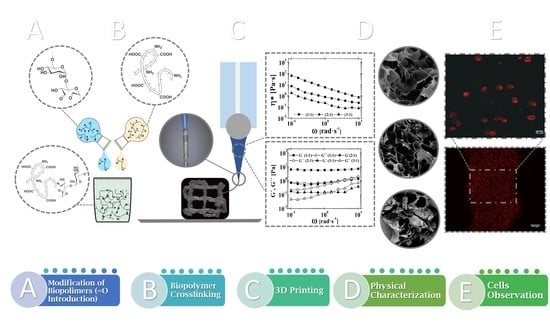
Cross-Linked Gelatine by Modified Dextran as a Potential Bioink Prepared by a Simple and Non-Toxic Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Dextran Oxidation
2.3. Polymers Characterisation
2.4. Hydrogels Preparation and Characterization
2.5. Cytotoxicity
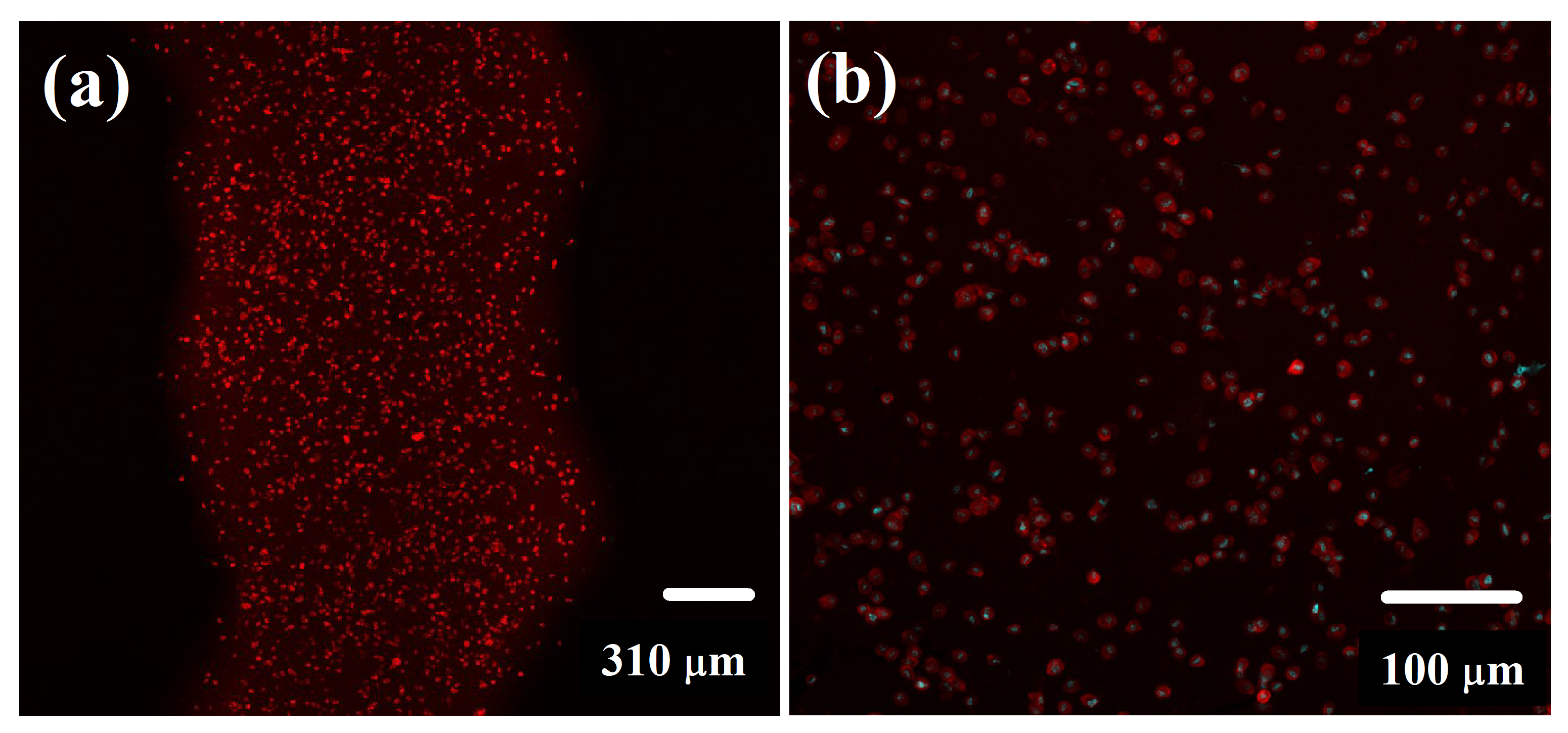
2.6. Cell Distribution within 3D-Printed Structure
3. Results and Discussion
3.1. Polysaccharide Oxidation and Hydrogel Formation
3.2. Reaction Kinetics
3.3. Rheology
3.4. 3D Printing
3.5. Swelling Tests
3.6. Inner Porosity
3.7. Cytotoxicity
3.8. Cell Distribution within 3D-Printed Structure Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, L.; Jovina, T.; Wai, Y.; May, W. Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication 2018, 10, 1758–5090. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Vlierberghe, S.V.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseini, A. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater. Today Bio 2019, 1, 100008. [Google Scholar] [CrossRef] [PubMed]
- Gungor-Ozkerim, P.; Inci, I.; Zhang, Y.; Khademhosseini, S.; Dokmeci, M. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorishetty, P.; Dutta, N.; Choudhury, N. Bioprintable tough hydrogels for tissue engineering applications. Adv. Colloid Interface Sci. 2020, 281, 102163. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Kaunas, R.; Gaharwar, A. Hydrogel Bioink Reinforcement for Additive Manufacturing: A Focused Review of Emerging Strategies. Adv. Mater. 2020, 32, 1902026–1902048. [Google Scholar] [CrossRef]
- Chung, S.H.; Son, S.; Min, J. The nanostructure effect on the adhesion and growth rates of epithelial cells with well-defined nanoporous alumina substrates. Nanotechnology 2010, 21, 125104. [Google Scholar] [CrossRef]
- Ermis, M.; Antmen, E.; Hasirci, V. Micro and Nanofabrication methods to control cell-substrate interactions and cell behavior: A review from the tissue engineering perspective. Bioact. Mater. 2018, 3, 355–369. [Google Scholar] [CrossRef]
- Buskermolen, A.; Suresh, H.; Shishvan, S.; Vigliotti, A.; DeSimone, A.; Kurniawan, N.; Bouten, C.; Deshpande, V. Entropic Forces Drive Cellular Contact Guidance. Biophys. J. 2019, 116, 1994–2008. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.; Stone, A.; Parkhill, R.; Stewart, R.; Simpkins, M.; Kachurin, A.; Warren, W.; Williams, S. Three-Dimensional BioAssembly Tool for Generating Viable Tissue-Engineered Constructs. Tissue Eng. 2004, 10, 1566–1576. [Google Scholar] [CrossRef]
- Blaeser, A.; Campos, D.D.; Puster, U.; Richtering, W.; Stevens, M.; Fischer, H. Controlling Shear Stress in 3D Bioprinting is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Vlierberghe, S.V.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002–032020. [Google Scholar] [CrossRef]
- Mackay, M. The importance of rheological behavior in the additive manufacturing technique material extrusion. J. Rheol. 2018, 62, 1549–1561. [Google Scholar] [CrossRef]
- Mori, A.D.; Fernández, M.P.; Blunn, G.; Tozzi, G.; Roldo, M. 3D Printing and Electrospinning of Composite Hydrogels for Cartilage and Bone Tissue Engineering. Polymers 2018, 10, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2019, 11, 015003–015014. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Gřundělová, L.; Gregorova, A.; Mráček, A.; Vícha, R.; Smolka, P.; Minařík, A. Viscoelastic and mechanical properties of hyaluronan films and hydrogels modified by carbodiimide. Carbohydr. Polym. 2015, 119, 142–148. [Google Scholar] [CrossRef]
- Dababneh, A.; Ozbolat, I. Bioprinting Technology: A Current State-of-the-Art Review. J. Manuf. Sci. Eng. 2014, 136, 061016. [Google Scholar] [CrossRef]
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and Molecular Design Criteria for 3D Printable Hydrogels. Chem. Rev. 2016, 116, 1496–1539. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 2041731417726464–2041731417726479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musilová, L.; Mráček, A.; Kovalcik, A.; Smolka, P.; Minařík, A.; Humpolíček, P.; Vícha, R.; Ponížil, P. Hyaluronan hydrogels modified by glycinated Kraft lignin: Morphology, swelling, viscoelastic properties and biocompatibility. Carbohydr. Polym. 2018, 181, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Poldervaart, M.; Goversen, B.; de Ruijter, M.; Abbadessa, A.; Melchels, F.; Öner, F.; Dhert, W.; Vermonden, T.; Alblas, J.; Yamamoto, M. 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS ONE 2017, 12, e0177628. [Google Scholar] [CrossRef] [Green Version]
- Skardal, A.; Zhang, J.; McCoard, L.; Xu, X.; Oottamasathien, S.; Prestwich, G. Photocrosslinkable Hyaluronan-Gelatin Hydrogels for Two-Step Bioprinting. Tissue Eng. Part A 2010, 16, 2675–2685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, S.; Ohi, H.; Taya, M. Gelatin/Hyaluronic Acid Content in Hydrogels Obtained through Blue Light-Induced Gelation Affects Hydrogel Properties and Adipose Stem Cell Behaviors. Biomolecules 2019, 9, 342. [Google Scholar] [CrossRef] [Green Version]
- Dobos, A.; Hoorick, J.V.; Steiger, W.; Gruber, P.; Markovic, M.; Andriotis, O.; Rohatschek, A.; Dubruel, P.; Thurner, P.; Vlierberghe, S.V.; et al. Thiol–Gelatin–Norbornene Bioink for Laser-Based High-Definition Bioprinting. Adv. Healthc. Mater. 2019, 9, 1900752–1900761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, I.; Kim, N.; Tran, H.; Lee, J.; Lee, C. 3D printable hyaluronic acid-based hydrogel for its potential application as a bioink in tissue engineering. Biomater. Res. 2019, 23, 3. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, S.; Melo, B.; Hirano, M.; Keung, W.; Li, R.; Mandal, B.; Shin, S. Nonmulberry Silk Based Ink for Fabricating Mechanically Robust Cardiac Patches and Endothelialized Myocardium-on-a-Chip Application. Adv. Funct. Mater. 2020, 30, 1907436. [Google Scholar] [CrossRef] [PubMed]
- Petta, D.; Armiento, A.; Grijpma, D.; Alini, M.; Eglin, D.; D’Este, M. 3D bioprinting of a hyaluronan bioink through enzymatic-and visible light-crosslinking. Biofabrication 2018, 10, 044104–044114. [Google Scholar] [CrossRef] [PubMed]
- Kajave, N.; Schmitt, T.; Nguyen, T.U.; Kishore, V. Dual crosslinking strategy to generate mechanically viable cell-laden printable constructs using methacrylated collagen bioinks. Mater. Sci. Eng. C 2020, 107, 110290–110301. [Google Scholar] [CrossRef]
- Yu, J.H.; Fridrikh, S.V.; Rutledge, G.C. The role of elasticity in the formation of electrospun fibers. Polymer 2006, 47, 4789–4797. [Google Scholar] [CrossRef]
- Angel Martinez-Ortiz, M.; Delia Hernandez-Fuentes, A.; Pimentel-Gonzalez, D.J.; Campos-Montiel, R.G.; Vargas-Torres, A.; Aguirre-Alvarez, G. Extraction and characterization of collagen from rabbit skin: Partial characterization. CYTA-J. Food 2015, 13, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, A.M.; Smucker, B.; Naber, A.; Wyrick, C.; Shaw, C.; Bennett, K.; Szekely, S.; Focke, C.; Wood, K. Controlling the extrudate swell in melt extrusion additive manufacturing of 3D scaffolds: A designed experiment. J. Biomater. Sci. 2017, 29, 195–216. [Google Scholar] [CrossRef]
- Tanner, R. A theory of die-swell. J. Polym. Sci. Part A-2 Polym. Phys. 1970, 8, 2067–2078. [Google Scholar] [CrossRef]
- Emmermacher, J.; Spura, D.; Cziommer, J.; Kilian, D.; Wollborn, T.; Fritsching, U.; Steingroewer, J.; Walther, T.; Gelinsky, M.; Lode, A. Engineering considerations on extrusion-based bioprinting: Interactions of material behavior, mechanical forces and cells in the printing needle. Biofabrication 2020, 12, 025022. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Yang, B.; Mohabatpour, F.; Betancourt, N.; Sarker, M.; Papagerakis, P.; Chen, X. Process-induced cell damage: Pneumatic versus screw-driven bioprinting. Biofabrication 2020, 12, 025011. [Google Scholar] [CrossRef] [PubMed]
- Mokrejš, P.; Gál, R.; Mrázek, P. Biotechnology-Based Production of Food Gelatine from Poultry by-Products. Patent number: CZ 307665, 16 May 2019. [Google Scholar]
- Mokrejš, P.; Mrázek, P.; Robert, R.G.; Pavlačková, J. Biotechnological Preparation of Gelatines from Chicken Feet. Polymers 2019, 11, 1060. [Google Scholar] [CrossRef] [Green Version]
- Gál, R.; Mokrejš, P.; Mrázek, P.; Pavlačková, J.; Janáčová, D.; Orsavová, J. Chicken Heads as a Promising By-Product for Preparation of Food Gelatins. Molecules 2020, 25, 494. [Google Scholar] [CrossRef] [Green Version]
- Maia, J.; Carvalho, R.; Coelho, J.; Simoes, P.; Gil, M. Insight on the Periodate Oxidation of Dextran and Its Structural Vicissitudes. Polymer 2011, 52, 258–265. [Google Scholar] [CrossRef]
- Zhao, H.; Heindel, N. Determination of Degree of Substitution of Formyl Groups in Polyaldehyde Dextran by the Hydroxylamine Hydrochloride Method. Pharm. Res. 1991, 8, 400–402. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020–035033. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R. Collagen-based biomaterilas for wound healing. Biopolymers 2014, 8, 821–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenbarth, E. Biomaterials for Tissue Engineering. Adv. Eng. Mater. 2007, 9, 1051–1060. [Google Scholar] [CrossRef]
- Young, A.T.; White, O.C.; Daniele, M.A. Rheological Properties of Coordinated Physical Gelation and Chemical Crosslinking in Gelatin Methacryloyl (GelMA) Hydrogels. Macromol. Biosci. 2020, 20, 2000183. [Google Scholar] [CrossRef] [PubMed]
- Draye, J.P.; Delaey, B.; de Voorde, A.V.; Bulcke, A.V.D.; Reu, B.D.; Schacht, E. In vitro and in vivo biocompatibility of dextran dialdehyde cross-linked gelatin hydrogel films. Biomaterials 1998, 19, 1677–1687. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Jayakrishnan, A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials 2005, 26, 3941–3951. [Google Scholar] [CrossRef]
- Kristiansen, K.; Potthast, A.; Christensen, B. Periodate oxidation of polysaccharides for modification of chemical and physical properties. Carbohydr. Res. 2010, 345, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Li, W.; Lian, W.; Kemell, M.; Hietala, S.; Figueiredo, P.; Li, L.; Mäkilä, E.; Ma, M.; et al. Close-loop dynamic nanohybrids on collagen-ark with in situ gelling transformation capability for biomimetic stage-specific diabetic wound healing. Mater. Horiz. 2019, 6, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Nonsuwan, P.; Matsugami, A.; Hayashi, F.; Hyon, S.H.; Matsumura, K. Controlling the degradation of an oxidized dextran-based hydrogel independent of the mechanical properties. Carbohydr. Polym. 2019, 204, 131–141. [Google Scholar] [CrossRef]
- Winter, H. Chapter Physical and Chemical Gelation. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Wu, D.; Yu, Y.; Tan, J.; Huang, L.; Luo, B.; Lu, L.; Zhou, C. 3D bioprinting of gellan gum and poly (ethylene glycol) diacrylate based hydrogels to produce human-scale constructs with high-fidelity. Mater. Des. 2018, 160, 486–495. [Google Scholar] [CrossRef]
- Khorshidi, S.; Karkhaneh, A.; Bonakdar, S.; Omidian, M. High-strength functionalized pectin/fibroin hydrogel with tunable properties: A structure–property relationship study. J. Appl. Polym. Sci. 2019, 137, 48859–48872. [Google Scholar] [CrossRef]
- Zehnder, T.; Freund, T.; Demir, M.; Detsch, R.; Boccaccini, A. Fabrication of Cell-Loaded Two-Phase 3D Constructs for Tissue Engineering. Materials 2016, 9, 887. [Google Scholar] [CrossRef] [Green Version]
- McIlroy, C.; Olmsted, P. Deformation of an amorphous polymer during the fused-filament-fabrication method for additive manufacturing. J. Rheol. 2017, 61, 379–397. [Google Scholar] [CrossRef] [Green Version]
- Comminal, R.; Serdeczny, M.; Pedersen, D.; Spangenberg, J. Numerical modeling of the strand deposition flow in extrusion-based additive manufacturing. Addit. Manuf. 2018, 20, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Serdeczny, M.; Comminal, R.; Pedersen, D.; Spangenberg, J. Experimental validation of a numerical model for the strand shape in material extrusion additive manufacturing. Addit. Manuf. 2018, 24, 145–153. [Google Scholar] [CrossRef]
- Xia, H.; Lu, J.; Tryggvason, G. A numerical study of the effect of viscoelastic stresses in fused filament fabrication. Comput. Methods Appl. Mech. Eng. 2019, 346, 242–259. [Google Scholar] [CrossRef]
- Hebda, M.; McIlroy, C.; Whiteside, B.; Caton-Rose, F.; Coates, P. A method for predicting geometric characteristics of polymer deposition during fused-filament-fabrication. Addit. Manuf. 2019, 27, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Coogan, T.; Kazmer, D. Modeling of interlayer contact and contact pressure during fused filament fabrication. J. Rheol. 2019, 63, 655–672. [Google Scholar] [CrossRef]
- Gopi, S.; Kontopoulou, M. Investigation of thermoplastic melt flow and dimensionless groups in 3D bioplotting. Rheol. Acta 2020, 59, 83–93. [Google Scholar] [CrossRef]
- Ahmed, E. Hydrogel: Preparation, characterization, and applications. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Trautmann, A.; Rüth, M.; Lemke, H.D.; Walther, T.; Hellmann, R. Two-photon polymerization based large scaffolds for adhesion and proliferation studies of human primary fibroblasts. Opt. Laser Technol. 2018, 106, 474–480. [Google Scholar] [CrossRef]
- Choksakulnimitr, S.; Masuda, S.; Tokuda, H.; Takakura, Y.; Hashida, M. In vitro cytotoxicity of macromolecules in different cell culture systems. J. Control. Release 1995, 34, 233–241. [Google Scholar] [CrossRef]
- Groot, C.D.; Luyn, M.V.; Dijk-Wolthuis, W.V.; Cadee, J.; Plantinga, J.; Otter, W.D.; Hennink, W. In vitro biocompatibility of biodegradable dextran-based hydrogels tested with human fibroblasts. Biomaterials 2001, 22, 1197–1203. [Google Scholar] [CrossRef]
- Poursamar, S.; Hatami, J.; Lehner, A.; da Silva, C.; Ferreira, F.; Antunes, A. Gelatin porous scaffolds fabricated using a modified gas foaming technique: Characterisation and cytotoxicity assessment. Mater. Sci. Eng. C 2015, 48, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pronina, E.; Vorotnikov, Y.; Pozmogova, T.; Solovieva, A.; Miroshnichenko, S.; Plyusnin, P.; Pishchur, D.; Eltsov, I.; Edeleva, M.; Efremova, M.S.O. No Catalyst Added Hydrogen Peroxide Oxidation of Dextran: An Environmentally Friendly Route to Multifunctional Polymers. ACS Sustain. Chem. Eng. 2020, 8, 5371–5379. [Google Scholar] [CrossRef]
- Artzi, N.; Shazly, T.; Crespo, C.; Ramos, A.; Chenault, H.; Edelman, E. Characterization of Star Adhesive Sealants Based on PEG/Dextran Hydrogels. Macromol. Biosci. 2009, 9, 754–765. [Google Scholar] [CrossRef]





| Gel:Dex-Ox Solution Ratio | Reaction Rate Coefficient (h) | Coefficient of Determination (1) | Gelation Point (min) |
|---|---|---|---|
| Gel-B | |||
| 1:1 | 11.6 | 0.998 | <1 |
| 2:1 | 8.3 | 0.990 | 2 |
| 3:1 | 10.6 | 0.986 | 2 |
| Gel-R | |||
| 1:1 | 11.1 | 0.993 | <1 |
| 2:1 | 7.6 | 0.994 | 2 |
| 3:1 | 8.7 | 0.990 | 2.5 |
| Gel-C | |||
| 1:1 | 2.0 | 0.997 | 2 |
| 2:1 | 1.2 | 0.999 | 13 |
| 3:1 | 2.4 | 0.994 | >30 |
| Gel:Dex-Ox Solution Ratio | Die Swell (1) | Relative Standard Deviation (RSD) of Die Swell (%) | Printability (Pr) (1) |
|---|---|---|---|
| Gel-B | |||
| 1:1 | 3.0 | 13 | 1.0 ± 0.2 |
| 2:1 | 3.2 | 11 | 1.0 ± 0.2 |
| 3:1 | 3.0 | 14 | / |
| Gel-R | |||
| 1:1 | 2.9 | 10 | 0.90 ± 0.09 |
| 2:1 | 3.3 | 6 | 0.873 ± 0.009 |
| 3:1 | 3.2 | 10 | 0.90 ± 0.07 |
| Gel-C | |||
| 1:1 | 2.4 | 7 | 1.0 ± 0.1 |
| 2:1 | 2.6 | 9 | 1.0 ± 0.2 |
| 3:1 | 2.7 | 8 | 0.92 ± 0.09 |
| Gel:Dex-Ox Solution Ratio | Average Pore Size (mm) | Relative Pore Area (%) |
|---|---|---|
| Gel-B | ||
| 1:1 | 0.014 ± 0.009 | 40–70 |
| 2:1 | 0.017 ± 0.005 | 45–80 |
| 3:1 | 0.020 ± 0.009 | 60–75 |
| Gel-R | ||
| 1:1 | 0.017 ± 0.006 | 40–80 |
| 2:1 | 0.011 ± 0.003 | 35–65 |
| 3:1 | 0.009 ± 0.004 | 35–50 |
| Gel-C | ||
| 1:1 | 0.010 ± 0.004 | 35–45 |
| 2:1 | 0.036 ± 0.008 | 45–50 |
| 3:1 | 0.04 ± 0.02 | 30–50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musilová, L.; Achbergerová, E.; Vítková, L.; Kolařík, R.; Martínková, M.; Minařík, A.; Mráček, A.; Humpolíček, P.; Pecha, J. Cross-Linked Gelatine by Modified Dextran as a Potential Bioink Prepared by a Simple and Non-Toxic Process. Polymers 2022, 14, 391. https://doi.org/10.3390/polym14030391
Musilová L, Achbergerová E, Vítková L, Kolařík R, Martínková M, Minařík A, Mráček A, Humpolíček P, Pecha J. Cross-Linked Gelatine by Modified Dextran as a Potential Bioink Prepared by a Simple and Non-Toxic Process. Polymers. 2022; 14(3):391. https://doi.org/10.3390/polym14030391
Chicago/Turabian StyleMusilová, Lenka, Eva Achbergerová, Lenka Vítková, Roman Kolařík, Martina Martínková, Antonín Minařík, Aleš Mráček, Petr Humpolíček, and Jiří Pecha. 2022. "Cross-Linked Gelatine by Modified Dextran as a Potential Bioink Prepared by a Simple and Non-Toxic Process" Polymers 14, no. 3: 391. https://doi.org/10.3390/polym14030391
APA StyleMusilová, L., Achbergerová, E., Vítková, L., Kolařík, R., Martínková, M., Minařík, A., Mráček, A., Humpolíček, P., & Pecha, J. (2022). Cross-Linked Gelatine by Modified Dextran as a Potential Bioink Prepared by a Simple and Non-Toxic Process. Polymers, 14(3), 391. https://doi.org/10.3390/polym14030391