Physical, Mechanical, and Morphological Performances of Arrowroot (Maranta arundinacea) Fiber Reinforced Arrowroot Starch Biopolymer Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Development of Thermoplastic Arrowroot Starch (TPAS)/Arrowroot Fiber (AF) Biopolymer Composites
2.3. Characterization of Biocomposites
2.3.1. The Thickness of Biopolymer Composite Films
2.3.2. Density
2.3.3. Water Contents (WC)
2.3.4. Water Solubility (WS)
2.3.5. Water Absorption (WA)
2.3.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.7. Field Emission Scanning Electron Microscopy (FESEM)
2.3.8. Mechanical Testing
2.3.9. Statistical Analysis
3. Results and Discussion
3.1. Thicknesses and Densities of Biopolymer Composite Film
3.2. Water Contents (Wc)
3.3. Water Solubility (Ws)
3.4. Water Absorption (WA)
3.5. Fourier Transform Infrared (FTIR) Spectroscopy
3.6. Morphological Properties
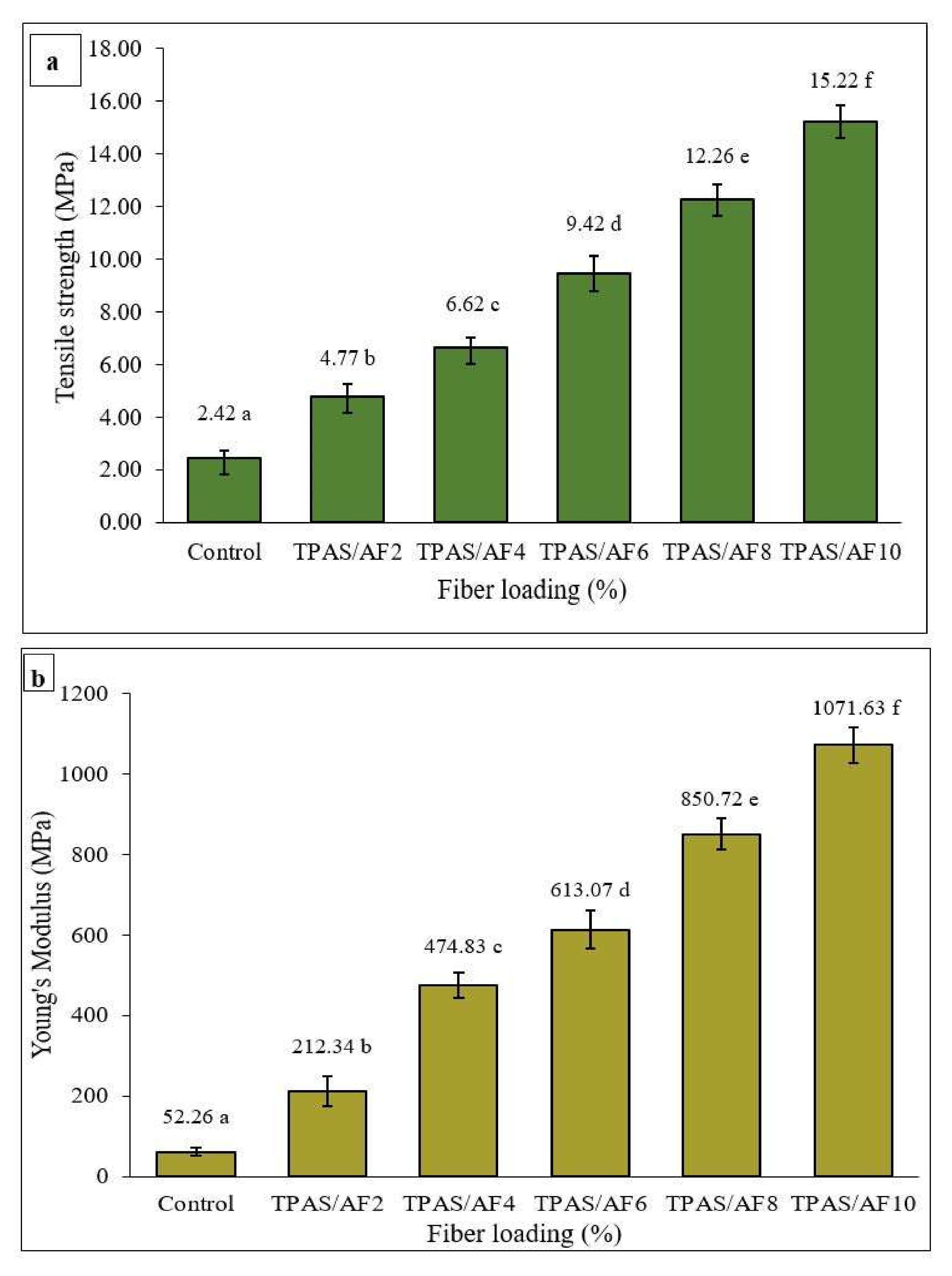
3.7. Tensile Properties
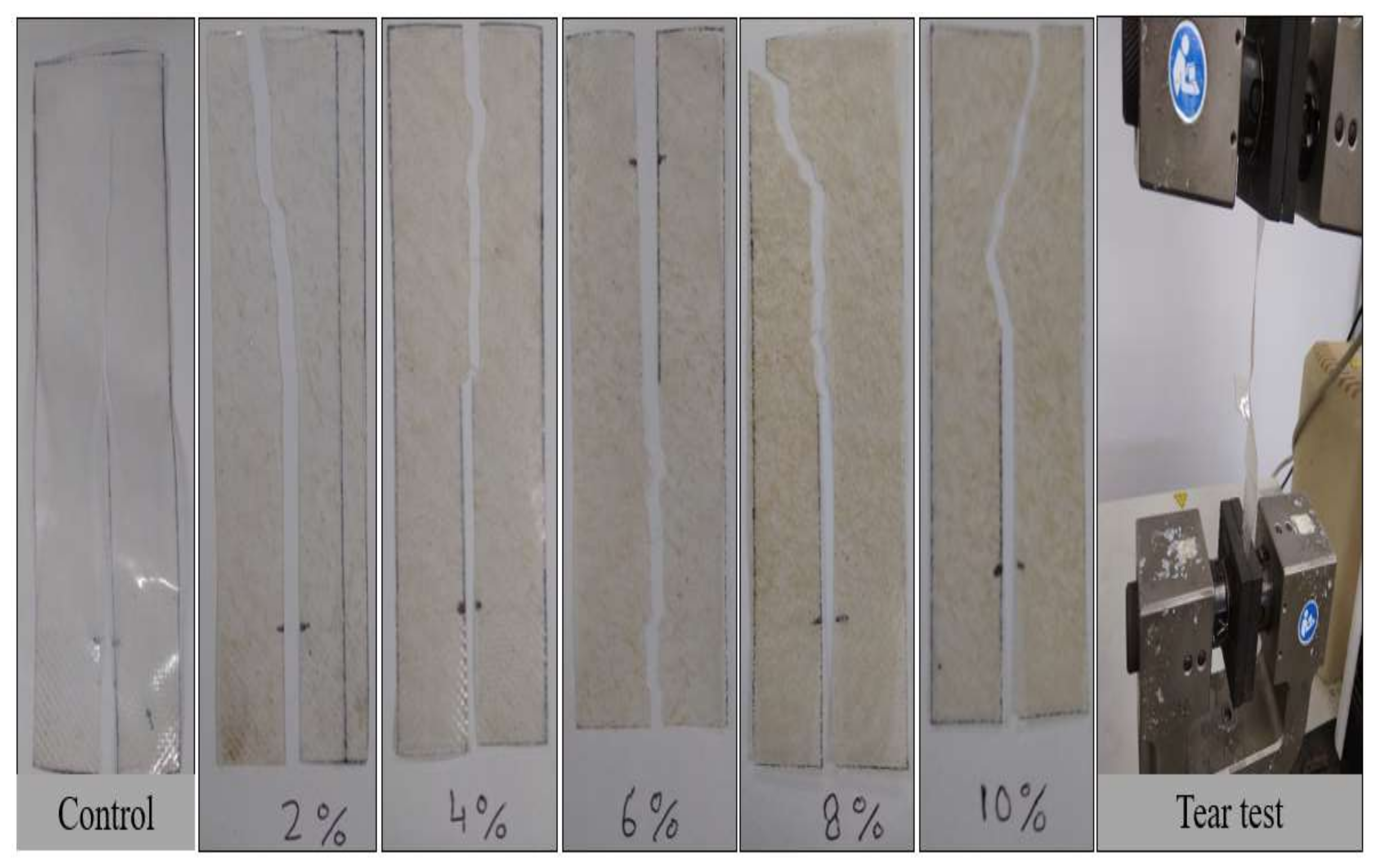
3.8. Tear Strength
3.9. Effect of the Fiber Loadings on the Physical and Mechanical Properties of TPAS and TPAS/AF Biocomposites: Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Development and characterization of sugar palm nanocrystalline cellulose reinforced sugar palm starch bionanocomposites. Carbohydr. Polym. 2018, 202, 186–202. [Google Scholar] [CrossRef]
- Abral, H.; Atmajaya, A.; Mahardika, M.; Hafizulhaq, F.; Kadriadi; Handayani, D.; Sapuan, S.M.; Ilyas, R.A. Effect of ultrasonication duration of polyvinyl alcohol (PVA) gel on characterizations of PVA film. J. Mater. Res. Technol. 2020, 9, 2477–2486. [Google Scholar] [CrossRef]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Koloor, S.S.R.; Petrů, M. Micro-and nanocellulose in polymer composite materials: A review. Polymers 2021, 13, 1–30. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on tensile, thermal and barrier properties of biodegradable films based on sugar palm (Arenga pinnata) starch. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Potential of using multiscale corn husk fiber as reinforcing filler in cornstarch-based biocomposites. Int. J. Biol. Macromol. 2019, 139, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Preparation and characterization of cassava bagasse reinforced thermoplastic cassava starch. Fibers Polym. 2017, 18, 162–171. [Google Scholar] [CrossRef]
- Acquavia, M.A.; Pascale, R.; Martelli, G.; Bondoni, M.; Bianco, G. Natural polymeric materials: A solution to plastic pollution from the agro-food sector. Polymers 2021, 13, 1–39. [Google Scholar] [CrossRef]
- Hazrol, M.D.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M.; Abdul Wahab, N.I. Corn Starch (Zea mays) Biopolymer Plastic Reaction in Combination with Sorbitol and Glycerol. Polymers 2021, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Atiqah, A.; Atikah, M.S.N.; Syafri, E.; Asrofi, M.; et al. Thermal, Biodegradability and Water Barrier Properties of Bio-Nanocomposites Based on Plasticised Sugar Palm Starch and Nanofibrillated Celluloses from Sugar Palm Fibres. J. Biobased Mater. Bioenergy 2020, 14, 234–248. [Google Scholar] [CrossRef]
- Lee, M.; Park, J.K.; Lee, H.S.; Lane, O.; Moore, R.B.; McGrath, J.E.; Baird, D.G. Effects of block length and solution-casting conditions on the final morphology and properties of disulfonated poly(arylene ether sulfone) multiblock copolymer films for proton exchange membranes. Polymer 2009, 50, 6129–6138. [Google Scholar] [CrossRef]
- Langhe, D.; Ponting, M. Coextrusion Processing of Multilayered Films. In Manufacturing and Novel Applications of Multilayer Polymer Films; Elsevier: Amsterdam, The Netherlands, 2016; pp. 16–45. ISBN 9780323371254. [Google Scholar]
- Lisiecka, K.; Wójtowicz, A.; Gancarz, M. Characteristics of newly developed extruded products supplemented with plants in a form of microwave-expanded snacks. Materials 2021, 14, 2791. [Google Scholar] [CrossRef] [PubMed]
- Jozinović, A.; Ačkar, D.; Jokić, S.; Babić, J.; Balentić, J.P.; Banožić, M.; Šubarić, D. Optimisation of extrusion variables for the production of corn snack products enriched with defatted hemp cake. Czech, J. Food Sci. 2017, 35, 507–516. [Google Scholar]
- Rangappa, S.M.; Siengchin, S.; Dhakal, H.N. Green-composites: Ecofriendly and sustainability. Appl. Sci. Eng. Prog. 2020, 13, 183–184. [Google Scholar] [CrossRef]
- Kumar, T.S.M.; Chandrasekar, M.; Senthilkumar, K.; Ilyas, R.A.; Sapuan, S.M.; Hariram, N.; Rajulu, A.V.; Rajini, N.; Siengchin, S. Characterization, Thermal and Antimicrobial Properties of Hybrid Cellulose Nanocomposite Films with in-Situ Generated Copper Nanoparticles in Tamarindus indica Nut Powder. J. Polym. Environ. 2021, 29, 1134–1142. [Google Scholar] [CrossRef]
- Syafri, E.; Sudirman; Mashadi; Yulianti, E.; Deswita; Asrofi, M.; Abral, H.; Sapuan, S.M.; Ilyas, R.A.; Fudholi, A. Effect of sonication time on the thermal stability, moisture absorption, and biodegradation of water hyacinth (Eichhornia crassipes) nanocellulose-filled bengkuang (Pachyrhizus erosus) starch biocomposites. J. Mater. Res. Technol. 2019, 8, 6223–6231. [Google Scholar]
- Aisyah, H.A.; Paridah, M.T.; Sapuan, S.M.; Ilyas, R.A.; Khalina, A.; Nurazzi, N.M.; Lee, S.H.; Lee, C.H. A comprehensive review on advanced sustainable woven natural fibre polymer composites. Polymers 2021, 13, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Nurazzi, N.M.; Khalina, A.; Sapuan, S.M.; Ilyas, R.A.; Rafiqah, S.A.; Hanafee, Z.M. Thermal properties of treated sugar palm yarn/glass fiber reinforced unsaturated polyester hybrid composites. J. Mater. Res. Technol. 2020, 9, 1606–1618. [Google Scholar] [CrossRef]
- Sari, N.H.; Pruncu, C.I.; Sapuan, S.M.; Ilyas, R.A.; Catur, A.D.; Suteja, S.; Sutaryono, Y.A.; Pullen, G. The effect of water immersion and fibre content on properties of corn husk fibres reinforced thermoset polyester composite. Polym. Test. 2020, 91, 106751. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A.; Sherwani, S.F.K.; Yusuf, J.; Ilyas, R.A. Recent developments in sustainable arrowroot (Maranta arundinacea Linn) starch biopolymers, fibres, biopolymer composites and their potential industrial applications: A review. J. Mater. Res. Technol. 2021, 13, 1191–1219. [Google Scholar] [CrossRef]
- Sandoval Gordillo, C.A.; Ayala Valencia, G.; Vargas Zapata, R.A.; Agudelo Henao, A.C. Physicochemical characterization of arrowroot starch (maranta arundinacea linn) and glycerol/arrowroot starch membranes. Int. J. Food Eng. 2014, 10, 727–735. [Google Scholar] [CrossRef]
- Branco, F.P.; Naka, M.H.; Cereda, M.P. Granulometry and energy consumption as indicators of disintegration efficiency in a hammer mill adapted to extraction arrowroot starch (Maranta arundinacea) in comparison to starch extraction from cassava. Eng. Agrícola 2019, 39, 341–349. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Extraction and Characterization of a Novel Natural Lignocellulosic (Bagasse and Husk) Fibers from Arrowroot (Maranta Arundinacea). J. Nat. Fibers 2021, 1–17. [Google Scholar] [CrossRef]
- ASTM American Society for Testing and Materials. F2251. In ASTM Stand; Annu., B., Ed.; ASTM: Philadelphia, PA, USA, 2013. [Google Scholar]
- ASTM. Standard Test Methods for Density and Specific Gravity (Relative Density) of Plastics by Displacement. ASTM Int. 2013, 15, 145–149. [Google Scholar] [CrossRef]
- ASTM. Standard Test Methods for Moisture Content of Paper and Paperboard by Oven Drying. In Annual Book of ASTM Standards; Standard D644-07; ASTM: Philadelphia, PA, USA, 2007. [Google Scholar]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Ojagh, S.M.; Hosseini, S.M.; Khaksar, R. Characterization of antioxidant-antimicrobial κ-carrageenan films containing Satureja hortensis essential oil. Int. J. Biol. Macromol. 2013, 52, 116–124. [Google Scholar] [CrossRef]
- ASTM. ASTM D570 “Standard Test Method for Water Absorption of Plastics”; ASTM Standard; ASTM: Philadelphia, PA, USA, 2014; Volume 98, pp. 25–28. [Google Scholar] [CrossRef]
- Sheltami, R.M.; Abdullah, I.; Ahmad, I.; Dufresne, A.; Kargarzadeh, H. Extraction of cellulose nanocrystals from mengkuang leaves (Pandanus tectorius). Carbohydr. Polym. 2012, 88, 772–779. [Google Scholar] [CrossRef]
- ASTM Standard test method for tensile properties of thin plastic sheeting. D882-02. In Annual Book of ASTM Standards; ASTM: Philadelphia, PA, USA, 2002. [Google Scholar]
- ASTM. Standard test method for tear-propagation resistance (Trouser Tear) of plastic film and thin sheeting by a single-tear method. In Annual book of ASTM; D1938-08; ASTM: Philadelphia, PA, USA, 2008. [Google Scholar]
- Versino, F.; García, M.A. Cassava (Manihot esculenta) starch films reinforced with natural fibrous filler. Ind. Crops Prod. 2014, 58, 305–314. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of sugar palm-derived cellulose reinforcement on the mechanical and water barrier properties of sugar palm starch biocomposite films. BioResources 2016, 11, 4134–4145. [Google Scholar] [CrossRef] [Green Version]
- Jumaidin, R.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Thermal, mechanical, and physical properties of seaweed/sugar palm fibre reinforced thermoplastic sugar palm Starch/Agar hybrid composites. Int. J. Biol. Macromol. 2017, 97, 606–615. [Google Scholar] [CrossRef]
- Soykeabkaew, N.; Supaphol, P.; Rujiravanit, R. Preparation and characterization of jute-and flax-reinforced starch-based composite foams. Carbohydr. Polym. 2004, 58, 53–63. [Google Scholar] [CrossRef]
- Kaisangsri, N.; Kerdchoechuen, O.; Laohakunjit, N. Biodegradable foam tray from cassava starch blended with natural fiber and chitosan. Ind. Crops Prod. 2012, 37, 542–546. [Google Scholar] [CrossRef]
- Thymi, S.; Krokida, M.K.; Pappa, A.; Maroulis, Z.B. Structural properties of extruded corn starch. J. Food Eng. 2005, 68, 519–526. [Google Scholar] [CrossRef]
- Luo, S.; Chan, E.; Masatcioglu, M.T.; Erkinbaev, C.; Paliwal, J.; Koksel, F. Effects of extrusion conditions and nitrogen injection on physical, mechanical, and microstructural properties of red lentil puffed snacks. Food Bioprod. Process. 2020, 121, 143–153. [Google Scholar] [CrossRef]
- Nair, S.B.; Alummoottil, N.J.; Moothandasserry, S.S. Chitosan-konjac glucomannan-cassava starch-nanosilver composite films with moisture resistant and antimicrobial properties for food-packaging applications. Starch/Staerke 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Sugar palm nanocrystalline cellulose reinforced sugar palm starch composite: Degradation and water-barrier properties. IOP Conf. Ser. Mater. Sci. Eng. 2018, 368, 012006. [Google Scholar] [CrossRef] [Green Version]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Ismarrubie Zahari, N. Preparation and Characterization of Cassava Starch/Peel Composite Film. Polym. Compos. 2018, 39, 1704–1715. [Google Scholar] [CrossRef]
- Cao, X.; Chen, Y.; Chang, P.R.; Stumborg, M.; Huneault, M.A. Green composites reinforced with hemp nanocrystals in plasticized starch. J. Appl. Polym. Sci. 2008, 109, 3804–3810. [Google Scholar] [CrossRef] [Green Version]
- Rhim, J.W.; Ng, P.K.W. Natural biopolymer-based nanocomposite films for packaging applications. Crit. Rev. Food Sci. Nutr. 2007, 47, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Dularia, C.; Sinhmar, A.; Thory, R.; Pathera, A.K.; Nain, V. Development of starch nanoparticles based composite films from non-conventional source—Water chestnut (Trapa bispinosa). Int. J. Biol. Macromol. 2019, 136, 1161–1168. [Google Scholar] [CrossRef]
- Jha, P. Effect of plasticizer and antimicrobial agents on functional properties of bionanocomposite films based on corn starch-chitosan for food packaging applications. Int. J. Biol. Macromol. 2020, 160, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Vilay, V.; Mariatti, M.; Mat Taib, R.; Todo, M. Effect of fiber surface treatment and fiber loading on the properties of bagasse fiber-reinforced unsaturated polyester composites. Compos. Sci. Technol. 2008, 68, 631–638. [Google Scholar] [CrossRef]
- Hazrati, K.Z.; Sapuan, S.M.; Zuhri, M.Y.M.; Jumaidin, R. Preparation and characterization of starch-based biocomposite films reinforced by Dioscorea hispida fibers. J. Mater. Res. Technol. 2021, 15, 1342–1355. [Google Scholar] [CrossRef]
- Silva, R.; Haraguchi, S.K.; Muniz, E.C.; Rubira, A.F. Applications of Lignocellulosic Fibers in Polymer Chemistry and in Composites. Quim. Nova 2009, 32, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Wu, Y.; Geng, F.; Chang, P.R.; Yu, J.; Ma, X. Effect of agar on the microstructure and performance of potato starch film. Carbohydr. Polym. 2009, 76, 299–304. [Google Scholar] [CrossRef]
- Prachayawarakorn, J.; Chaiwatyothin, S.; Mueangta, S.; Hanchana, A. Effect of jute and kapok fibers on properties of thermoplastic cassava starch composites. Mater. Des. 2013, 47, 309–315. [Google Scholar] [CrossRef]
- Kaewtatip, K.; Thongmee, J. Effect of kraft lignin and esterified lignin on the properties of thermoplastic starch. Mater. Des. 2013, 49, 701–704. [Google Scholar] [CrossRef]
- Combrzyński, M.; Oniszczuk, T.; Kupryaniuk, K.; Wójtowicz, A.; Mitrus, M.; Milanowski, M.; Soja, J.; Budziak-Wieczorek, I.; Karcz, D.; Kamiński, D.; et al. Physical properties, spectroscopic, microscopic, x-ray, and chemometric analysis of starch films enriched with selected functional additives. Materials 2021, 14, 2673. [Google Scholar] [CrossRef] [PubMed]
- Aloui, H.; Deshmukh, A.R.; Khomlaem, C.; Kim, B.S. Novel composite films based on sodium alginate and gallnut extract with enhanced antioxidant, antimicrobial, barrier and mechanical properties. Food Hydrocoll. 2021, 113, 106508. [Google Scholar] [CrossRef]
- Fang, J.M.; Fowler, P.A.; Tomkinson, J.; Hill, C.A.S. The preparation and characterisation of a series of chemically modified potato starches. Carbohydr. Polym. 2002, 47, 245–252. [Google Scholar] [CrossRef]
- Sahari, J.; Sapuan, S.M.; Zainudin, E.S.; Maleque, M.A. Thermo-mechanical behaviors of thermoplastic starch derived from sugar palm tree (Arenga pinnata). Carbohydr. Polym. 2013, 92, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Lomelí Ramírez, M.G.; Satyanarayana, K.G.; Iwakiri, S.; De Muniz, G.B.; Tanobe, V.; Flores-Sahagun, T.S. Study of the properties of biocomposites. Part, I. Cassava starch-green coir fibers from Brazil. Carbohydr. Polym. 2011, 86, 1712–1722. [Google Scholar] [CrossRef] [Green Version]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Development and characterization of sugar palm starch and poly(lactic acid) bilayer films. Carbohydr. Polym. 2016, 146, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.; Jiménez, A.; Cháfer, M.; González-Martínez, C.; Chiralt, A. Physical properties and stability of starch-gelatin based films as affected by the addition of esters of fatty acids. Food Hydrocoll. 2015, 49, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Sholichah, E.; Nugroho, P.; Purwono, B. Preparation and characterization of active film made from arrowroot starch/PVA film and isolated quercetin from shallot (Allium cepa L. var, aggregatum). AIP Conf. Proc. 2018, 2024, 020013. [Google Scholar]
- Ng, H.M.; Sin, L.T.; Tee, T.T.; Bee, S.T.; Hui, D.; Low, C.Y.; Rahmat, A.R. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part B Eng. 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Rahman, M.R.; Huque, M.M.; Islam, M.N.; Hasan, M. Improvement of physico-mechanical properties of jute fiber reinforced polypropylene composites by post-treatment. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1739–1747. [Google Scholar] [CrossRef]
- Dias, A.B.; Müller, C.M.O.; Larotonda, F.D.S.; Laurindo, J.B. Mechanical and barrier properties of composite films based on rice flour and cellulose fibers. LWT—Food Sci. Technol. 2011, 44, 535–542. [Google Scholar] [CrossRef]
- Müller, C.M.O.; Laurindo, J.B.; Yamashita, F. Effect of cellulose fibers on the crystallinity and mechanical properties of starch-based films at different relative humidity values. Carbohydr. Polym. 2009, 77, 293–299. [Google Scholar] [CrossRef]
- Fahma, F.; Iwamoto, S.; Hori, N.; Iwata, T.; Takemura, A. Effect of pre-acid-hydrolysis treatment on morphology and properties of cellulose nanowhiskers from coconut husk. Cellulose 2011, 18, 443–450. [Google Scholar] [CrossRef]
- Sahari, J.; Sapuan, S.M.; Zainudin, E.S.; Maleque, M.A. Mechanical and thermal properties of environmentally friendly composites derived from sugar palm tree. Mater. Des. 2013, 49, 285–289. [Google Scholar] [CrossRef]
- Versino, F.; López, O.V.; García, M.A. Sustainable use of cassava (Manihot esculenta) roots as raw material for biocomposites development. Ind. Crops Prod. 2015, 65, 79–89. [Google Scholar] [CrossRef]
- Chen, D.; Lawton, D.; Thompson, M.R.; Liu, Q. Biocomposites reinforced with cellulose nanocrystals derived from potato peel waste. Carbohydr. Polym. 2012, 90, 709–716. [Google Scholar] [CrossRef] [Green Version]
- Zavareze, E.D.R.; Pinto, V.Z.; Klein, B.; El Halal, S.L.M.; Elias, M.C.; Prentice-Hernández, C.; Dias, A.R.G. Development of oxidised and heat-moisture treated potato starch film. Food Chem. 2012, 132, 344–350. [Google Scholar] [CrossRef] [Green Version]
- López, O.V.; Castillo, L.A.; García, M.A.; Villar, M.A.; Barbosa, S.E. Food packaging bags based on thermoplastic corn starch reinforced with talc nanoparticles. Food Hydrocoll. 2015, 43, 18–24. [Google Scholar] [CrossRef]
- Wawro, D.; Kazimierczak, J. Forming Conditions and Mechanical Properties of Potato Starch Films. Fibres Text. East. Eur. 2008, 16, 106–112. [Google Scholar]
- Ismail, I.; Osman, A.F.; Leong Ping, T. Effects of ultrasonication process on crystallinity and tear strength of thermoplastic starch/cellulose biocomposites. IOP Conf. Ser. Mater. Sci. Eng. 2019, 701, 012045. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]








| Composite | Starch (g) | Fiber Loading (%) | Glycerol (g) | Distilled Water (mL) |
|---|---|---|---|---|
| Control | 5 | 0 | 1.5 | 100 |
| TPAS/AF-2 | 5 | 2 | 1.5 | 100 |
| TPAS/AF-4 | 5 | 4 | 1.5 | 100 |
| TPAS/AF-6 | 5 | 6 | 1.5 | 100 |
| TPAS/AF-8 | 5 | 8 | 1.5 | 100 |
| TPAS/AF-10 | 5 | 10 | 1.5 | 100 |
| Composite | Thickness (μm) | Density (g/cm3) | Water Content (%) | Water Solubility (%) |
|---|---|---|---|---|
| Control | 163 ± 1.53 a | 1.33 ± 0.02 c | 9.77 ± 0.54 a | 32.62 ± 3.98 a |
| TPAS/AF-2 | 169 ± 1.53 a,b | 1.32 ± 0.01 c | 9.82 ± 0.20 a | 33.19 ± 0.51 d |
| TPAS/AF-4 | 174 ± 1.53 b | 1.29 ± 0.01 b | 10.51 ± 0.41 a,b | 31.76 ± 1.06 c,d |
| TPAS/AF-6 | 181 ± 1.00 c | 1.28 ± 0.02 b | 11.19 ± 0.67 b,c | 29.16 ± 0.53 b,c |
| TPAS/AF-8 | 186 ± 5.77 c | 1.26 ± 0.02 a,b | 11.65 ± 0.17 c | 26.29 ± 0.46 b |
| TPAS/AF-10 | 203 ± 5.77 d | 1.25 ± 0.03 a | 12.71 ± 0.54 d | 22.56 ± 0.44 a |
| Composite | Water Absorption (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Types of Water | Distilled Water | Seawater | Rainwater | ||||||
| Time | 30 min | 60 min | 180 min | 30 min | 60 min | 180 min | 30 min | 60 min | 180 min |
| Control | 166.2 ± 4.1 c | 175.5 ± 5.8 c | 189.6 ± 7.5 c | 36.4 ± 0.8 a | 40.9 ± 0.8 a | 48.5 ± 0.5 a | 31.8 ± 0.8 a | 38.8 ± 0.5 a | 40.9± 0.8 a |
| TPAS/AF-2 | 133.7 ± 1.4 a | 149.6 ± 1.4 a | 171.7 ± 0.5 a | 120.0 ± 2.2 d | 128.1 ± 2.5 d | 140.0 ± 0.8 d | 108.7 ± 0.8 b | 121.4 ± 0.8 b, c | 127.3 ± 0.9 b |
| TPAS/AF-4 | 111.1 ± 4.9 b | 117.8 ± 5.7 b | 133.3 ± 5.7 b | 110.4 ± 0.8 b | 115.2 ± 0.5 b | 120.9 ± 0.9 b | 107.1 ± 0.8 b | 117.1 ± 1.2 b | 125.2 ± 1.2 b |
| TPAS/AF-6 | 94.4 ± 1.6 b | 100.0 ± 2.9 b | 112.7 ± 4.1 b | 138.9 ± 0.8 d | 150.6 ± 1.6 d | 157.6 ± 0.8 d | 62.7 ± 5.6 d | 138.5 ± 6.4 d | 148.0 ± 5.4 d |
| TPAS/AF-8 | 89.8 ± 5.7 b | 95.2 ± 7.4 b | 110.2 ± 7.7 b | 104.6 ± 2.9 c | 115.0 ± 4.0 c | 120.6 ± 3.6 c | 96.6 ± 2.2 b | 101.4 ± 2.2 b | 107.0 ± 1.7 b |
| TPAS/AF-10 | 91.2 ± 2.5 b | 92.3 ± 2.9 b | 100.0 ± 4.1 b | 100.1 ± 3.1 d | 108.6 ± 2.2 d | 117.7 ± 3.1 d | 96.5 ± 7.5 c | 105.0 ± 8.2 c | 112.8 ± 8.4 c |
| Variables | df | Tensile Strength | Young Modulus | Elongation at Break | Tear Strength |
|---|---|---|---|---|---|
| Mixture | 4 | 0.00 * | 0.00 * | 0.00 * | 0.00 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarique, J.; Zainudin, E.S.; Sapuan, S.M.; Ilyas, R.A.; Khalina, A. Physical, Mechanical, and Morphological Performances of Arrowroot (Maranta arundinacea) Fiber Reinforced Arrowroot Starch Biopolymer Composites. Polymers 2022, 14, 388. https://doi.org/10.3390/polym14030388
Tarique J, Zainudin ES, Sapuan SM, Ilyas RA, Khalina A. Physical, Mechanical, and Morphological Performances of Arrowroot (Maranta arundinacea) Fiber Reinforced Arrowroot Starch Biopolymer Composites. Polymers. 2022; 14(3):388. https://doi.org/10.3390/polym14030388
Chicago/Turabian StyleTarique, J., E. S. Zainudin, S. M. Sapuan, R. A. Ilyas, and A. Khalina. 2022. "Physical, Mechanical, and Morphological Performances of Arrowroot (Maranta arundinacea) Fiber Reinforced Arrowroot Starch Biopolymer Composites" Polymers 14, no. 3: 388. https://doi.org/10.3390/polym14030388









