Interpolymer Complexes of Poly(methacryloyloxyethyl phosphorylcholine) and Polyacids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
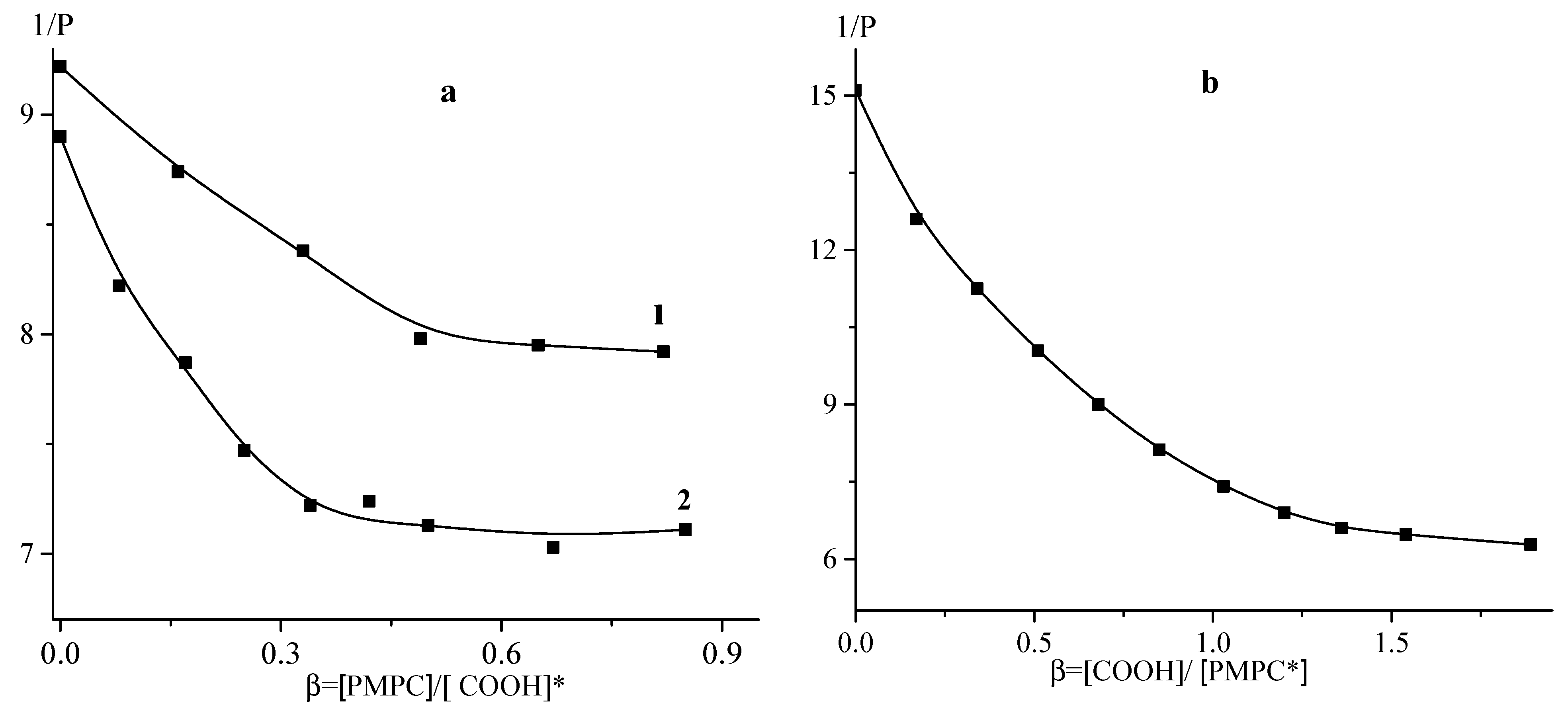
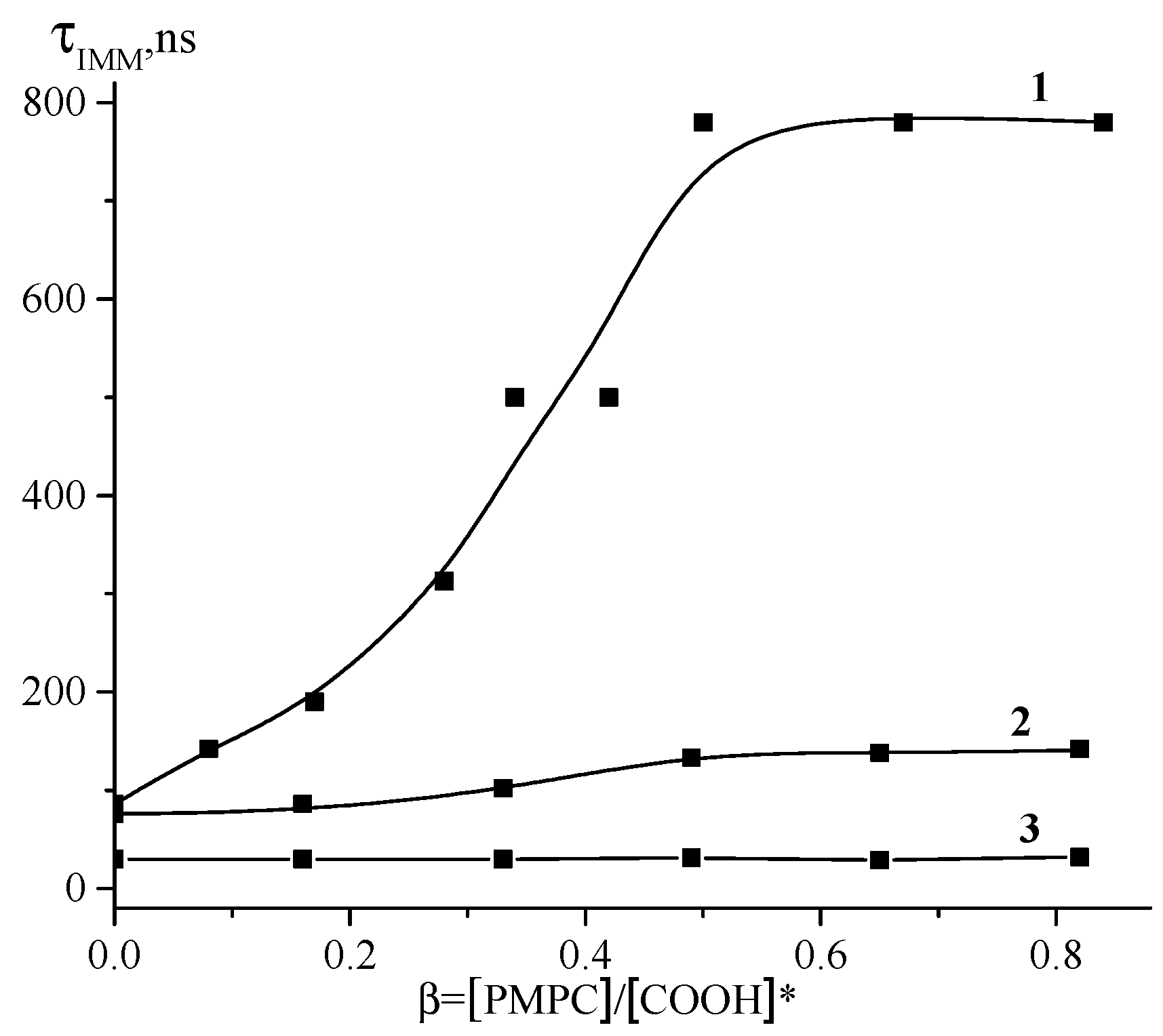
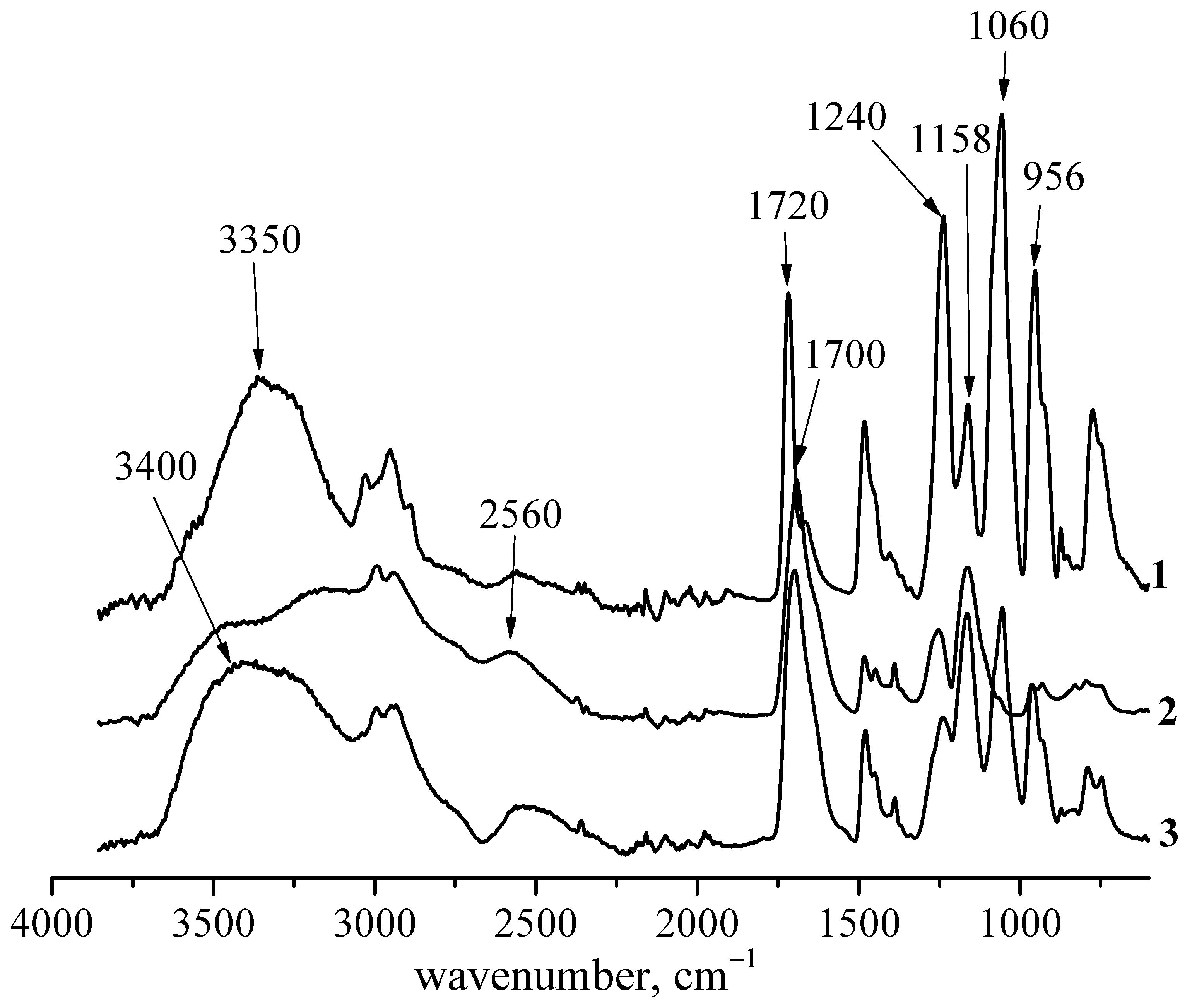
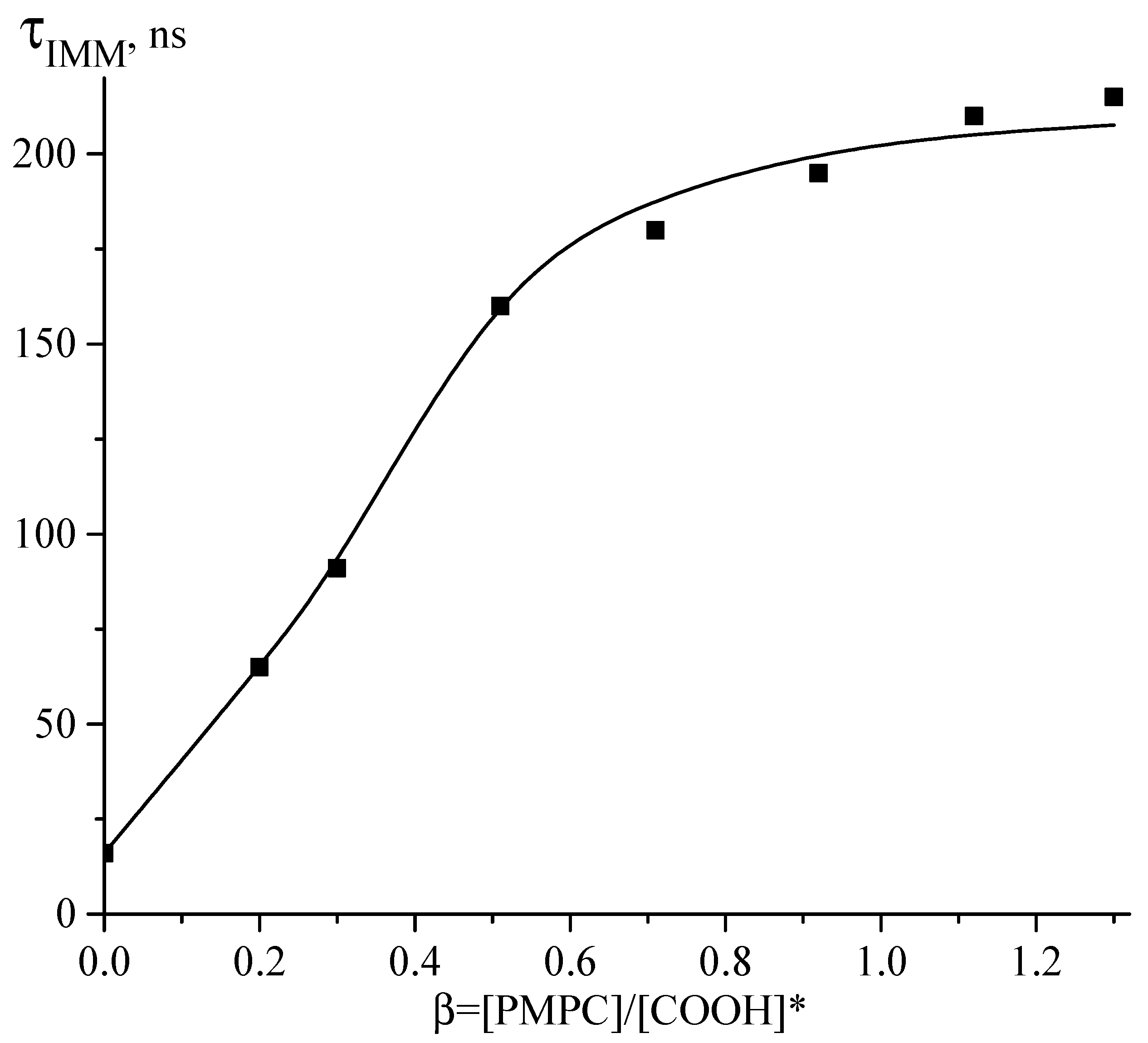
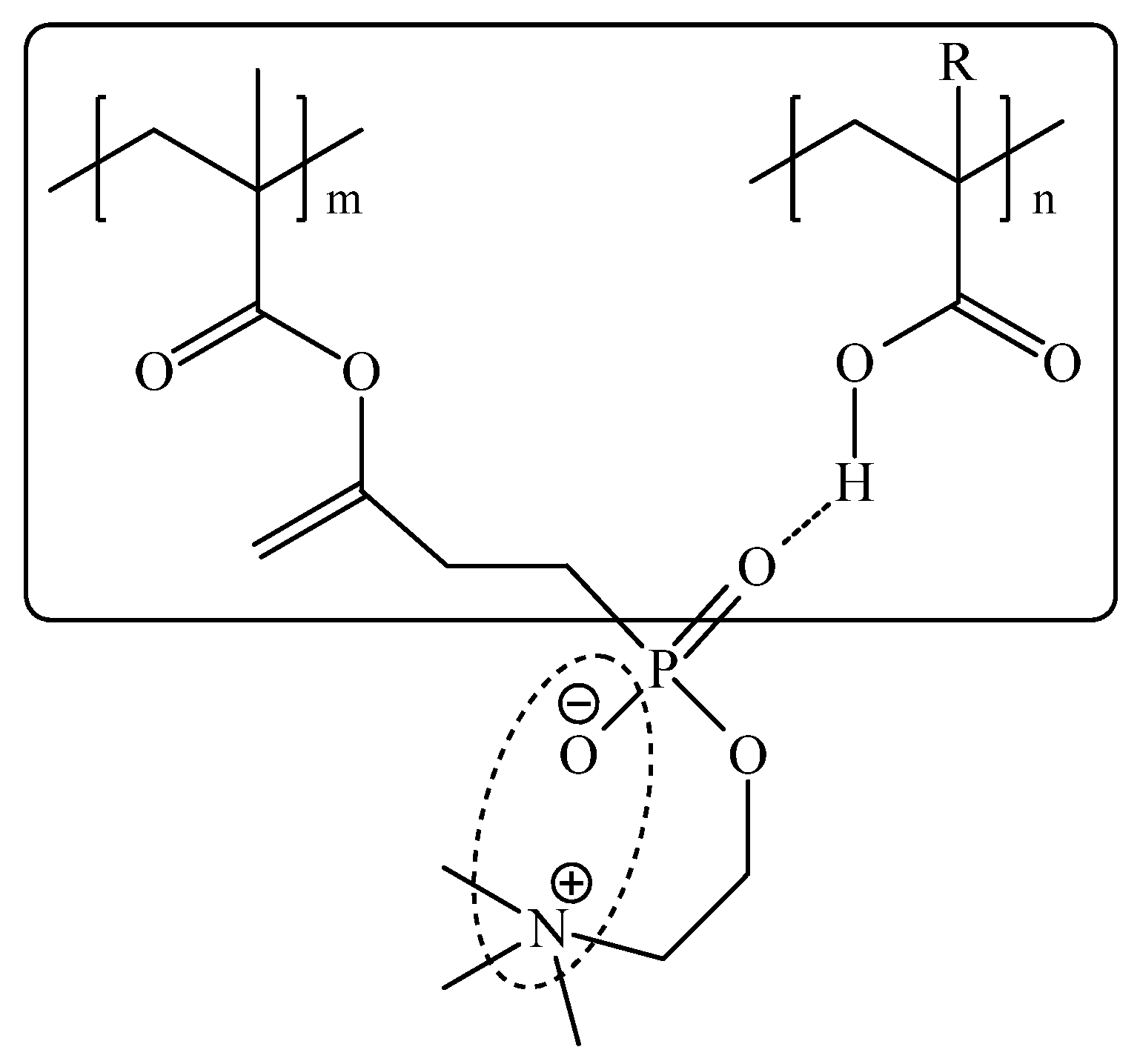
3.1. Interaction between PMPC and Non-Ionized Poly(carboxylic acids)
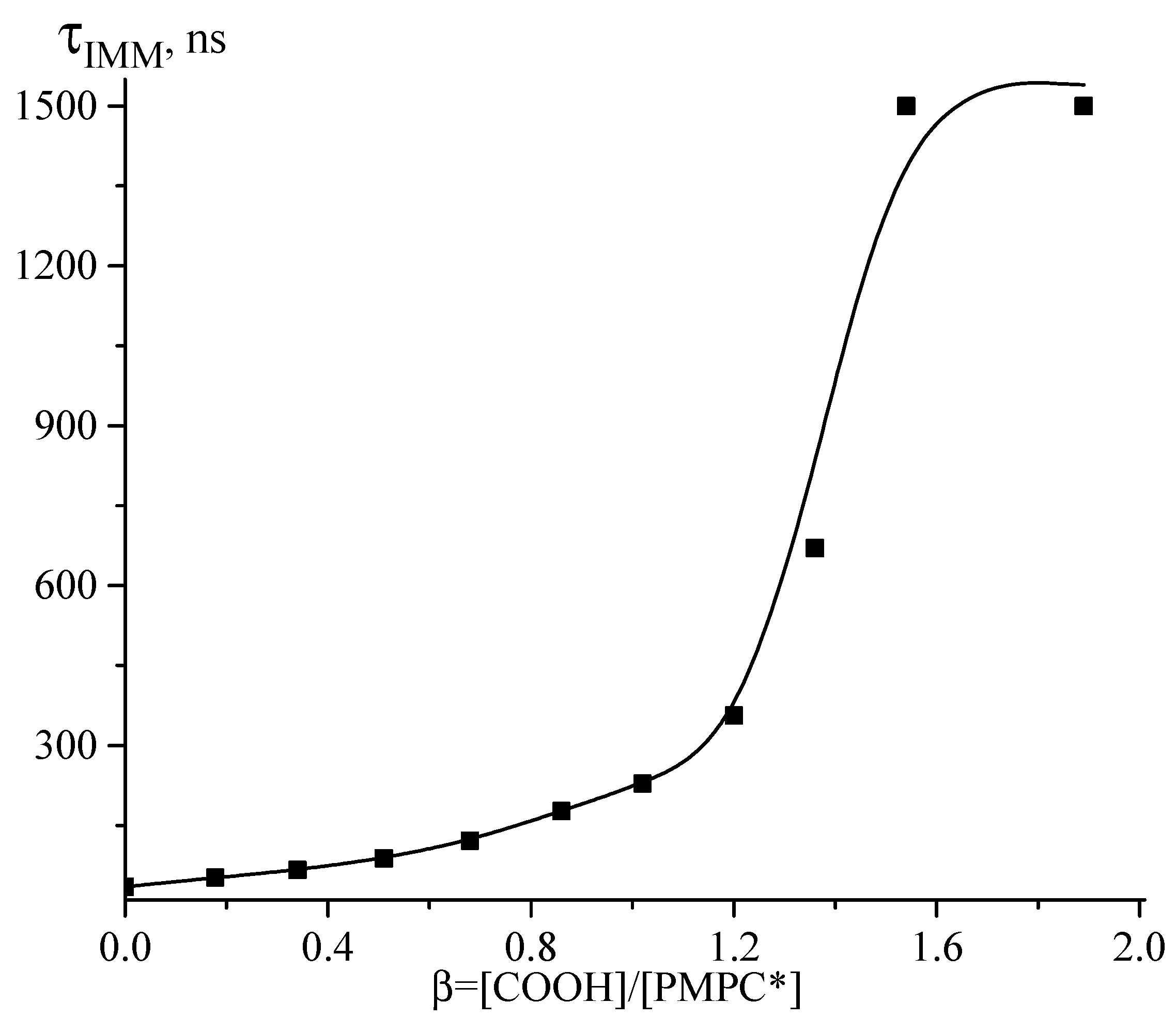
3.2. Interaction between PMPC and Ionized Poly(carboxylic acids)
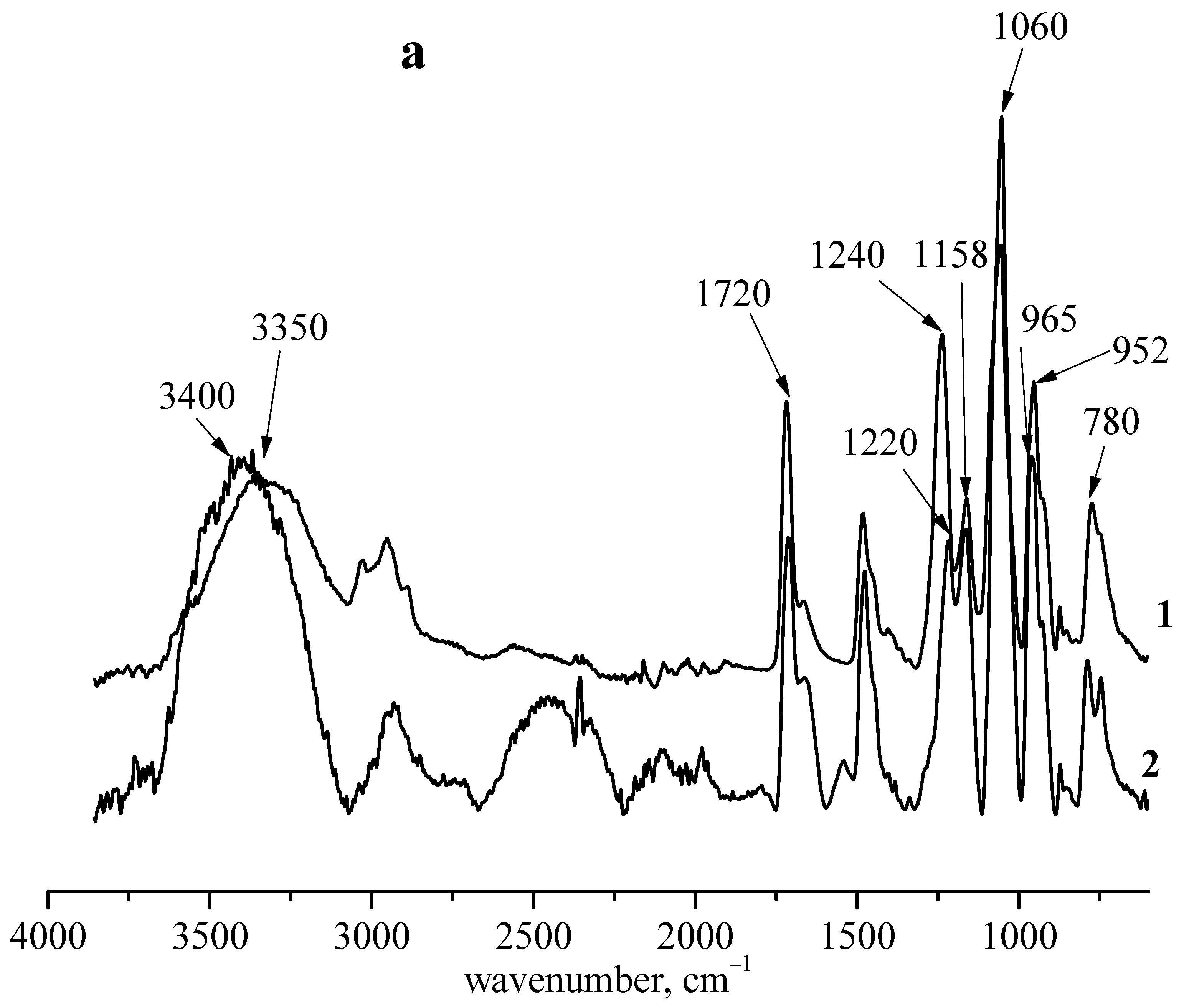
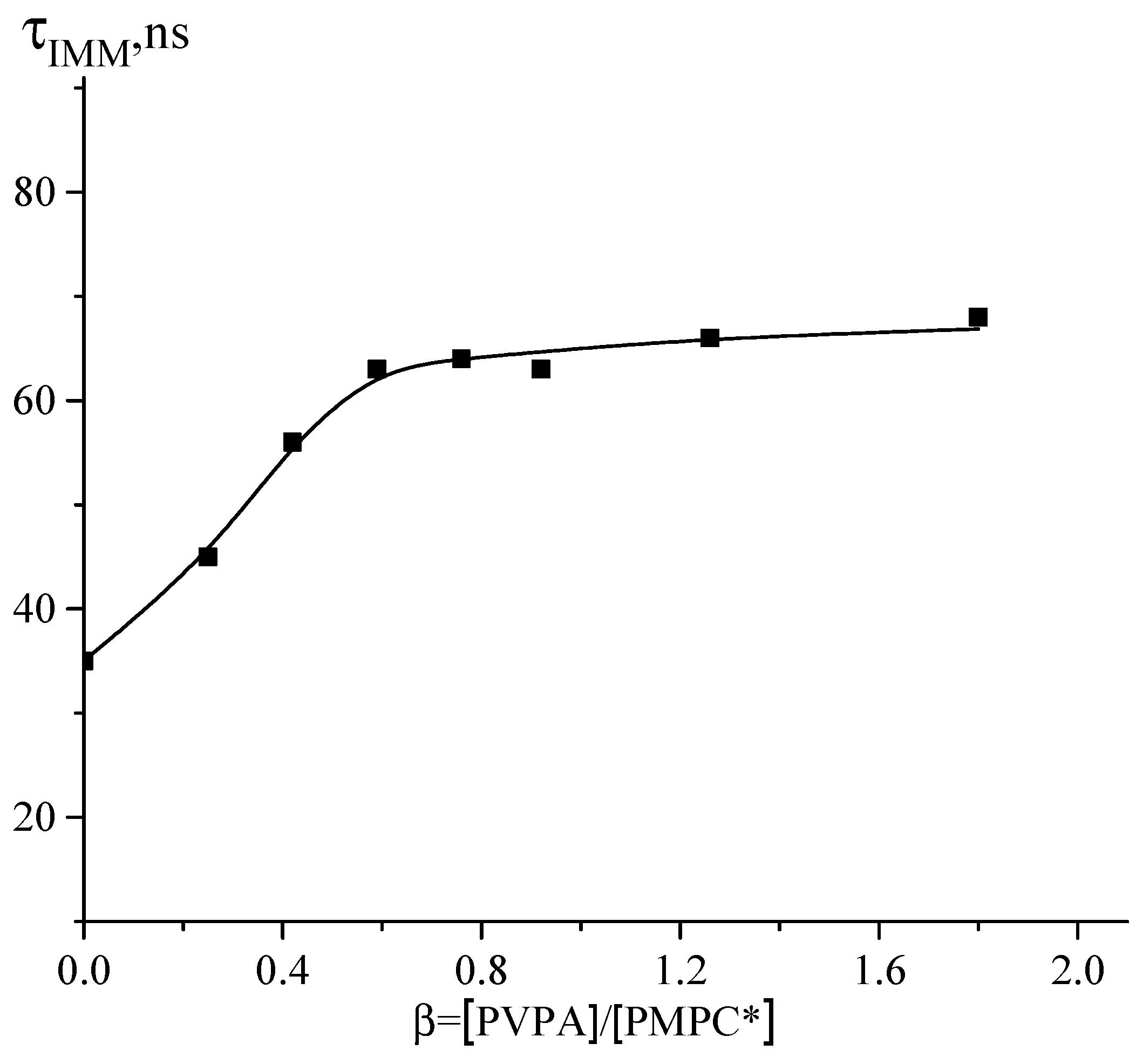
3.3. Interaction between PMPC and Poly(vinylphosphonic acid)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Khutoryanskiy, V.V.; Staikos, G. Hydrogen-Bonded Interpolymer Complexes: Formation, Structure and Applications; World Scientific: Hackensack, NJ, USA; Singapore, 2009; ISBN 978-981-270-785-7. [Google Scholar]
- Pergushov, D.V.; Zezin, A.A.; Zezin, A.B.; Müller, A.H.E. Advanced Functional Structures Based on Interpolyelectrolyte Complexes. In Polyelectrolyte Complexes in the Dispersed and Solid State I; Müller, M., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2013; Volume 255, pp. 173–225. ISBN 978-3-642-40733-8. [Google Scholar]
- De Leo, V.; Milano, F.; Agostiano, A.; Catucci, L. Recent Advancements in Polymer/Liposome Assembly for Drug Delivery: From Surface Modifications to Hybrid Vesicles. Polymers 2021, 13, 1027. [Google Scholar] [CrossRef]
- Erfani, A.; Seaberg, J.; Aichele, C.P.; Ramsey, J.D. Interactions between Biomolecules and Zwitterionic Moieties: A Review. Biomacromolecules 2020, 21, 2557–2573. [Google Scholar] [CrossRef]
- Fernández-Peña, L.; Guzmán, E.; Ortega, F.; Bureau, L.; Leonforte, F.; Velasco, D.; Rubio, R.G.; Luengo, G.S. Physico-Chemical Study of Polymer Mixtures Formed by a Polycation and a Zwitterionic Copolymer in Aqueous Solution and upon Adsorption onto Negatively Charged Surfaces. Polymer 2021, 217, 123442. [Google Scholar] [CrossRef]
- Li, M.; Zhuang, B.; Yu, J. Functional Zwitterionic Polymers on Surface: Structures and Applications. Chem. Asian J. 2020, 15, 2060–2075. [Google Scholar] [CrossRef]
- Schwarz, S.; Jaeger, W.; Bratskaya, S.; Bohrisch, J.; Schimmel, T.; Mende, M.; Oelmann, M.; Boyko, V. Formation of Polyelectrolyte Complexes in a Polycarboxybetaine/Weak Polyanion System. Colloids Surf. A Physicochem. Eng. Asp. 2006, 276, 65–71. [Google Scholar] [CrossRef]
- Mary, P.; Bendejacq, D.D. Interactions between Sulfobetaine-Based Polyzwitterions and Polyelectrolytes. J. Phys. Chem. B 2008, 112, 2299–2310. [Google Scholar] [CrossRef]
- Chen, L.; Honma, Y.; Mizutani, T.; Liaw, D.-J.; Gong, J.P.; Osada, Y. Effects of Polyelectrolyte Complexation on the UCST of Zwitterionic Polymer. Polymer 2000, 41, 141–147. [Google Scholar] [CrossRef]
- Izumrudov, V.A.; Zelikin, A.N.; Zhiryakova, M.V.; Jaeger, W.; Bohrisch, J. Interpolyelectrolyte Reactions in Solutions of Polycarboxybetaines. J. Phys. Chem. B 2003, 107, 7982–7986. [Google Scholar] [CrossRef]
- Lowe, A.B.; McCormick, C.L. Synthesis and Solution Properties of Zwitterionic Polymers. Chem. Rev. 2002, 102, 4177–4190. [Google Scholar] [CrossRef]
- Laschewsky, A. Structures and Synthesis of Zwitterionic Polymers. Polymers 2014, 6, 1544–1601. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Madsen, J.; Warren, N.J.; Mears, M.; Leggett, G.J.; Lewis, A.L.; Geoghegan, M. Influence of Salt on the Solution Dynamics of a Phosphorylcholine-Based Polyzwitterion. Eur. Polym. J. 2017, 87, 449–457. [Google Scholar] [CrossRef]
- Hildebrand, V.; Heydenreich, M.; Laschewsky, A.; Möller, H.M.; Müller-Buschbaum, P.; Papadakis, C.M.; Schanzenbach, D.; Wischerhoff, E. “Schizophrenic” Self-Assembly of Dual Thermoresponsive Block Copolymers Bearing a Zwitterionic and a Non-Ionic Hydrophilic Block. Polymer 2017, 122, 347–357. [Google Scholar] [CrossRef]
- Clark, A.; Taylor, M.E.; Panzer, M.J.; Cebe, P. Interactions between Ionic Liquid and Fully Zwitterionic Copolymers Probed Using Thermal Analysis. Thermochim. Acta 2020, 691, 178710. [Google Scholar] [CrossRef]
- Anufrieva, E.V.; Gotlib, Y.Y. Investigation of Polymers in Solution by Polarized Luminescence. In Luminescence; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1981; Volume 40, pp. 1–68. ISBN 978-3-540-10550-3. [Google Scholar]
- Procházka, K. Historical Perspective of Advances in Fluorescence Research on Polymer Systems. In Fluorescence Studies of Polymer Containing Systems; Procházka, K., Ed.; Springer Series on Fluorescence; Springer International Publishing: Cham, Switzerland, 2016; Volume 16, pp. 151–202. ISBN 978-3-319-26786-9. [Google Scholar]
- Pautov, V.D.; Nekrasova, T.N.; Anan’eva, T.D.; Smyslov, R.Y. Polarized Luminescence Studies of Nanosecond Dynamics of Thermosensitive Polymers in Aqueous Solutions. In Temperature-Responsive Polymers; Khutoryanskiy, V.V., Georgiou, T.K., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2018; pp. 249–277. ISBN 978-1-119-15783-0. [Google Scholar]
- Nekrasova, T.N.; Pautov, V.D.; Anan’eva, T.D.; Meleshko, T.K.; Ivanov, I.V.; Yakimansky, A.V. Structural Organization of Interpolymer Complexes Formed by Poly(N-Vinylamides) and Poly(Methacrylic Acid) Chains Regularly Grafted to Polyimide in Aqueous Solutions. Polym. Sci. Ser. C 2018, 60, 172–178. [Google Scholar] [CrossRef]
- Nekrasova, T.N.; Kirila, T.Y.; Kurlykin, M.P.; Ten’kovtsev, A.V.; Filippov, A.P. Interpolymer Complexes of Star-Shaped Copolymers of Polyoxazoline with the Calixarene Core and Linear Polyacids in Solution. Polym. Sci. Ser. B 2021, 63, 116–125. [Google Scholar] [CrossRef]
- Guillet, J. Polymer Photophysics and Photochemistry: An Introduction to the Study of Photoprocesses in Macromolecules; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1985; ISBN 978-0-521-23506-8. [Google Scholar]
- Nazarova, O.; Chernova, E.; Dobrodumov, A.; Zolotova, Y.; Bezrukova, M.; Nekrasova, T.; Vlasova, E.; Panarin, E. New water-soluble copolymers of 2-methacryloyloxyethyl phosphorylcholine for surface modification. J. Appl. Polym. Sci. 2021, 138, 50272. [Google Scholar] [CrossRef]
- Nazarova, O.V.; Levit, M.L.; Nekrasova, T.N.; Bel’nikevich, N.G.; Dobrodumov, A.V.; Panarin, E.F. Copolymers of 2-Deoxy-2-Methacrylamido-d-Glucose and Unsaturated Acids. Polym. Sci. Ser. B 2009, 51, 321–326. [Google Scholar] [CrossRef]
- Levit, M.L.; Nazarova, O.V.; Nekrasova, T.N.; Dobrodumov, A.V.; Anan’eva, T.D.; Nikiticheva, A.A.; Vlasova, E.N.; Pautov, V.D.; Panarin, E.F. Water-Soluble Polymer Derivatives of Cholesterol. Polym. Sci. Ser. B 2010, 52, 648–655. [Google Scholar] [CrossRef]
- TSvetkov, V.N. Rigid-Chain Polymers: Hydrodynamic and Optical Properties in Solution; Macromolecular Compounds; Consultants Bureau: New York, NY, USA, 1989; ISBN 978-0-306-11020-7. [Google Scholar]
- Anufrieva, E.; Pautov, V.; Stepanov, V.; Skorokhodov, S. Intermolecular complexes of poly(1,2-dimethoxyethylene) and poly(p-dioxene) with poly(methacrylic acid). Makromol. Chem. 1979, 180, 1843–1844. [Google Scholar] [CrossRef]
- Ishihara, K.; Mu, M.; Konno, T.; Inoue, Y.; Fukazawa, K. The Unique Hydration State of Poly(2-Methacryloyloxyethyl Phosphorylcholine). J. Biomater. Sci. Polym. Ed. 2017, 28, 884–899. [Google Scholar] [CrossRef]
- Bellamy, L.J. Infra-Red Spectra of Complex Molecules.; Springer: Dordrecht, The Netherlands, 2013; ISBN 978-94-011-6017-9. [Google Scholar]
- Anufrieva, E.V.; Birshtein, T.M.; Nekrasova, T.N.; Ptitsyn, O.B.; Sheveleva, T.V. The Models of the Denaturation of Globular Proteins. II. Hydrophobic Interactions and Conformational Transition in Polymethacrylic Acid: Denaturation of Globular Proteins. II. J. Polym. Sci. C Polym. Symp. 1967, 16, 3519–3531. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S. Influence of Charged Groups on the Properties of Zwitterionic Moieties: A Molecular Simulation Study. J. Phys. Chem. B 2014, 118, 7630–7637. [Google Scholar] [CrossRef] [PubMed]
- Bingöl, B.; Meyer, W.H.; Wagner, M.; Wegner, G. Synthesis, Microstructure, and Acidity of Poly(Vinylphosphonic Acid). Macromol. Rapid Commun. 2006, 27, 1719–1724. [Google Scholar] [CrossRef]
- Taherkhani, Z.; Abdollahi, M.; Sharif, A. Synthesis and Microstructural Characterization of Low to High Molecular Weight Poly(Vinylphosphonic Acid)s: Effect of Molecular Weight and Temperature on Acidity and Polyelectrolyte Behavior. J. Polym. Res. 2017, 24, 132. [Google Scholar] [CrossRef]
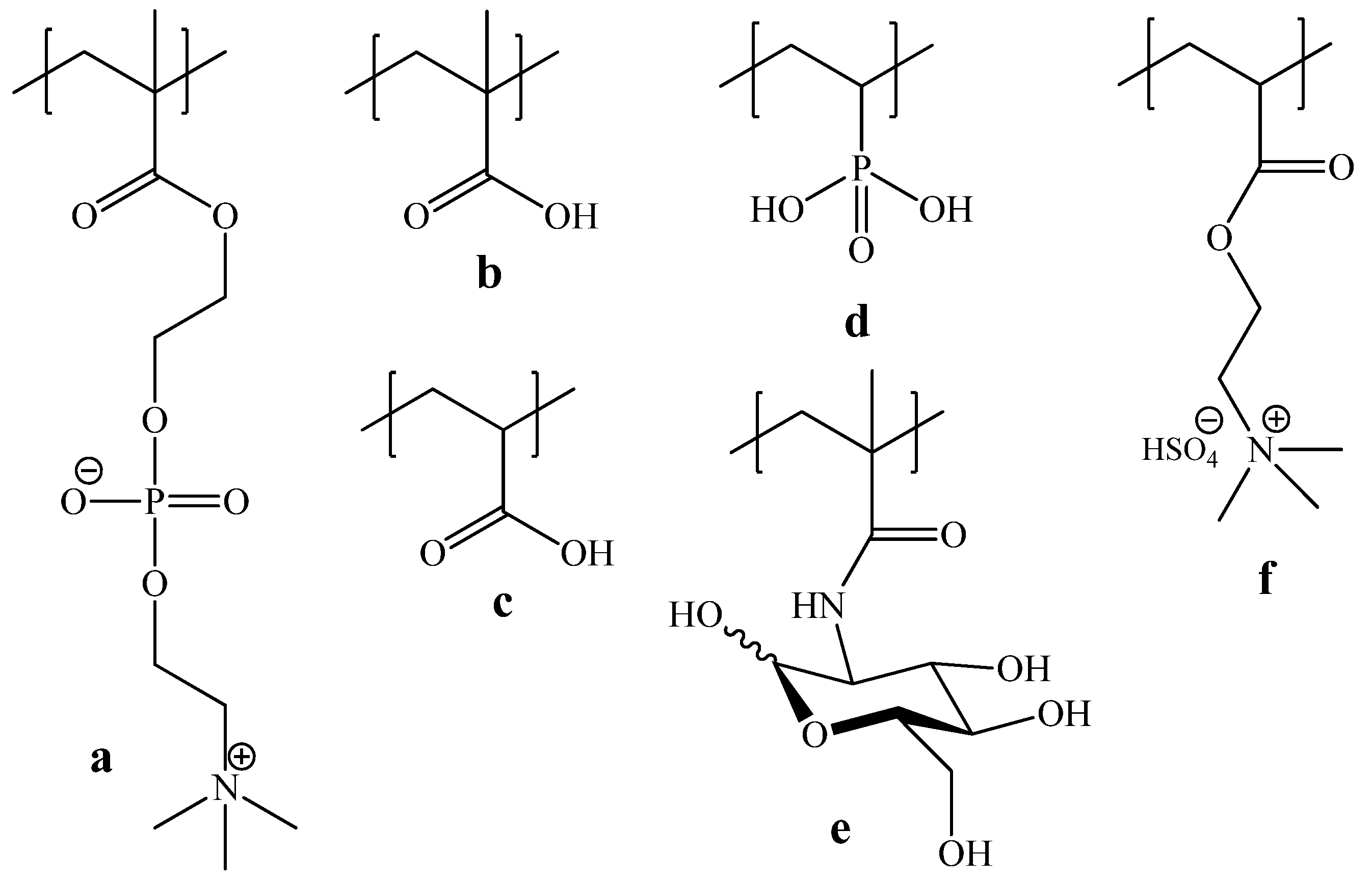

| (co)polymer | MM × 10−3 | [(co)polymer unit]:[LM] |
|---|---|---|
| PMPC | 463 | |
| PMPC * | 280 | 450 |
| PMAA | 306 | |
| PMAA * | 267 | 670 |
| Copolymer (MAG-MAA70) * | 67 | 420 |
| Copolymer (MAG-MAA26) * | 97 | 600 |
| PAA | 130 | |
| PAA * | 130 | 300 |
| PVPA | 31 | |
| Copolymer (MAG-TMAEM47) * | 65 | 380 |
| Functional Group | ν, cm−1 Polymer | ν, cm−1 Complex |
|---|---|---|
| OH | 3400, 3500 | No change |
| Bonded OH | 2560 | Intensity decrease |
| COOH | 1700 | No change |
| COO– (ester) | 1720 | Broaden |
| P=O | 1240 | 1220 |
| P–O–C2H5 | 1170–1150 | No change |
| P–O–C | 1060–990 | 1040 |
| P–O–C | 965 | 952 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekrasova, T.; Nazarova, O.; Vlasova, E.; Fischer, A.; Zolotova, Y.; Bezrukova, M.; Panarin, E. Interpolymer Complexes of Poly(methacryloyloxyethyl phosphorylcholine) and Polyacids. Polymers 2022, 14, 407. https://doi.org/10.3390/polym14030407
Nekrasova T, Nazarova O, Vlasova E, Fischer A, Zolotova Y, Bezrukova M, Panarin E. Interpolymer Complexes of Poly(methacryloyloxyethyl phosphorylcholine) and Polyacids. Polymers. 2022; 14(3):407. https://doi.org/10.3390/polym14030407
Chicago/Turabian StyleNekrasova, Tatiana, Olga Nazarova, Elena Vlasova, Andrei Fischer, Yuliya Zolotova, Marina Bezrukova, and Evgeniy Panarin. 2022. "Interpolymer Complexes of Poly(methacryloyloxyethyl phosphorylcholine) and Polyacids" Polymers 14, no. 3: 407. https://doi.org/10.3390/polym14030407






