic CsPbBr3 perovskite quantum-dot nanocomposites (MP-PQDs) with high stability and wide color gamut used for backlight display. Angew. Chem. Int. Ed. 2016, 55, 7924–7929. [Google Scholar] [CrossRef]
- Hai, J.; Li, H.; Zhao, Y.; Chen, F.; Peng, Y.; Wang, B. Designing of blue, green, and red CsPbX3 perovskite-codoped flexible films with water resistant property and elimination of anion-exchange for tunable white light emission. Chem. Commun. 2017, 53, 5400–5403. [Google Scholar] [CrossRef]
- Lv, W.; Li, L.; Xu, M.; Hong, J.; Tang, X.; Xu, L.; Wu, Y.; Zhu, R.; Chen, R.; Huang, W. Improving the stability of metal halide perovskite quantum dots by encapsulation. Adv. Mater. 2019, 31, 1900682. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [Green Version]
- Raja, S.N.; Bekenstein, Y.; Koc, M.A.; Fischer, S.; Zhang, D.; Lin, L.; Ritchie, R.O.; Yang, P.; Alivisatos, A.P. Encapsulation of perovskite nanocrystals into macroscale polymer matrices: Enhanced stability and polarization. ACS Appl. Mater. Interfaces 2016, 8, 35523–35533. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Chen, J.; Song, J.; Li, J.; Xue, J.; Dong, Y.; Cai, B.; Shan, Q.; Han, B.; Zeng, H. Double-protected all-inorganic perovskite nanocrystals by crystalline matrix and silica for triple-modal anti-counterfeiting codes. ACS Appl. Mater. Interfaces 2017, 9, 26556–26564. [Google Scholar] [CrossRef]
- Wei, Y.; Deng, X.; Xie, Z.; Cai, X.; Liang, S.; Ma, P.; Hou, Z.; Cheng, Z.; Lin, J. Enhancing the stability of perovskite quantum dots by encapsulation in crosslinked polystyrene beads via a swelling–shrinking strategy toward superior water resistance. Adv. Funct. Mater. 2017, 27, 1703535. [Google Scholar] [CrossRef]
- Yang, X.; Xu, T.; Zhu, Y.; Cai, J.; Gu, K.; Zhu, J.; Wang, Y.; Shen, J.; Li, C. Preparation of CsPbBr3@PS composite microspheres with high stability by electrospraying. J. Mater. Chem. C 2018, 6, 7971–7975. [Google Scholar] [CrossRef]
- Zhou, H.; Park, J.; Lee, Y.; Park, J.-M.; Kim, J.-H.; Kim, J.S.; Lee, H.-D.; Jo, S.H.; Cai, X.; Li, L.; et al. Water passivation of perovskite nanocrystals enables air-stable intrinsically stretchable color-conversion layers for stretchable displays. Adv. Mater. 2020, 32, 2001989. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ge, W.; Gao, W.; Xu, M.; Zhu, J.; Li, Y. Enhanced thermal stability of halide perovskite CsPbX3 nanocrystals by a facile TPU encapsulation. Adv. Optical Mater. 2020, 8, 1901516. [Google Scholar] [CrossRef]
- Zhu, L.; Yuan, S.; Cheng, J.; Chen, L.; Liu, C.; Tong, H.; Zeng, H.; Cheng, Q. Room temperature in-situ synthesis of inorganic lead halide perovskite nanocrystals sol using ultraviolet polymerized acrylic monomers as solvent and their composites with high stability. Appl. Sci. 2020, 10, 3325. [Google Scholar] [CrossRef]
- Xin, Y.; Shen, W.; Deng, Z.; Zhang, J. Highly emissive and color-tunable perovskite cross-linkers for luminescent polymer networks. ACS Appl. Mater. Interfaces 2018, 10, 28971–28978. [Google Scholar] [CrossRef]
- Tong, J.; Wu, J.; Shen, W.; Zhang, Y.; Liu, Y.; Zhang, T.; Nie, S.; Deng, Z. Direct hot-injection synthesis of lead halide perovskite nanocubes in acrylic monomers for ultrastable and bright nanocrystal−polymer composite films. ACS Appl. Mater. Interfaces 2019, 11, 9317–9325. [Google Scholar] [CrossRef]
- Xin, Y.; Zhao, H.; Zhang, J. Highly stable and luminescent perovskite−polymer composites from a convenient and universal strategy. ACS Appl. Mater. Interfaces 2018, 10, 4971–4980. [Google Scholar] [CrossRef]
- Sun, H.; Yang, Z.; Wei, M.; Sun, W.; Li, X.; Ye, S.; Zhao, Y.; Tan, H.; Kynaston, E.L.; Schon, T.B.; et al. Chemically addressable perovskite nanocrystals for light-emitting applications. Adv. Mater. 2017, 29, 1701153. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Mod. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MOPAC2016. Available online: http://openmopac.net/MOPAC2016.html (accessed on 20 October 2021).
- Jing, Q.; Xu, Y.; Su, Y.; Xing, X.; Lu, Z. A systematic study of the synthesis of cesium lead halide nanocrystals: Does Cs4PbBr6 or CsPbBr3 form? Nanoscale 2019, 11, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, C.; Li, J.; Wu, J.; Zhang, S.; Kuo, H.-C.; Shao, L.; Zhao, S.; Zhang, J.; Kang, F.; et al. CsPbBr3-Cs4PbBr6 composite nanocrystals for highly efficient pure green light emission. Nanoscale 2019, 11, 22899–22906. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tang, Y.; Rao, L.; Li, Z.; Ding, X.; Song, C.; Yu, B. Investigating the transformation of CsPbBr3 nanocrystals into highly stable CsPbBr3/Cs4PbBr6 nanocrystals using ethyl acetate in a microchannel reactor. Nanotechnology 2019, 30, 295603. [Google Scholar] [CrossRef]
- Gao, H.; Liu, S.; Xue, Z.; Liu, W.; Nie, Y.; Chen, G.; Li, X. Synthesis and photoluminescence properties of CsPbBr3 quantum dots by using para-xylene as the anti-solvent. J. Lumines. 2019, 215, 116584. [Google Scholar] [CrossRef]
- Adhikari, G.C.; Thapa, S.; Zhu, H.; Zhu, P. UV resin enhanced stability of metal halide perovskite nanocrystals for white light-emitting diodes. ACS Appl. Electron. Mater. 2020, 2, 35–40. [Google Scholar] [CrossRef]
- Wang, X.; Bao, Z.; Chang, Y.-C.; Liu, R.-S. Perovskite quantum dots for application in high color gamut backlighting display of light-emitting diodes. ACS Energy Lett. 2020, 5, 3374–3396. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.H.; Bae, J.; Kim, K.-P.; Woo, S. Wavelength-Tunable and Water-Stable Cesium–Lead-Based All-Bromide Nanocrystal–Polymer Composite Films Using Ultraviolet-Curable Prepolymer as an Anti-Solvent. Polymers 2022, 14, 381. https://doi.org/10.3390/polym14030381
Kim WH, Bae J, Kim K-P, Woo S. Wavelength-Tunable and Water-Stable Cesium–Lead-Based All-Bromide Nanocrystal–Polymer Composite Films Using Ultraviolet-Curable Prepolymer as an Anti-Solvent. Polymers. 2022; 14(3):381. https://doi.org/10.3390/polym14030381
Chicago/Turabian StyleKim, Wook Hyun, Jungyoun Bae, Kang-Pil Kim, and Sungho Woo. 2022. "Wavelength-Tunable and Water-Stable Cesium–Lead-Based All-Bromide Nanocrystal–Polymer Composite Films Using Ultraviolet-Curable Prepolymer as an Anti-Solvent" Polymers 14, no. 3: 381. https://doi.org/10.3390/polym14030381




