Designing Versatile Polymers for Lithium-Ion Battery Applications: A Review
Abstract
:1. Introduction
2. Overview
3. Performance Metrics
3.1. High Ionic Conductivity
3.2. Decrease Glass Transition Temperature and Crystallinity
3.3. Increase Ion-Pair Dissociation
3.4. Lithium Transference Number
3.5. Electrochemical Window of Stability
3.6. Mechanical Stability and Shear Modulus
3.7. Chemical and Thermal Stability Range
4. Design of Solid- and Gel-Polymer Electrolytes
4.1. Novel and Versatile Polymer Electrolytes with Advanced Properties
4.1.1. Polymer Matrices and Blending
4.1.2. Copolymers
4.1.3. Crosslinking
4.1.4. SIC (Single-Ion Conducting)
5. Polymer Composite Electrolytes
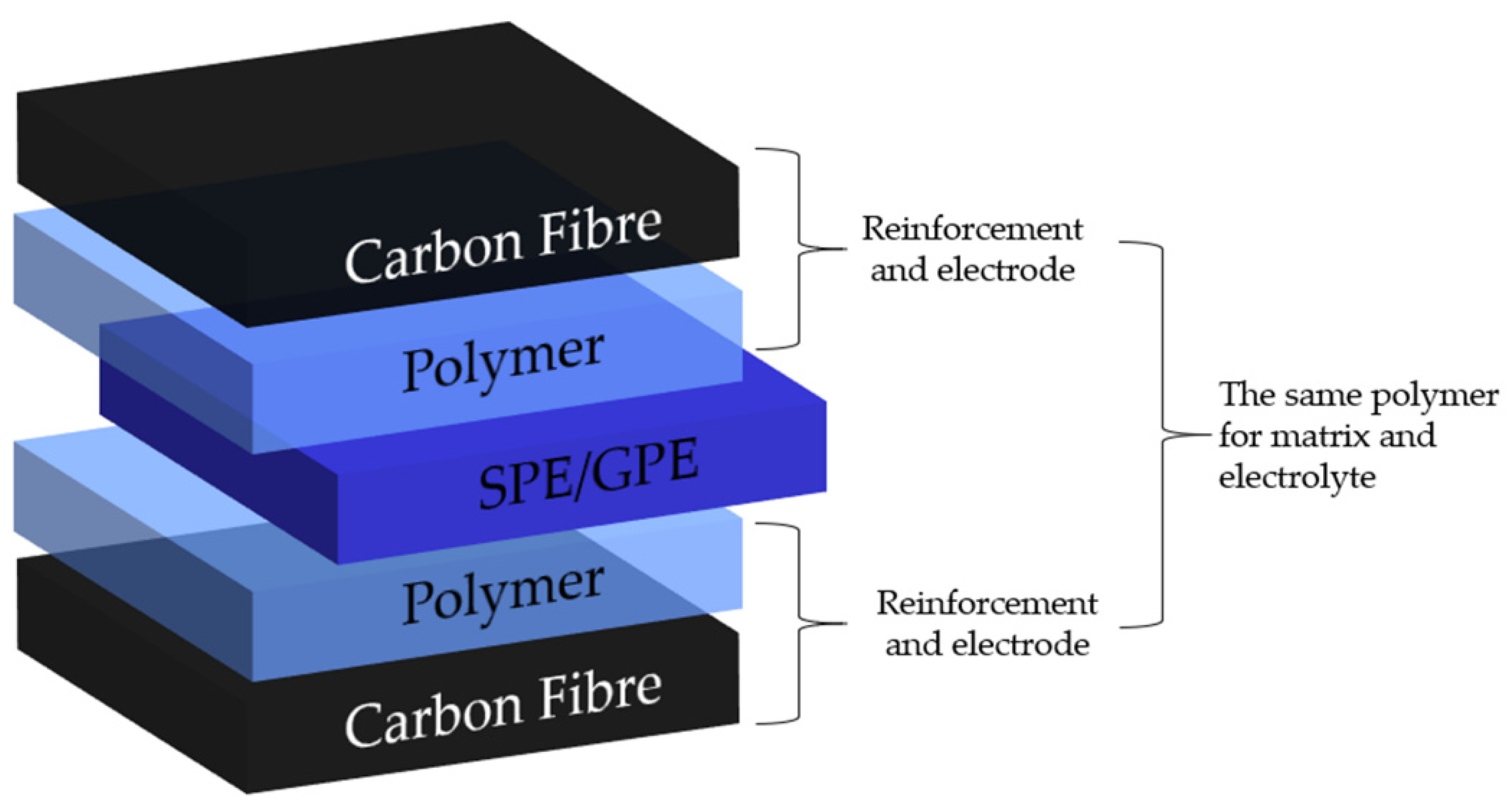
6. Application of Polymer Electrolytes for Structural Batteries
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Danzi, F.; Salgado, R.M.; Oliveira, J.E.; Arteiro, A.; Camanho, P.P.; Braga, M.H. Structural Batteries: A Review. Molecules 2021, 26, 2203. [Google Scholar] [CrossRef]
- Sequeira, C.; Santos, D. Introduction to Polymer Electrolyte Materials; Woodhead Publishing: Cambridge, UK, 2010; pp. 3–61. [Google Scholar]
- Asp, L.; Johansson, M.; Lindbergh, G.; Xu, J.; Zenkert, D. Structural Battery Composites: A Review. Funct. Compos. Struct. 2019, 1, 042001. [Google Scholar] [CrossRef]
- Hellqvist Kjell, M. Performance of Conventional and Structural Lithium-Ion Batteries. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2013. [Google Scholar]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- De Souza, F.L.; Leite, E.R. Hybrid Polymer Electrolytes for Electrochemical Devices; Sequeira, C., Santos, D.B.T.P.E., Eds.; Woodhead Publishing: Cambridge, UK, 2010; pp. 583–602. [Google Scholar]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Mauger, A.; Armand, M.; Julien, C.M.; Zaghib, K. Challenges and issues facing lithium metal for solid-state rechargeable batteries. J. Power Sources 2017, 353, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Kotobuki, M.; Yan, B.; Pan, F.; Lu, L.; Savilov, S.; Aldoshin, S. Low temperature sintering of crystallized Li1.5Al0.5Ge1.5(PO4)3 using hot-press technique. Mater. Today Proc. 2019, 17, 408–415. [Google Scholar] [CrossRef]
- Kotobuki, M.; Suzuki, Y.; Kanamura, K.; Sato, Y.; Yamamoto, K.; Yoshida, T. A novel structure of ceramics electrolyte for future lithium battery. J. Power Sources 2011, 196, 9815–9819. [Google Scholar] [CrossRef]
- Kotobuki, M. Polymer Electrolytes. In Polymer Electrolytes; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 1–21. [Google Scholar]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef]
- Gebert, F.; Knott, J.; Gorkin, R.; Chou, S.-L.; Dou, S.-X. Polymer electrolytes for sodium-ion batteries. Energy Storage Mater. 2021, 36, 10–30. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and prospects of PVDF based polymer electrolytes. J. Energy Chem. 2022, 64, 62–84. [Google Scholar] [CrossRef]
- Or, T.; Gourley, S.W.D.; Kaliyappan, K.; Yu, A.; Chen, Z. Recycling of mixed cathode lithium-ion batteries for electric vehicles: Current status and future outlook. Carbon Energy 2020, 2, 6–43. [Google Scholar] [CrossRef] [Green Version]
- Wright, P.V. Electrical conductivity in ionic complexes of poly(ethylene oxide). Br. Polym. J. 1975, 7, 319–327. [Google Scholar] [CrossRef]
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Armand, M. Polymer solid electrolytes—An overview. Solid State Ion. 1983, 9–10, 745–754. [Google Scholar] [CrossRef]
- Feuillade, G.; Perche, P. Ion-conductive macromolecular gels and membranes for solid lithium cells. J. Appl. Electrochem. 1975, 5, 63–69. [Google Scholar] [CrossRef]
- Skaarup, S.; West, K.; Zachau-Christiansen, B. Mixed phase solid electrolytes. Solid State Ion. 1988, 28–30, 975–978. [Google Scholar] [CrossRef]
- Wieczorek, W.; Such, K.; Wyciślik, H.; Płocharski, J. Modifications of crystalline structure of peo polymer electrolytes with ceramic additives. Solid State Ion. 1989, 36, 255–257. [Google Scholar] [CrossRef]
- Niu, H.; Wang, L.; Guan, P.; Zhang, N.; Yan, C.; Ding, M.; Guo, X.; Huang, T.; Hu, X. Recent Advances in Application of Ionic Liquids in Electrolyte of Lithium Ion Batteries. J. Energy Storage 2021, 40, 102659. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Yan, Z.; Cheng, F.; Chen, J. Structure design and mechanism analysis of silicon anode for lithium-ion batteries. Sci. China Mater. 2019, 62, 1515–1536. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Ku, L.; Wang, L.; Ma, Y.; Hongfei, Z.; Xu, W.; Han, J.; Qu, B.; Chen, Y.; Xie, Q.; et al. Engineering oxygen vacancies in hierarchically Li-rich layered oxide porous microspheres for high-rate lithium ion battery cathode. Sci. China Mater. 2019, 62, 1374–1384. [Google Scholar] [CrossRef] [Green Version]
- Mendes, T.C.; Zhang, X.; Wu, Y.; Howlett, P.C.; Forsyth, M.; Macfarlane, D.R. Supported Ionic Liquid Gel Membrane Electrolytes for a Safe and Flexible Sodium Metal Battery. ACS Sustain. Chem. Eng. 2019, 7, 3722–3726. [Google Scholar] [CrossRef]
- Xue, Z.; He, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Aurbach, D.; Zinigrad, E.; Cohen, Y.; Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion. 2002, 148, 405–416. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.; Ding, F.; Liu, X. Recent advances in solid polymer electrolytes for lithium batteries. Nano Res. 2017, 10, 4139–4174. [Google Scholar] [CrossRef]
- Selis, L.A.; Seminario, J.M. Dendrite formation in silicon anodes of lithium-ion batteries. RSC Adv. 2018, 8, 5255–5267. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.-Y.; Hwang, G.S. Surface effects on the structure and lithium behavior in lithiated silicon: A first principles study. Surf. Sci. 2013, 612, 16–23. [Google Scholar] [CrossRef]
- Bieker, G.; Winter, M.; Bieker, P. Electrochemical in situ investigations of SEI and dendrite formation on the lithium metal anode. Phys. Chem. Chem. Phys. 2015, 17, 8670–8679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borzutzki, K.; Dong, K.; Nair, J.R.; Wolff, B.; Hausen, F.; Eichel, R.-A.; Winter, M.; Manke, I.; Brunklaus, G. Lithium deposition in single-ion conducting polymer electrolytes. Cell Rep. Phys. Sci. 2021, 2, 100496. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Zhao, C.-Z.; Archer, L.A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 2020, 5, 229–252. [Google Scholar] [CrossRef]
- Tikekar, M.D.; Choudhury, S.; Tu, Z.; Archer, L.A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 2016, 1, 16114. [Google Scholar] [CrossRef]
- Aziz, S.B.; Woo, T.J.; Kadir, M.F.Z.; Ahmed, H.M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Berthier, C.; Gorecki, W.; Minier, M.; Armand, M.B.; Chabagno, J.M.; Rigaud, P. Microscopic investigation of ionic conductivity in alkali metal salts-poly(ethylene oxide) adducts. Solid State Ion. 1983, 11, 91–95. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, H.; Zhou, C.; Zheng, L.; Cheng, P.; Nie, J.; Feng, W.; Hu, Y.-S.; Li, H.; Huang, X.; et al. Single Lithium-Ion Conducting Polymer Electrolytes Based on a Super-Delocalized Polyanion. Angew. Chem. Int. Ed. 2016, 55, 2521–2525. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, X.; Liu, J.; Miller, J.D. Natural halloysite nano-clay electrolyte for advanced all-solid-state lithium-sulfur batteries. Nano Energy 2017, 31, 478–485. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.; Stalin, S.; Khan, K.; Archer, L.A. Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries. Nat. Energy 2019, 4, 365–373. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Z.; Zhao, S.; Westover, A.S.; Belharouak, I.; Cao, P.-F. Single-Ion Conducting Polymer Electrolytes for Solid-State Lithium–Metal Batteries: Design, Performance, and Challenges. Adv. Energy Mater. 2021, 11, 2003836. [Google Scholar] [CrossRef]
- Nykaza, J.R.; Savage, A.; Pan, Q.; Wang, S.; Beyer, F.; Tang, M.H.; Li, C.Y.; Elabd, Y.J.P. Polymerized ionic liquid diblock copolymer as solid-state electrolyte and separator in lithium-ion battery. Polymer 2016, 101, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Pelz, A.; Dörr, T.S.; Zhang, P.; de Oliveira, P.W.; Winter, M.; Wiemhöfer, H.-D.; Kraus, T. Self-Assembled Block Copolymer Electrolytes: Enabling Superior Ambient Cationic Conductivity and Electrochemical Stability. Chem. Mater. 2019, 31, 277–285. [Google Scholar] [CrossRef]
- Yue, L.; Ma, J.; Zhang, J.; Zhao, J.; Dong, S.; Liu, Z.; Cui, G.; Chen, L. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater. 2016, 5, 139–164. [Google Scholar] [CrossRef]
- Ramesh, S.; Ng, H.M. An investigation on PAN–PVC–LiTFSI based polymer electrolytes system. Solid State Ion. 2011, 192, 2–5. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Yang, W. Enhancement of electrochemical properties of hot-pressed poly(ethylene oxide)-based nanocomposite polymer electrolyte films for all-solid-state lithium polymer batteries. Electrochim. Acta 2010, 55, 1895–1899. [Google Scholar] [CrossRef]
- Rivas, B.L.; Pereira, E.D.; Moreno-Villoslada, I. Water-soluble polymer–metal ion interactions. Prog. Polym. Sci. 2003, 28, 173–208. [Google Scholar] [CrossRef]
- Klongkan, S.; Pumchusak, J. Effects of Nano Alumina and Plasticizers on Morphology, Ionic Conductivity, Thermal and Mechanical Properties of PEO-LiCF3SO3 Solid Polymer Electrolyte. Electrochim. Acta 2015, 161, 171–176. [Google Scholar] [CrossRef]
- Chaurasia, S.K.; Shalu; Gupta, A.K.; Verma, Y.L.; Singh, V.K.; Tripathi, A.K.; Saroj, A.L.; Singh, R.K. Role of ionic liquid [BMIMPF6] in modifying the crystallization kinetics behavior of the polymer electrolyte PEO-LiClO4. RSC Adv. 2015, 5, 8263–8277. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, W.-H. Development of Electrolytes towards Achieving Safe and High-Performance Energy-Storage Devices: A Review. ChemElectroChem 2015, 2, 22–36. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Ji, J.; Eyler, A.; Zhong, W.-H. A Gum-Like Electrolyte: Safety of a Solid, Performance of a Liquid. Adv. Energy Mater. 2013, 3, 1557–1562. [Google Scholar] [CrossRef]
- Shen, Y. Porous PVDF with LiClO4 complex as “solid” and “wet” polymer electrolyte. Solid State Ion. 2004, 175, 747–750. [Google Scholar] [CrossRef]
- Jeong, H.-S.; Kim, J.H.; Lee, S.-Y. A novel poly(vinylidene fluoride-hexafluoropropylene)/poly(ethylene terephthalate) composite nonwoven separator with phase inversion-controlled microporous structure for a lithium-ion battery. J. Mater. Chem. 2010, 20, 9180–9186. [Google Scholar] [CrossRef]
- Subramania, A.; Kalyana Sundaram, N.T.; Vijaya Kumar, G.; Vasudevan, T. New polymer electrolyte based on (PVA–PAN) blend for Li-ion battery applications. Ionics 2006, 12, 175–178. [Google Scholar] [CrossRef]
- Lin, C.; Hung, C.; Venkateswarlu, M.; Hwang, B. Influence of TiO2 nano-particles on the transport properties of composite polymer electrolyte for lithium-ion batteries. J. Power Sources 2005, 146, 397–401. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, W.; Cong, L.; Liu, J.; Sun, L.; Mauger, A.; Julien, C.M.; Xie, H.; Liu, J. Cross-linking network based on Poly(ethylene oxide): Solid polymer electrolyte for room temperature lithium battery. J. Power Sources 2019, 420, 63–72. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Z.; Yin, L.; Xu, S.; Ghazi, Z.A.; Shi, Y.; An, B.; Sun, Z.; Cheng, H.-M.; Li, F. Fast lithium ion transport in solid polymer electrolytes from polysulfide-bridged copolymers. Nano Energy 2020, 75, 104976. [Google Scholar] [CrossRef]
- Kalnaus, S.; Asp, L.E.; Li, J.; Veith, G.M.; Nanda, J.; Daniel, C.; Chen, X.C.; Westover, A.; Dudney, N.J. Multifunctional approaches for safe structural batteries. J. Energy Storage 2021, 40, 102747. [Google Scholar] [CrossRef]
- Dias, F.B.; Plomp, L.; Veldhuis, J.B.J. Trends in polymer electrolytes for secondary lithium batteries. J. Power Sources 2000, 88, 169–191. [Google Scholar] [CrossRef]
- Ding, P.; Lin, Z.; Guo, X.; Wu, L.; Wang, Y.; Guo, H.; Li, L.; Yu, H. Polymer electrolytes and interfaces in solid-state lithium metal batteries. Mater. Today 2021, 51, 449–474. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.; Li, Z.; Xie, H.; Fu, J.; Wei, L.; Yang, H.; Guo, X. Hybrid electrolytes with an ultrahigh Li-ion transference number for lithium-metal batteries with fast and stable charge/discharge capability. J. Mater. Chem. A 2021, 9, 18239–18246. [Google Scholar] [CrossRef]
- Silva, M.; Bermudez, V.; Pawlicka, A. Insight on Polymer Electrolytes for Electrochemical Devices Applications; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 113–136. [Google Scholar]
- Hu, J.; Wang, W.H.; Zhu, X.J.; Liu, S.B.; Wang, Y.J.; Xu, Y.J.; Zhou, S.K.; He, X.C.; Xue, Z.G. Composite polymer electrolytes reinforced by hollow silica nanotubes for lithium metal batteries. J. Membr. Sci. 2021, 618, 15. [Google Scholar] [CrossRef]
- Cao, J.; He, R.; Kyu, T. Fire retardant, superionic solid state polymer electrolyte membranes for lithium ion batteries. Curr. Opin. Chem. Eng. 2017, 15, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Johan, M.R.; Fen, L.B. Combined effect of CuO nanofillers and DBP plasticizer on ionic conductivity enhancement in the solid polymer electrolyte PEO–LiCF3SO3. Ionics 2010, 16, 335–338. [Google Scholar] [CrossRef]
- Ye, F.; Liao, K.; Ran, R.; Shao, Z. Recent Advances in Filler Engineering of Polymer Electrolytes for Solid-State Li-Ion Batteries: A Review. Energy Fuels 2020, 34, 9189–9207. [Google Scholar] [CrossRef]
- Huang, H.; Ding, F.; Zhong, H.; Li, H.; Zhang, W.; Liu, X.; Xu, Q. Nano-SiO2-embedded poly(propylene carbonate)-based composite gel polymer electrolyte for lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 9539–9549. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, J.; Chen, H.; Yu, Q.; Li, B. High electrochemical stability of a 3D cross-linked network PEO@nano-SiO2 composite polymer electrolyte for lithium metal batteries. J. Mater. Chem. A 2019, 7, 6832–6839. [Google Scholar] [CrossRef]
- Khurana, R.; Schaefer, J.L.; Archer, L.A.; Coates, G.W. Suppression of lithium dendrite growth using cross-linked polyethylene/poly(ethylene oxide) electrolytes: A new approach for practical lithium-metal polymer batteries. J. Am. Chem. Soc. 2014, 136, 7395–7402. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sheng, L.; Yasin, G.; Wang, L.; Xu, H.; He, X. Reviewing the current status and development of polymer electrolytes for solid-state lithium batteries. Energy Storage Mater. 2020, 33, 188–215. [Google Scholar] [CrossRef]
- Pan, X.; Yang, P.; Guo, Y.; Zhao, K.; Xi, B.; Lin, F.; Xiong, S. Electrochemical and Nanomechanical Properties of TiO2 Ceramic Filler Li-Ion Composite Gel Polymer Electrolytes for Li Metal Batteries. Adv. Mater. Interfaces 2021, 8, 2100669. [Google Scholar] [CrossRef]
- Irfan, M.; Atif, M.; Yang, Z.; Zhang, W. Recent advances in high performance conducting solid polymer electrolytes for lithium-ion batteries. J. Power Sources 2021, 486, 229378. [Google Scholar] [CrossRef]
- Deng, K.; Zeng, Q.; Wang, D.; Liu, Z.; Qiu, Z.; Zhang, Y.; Xiao, M.; Meng, Y. Single-ion conducting gel polymer electrolytes: Design, preparation and application. J. Mater. Chem. A 2020, 8, 1557–1577. [Google Scholar] [CrossRef]
- Putri, R.M.; Sundari, C.D.D.; Floweri, O.; Mayangsari, T.R.; Ivansyah, A.L.; Santosa, S.P.; Arcana, I.M.; Iskandar, F. PEO/PVA/LiOH Solid Polymer Electrolyte Prepared via Ultrasound-assisted Solution Cast Method. J. Non-Cryst. Solids 2021, 556, 120549. [Google Scholar] [CrossRef]
- Luo, K.; Shao, D.; Yang, L.; Liu, L.; Chen, X.; Zou, C.; Wang, D.; Luo, Z.; Wang, X. Semi-interpenetrating gel polymer electrolyte based on PVDF-HFP for lithium ion batteries. J. Appl. Polym. Sci. 2021, 138, 49993. [Google Scholar] [CrossRef]
- Swiderska-Mocek, A.; Kubis, A. Preparation and electrochemical properties of polymer electrolyte containing lithium difluoro(oxalato)borate or lithium bis(oxalate)borate for Li-ion polymer batteries. Solid State Ion. 2021, 364, 115628. [Google Scholar] [CrossRef]
- Gong, X.; Luo, H.; Liu, G.; Luo, C.; Niu, Y.; Li, G. High-performance gel polymer electrolytes derived from PAN-POSS/PVDF composite membranes with ionic liquid for lithium ion batteries. Ionics 2021, 27, 2945–2953. [Google Scholar] [CrossRef]
- He, Y.; Liu, N.; Kohl, P.A. Difunctional block copolymer with ion solvating and crosslinking sites as solid polymer electrolyte for lithium batteries. J. Power Sources 2021, 481, 228832. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, T.; Zhao, B.; Shen, F.; Jin, H.; Han, X. Recent advances in organic-inorganic composite solid electrolytes for all-solid-state lithium batteries. Energy Storage Mater. 2021, 34, 388–416. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Pan, X.; Liu, J.; Doyle-Davis, K.; Sun, L.; Liu, J.; Jiao, X.; Jie, J.; Xie, H.; et al. Dendrite-free lithium metal solid battery with a novel polyester based triblock copolymer solid-state electrolyte. Nano Energy 2020, 72, 104690. [Google Scholar] [CrossRef]
- Mallela, Y.L.N.K.; Kim, S.; Seo, G.; Kim, J.W.; Kumar, S.; Lee, J.; Lee, J.-S. Crosslinked poly(allyl glycidyl ether) with pendant nitrile groups as solid polymer electrolytes for Li–S batteries. Electrochim. Acta 2020, 362, 137141. [Google Scholar] [CrossRef]
- Tseng, Y.-C.; Hsiang, S.-H.; Tsao, C.-H.; Teng, H.; Hou, S.-S.; Jan, J.-S. In situ formation of polymer electrolytes using a dicationic imidazolium cross-linker for high-performance lithium ion batteries. J. Mater. Chem. A 2021, 9, 5796–5806. [Google Scholar] [CrossRef]
- Deng, K.; Zhou, S.; Xu, Z.; Xiao, M.; Meng, Y. A high ion-conducting, self-healing and nonflammable polymer electrolyte with dynamic imine bonds for dendrite-free lithium metal batteries. Chem. Eng. J. 2022, 428, 131224. [Google Scholar] [CrossRef]
- Liang, L.; Yuan, W.; Chen, X.; Liao, H. Flexible, nonflammable, highly conductive and high-safety double cross-linked poly(ionic liquid) as quasi-solid electrolyte for high performance lithium-ion batteries. Chem. Eng. J. 2021, 421, 130000. [Google Scholar] [CrossRef]
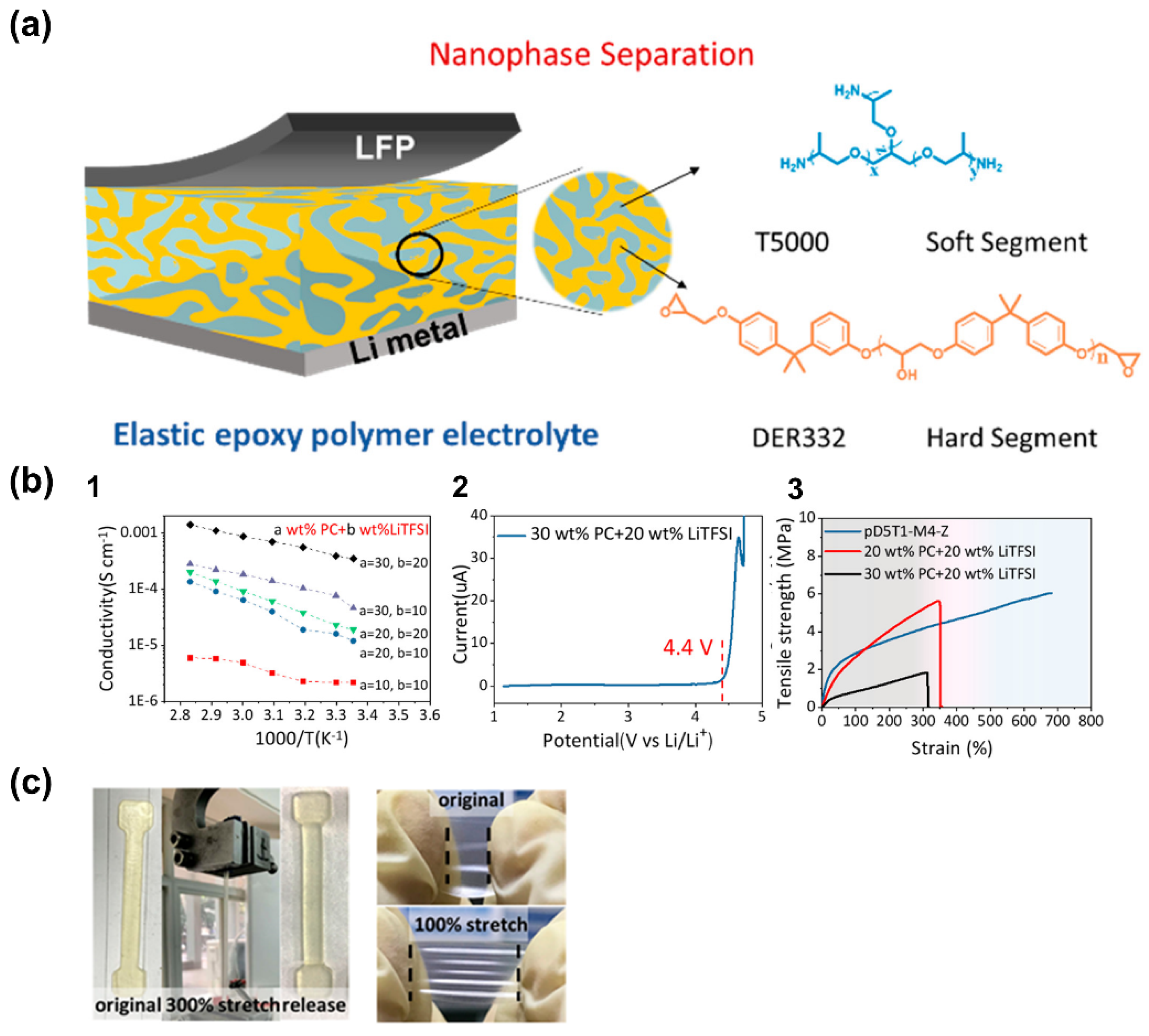
- Zeng, Z.; Chen, X.; Sun, M.; Jiang, Z.; Hu, W.; Yu, C.; Cheng, S.; Xie, J. Nanophase-Separated, Elastic Epoxy Composite Thin Film as an Electrolyte for Stable Lithium Metal Batteries. Nano Lett. 2021, 21, 3611–3618. [Google Scholar] [CrossRef]
- Liu, Z.; Chai, J.; Xu, G.; Wang, Q.; Cui, G. Functional lithium borate salts and their potential application in high performance lithium batteries. Coord. Chem. Rev. 2015, 292, 56–73. [Google Scholar] [CrossRef]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef]
- Hu, P.; Chai, J.; Duan, Y.; Liu, Z.; Cui, G.; Chen, L. Progress in nitrile-based polymer electrolytes for high performance lithium batteries. J. Mater. Chem. A 2016, 4, 10070–10083. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Y.; Cao, H.; Zhao, L.; Huang, Y.; Song, A.; Lin, Y.; Li, X.; Wang, M. A novel porous gel polymer electrolyte based on poly(acrylonitrile-polyhedral oligomeric silsesquioxane) with high performances for lithium-ion batteries. J. Membr. Sci. 2018, 545, 140–149. [Google Scholar] [CrossRef]
- Kim, D.-G.; Sohn, H.-S.; Kim, S.-K.; Lee, A.; Lee, J.-C. Star-shaped polymers having side chain poss groups for solid polymer electrolytes; synthesis, thermal behavior, dimensional stability, and ionic conductivity. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3618–3627. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, C.; Liu, J.; Chen, L.; Pan, A.; Wei, W. Solid polymer electrolyte membranes based on organic/inorganic nanocomposites with star-shaped structure for high performance lithium ion battery. J. Membr. Sci. 2016, 509, 138–148. [Google Scholar] [CrossRef]
- Giles, J.; Gray, F.; MacCallum, J.; Vincent, C. Synthesis and characterization of ABA block copolymer-based polymer electrolytes. Polymer 1987, 28, 1977–1981. [Google Scholar] [CrossRef]
- Gray, F.; MacCallum, J.; Vincent, C.; Giles, J. Novel polymer electrolytes based on ABA block copolymers. Macromolecules 1988, 21, 392–397. [Google Scholar] [CrossRef]
- Zhou, D.; He, Y.B.; Liu, R.; Liu, M.; Du, H.; Li, B.; Cai, Q.; Yang, Q.H.; Kang, F. In Situ Synthesis of a Hierarchical All-Solid-State Electrolyte Based on Nitrile Materials for High-Performance Lithium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1500353. [Google Scholar] [CrossRef]
- Cho, Y.G.; Hwang, C.; Cheong, D.S.; Kim, Y.S.; Song, H.K. Gel/solid polymer electrolytes characterized by in situ gelation or polymerization for electrochemical energy systems. Adv. Mater. 2019, 31, 1804909. [Google Scholar] [CrossRef]
- Han, J.G.; Kim, K.; Lee, Y.; Choi, N.S. Scavenging Materials to Stabilize LiPF6-Containing Carbonate-Based Electrolytes for Li-Ion Batteries. Adv. Mater. 2019, 31, 1804822. [Google Scholar] [CrossRef]
- Chen, S.; Che, H.; Feng, F.; Liao, J.; Wang, H.; Yin, Y.; Ma, Z.-F. Poly (vinylene carbonate)-based composite polymer electrolyte with enhanced interfacial stability to realize high-performance room-temperature solid-state sodium batteries. ACS Appl. Mater. Interfaces 2019, 11, 43056–43065. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Theerthagiri, J.; Vikraman, D.; Yim, C.-J.; Hussain, S.; Sharma, R.; Maiyalagan, T.; Qin, J.; Kim, H.-S. Ionic Liquid-Based Electrolytes for Energy Storage Devices: A Brief Review on Their Limits and Applications. Polymers 2020, 12, 918. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Yi, Y.; Yang, P.; Liu, P.; Qu, L.; Li, M.; Hu, Y.-s.; Yang, B. High-Charge Density Polymerized Ionic Networks Boosting High Ionic Conductivity as Quasi-Solid Electrolytes for High-Voltage Batteries. ACS Appl. Mater. Interfaces 2019, 11, 4001–4010. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; He, Y.-B.; Yu, Q.; Li, B.; Kaneti, Y.V.; Yao, Y.; Kang, F.; Yang, Q.-H. Dendrite-Free, High-Rate, Long-Life Lithium Metal Batteries with a 3D Cross-Linked Network Polymer Electrolyte. Adv. Mater. 2017, 29, 1604460. [Google Scholar] [CrossRef]
- Matsumoto, K.; Endo, T. Design and synthesis of ionic-conductive epoxy-based networked polymers. React. Funct. Polym. 2013, 73, 278–282. [Google Scholar] [CrossRef]
- Zhou, J.; Ji, H.; Liu, J.; Qian, T.; Yan, C. A new high ionic conductive gel polymer electrolyte enables highly stable quasi-solid-state lithium sulfur battery. Energy Storage Mater. 2019, 22, 256–264. [Google Scholar] [CrossRef]
- Andrews, W.T.; Liebig, A.; Cook, J.; Marsh, P.; Ciocanel, C.; Lindberg, G.E.; Browder, C.C. Development of a PEO-based lithium ion conductive epoxy resin polymer electrolyte. Solid State Ion. 2018, 326, 150–158. [Google Scholar] [CrossRef]
- Schulze, M.W.; McIntosh, L.D.; Hillmyer, M.A.; Lodge, T.P. High-Modulus, High-Conductivity Nanostructured Polymer Electrolyte Membranes via Polymerization-Induced Phase Separation. Nano Lett. 2014, 14, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Johannisson, W.; Johansen, M.; Liu, F.; Zenkert, D.; Lindbergh, G.; Asp, L.E. Characterization of the adhesive properties between structural battery electrolytes and carbon fibers. Compos. Sci. Technol. 2020, 188, 107962. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kang, D.A.; Kim, N.U.; Lee, J.M.; Kim, J.H. Bicontinuously crosslinked polymer electrolyte membranes with high ion conductivity and mechanical strength. J. Membr. Sci. 2019, 589, 117250. [Google Scholar] [CrossRef]
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.-P.; Phan, T.N.T.; Bertin, D.; Gigmes, D.; Devaux, D.; et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 2013, 12, 452–457. [Google Scholar] [CrossRef]
- Jeong, K.; Park, S.; Lee, S.-Y. Revisiting polymeric single lithium-ion conductors as an organic route for all-solid-state lithium ion and metal batteries. J. Mater. Chem. A 2019, 7, 1917–1935. [Google Scholar] [CrossRef]
- Tsuchida, E.; Ohno, H.; Kobayashi, N.; Ishizaka, H. Poly[(ι-carboxy)oligo(oxyethylene) methacrylate] as a new type of polymeric solid electrolyte for alkali-metal ion transport. Macromolecules 1989, 22, 1771–1775. [Google Scholar] [CrossRef]
- Tada, Y.; Sato, M.; Takeno, N.; Nakacho, Y.; Shigehara, K. Attempts at lithium single-ionic conduction by anchoring sulfonate anions as terminating groups of oligo(oxyethylene) side chains in comb-type polyphosphazenes. Chem. Mater. 1994, 6, 27–30. [Google Scholar] [CrossRef]
- Gao, J.; Sun, C.; Xu, L.; Chen, J.; Wang, C.; Guo, D.; Chen, H. Lithiated Nafion as polymer electrolyte for solid-state lithium sulfur batteries using carbon-sulfur composite cathode. J. Power Sources 2018, 382, 179–189. [Google Scholar] [CrossRef]
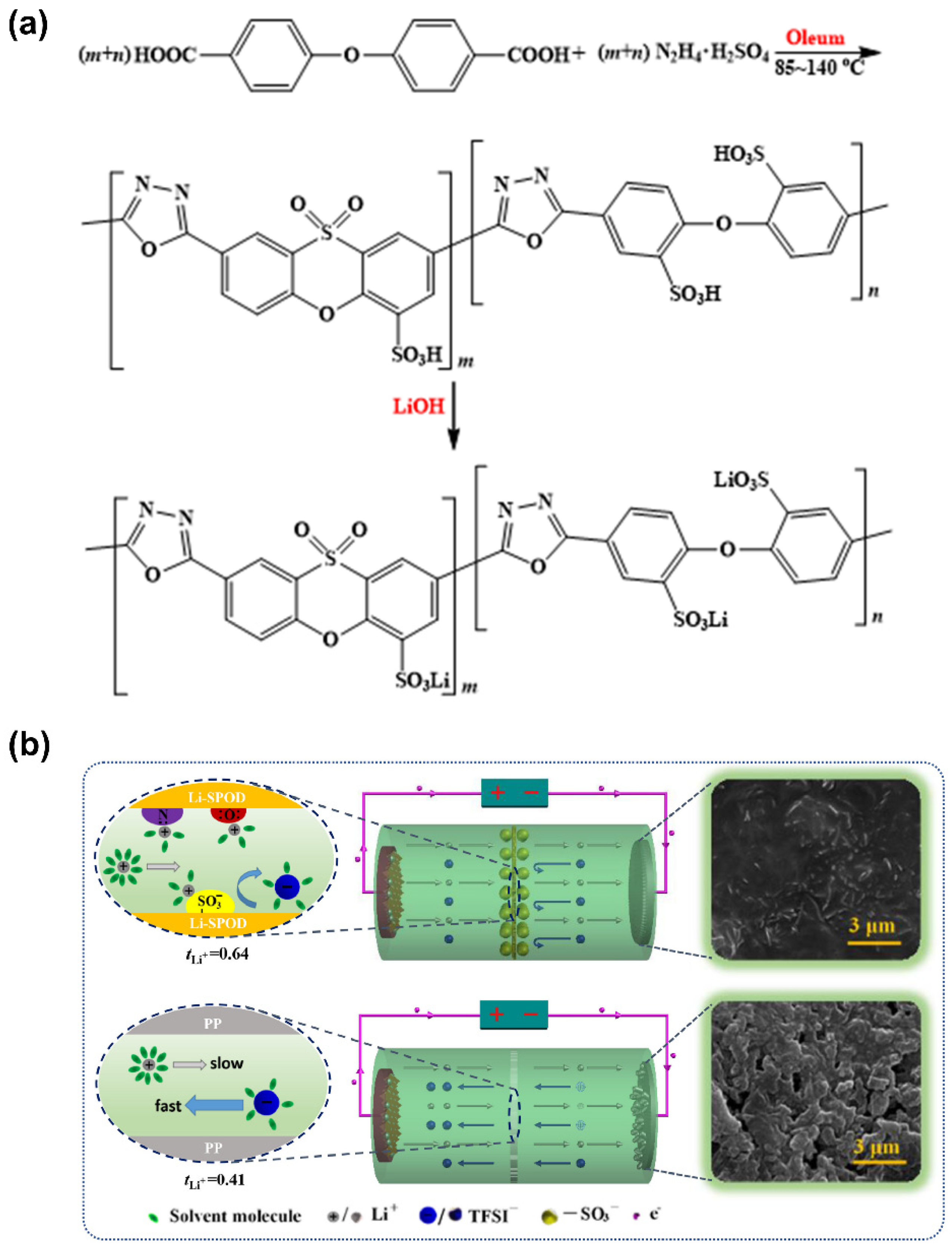
- Lee, J.; Song, J.; Lee, H.; Noh, H.; Kim, Y.-J.; Kwon, S.H.; Lee, S.G.; Kim, H.-T. A Nanophase-Separated, Quasi-Solid-State Polymeric Single-Ion Conductor: Polysulfide Exclusion for Lithium–Sulfur Batteries. ACS Energy Lett. 2017, 2, 1232–1239. [Google Scholar] [CrossRef]
- Siska, D.P.; Shriver, D.F. Li+ Conductivity of Polysiloxane−Trifluoromethylsulfonamide Polyelectrolytes. Chem. Mater. 2001, 13, 4698–4700. [Google Scholar] [CrossRef]
- Meziane, R.; Bonnet, J.-P.; Courty, M.; Djellab, K.; Armand, M. Single-ion polymer electrolytes based on a delocalized polyanion for lithium batteries. Electrochim. Acta 2011, 57, 14–19. [Google Scholar] [CrossRef]
- Rohan, R.; Pareek, K.; Chen, Z.; Cai, W.; Zhang, Y.; Xu, G.; Gao, Z.; Cheng, H. A high performance polysiloxane-based single ion conducting polymeric electrolyte membrane for application in lithium ion batteries. J. Mater. Chem. A 2015, 3, 20267–20276. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Wang, X.J.; Hou, Y.Y.; Gao, X.W.; Liu, L.L.; Wu, Y.P.; Shimizu, M. A new single-ion polymer electrolyte based on polyvinyl alcohol for lithium ion batteries. Electrochim. Acta 2013, 87, 113–118. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Rohan, R.; Cai, W.; Cheng, H. Synthesis, Characterization and Battery Performance of A Lithium Poly (4-vinylphenol) Phenolate Borate Composite Membrane. Electrochim. Acta 2014, 139, 264–269. [Google Scholar] [CrossRef]
- He, Y.; Liu, N.; Kohl, P.A. Lithium Ion Conduction in Diblock Polymer Electrolyte with Tethered Anion. ChemistrySelect 2021, 6, 595–599. [Google Scholar] [CrossRef]
- Wang, C.; Li, R.; Chen, P.; Fu, Y.; Ma, X.; Shen, T.; Zhou, B.; Chen, K.; Fu, J.; Bao, X.; et al. Highly stretchable, non-flammable and notch-insensitive intrinsic self-healing solid-state polymer electrolyte for stable and safe flexible lithium batteries. J. Mater. Chem. A 2021, 9, 4758–4769. [Google Scholar] [CrossRef]
- Li, D.; Luo, L.; Zhu, J.; Qin, H.; Liu, P.; Sun, Z.; Lei, Y.; Jiang, M. A hybrid lithium sulfonated polyoxadiazole derived single-ion conducting gel polymer electrolyte enabled effective suppression of dendritic lithium growth. Chin. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Cao, C.; Li, Y.; Feng, Y.; Peng, C.; Li, Z.; Feng, W. A solid-state single-ion polymer electrolyte with ultrahigh ionic conductivity for dendrite-free lithium metal batteries. Energy Storage Mater. 2019, 19, 401–407. [Google Scholar] [CrossRef]
- Pan, Q.; Jiang, S.; Li, Z.; Liu, Y.; Du, Y.; Zhao, N.; Zhang, Y.; Liu, J.-M. Highly porous single ion conducting membrane via a facile combined “structural self-assembly” and in-situ polymerization process for high performance lithium metal batteries. J. Membr. Sci. 2021, 636, 119601. [Google Scholar] [CrossRef]
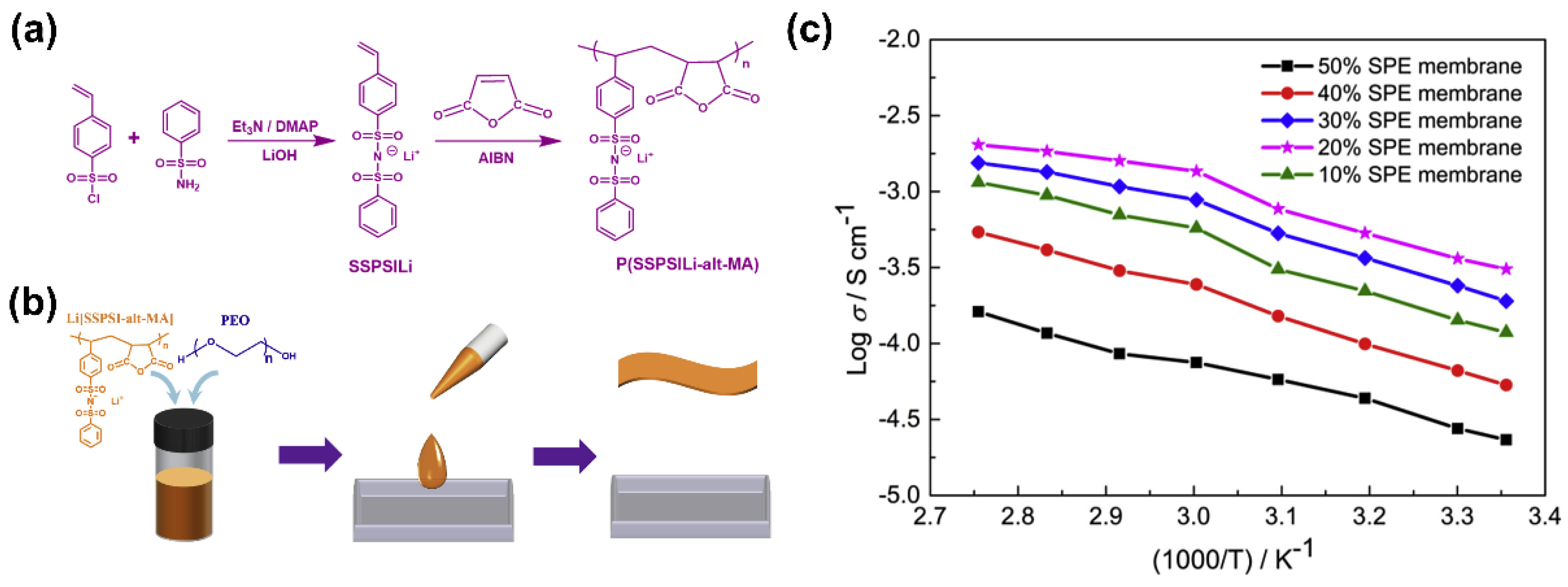
- Zhang, J.; Wang, S.; Han, D.; Xiao, M.; Sun, L.; Meng, Y. Lithium (4-styrenesulfonyl) (trifluoromethanesulfonyl) imide based single-ion polymer electrolyte with superior battery performance. Energy Storage Mater. 2020, 24, 579–587. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Tan, C.; He, Y.; Chen, Y.; Huo, S.; Zeng, D.; Li, C.; Cheng, H. Fire-retardant sp3 boron-based single ion conducting polymer electrolyte for safe, high efficiency and dendrite-free Li-metal batteries. J. Membr. Sci. 2021, 620, 118921. [Google Scholar] [CrossRef]
- Cao, C.; Li, Y.; Feng, Y.; Long, P.; An, H.; Qin, C.; Han, J.; Li, S.; Feng, W. A sulfonimide-based alternating copolymer as a single-ion polymer electrolyte for high-performance lithium-ion batteries. J. Mater. Chem. A 2017, 5, 22519–22526. [Google Scholar] [CrossRef]
- Van Humbeck, J.F.; Aubrey, M.L.; Alsbaiee, A.; Ameloot, R.; Coates, G.W.; Dichtel, W.R.; Long, J.R. Tetraarylborate polymer networks as single-ion conducting solid electrolytes. Chem. Sci. 2015, 6, 5499–5505. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.-M.; Bachman, J.E.; Taylor, M.K.; Kamcev, J.; Park, J.G.; Ziebel, M.E.; Velasquez, E.; Jarenwattananon, N.N.; Sethi, G.K.; Cui, Y.; et al. A Single-Ion Conducting Borate Network Polymer as a Viable Quasi-Solid Electrolyte for Lithium Metal Batteries. Adv. Mater. 2020, 32, 1905771. [Google Scholar] [CrossRef]
- Weston, J.E.; Steele, B.C.H. Effects of inert fillers on the mechanical and electrochemical properties of lithium salt-poly(ethylene oxide) polymer electrolytes. Solid State Ion. 1982, 7, 75–79. [Google Scholar] [CrossRef]
- Fan, P.; Liu, H.; Marosz, V.; Samuels, N.T.; Suib, S.L.; Sun, L.; Liao, L. High Performance Composite Polymer Electrolytes for Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101380. [Google Scholar] [CrossRef]
- Hoang Huy, V.P.; So, S.; Hur, J. Inorganic Fillers in Composite Gel Polymer Electrolytes for High-Performance Lithium and Non-Lithium Polymer Batteries. Nanomaterials 2021, 11, 614. [Google Scholar] [CrossRef]
- Shen, Z.; Cheng, Y.; Sun, S.; Ke, X.; Liu, L.; Shi, Z. The critical role of inorganic nanofillers in solid polymer composite electrolyte for Li+ transportation. Carbon Energy 2021, 3, 482–508. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Qin, Y.; Ji, S.; Huo, Y.; Wang, Z.; Liu, Z.; Shen, J.; Liu, J. Recent Progress in Organic–Inorganic Composite Solid Electrolytes for All-Solid-State Lithium Batteries. Chem.—A Eur. J. 2020, 26, 1720–1736. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, L.; Zhou, Y.; Liang, Z.; Tavajohi, N.; Li, B.; Li, T. Solid Polymer Electrolytes with High Conductivity and Transference Number of Li Ions for Li-Based Rechargeable Batteries. Adv. Sci. 2021, 8, 2003675. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, M.; Krishna, R.H.; Raja, M.; Therese, H.A.; Balakrishnan, N.T.M.; Raghavan, P.; Sivakumar, P. Titanium dioxide nano-ceramic filler in solid polymer electrolytes: Strategy towards suppressed dendrite formation and enhanced electrochemical performance for safe lithium ion batteries. J. Alloys Compd. 2021, 882, 160709. [Google Scholar] [CrossRef]
- Zhan, H.; Wu, M.; Wang, R.; Wu, S.; Li, H.; Tian, T.; Tang, H. Excellent Performances of Composite Polymer Electrolytes with Porous Vinyl-Functionalized SiO2 Nanoparticles for Lithium Metal Batteries. Polymers 2021, 13, 2468. [Google Scholar] [CrossRef]
- Xu, H.M.; Jing, M.X.; Li, J.; Huang, Z.H.; Wang, T.F.; Yuan, W.Y.; Ju, B.W.; Shen, X.Q. Safety-Enhanced Flexible Polypropylene Oxide-ZrO2 Composite Solid Electrolyte Film with High Room-Temperature Ionic Conductivity. ACS Sustain. Chem. Eng. 2021, 9, 11118–11126. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, S.; Li, G.; Wang, C.; Li, B.; Li, M.; Wang, Y.; Ming, H.; Wen, Y.; Qiu, J.; et al. A cross-linked gel polymer electrolyte employing cellulose acetate matrix and layered boron nitride filler prepared via in situ thermal polymerization. J. Power Sources 2021, 484, 229235. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Fu, D.; Zhang, F.; Wang, Z.; Chen, X.; Xu, J.; Hu, J.; Wu, X. Flexible Composite Solid Electrolyte with an Active Inorganic Filler. ACS Sustain. Chem. Eng. 2021, 9, 2237–2245. [Google Scholar] [CrossRef]
- Zhou, P.; Yao, D.; Zuo, K.; Xia, Y.; Yin, J.; Liang, H.; Zeng, Y.-P. Highly dispersible silicon nitride whiskers in asymmetric porous separators for high-performance lithium-ion battery. J. Membr. Sci. 2021, 621, 119001. [Google Scholar] [CrossRef]
- Tian, L.; Li, A.; Huang, Q.; Zhang, Y.; Long, D. Homogenously dispersed ultrasmall niobium(V) oxide nanoparticles enabling improved ionic conductivity and interfacial compatibility of composite polymer electrolyte. J. Colloid Interface Sci. 2021, 586, 855–865. [Google Scholar] [CrossRef]
- Hu, J.; Chen, K.; Yao, Z.; Li, C. Unlocking solid-state conversion batteries reinforced by hierarchical microsphere stacked polymer electrolyte. Sci. Bull. 2021, 66, 694–707. [Google Scholar] [CrossRef]
- Li, Z.; Liu, F.; Chen, S.; Zhai, F.; Li, Y.; Feng, Y.; Feng, W. Single Li ion conducting solid-state polymer electrolytes based on carbon quantum dots for Li-metal batteries. Nano Energy 2021, 82, 105698. [Google Scholar] [CrossRef]
- Asp, L.E.; Bouton, K.; Carlstedt, D.; Duan, S.; Harnden, R.; Johannisson, W.; Johansen, M.; Johansson, M.K.G.; Lindbergh, G.; Liu, F.; et al. A Structural Battery and its Multifunctional Performance. Adv. Energy Sustain. Res. 2021, 2, 2000093. [Google Scholar] [CrossRef]
- Jamal, H.; Khan, F.; Hyun, S.; Min, S.W.; Kim, J.H. Enhancement of the ionic conductivity of a composite polymer electrolyte via surface functionalization of SSZ-13 zeolite for all-solid-state Li-metal batteries. J. Mater. Chem. A 2021, 9, 4126–4137. [Google Scholar] [CrossRef]
- Hu, S.; Du, L.; Zhang, G.; Zou, W.; Zhu, Z.; Xu, L.; Mai, L. Open-Structured Nanotubes with Three-Dimensional Ion-Accessible Pathways for Enhanced Li+ Conductivity in Composite Solid Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 13183–13190. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zong, X.; Zhang, X.; Liu, C.; Li, D.; Jia, Z.; Li, G.; Zhou, X.; Wei, J.; Xiong, Y. Interface regulation enabling three-dimensional Li1.3Al0.3Ti1.7(PO4)3-reinforced composite solid electrolyte for high-performance lithium batteries. J. Power Sources 2021, 501, 230027. [Google Scholar] [CrossRef]
- Walle, K.Z.; Musuvadhi Babulal, L.; Wu, S.H.; Chien, W.C.; Jose, R.; Lue, S.J.; Chang, J.K.; Yang, C.C. Electrochemical Characteristics of a Polymer/Garnet Trilayer Composite Electrolyte for Solid-State Lithium-Metal Batteries. ACS Appl. Mater Interfaces 2021, 13, 2507–2520. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.; Zhang, G.; Chao, L. Three–dimensional fiber network reinforced polymer electrolyte for dendrite–free all–solid–state lithium metal batteries. Energy Storage Mater. 2021, 41, 631–641. [Google Scholar] [CrossRef]
- Shen, X.; Li, R.; Ma, H.; Peng, L.; Huang, B.; Zhang, P.; Zhao, J. Enhancing Li+ transport kinetics of PEO-based polymer electrolyte with mesoporous silica-derived fillers for lithium-ion batteries. Solid State Ion. 2020, 354, 115412. [Google Scholar] [CrossRef]
- He, H.; Chai, Y.; Zhang, X.; Shi, P.; Fan, J.; Xu, Q.; Min, Y. A 2D–3D co-conduction effect in PEO-based all-solid-state batteries for long term cycle stability. J. Mater. Chem. A 2021, 9, 9214–9227. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Gonçalves, R.; Costa, C.M.; de Zea Bermudez, V.; Fidalgo-Marijuan, A.; Zhang, Q.; Lanceros-Méndez, S. Metal–organic frameworks and zeolite materials as active fillers for lithium-ion battery solid polymer electrolytes. Mater. Adv. 2021, 2, 3790–3805. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.; Gao, H.; Li, C.; Hang, J.; Liu, P. MOF-derived multifunctional filler reinforced polymer electrolyte for solid-state lithium batteries. J. Energy Chem. 2021, 60, 259–271. [Google Scholar] [CrossRef]
- Wu, X.; Chen, K.; Yao, Z.; Hu, J.; Huang, M.; Meng, J.; Ma, S.; Wu, T.; Cui, Y.; Li, C. Metal organic framework reinforced polymer electrolyte with high cation transference number to enable dendrite-free solid state Li metal conversion batteries. J. Power Sources 2021, 501, 229946. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.; Li, C.; Li, X. Metal–Organic Framework-Supported Poly(ethylene oxide) Composite Gel Polymer Electrolytes for High-Performance Lithium/Sodium Metal Batteries. ACS Appl. Mater. Interfaces 2021, 13, 37262–37272. [Google Scholar] [CrossRef]
- Lv, F.; Liu, K.; Wang, Z.; Zhu, J.; Zhao, Y.; Yuan, S. Ultraviolet-cured polyethylene oxide-based composite electrolyte enabling stable cycling of lithium battery at low temperature. J. Colloid Interface Sci. 2021, 596, 257–266. [Google Scholar] [CrossRef]
- Kuai, Y.; Wang, F.; Yang, J.; Lu, H.; Xu, Z.; Xu, X.; NuLi, Y.; Wang, J. Silica-nanoresin crosslinked composite polymer electrolyte for ambient-temperature all-solid-state lithium batteries. Mater. Chem. Front. 2021, 5, 6502–6511. [Google Scholar] [CrossRef]
- Didwal, P.N.; Singhbabu, Y.N.; Verma, R.; Sung, B.-J.; Lee, G.-H.; Lee, J.-S.; Chang, D.R.; Park, C.-J. An advanced solid polymer electrolyte composed of poly(propylene carbonate) and mesoporous silica nanoparticles for use in all-solid-state lithium-ion batteries. Energy Storage Mater. 2021, 37, 476–490. [Google Scholar] [CrossRef]
- Wu, J.; Chen, J.; Wang, X.; Zhou, A.; Yang, Z. Applying multi-scale silica-like three-dimensional networks in a PEO matrix via in situ crosslinking for high-performance solid composite electrolytes. Mater. Chem. Front. 2021, 5, 7767–7777. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, W.; Zhou, L.; Xu, Q.; Min, Y. Surface-modified boron nitride as a filler to achieve high thermal stability of polymer solid-state lithium-metal batteries. J. Mater. Chem. A 2021, 9, 20530–20543. [Google Scholar] [CrossRef]
- Ramachandran, M.; Subadevi, R.; Rajkumar, P.; Muthupradeepa, R.; Sivakumar, M. Electrochemical analyses of ZrO2 dispersoid incorporated poly (styrene-methyl methacrylate) blend gel electrolytes for lithium-ion battery. J. Appl. Polym. Sci. 2021, 138, 9. [Google Scholar] [CrossRef]
- Beshahwured, S.L.; Wu, Y.-S.; Wu, S.-h.; Chien, W.-C.; Jose, R.; Lue, S.J.; Yang, C.-C. Flexible hybrid solid electrolyte incorporating ligament-shaped Li6.25Al0.25La3Zr2O12 filler for all-solid-state lithium-metal batteries. Electrochim. Acta 2021, 366, 137348. [Google Scholar] [CrossRef]
- Chen, F.; Jing, M.-X.; Yang, H.; Yuan, W.-Y.; Liu, M.-Q.; Ji, Y.-S.; Hussain, S.; Shen, X.-Q. Improved ionic conductivity and Li dendrite suppression of PVDF-based solid electrolyte membrane by LLZO incorporation and mechanical reinforcement. Ionics 2021, 27, 1101–1111. [Google Scholar] [CrossRef]
- Mu, S.; Huang, W.; Sun, W.; Zhao, N.; Jia, M.; Bi, Z.; Guo, X. Heterogeneous electrolyte membranes enabling double-side stable interfaces for solid lithium batteries. J. Energy Chem. 2021, 60, 162–168. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Y.; Goodenough, J.B.; Manthiram, A. Rationally Designed PEGDA–LLZTO Composite Electrolyte for Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2021, 13, 30703–30711. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Huang, Y.; He, M.; Hu, G.; Peng, Z.; Cao, Y.; Du, K. Multilayer PEO/LLZTO composite electrolyte enables high-performance solid-state Li-ion batteries. Ionics 2021, 27, 4127–4134. [Google Scholar] [CrossRef]
- Luo, S.; Zhao, E.; Gu, Y.; Huang, J.; Zhang, Z.; Yang, L.; Hirano, S.-I. Rational design of fireproof fiber-network reinforced 3D composite solid electrolyte for dendrite-free solid-state batteries. Chem. Eng. J. 2021, 421, 127771. [Google Scholar] [CrossRef]
- Siyal, S.H.; Shah, S.S.A.; Najam, T.; Javed, M.S.; Imran, M.; Lan, J.-L. Significant Reduction in Interface Resistance and Super-Enhanced Performance of Lithium-Metal Battery by In Situ Construction of Poly(vinylidene fluoride)-Based Solid-State Membrane with Dual Ceramic Fillers. ACS Appl. Energy Mater. 2021, 4, 8604–8614. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, Y.; Li, W.; Liu, Y.; Zhang, Y.; Li, Y.; Li, C. Solid electrolytes reinforced by infinite coordination polymer nano-network for dendrite-free lithium metal batteries. Energy Storage Mater. 2021, 41, 436–447. [Google Scholar] [CrossRef]
- Carlstedt, D.; Asp, L.E. Thermal and diffusion induced stresses in a structural battery under galvanostatic cycling. Compos. Sci. Technol. 2019, 179, 69–78. [Google Scholar] [CrossRef]
- Snyder, J.F.; Carter, R.H.; Wetzel, E.D. Electrochemical and Mechanical Behavior in Mechanically Robust Solid Polymer Electrolytes for Use in Multifunctional Structural Batteries. Chem. Mater. 2007, 19, 3793–3801. [Google Scholar] [CrossRef]
- Guigon, M.; Oberlin, A.; Desarmot, G. Microtexture and structure of some high-modulus, PAN-base carbon fibres. Fibre Sci. Technol. 1984, 20, 177–198. [Google Scholar] [CrossRef]
- Tanaka, F.; Okabe, T.; Okuda, H.; Kinloch, I.A.; Young, R.J. Factors controlling the strength of carbon fibres in tension. Compos. Part A Appl. Sci. Manuf. 2014, 57, 88–94. [Google Scholar] [CrossRef]
- Snyder, J.F.; Wong, E.L.; Hubbard, C.W. Evaluation of Commercially Available Carbon Fibers, Fabrics, and Papers for Potential Use in Multifunctional Energy Storage Applications. J. Electrochem. Soc. 2009, 156, A215. [Google Scholar] [CrossRef]
- Snyder, J.; O’Brien, D.; Baechle, D.; Mattson, D.; Wetzel, E. Structural Composite Capacitors, Supercapacitors, and Batteries for U.S. Army Applications. In Proceedings of the ASME 2008 Conference on Smart Materials, Adaptive Structures and Intelligent Systems. Smart Materials, Adaptive Structures and Intelligent Systems, Ellicott City, MD, USA, 28–30 October 2008; Volume 1. [Google Scholar]
- Liu, P.; Sherman, E.; Jacobsen, A. Design and fabrication of multifunctional structural batteries. J. Power Sources 2009, 189, 646–650. [Google Scholar] [CrossRef]
- Ekstedt, S.; Wysocki, M.; Asp, L. Structural batteries made from fibre reinforced composites. Plast. Rubber Compos. 2010, 39, 148–150. [Google Scholar] [CrossRef] [Green Version]
- Danzi, F.; Camanho, P.P.; Braga, M.H. An all-solid-state coaxial structural battery using sodium-based electrolyte. Molecules 2021, 26, 5226. [Google Scholar] [CrossRef]
- Salgado, R.M.; Danzi, F.; Oliveira, J.E.; El-Azab, A.; Camanho, P.P.; Braga, M.H. The latest trends in Electric Vehicles batteries. Molecules 2021, 26, 3188. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Wang, Z.; Shi, L.; Zhu, J.; Edström, K.; Mindemark, J.; Yuan, S. Challenges and development of composite solid-state electrolytes for high-performance lithium ion batteries. J. Power Sources 2019, 441, 227175. [Google Scholar] [CrossRef]
- Duan, H.; Fan, M.; Chen, W.-P.; Li, J.-Y.; Wang, P.-F.; Wang, W.-P.; Shi, J.-L.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Extended Electrochemical Window of Solid Electrolytes via Heterogeneous Multilayered Structure for High-Voltage Lithium Metal Batteries. Adv. Mater. 2019, 31, 1807789. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Zhu, B.; Zhai, H.; Liao, X.; Zhu, Y.; Xu, W.; Cheng, Q.; Jayyosi, C.; Li, Z.; Zhu, J.; et al. PVDF/Palygorskite Nanowire Composite Electrolyte for 4 V Rechargeable Lithium Batteries with High Energy Density. Nano Lett 2018, 18, 6113–6120. [Google Scholar] [CrossRef]
- Xu, D.; Su, J.; Jin, J.; Sun, C.; Ruan, Y.; Chen, C.; Wen, Z. In Situ Generated Fireproof Gel Polymer Electrolyte with Li6.4Ga0.2La3Zr2O12 As Initiator and Ion-Conductive Filler. Adv. Energy Mater. 2019, 9, 1900611. [Google Scholar] [CrossRef]
- Li, Z.; Sha, W.-X.; Guo, X. Three-Dimensional Garnet Framework-Reinforced Solid Composite Electrolytes with High Lithium-Ion Conductivity and Excellent Stability. ACS Appl. Mater. Interfaces 2019, 11, 26920–26927. [Google Scholar] [CrossRef]
- Niu, C.; Liu, J.; Chen, G.; Liu, C.; Qian, T.; Zhang, J.; Cao, B.; Shang, W.; Chen, Y.; Han, J.; et al. Anion-regulated solid polymer electrolyte enhances the stable deposition of lithium ion for lithium metal batteries. J. Power Sources 2019, 417, 70–75. [Google Scholar] [CrossRef]
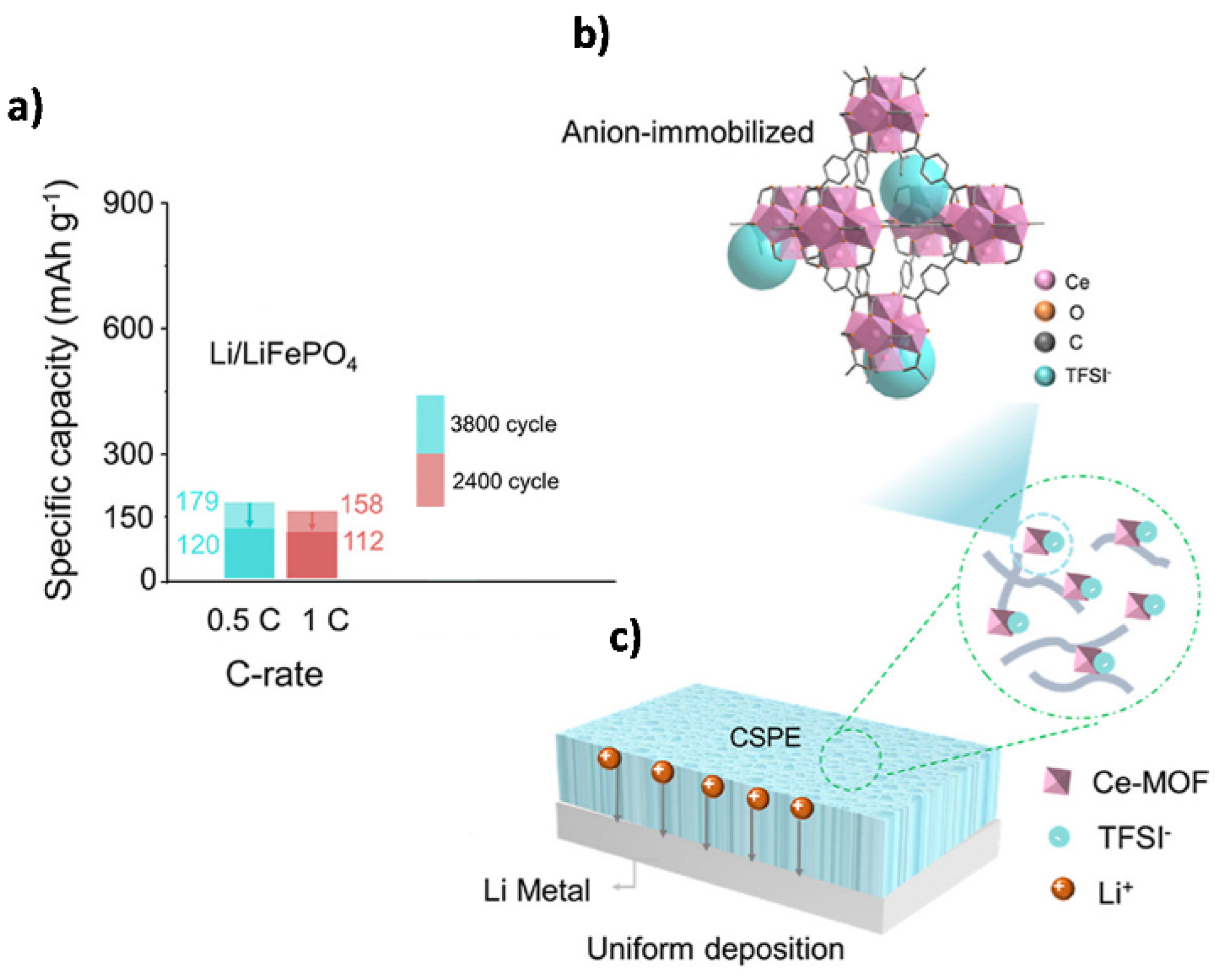
- Huo, H.; Wu, B.; Zhang, T.; Zheng, X.; Ge, L.; Xu, T.; Guo, X.; Sun, X. Anion-immobilized polymer electrolyte achieved by cationic metal-organic framework filler for dendrite-free solid-state batteries. Energy Storage Mater. 2019, 18, 59–67. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.; Zhang, S.; Shi, L.; Wu, H.; Bu, H.; Ding, S. g-C3N4 nanosheets enhanced solid polymer electrolytes with excellent electrochemical performance, mechanical properties, and thermal stability. J. Mater. Chem. A 2019, 7, 11069–11076. [Google Scholar] [CrossRef]
- Ihrner, N.; Johannisson, W.; Sieland, F.; Zenkert, D.; Johansson, M. Structural lithium ion battery electrolytes via reaction induced phase-separation. J. Mater. Chem. A 2017, 5, 25652–25659. [Google Scholar] [CrossRef] [Green Version]
- Schneider, L.M.; Ihrner, N.; Zenkert, D.; Johansson, M. Bicontinuous Electrolytes via Thermally Initiated Polymerization for Structural Lithium Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 4362–4369. [Google Scholar] [CrossRef] [Green Version]
- Moyer, K.; Meng, C.; Marshall, B.; Assal, O.; Eaves, J.; Perez, D.; Karkkainen, R.; Roberson, L.; Pint, C.L. Carbon fiber reinforced structural lithium-ion battery composite: Multifunctional power integration for CubeSats. Energy Storage Mater. 2020, 24, 676–681. [Google Scholar] [CrossRef]
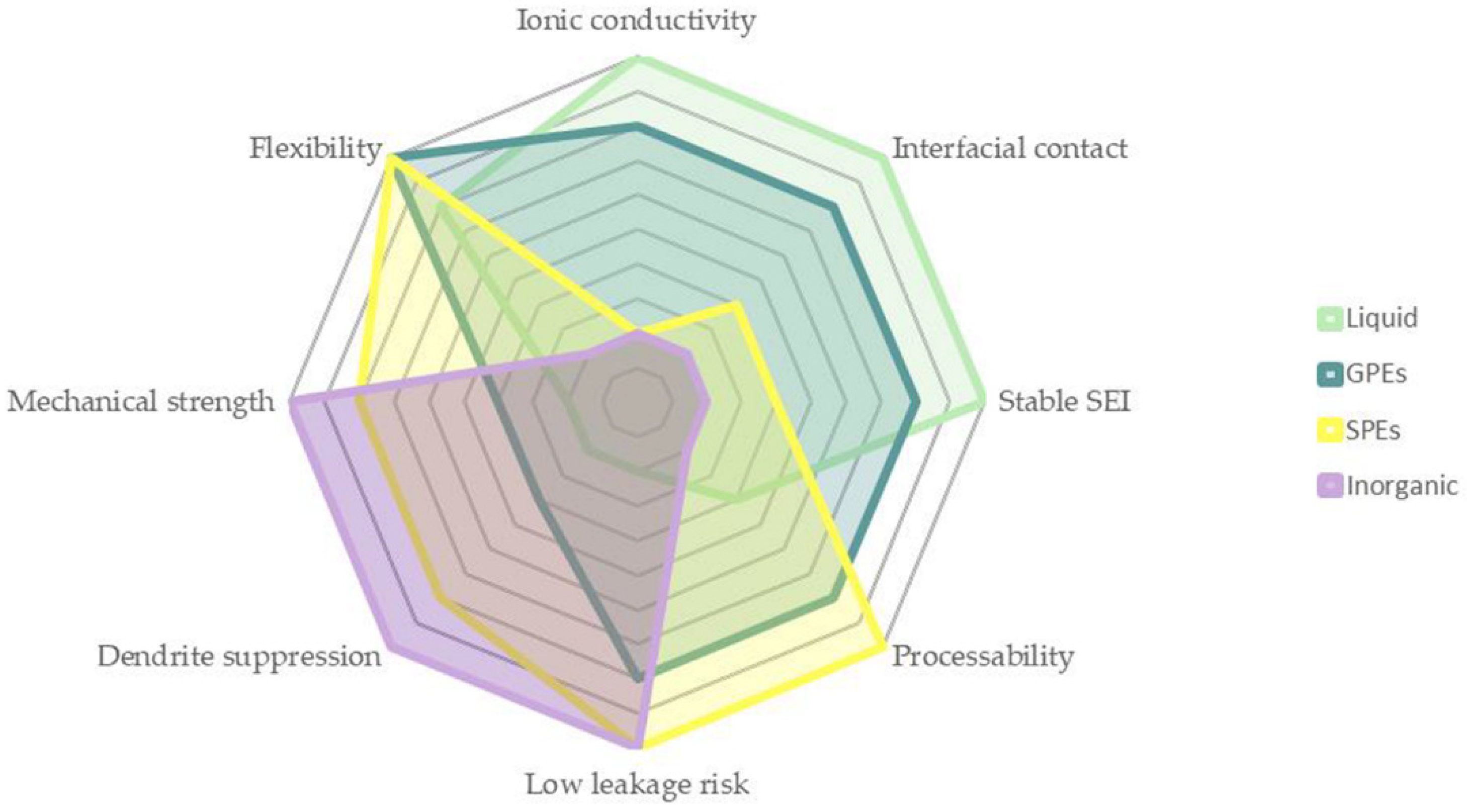
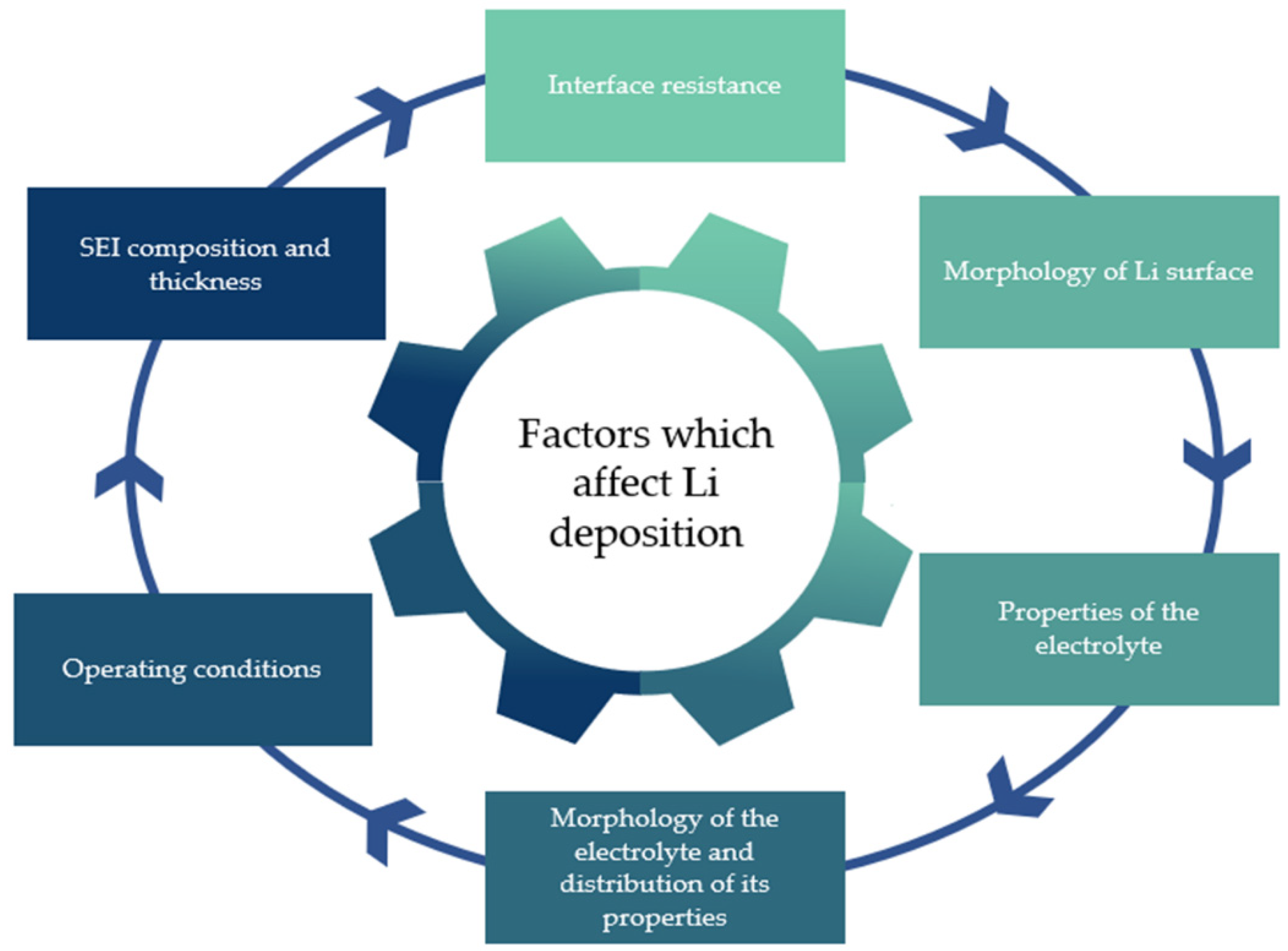


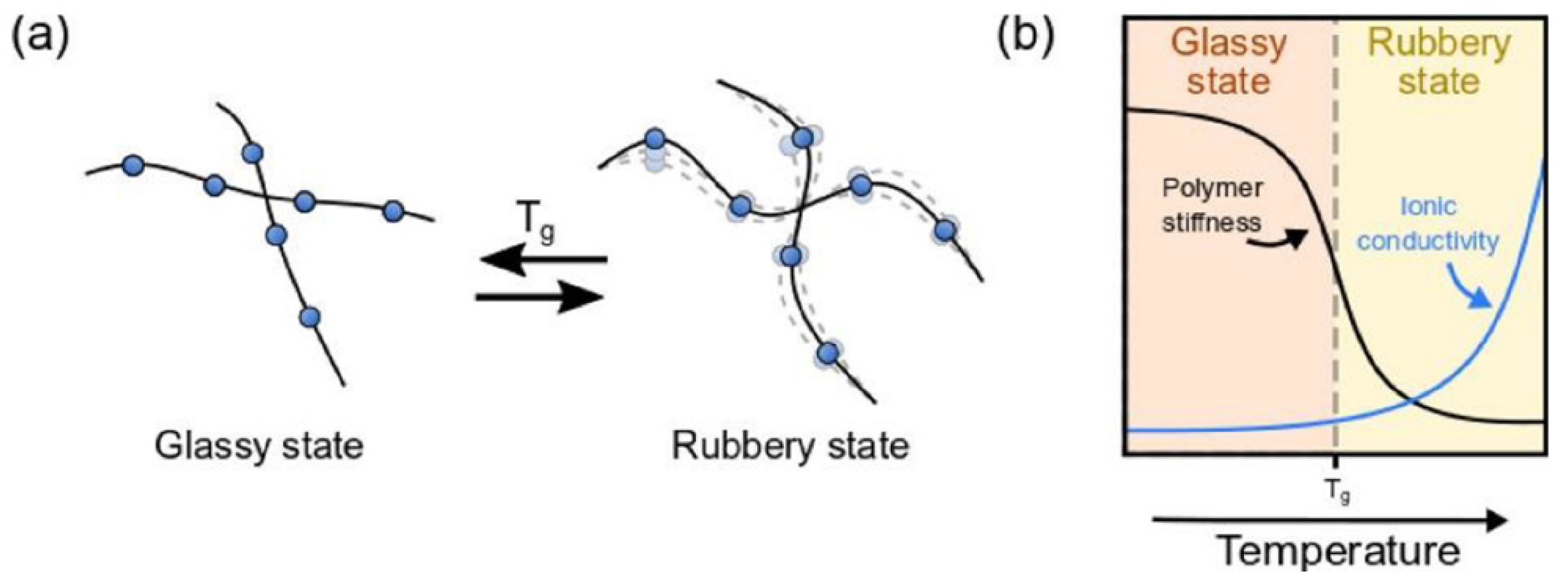
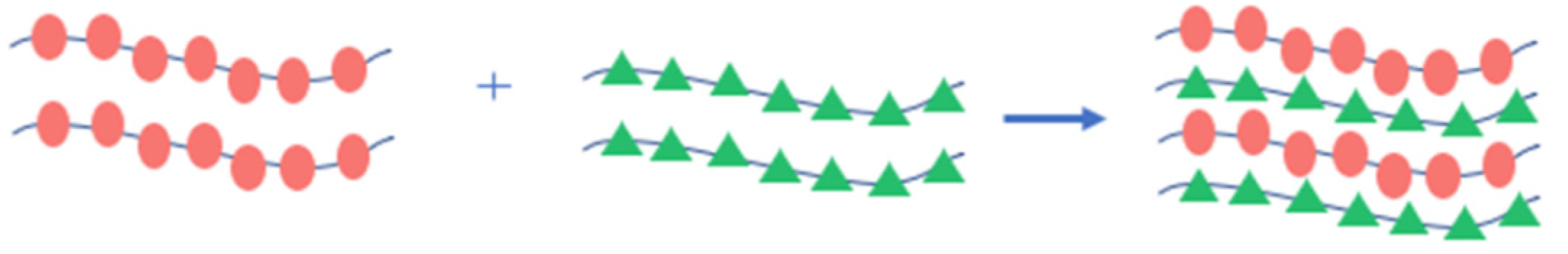
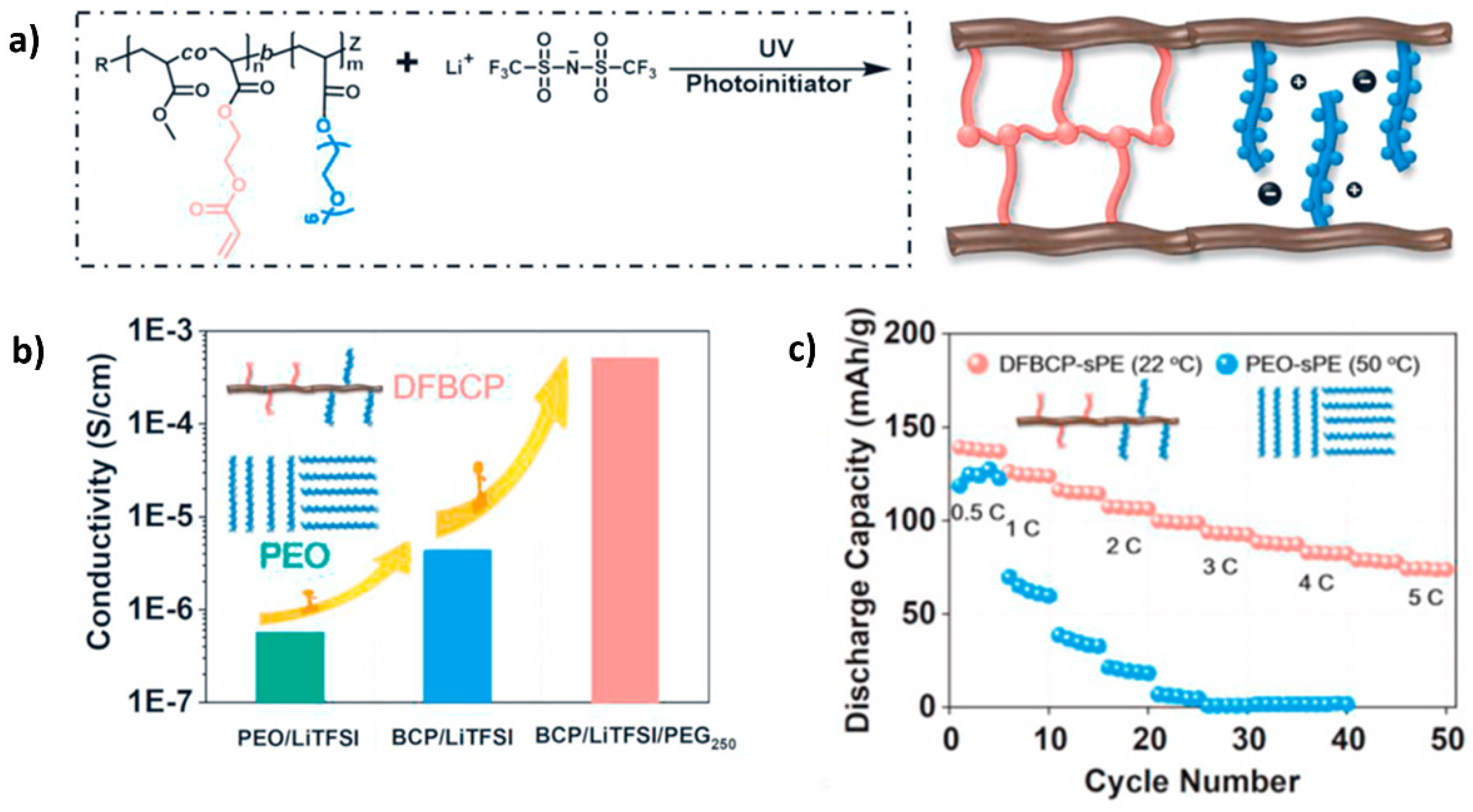
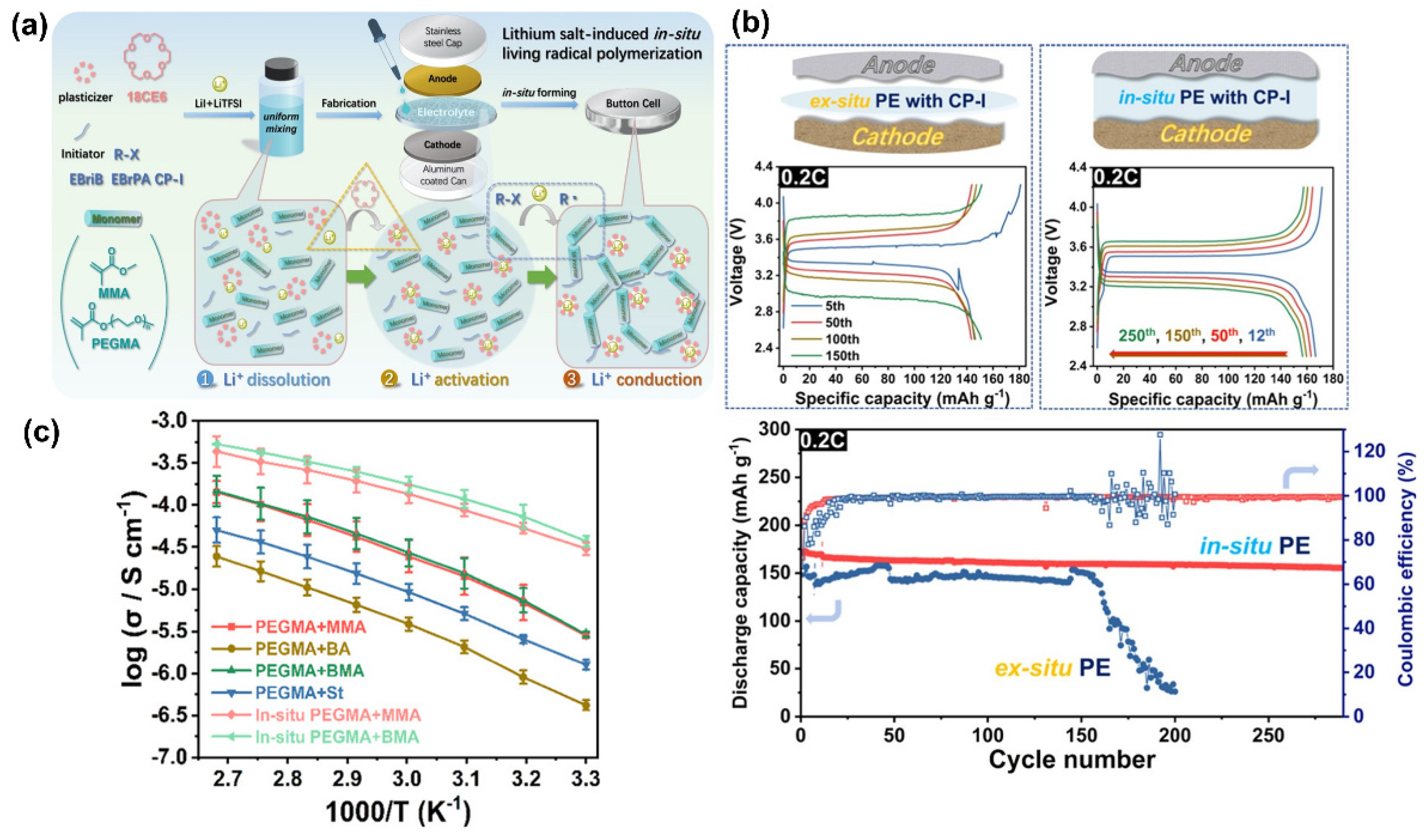

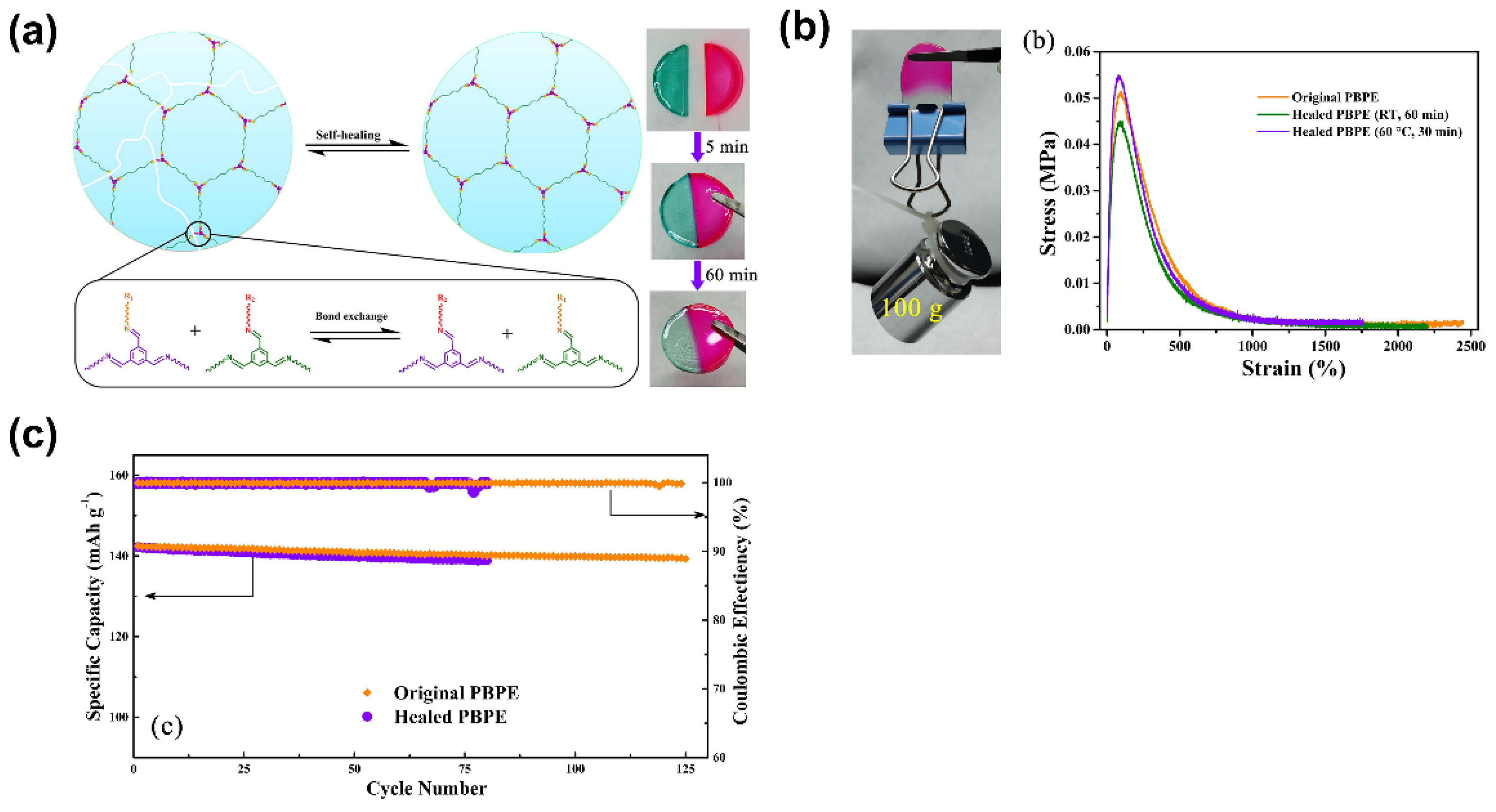
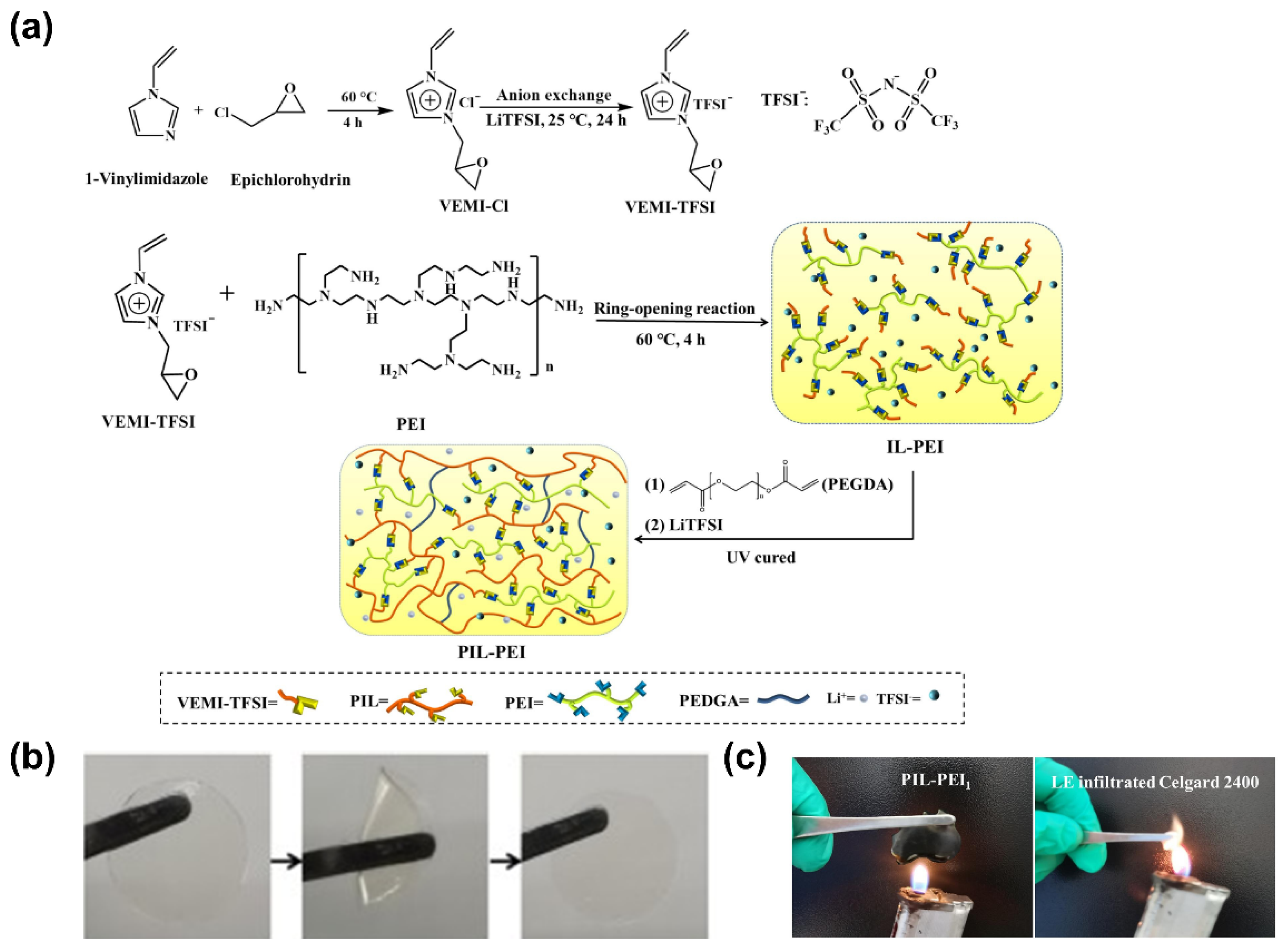




| Electrolyte Type | Ionic Conductivity (RT) | Interfacial Properties | Thermal Stability | Electrochemical Stability | Mechanical Strength | Safety |
|---|---|---|---|---|---|---|
| Liquid | >10−3 S·cm−1 | Good | Poor | Poor | Poor | Poor |
| Solid polymer | <10−4 S·cm−1 | Poor | Good | Good | Good | Good |
| Gel polymer | >10−4 S·cm−1 | Medium | Medium | Poor | Medium | Medium |
| Polymer | Optimization Strategy | Ionic Conductivity (S·cm−1) | EWS (V vs. Li/Li+) | tLi+ | Year | Ref. |
|---|---|---|---|---|---|---|
| PEO/LiClO4 | PEO/LiX (X:ClO4−) | 1.03 × 10−5, 30 °C | - | 0.21 | 2005 | [60] |
| (PEO-TEGDMA-TEGDME)/LiTFSI | Crosslinking | 2.70 × 10−4, 24 °C | 5 | 0.56 | 2019 | [61] |
| (PEO-sulfur-PEGMA)/LiTFSI | Polymer blending | 2.13 × 10−4, 50 °C | 5.4 | 0.61 | 2020 | [62] |
| (PEG-HDIt)/LiTFSI | Copolymerization | 6.51 × 10−5, 25 °C | 4.65 | 0.49 | 2021 | [63] |
| Structure | σ (S·cm−1) (°C) | tLi+ | Cell Type | Initial Capacity (mAh·g−1) (Conditions) | Cycle Stability (Capacity Retention) | Ref. |
|---|---|---|---|---|---|---|
| PEO/PVA | 1.25 × 10−4 (25 °C) | - | - | - | - | [79] |
| PVDF-HFP/PETEA | 5 × 10−4 (25 °C) | - | Li/LiFePO4 | 151 (0.5 C, 25 °C) | 50 (98%) | [80] |
| PVDF/TMS | 3.21 × 10−3 (25 °C) | 0.03 | Li/LiFePO4 | 181 (0.1 C, 25 °C) | 50 (91%) | [81] |
| PVDF/PAN-POSS | 1.91 × 10−3 (20 °C) | - | Li/LiFePO4 | 112 (0.1 C, 20 °C) | 25 (~90%) | [82] |
| P(HOEA-co-MA)-PEG | 6.0 × 10−4 (25 °C) | 0.35 | Li/LiFePO4 | 101.4 (2.0 C, 22 °C) | 1000 (66%) | [83] |
| P(PEGMA-co-MMA) | 3.02 × 10−5 (30 °C) | 0.37 | Li/LiFePO4 | 166.5 (0.2 C, 60 °C) | 290 (93%) | [84] |
| PCL-PPC-PCL | 3 × 10−5 (30 °C) | 0.4 | Li/LiFePO4 | 142 (0.05 C, 30 °C) | 200 (90%) | [85] |
| CN/FM-PAGE | 1.01 × 10−4 (30 °C) | - | Li/S | 944 (0.2 C, 60 °C) | 80 (~60%) | [86] |
| PEGMEA-PEGDME-XVIm-TFSI | 3.16 × 10−4 (25 °C) | - | Li/LiFePO4 | 160 (0.2 C, 25 °C) | 150 (93.8%) | [87] |
| PEG-BTA | 4.79 × 10−3 (30 °C) | 0.38 | Li/LiFePO4 | 118.2 (5 C, 30 °C) | 125 (97.8%) | [88] |
| VEMI-TFSI-PEI-PEGDGA | 1.03 × 10−3 (25 °C) | 0.47 | Li/LiFePO4 | 165.6 (0.1 C, 25 °C) | 200 (97.3%) | [89] |
| T5000-DER332 (epoxy resin) | 3.5 × 10−4 (25 °C) | - | Li/LiFePO4 | 130 (0.1 C, 25 °C) | 120 (93%) | [90] |
| Anion | σ (S·cm−1) (°C) | tLi+ | Cell Type | Initial Capacity (mAh·g−1) (Conditions) | Cycle Stability (Capacity Retention) | Ref. |
|---|---|---|---|---|---|---|
| –SO3 | 1.95 × 10−6 (25 °C) | 0.83 | Li/LiFePO4 | - | - | [123] |
| –SO3 | 1.2 × 10−5 (25 °C) | 0.37 | Li/LiFePO4 | 144.8 (0.2 C, 25 °C) | 100 (82%) | [124] |
| –SO3 | 2.03 × 10−3 (25 °C) | 0.64 | Li/LiFePO4 | 125 (2.0 C, 25 °C) | 300 (99%) | [125] |
| –N(SO2)2 | 3.08 × 10−4 (25 °C) | 0.97 | Li/LiFePO4 | 152.3 (0.02 C, 25 °C) | 100 (97.5%) | [126] |
| –N(SO2)2 | 2.3 × 10−4 (25 °C) | 0.9 | Li/LiFePO4 | 165 (0.05 C, 25 °C) | 200 (95.5%) | [127] |
| –N(SO2)2 | 8.4 × 10−4 (25 °C) | 0.93 | Li/LiFePO4 | 133 (1.0 C, 25 °C) | 400 (83%) | [128] |
| –BO4 | 6.2 × 10−4 (25 °C) | 0.85 | Li/LiFePO4 | 131.8 (0.1 C, 25 °C) | 200 (76.1%) | [129] |
| Filler | SPE | σ (S·cm−1) (°C) | tLi+ | EWS (V vs. Li/Li+) | Discharge Capacity (mAh·g−1) (Conditions) | Cycle Stability (Capacity Retention) | Tensile Strength (MPa) | Ref. |
|---|---|---|---|---|---|---|---|---|
| TiO2 | PVDF/PVA/LiTFSI | 2.69 × 10−3 (30 °C) | 0.53 | 5.4 | 154 (0.1 C; 40 °C) | 50 (94%) | 8.4 | [139] |
| TiO2 | PVDF/LiTFSI | 1.51 × 10−3 (30 °C) | 0.42 | - | 160 (0.1 C; 60 °C) | 50 (80%) | 0.9 | [76] |
| TiO2 Nanowires | PEO/LiTFSI | 1.10 × 10−4 (30 °C) | 0.36 | 5.5 | 151 (0.1 C; 60 °C) | 100 (91%) | - | [65] |
| SiO2 | PEO/LiTFSI | 7.5 × 10−6 (25 °C) 4.3 × 10−4 (60 C) | - | - | 150 (0.1 C; 60 °C) | 80 (88.4%) | 2.3 | [154] |
| SiO2 | PEO/PEGDA/LiTFSI | 5.08 × 10−3 (60 °C) | - | 5.2 | 155.1 (0.5 C; 60 °C) | 300 (91%) | 2.46 | [140] |
| SiO2 | PEO/PEGDA/LiTFSI | 3.37 × 10−4 (25 °C) 1.73 × 10−4 (0 °C) | 0.38 | 4.9 | 129.4 (0.2 C; 0 °C) | 150 (99%) | 26.9 | [160] |
| SiO2 | PVEC/LiTFSI | 1.65 × 10−4 (25 °C) | 0.63 | 5.3 | 150 (0.5 C; 25 °C) | 200 (79.4%) | - | [161] |
| SiO2 | PPC/LiTFSI | 8.48 × 10−4 (60 °C) | 0.86 | 4.8 | 171 (0.1 C; 60 °C) | 100 (86%) | 4.0 | [162] |
| SiO2 | PEO/AKP/LiTFSI | 2.52 × 10−4 (40 °C) | 0.22 | 5.1 | 154 (0.5 C; 50 °C) | 100 (97.9%) | 5.51 | [163] |
| SiO2@BN | PEO/LiTFSI | 4.53 × 10−4 (60 °C) | 0.54 | 4.71 | 150 (1.0 C; 60 °C) | 900 (87%) | 1.33 | [164] |
| Si Nanotubes | PEO/LiTFSI | 4.35 × 10−4 (30 °C) | 0.65 | 5.0 | 151 (0.1 C; 60 °C) | 100 (83.4%) | - | [25] |
| Al2O3 | PVDF/LiPF6 | 8.5 × 10−4 (25 °C) | 0.92 | 5.2 | 116 (2.0C; 25 °C) | 2000 (88%) | 27.1 | [66] |
| ZrO2 | PPO LiTFSI | 9.62 × 10−4 (25 °C) | 0.8 | 5.0 | 145 (0.5 C; 25 °C) | 140 (63%) | 47.5 | [141] |
| ZrO2 | P(S-MMA)/PVDF/LiClO4 | 1.2 × 10−2 (30 °C) | - | 4.6 | 144 (0.1 C; 25 °C) | 50 (74.3%) | - | [165] |
| CeO2 | PEO/LiTFSI | 1.1 × 10−3 (60 °C) | 0.47 | 5.1 | 164 (0.1 C; 60 °C) | 100 (98%) | 0.6 | [82] |
| BN | PEGDA/CA/LiPF6 | 8.8 × 10−3 (30 °C) | - | 5.5 | 113.2 (0.1 C; 25 °C) | 200 (77%) | - | [142] |
| MoS2 | PVDF/LiTFSI | 2.8 × 10−4 (25 °C) | 4.57 | 137 (0.54 C; 25 °C) | 200 (98%) | - | [143] | |
| SSZ-13 | PEO/LiTFSI | 6.16 × 10−4 (30 °C) 5.34 × 10−2 (70 °C) | 0.85 | 4.3 | 154 (0.1 C; 60 °C) | 80 (94%) | 5.3 | [149] |
| Si3N4 | PVDF/LiPF6 | 8.84 × 10−4 (25 °C) | - | 4.25 | 146.3 (0.5 C; 25 °C) | 100 (97.6%) | 3.13 | [144] |
| Nb2O5 | PVDF/LiTFSI | 6.6 × 10−5 (25 °C) | - | 5.1 | 151 (0.5 C; 30 °C) | 230 (~97%) | ~9.2 | [145] |
| g-C3N4 | PEO/LiTFSI | 3.18 × 10−5 (25 °C) 2.5 × 10−4 (60 °C) | 0.69 | 5.2 | 168 (0.3 C; 60 °C) | 400 (67%) | 3.97 | [146] |
| NS-CD | PEO/LiClO4 | 2.10 × 10−4 (25 °C) | 0.51 | 5.0 | 145.2 (0.5 C; 45 °C) | 200 (~95%) | 2.51 | [148] |
| QD | PEO/PLSS | 2.02 × 10−4 (25 °C) | 0.94 | 4.4 | 155.9 (0.2 C; 60 °C) 136.7 (2 C; 60 °C) | 100 (94.3%) 1000 (83.4%) | 5.1 | [147] |
| LLZO | PVDF/LiClO4 | 1.2 × 10−4 (25 °C) | 0.42 | 4.2 | 160.92 (0.1 C; 25 °C) | 100 (92.5%) | - | [166] |
| LLZO | PVDF/PAN/LiTFSI | 4.5 × 10−4 (25 °C) | 0.84 | 5.0 | 103.8 (1.0 C; 25 °C) | 300 (89.8%) | - | [152] |
| LLZO | PVDF/LiTFSI | 1.74 × 10−4 (25 °C) | 0.34 | 4.5 | 151 (0.5 C; 25 °C) | 100 (71.5%) | 95 | [167] |
| LLZTO | PAN/PEO/LiTFSI | 2.6 × 10−4 (30 °C) | - | 4.5 | 170.1 (0.1 C; 30 °C) | 100 (72.8%) | - | [168] |
| LLZTO | PEGDA/LiTFSI | 3.1 × 10−4 (25 °C) | 0.43 | 4.7 | 175 (0.2 C; 25 °C) | 200 (85.4%) | - | [169] |
| LLZTO | PEO/LiTFSI | 4.61 × 10−4 (60 °C) | - | 5.1 | 130.3 (0.5 C; 60 °C) | 200 (98.8%) | - | [170] |
| LLZTO | PPC/LiTFSI | 2.7 × 10−4 (30 °C) | 0.54 | 4.4 | 163 (0.2 C; 25 °C) | 140 (90.79%) | 11.78 | [171] |
| LLZTO | PEO/LiTFSI | 1.43 × 10−3 (25 °C) | - | 4.8 | 152 (1.0 C; 25 °C) | 1000 (90%) | 0.55 | [142] |
| LLZTO | PEO/LiTFSI | 1.76 × 10−4 (30 °C) | 0.53 | 5.2 | 155.3 (0.2 C; 60 °C) 136.1 (0.5 C; 60 °C) 120.7 (1.0 C; 60 °C) | 300 (93.2%) 400 (90.6%) 1000 (86.0%) | 9.47 | [153] |
| LLTO | PAN/LiClO4 | 3.6 × 10−4 (25 °C) | 0.38 | 5.0 | 142.5 (0.5 C; 25 °C) | 100 (90%) | 5.61 | [150] |
| LLGO | PEO/LiTFSI | 4.4 × 10−4 (60° C) | 0.69 | 4.8 | 120 (0.2 C; 60 °C) | 400 (80%) | 1.3 | [155] |
| LATP | PVDF/LiTFSI | 1.23 × 10−3 (25 °C) | 0.85 | 4.8 | 131.8 (0.5 C; 25 °C) | 150 (91.2%) | 5.68 | [151] |
| LATP-GF | PEO/LiTFSI | 6.3 × 10−5 (25 °C) | 0.37 | 4.4 | 148 (0.5 C; 60 °C) | 270 (68%) | 33.1 | [143] |
| LATP:LLTO | PVDF/LiClO4 | 2.08 × 10−3 (25 °C) | 0.88 | 5.3 | 159.7 (0.1 C; 30 °C) | 1000 (85%) | 4.5 | [172] |
| Co-MOF | PEO/LiTFSI | 1.91 × 10−4 (30 °C) | 0.5 | 5.2 | 140 (0.5 C; 60 °C) | 150 (95.9%) | 3.72 | [157] |
| Ce-MOF | PEO/LiTFSI | 2.76 × 10−4 (60 °C) | 0.47 | 4.8 | 161.3 (0.5 C; 60 °C) | 2000 (63%) | 1.0 | [173] |
| Ce-MOF | PEO/LiTFSI | 3.0 × 10−5 (30 °C) | 0.75 | 4.5 | 179 (0.5 C; 60 °C) | 3800 (67%) | 0.6 | [158] |
| Cu-MOF | PEO/LiPF6 | 4.99 × 10−3 (30 °C) | 0.61 | 5.25 | 144.6 (0.2 C; 25 °C) 138.3 (1.0 C; 25 °C) | 200 (92.3%) 700 (98.6%) | - | [159] |
| Electrolyte Type | Cathode Material and Mass Loading | σ (S·cm−1) (°C) | tLi+ | EWS (V) | Discharge Capacity (mAh·g−1) (Conditions) | Coulombic Efficiency | Cycle Stability (Capacity Retention) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Multilayer with PAN@LAGP-LiTFSI and PEGDA-LiTFSI | NMC811/NMC622/Super P/PVDF/PAN 3–5 mg·cm−2 | 3.7 × 10−6 (25 °C) | - | 5 | Li/NMC811 175 (25 °C, 0.5 C); Li/NMC622 180 (25 °C, 0.1 C); | -Li/NMC622 (25 °C, 0.5 C): 99.8% | Li/NMC811 81.5% Li/NMC622 97.7% | [185] |
| PVDF-5 wt% palygorskite ((Mg, Al)2Si4O10(OH)) nanowires-LiClO4 | NMC111/Super C65/PVDF/LiClO4 1.5 mg·cm−2 | 1.2 × 10−4 (25 °C) | 0.54 | 4.7 | 117.6 (25 °C, 0.3 C, 3–4.2 V) | Close to 100% | [186] | |
| PVDF-HFP-20% Li6.4Ga0.2La3Zr2O,- TEP and FEC (7:3, v/v)-LiFSI | NMC523/PVDF/Super-P 5 mg·cm−2 | 1.84 × 10−3 (20 °C) | 0.563 | 4.75 | 96.3 (25 °C, 0.5 C, 2.8–4.3 V) | 98% | 200 (94.08%) | [187] |
| PVDF-HFP-12.5 wt% LLZTO-LiTFSI | LFP/PVDF-HFP/acetylene black 1.5 mg·cm−2 | 1.2 × 10−4 (30 °C) | 0.33 | 5.6 | 60 °C, 0.5 C, 2.8–3.8 V | 99% | 50 (92.1%) | [188] |
| EGPEA-Nano fumed SiO2-LiTFSI | LFP/PVDF/KB 2.5 mg·cm−2 | 2.16 × 10−5 (25 °C) | 0.63 (55 °C) | 4.8 | 55 °C, 0.1 C, 2.5–4.0 V | - | 100 (95%) | [189] |
| PEO -12.5 vol% UiO-66-LiTFSI | LFMP/LFP/LiTFSI/PEO/Super-P 2 mg·cm−2 | 3.1 × 10−5 (25 °C) | 0.72 | 4.97 | Li/LFP (60 °C, 1 C, 2.8–3.8 V): Li/LFMP (60 °C, 1 C, 2.8–4.4 V) | - | Li/LFP300 (85.4%) Li/LFMP 100 (81.2%) | [190] |
| PEO-20% P(SSPSILi-alt-MA) | LFP/P(SSPSILi-alt-MA)/PEO/ carbon black | 3.08 × 10−4 (25 °C) | 0.97 (at 80°C) | 5.0 | 80 °C, 0.1 C, 2.5–4 V | ~100% | 100 (97.5%) | [126] |
| PEO-g-C3N4-LiTFSI | LFP/PVDF/carbon black 1.2 mg·cm−2 | 1.7 × 10−5 (30 °C) | 0.56 | 4.7 | 60 °C, 0.2 C, 2.8–4.0 V | 99.5% | 100 (96.2%) | [191] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maia, B.A.; Magalhães, N.; Cunha, E.; Braga, M.H.; Santos, R.M.; Correia, N. Designing Versatile Polymers for Lithium-Ion Battery Applications: A Review. Polymers 2022, 14, 403. https://doi.org/10.3390/polym14030403
Maia BA, Magalhães N, Cunha E, Braga MH, Santos RM, Correia N. Designing Versatile Polymers for Lithium-Ion Battery Applications: A Review. Polymers. 2022; 14(3):403. https://doi.org/10.3390/polym14030403
Chicago/Turabian StyleMaia, Beatriz Arouca, Natália Magalhães, Eunice Cunha, Maria Helena Braga, Raquel M. Santos, and Nuno Correia. 2022. "Designing Versatile Polymers for Lithium-Ion Battery Applications: A Review" Polymers 14, no. 3: 403. https://doi.org/10.3390/polym14030403







