Screen Printing of pH-Responsive Dye to Textile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabric
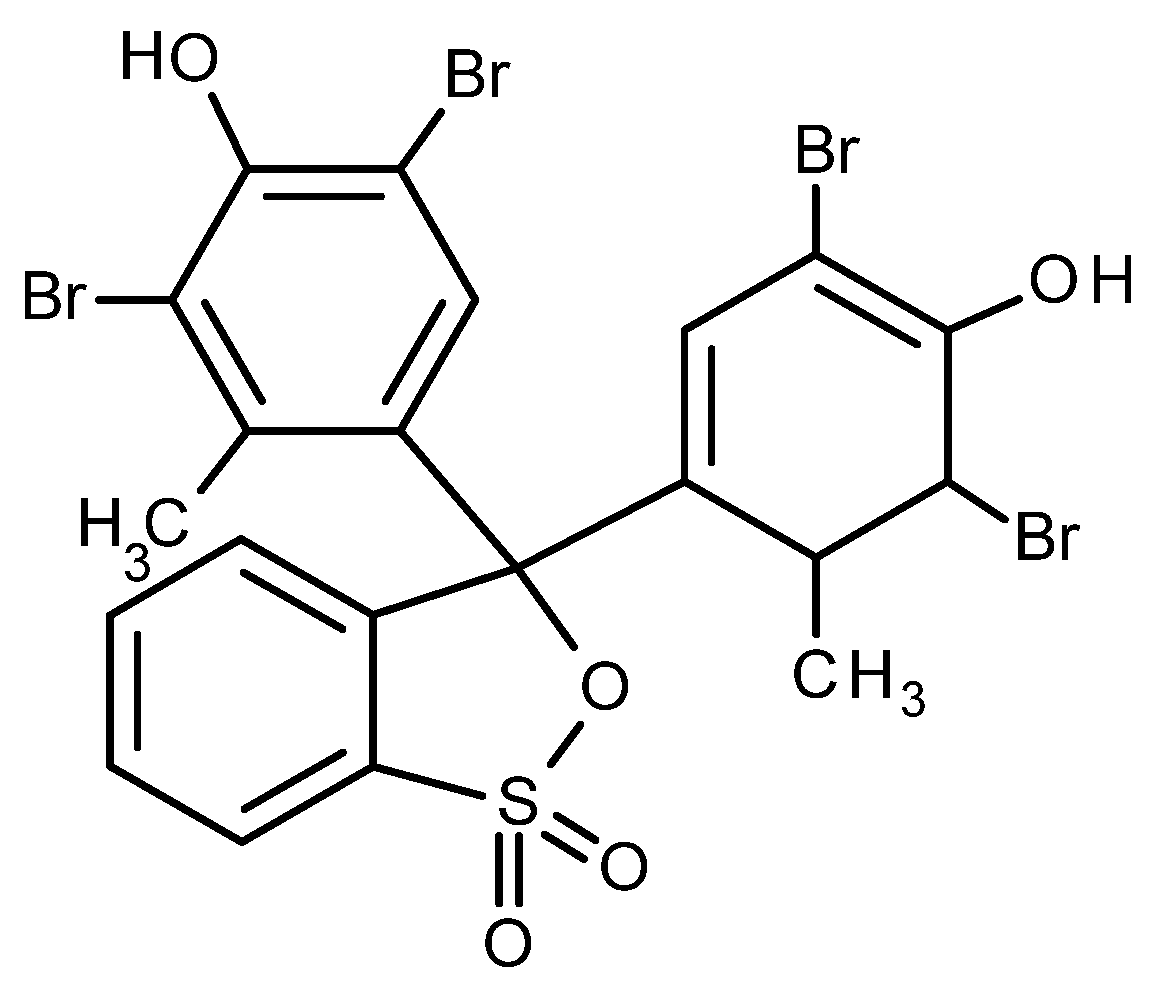
2.2. Dye
2.3. Auxiliaries
2.4. Preparation of Printing Pastes
2.5. Printing and Aftertreatments
2.6. Methods of Testing
2.6.1. Fabric Thickness
2.6.2. Mass per Unit Area
2.6.3. Stiffness
2.6.4. Breaking Force, Tensile Strength and Elongation
2.6.5. Air Permeability
2.6.6. Spectrophotometric Measurements
2.6.7. Colour Fastness to Rubbing
2.6.8. Colour Fastness to Washing
2.6.9. Colour Fastness to Light
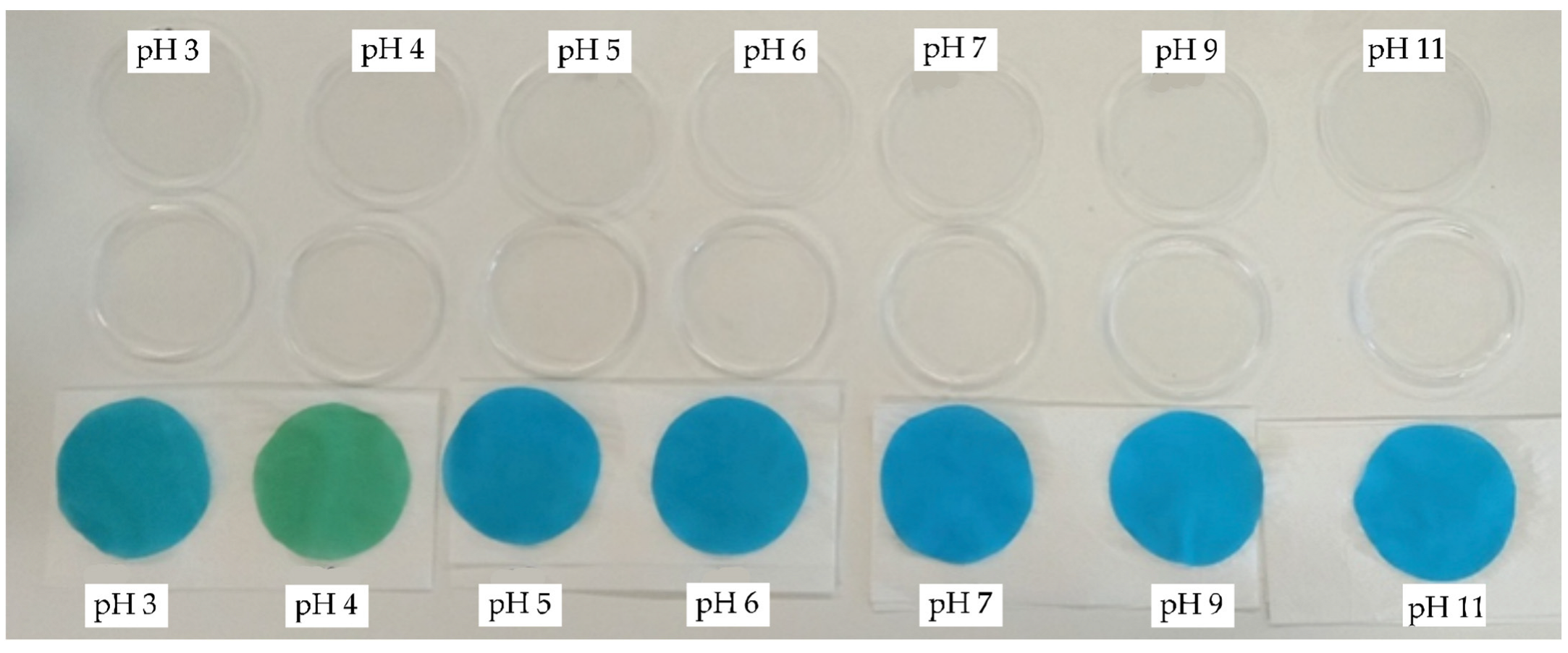
2.6.10. Determination of pH Response of Printed Samples to Colour Change
2.6.11. Determination of Response Time
3. Results and Discussion
3.1. Results of the Mechanical and Physical Properties of the Fabric
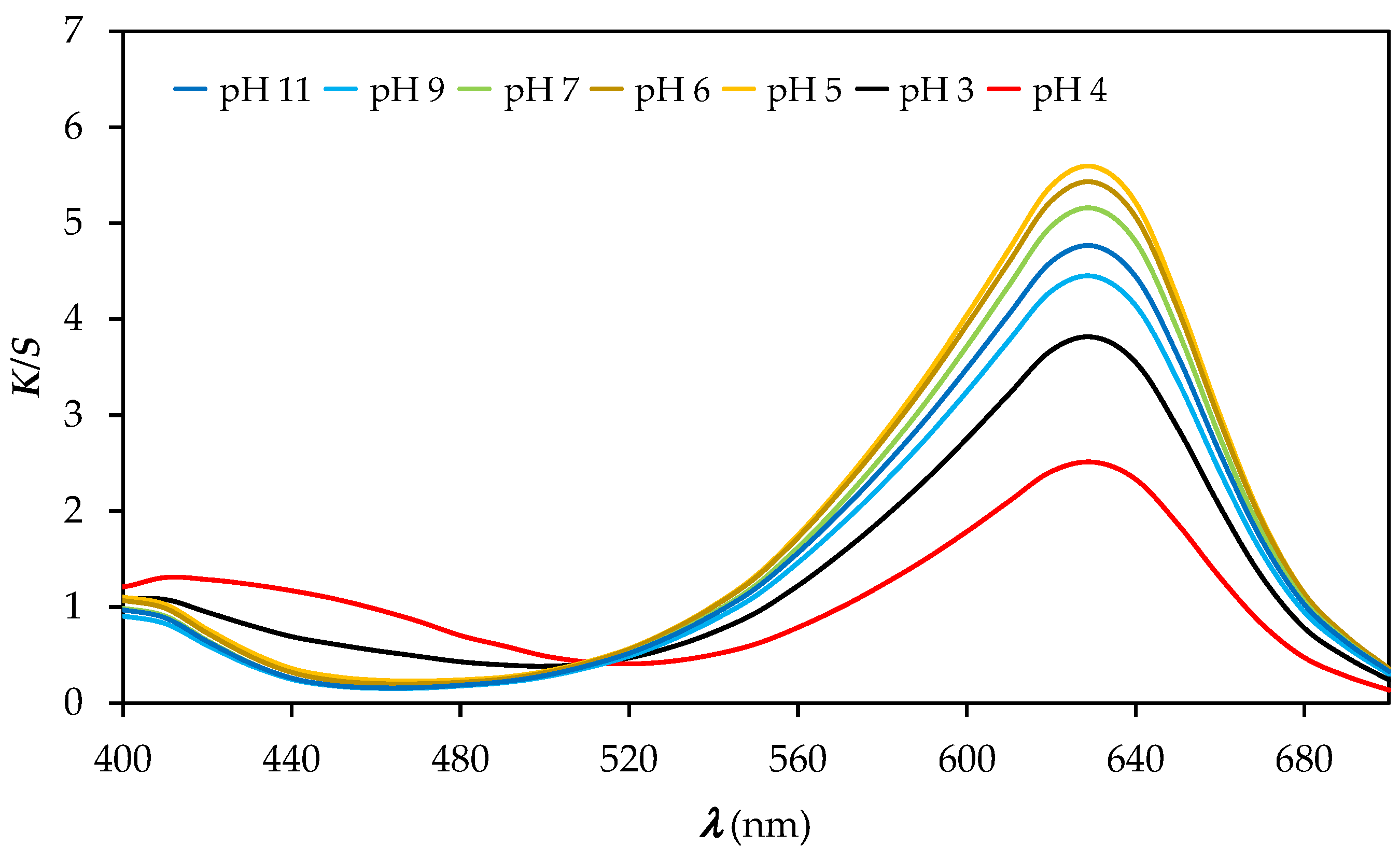
3.2. Colour and Colour Fastness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vik, M.; Periyasamy, A.P. Chromic Materials: Fundamentals. Measurements and Applications; Viková, M., Ed.; Apple Academic Press: New York, NY, USA, 2018; p. 422. [Google Scholar]
- Christie, R.M. Chromic materials for technical textile applications. In Advances in the Dyeing and Finishing of Technical Textiles; Woodhead Publishing: Oxford, UK; Cambridge, UK; Philadelphia, PA, USA; New Delhi, India, 2013; pp. 3–36. [Google Scholar]
- Bamfield, P. Chromic Phenomena: Technological Applications of Colour Chemistry; Royal Society of Chemistry: Cambridge, UK, 2001; pp. 1–8. [Google Scholar]
- Possanzini, L.; Decataldo, F.; Mariani, F.; Guilandi, I.; Tessarolo, M.; Scavetta, E.; Fraboni, B. Textile sensors platform for the selective and simultaneous detection of chloride ion and pH in sweat. Sci. Rep. 2020, 10, 17180. [Google Scholar] [CrossRef]
- Giachet, F.T.; Vineris, C.; Sanchez Ramirez, D.O.; Carletto, R.A.; Varesano, A.; Mazzuchetti, G. Reversible and washing resistant textile-based optical pH sensors by dyeing fabrics with curcuma. Fiber Polym. 2017, 18, 720–730. [Google Scholar] [CrossRef]
- De Meyer, T.; Steyaert, I.; Hemelsoet, K.; Hoogenboom, R.; Van Speybroeck, V.; De Clerck, K. Halochromic properties of sulfonphthaleine dyes in a textile environment: The influence of substituents. Dye. Pigment. 2016, 124, 249–257. [Google Scholar] [CrossRef]
- Steyaert, I.; Vancoillie, G.; Hoogenboom, R.; De Clerck, K. Dye immobilization in halochromic nanofibers through blend electrospinning of a dye-containing copolymer and polyamide-6. Polym. Chem. 2015, 6, 2685–2694. [Google Scholar] [CrossRef]
- Van der Schueren, L.; De Clerck, K.; Brancatelli, G.; Rosace, G.; Van Damme, E.; De Vos, W. Novel cellulose and polyamide halochromic textile sensors based on the encapsulation of Methyl Red into a sol-gel matrix. Sens. Actuator B Chem. 2012, 162, 27–34. [Google Scholar] [CrossRef]
- Manjakkal, L.; Derin, S.; Dahiya, R. Flexible potentiometric pH sensors for wearable systems. RSC Adv. 2020, 10, 8594–8617. [Google Scholar] [CrossRef] [Green Version]
- Van der Schueren, L.; De Clerck, K. The use of pH-indicator dyes for pH-sensitive textile materials. Text. Res. J. 2010, 80, 590–603. [Google Scholar] [CrossRef]
- Van der Schueren, L.; De Clerck, K. Coloration and application of pH-sensitive dyes on textile materials. Coloration Technol. 2012, 128, 82–90. [Google Scholar] [CrossRef]
- De Clerck, K.; Geltmeyer, J.; Steyaert, I.; Van der Schueren, L. Halochromic textile materials as innovative pH-sensors. Adv. Sci. Technol. 2012, 80, 47–52. [Google Scholar] [CrossRef]
- Schaude, C.; Fröhlich, E.; Meindl, C.; Attard, J.; Binder, B.; Mohr, G.J. The development of indicator cotton swabs for the detection of pH in wounds. Sensors 2017, 17, 1365. [Google Scholar] [CrossRef] [Green Version]
- Mohr, G.J.; Müller, H. Tailoring colour changes of optical sensor materials by combining indicator and inert dyes and their use in sensor layer, textiles and non-wovens. Sens. Actuator B Chem. 2015, 206, 788–793. [Google Scholar] [CrossRef]
- Kramar, A.D.; Ilic-Tomic, T.R.; Lađarević, J.M.; Nikodinovic-Runic, J.B.; Kostic, M.M. Hallochromic cellulose texile obtained via dyeing with biocolorant isolated from Streptomyces sp. strain NP4. Cellulose 2020, 28, 8771–8784. [Google Scholar] [CrossRef]
- Schaude, C.; Mohr, G.J. Indicator washcloth for detecting alkaline washing solutions to prevent dermatitis patients and babies from skin irritation. Fash Text. 2017, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Raheja, A.; Natarajan, T.S.; Chandra, T.S. Development of universal pH sensing electrospun nanofibers. Sens. Actuators B Chem. 2012, 161, 1097–1101. [Google Scholar] [CrossRef]
- Van der Schueren, L.; Mollet, T.; Ceylan, Ö.; De Clerck, K. The development of polyamide 6.6 nanofibres with pH-sensitive function by electrospinning. Eur. Polym. J. 2010, 46, 2229–2239. [Google Scholar] [CrossRef]
- Kassal, P.; Zubak, M.; Scheipl, G.; Mohr, G.J.; Steinberg, M.D.; Steinberg, I.M. Smart bandage with wireless connectivity for optical monitoring of pH. Sens. Actuator B Chem. 2017, 246, 455–460. [Google Scholar] [CrossRef]
- Pallás, I.; Marcos, M.D.; Martínez-Máñez, R.; Ros-Lis, J.V. Development of a textile nanocomposite as naked eye indicator of the exposition to strong acids. Sensors. 2017, 17, 2134. [Google Scholar] [CrossRef] [Green Version]
- Caldara, M.; Colleoni, C.; Guido, E.; Re, V.; Rosace, G. Optical monitoring of sweat pH by a textile fabric wearable sensor based on covalently bonded litmus-3-glycidoxypropyltrimethoxysilane coating. Sens. Actuator B Chem. 2016, 222, 213–220. [Google Scholar] [CrossRef]
- Salem, T.; Simon, F.; El-Sayed, A.A.; Salam, M. Plasma-assisted surface modification of polyester fabric for developing halochromic properties. Fiber Polym. 2017, 18, 731–740. [Google Scholar] [CrossRef]
- Shaikh, M.A. Water conservation in textile industry. Pak. Text. J. 2009, 58, 48–51. Available online: https://www.ptj.com.pk/Web-2009/11-09/PDF-November-2009/Ayaz-Shaikh.pdf (accessed on 10 January 2022).
- ISO 5084; Textiles—Determination of Thickness of Textiles and Textile Products. ISO: Geneva, Switzerland, 1996.
- EN 12127; Textiles—Fabrics—Determination of Mass per Unit Area Using Small Samples. CEN: Brussels, Belgium, 1997.
- ASTM D1388-18; Standard Test Method for Stiffness of Fabric. ASTM: West Conshohocken, PA, USA, 2018.
- ISO 13934; Textiles—Tensile Properties of Fabrics—Part 1: Determination of Maximum Force and Elongation at Maximum Force Using the Strip Method. ISO: Geneva, Switzerland, 2013.
- ISO 9237; Textiles—Determination of the Permeability of Fabrics to Air. ISO: Geneva, Switzerland, 1995.
- ISO 105-X12; Textiles—Tests for Colour Fastness—Part X12: Colour Fastness to Rubbing. ISO: Geneva, Switzerland, 2016.
- ISO 105-A03; Textiles—Tests for Colour Fastness—Part A03: Grey Scale for Assessing Staining. ISO: Geneva, Switzerland, 2019.
- ISO 105-C06; Textiles—Tests for Colour Fastness—Part C06: Colour Fastness to Domestic and Commercial Laundering. ISO: Geneva, Switzerland, 2010.
- EN 20105-A02:1994; Textiles—Tests for Colour Fastness—Part A02: Grey Scale for Assessing Change in Colour. CEN: Brussels, Belgium, 1994.
- ISO 105-B02; Textiles—Tests for Colour Fastness—Part B02: Colour Fastness to Artificial Light: Xenon Arc Fading Lamp Test. ISO: Geneva, Switzerland, 2014.
- Stojkoski, V.; Kert, M. Design of pH responsive textile as a sensor material for acid rain. Polymers 2020, 12, 2251. [Google Scholar] [CrossRef]
- Sekar, N. Acid dyes. In Handbook of Textile and Industrial Dyeing, Volume: 1 Principles, Processes and Types of Dyes; Clark, M., Ed.; Woodhead Publishing: Oxford, UK; Cambridge, UK; Philadelphia, PA, USA; New Delhi, India, 2011; pp. 505–509. [Google Scholar]

| Printing Paste for Printing of CO Fabric | Printing Paste for Printing of PA6 Fabric | ||
|---|---|---|---|
| Concentration (g/kg) | Component | Concentration (g/kg) | Component |
| 1 | dye BCG | 1 | dye BCG |
| 3 | ethanol | 3 | ethanol |
| 729 | H2O | 100 | urea |
| 3 | Antifoam W | 50 | H2O, cold |
| 34 | Clear CP | 236 | H2O, boiling |
| 200 | Legante SE | 50 | Glyezin A |
| 10 | Softner A/95 | 500 | Prisulon DCA 130 13% |
| 20 | Fixator NFO | 60 | (NH4)2SO4 (1:2) |
| Sample | d (mm) | W (g/m2) | G (mg × cm) | F (N) | σ (N/mm2) | ɛ (%) | Rp (mm/s) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gw | Gf | Go | Warp | Weft | Warp | Weft | Warp | Weft | ||||
| CO | 0.2321 | 119.65 | 271.55 | 114.81 | 176.57 | 376.96 | 211.57 | 64.97 | 36.46 | 12.13 | 13.24 | 442.67 |
| CO_P | 0.2564 | 141.86 | 463.49 | 217.53 | 88.28 | 384.33 | 210.27 | 59.96 | 32.80 | 12.04 | 13.92 | 37.32 |
| PA6 | 0.1318 | 75.82 | 94.91 | 82.12 | 317.52 | 366.38 | 296.14 | 111.19 | 89.88 | 38.21 | 46.06 | 344.19 |
| PA6_P | 0.1325 | 78.30 | 137.62 | 110.80 | 123.48 | 342.81 | 320.82 | 103.49 | 96.85 | 42.62 | 47.80 | 210.12 |
| Sample | L* | a* | b* | C*ab | hab (°) |
|---|---|---|---|---|---|
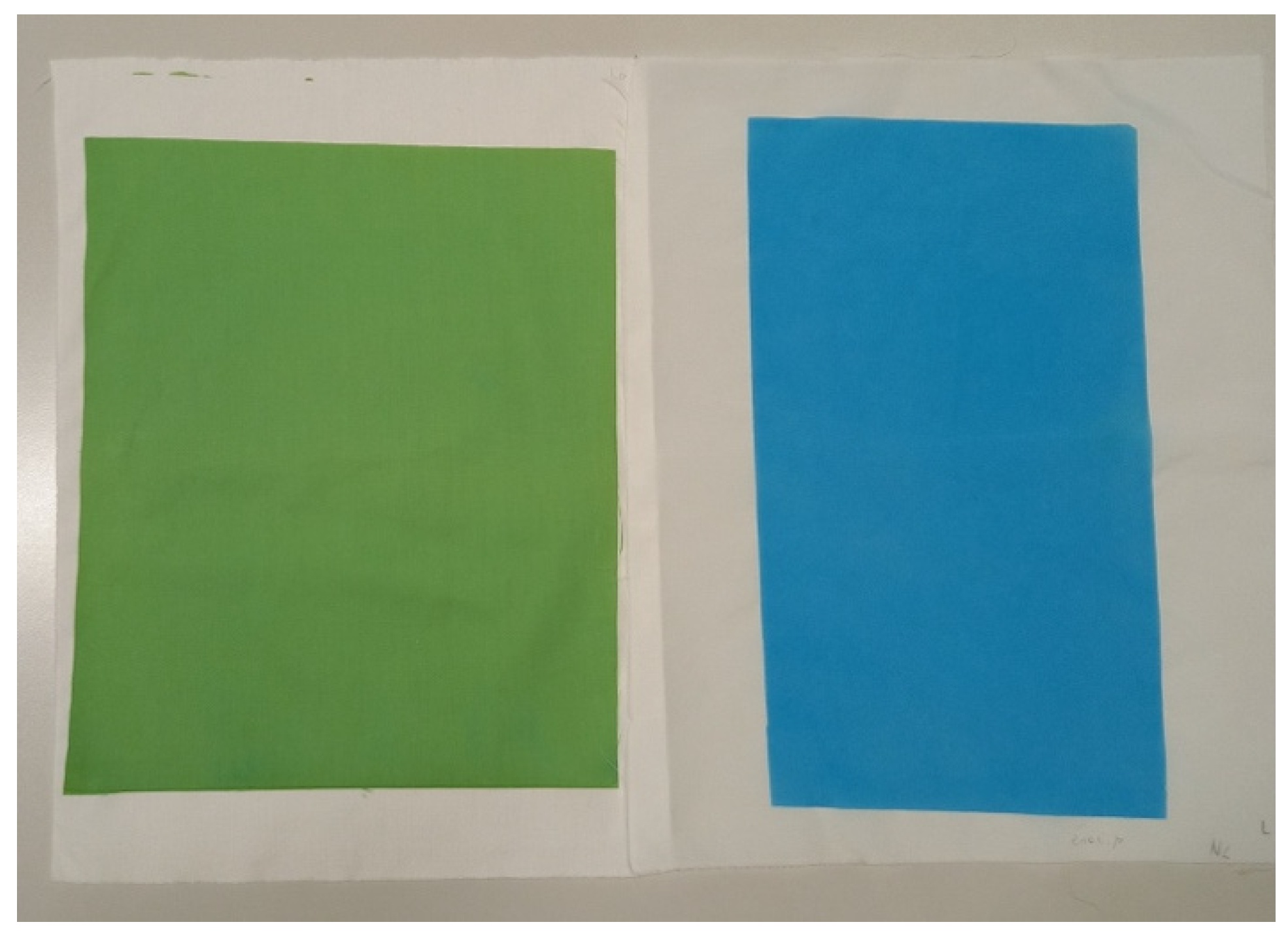
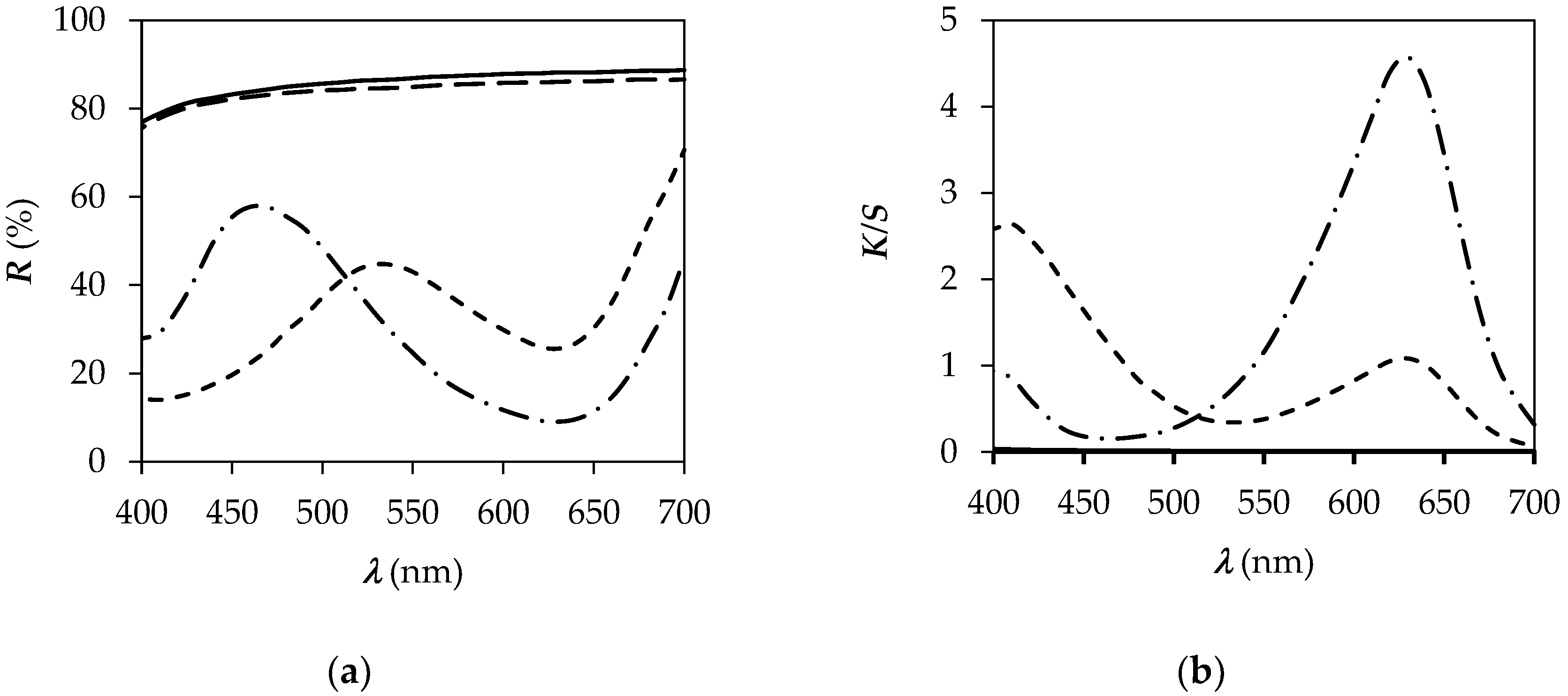
| CO | 94.63 | −0.14 | 2.70 | 2.70 | 92.95 |
| CO_P | 67.19 | −19.74 | 23.80 | 30.91 | 129.67 |
| PA6 | 93.84 | −0.09 | 2.20 | 2.20 | 92.43 |
| PA6_P | 59.23 | −20.93 | −29.93 | 36.52 | 235.03 |
| Sample | pH | L* | a* | b* | C*ab | hab (°) |
|---|---|---|---|---|---|---|
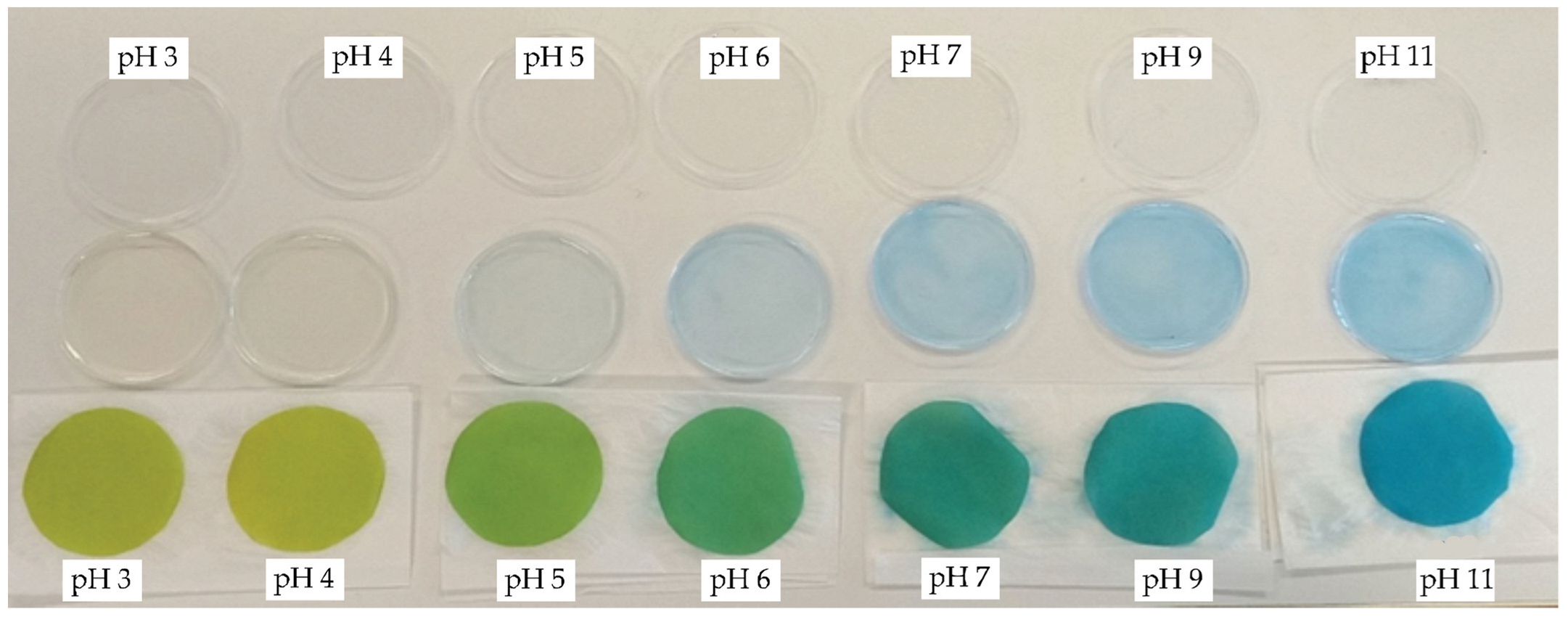
| CO_P | 3 | 75.83 | −12.30 | 38.80 | 40.70 | 107.60 |
| 4 | 76.67 | −11.61 | 39.72 | 41.38 | 106.30 | |
| 5 | 69.52 | −21.55 | 24.67 | 32.76 | 131.14 | |
| 6 | 64.13 | −26.11 | 7.77 | 27.24 | 163.43 | |
| 7 | 62.32 | −28.18 | −0.21 | 28.18 | 180.43 | |
| 9 | 61.89 | −28.06 | −7.64 | 29.08 | 195.23 | |
| 11 | 60.85 | −28.48 | −11.18 | 30.60 | 201.43 | |
| PA6_P | 3 | 59.14 | −26.73 | −11.16 | 28.97 | 202.65 |
| 4 | 62.08 | −26.33 | 5.43 | 26.93 | 168.34 | |
| 5 | 57.02 | −23.32 | −27.50 | 36.07 | 229.69 | |
| 6 | 57.42 | −22.35 | −29.05 | 36.65 | 232.43 | |
| 7 | 58.47 | −21.47 | −30.87 | 37.60 | 235.18 | |
| 9 | 59.54 | −20.87 | −29.60 | 36.22 | 234.82 | |
| 11 | 58.75 | −20.87 | −30.17 | 36.69 | 235.32 |
| Sample | Number of Washing Cycles | Visual Assessment Using Grey Scale | ||
|---|---|---|---|---|
| Staining of 1st Adjacent Fabric | Staining of 2nd Adjacent Fabric | Colour Change | ||
| CO_P | 1 | 5 | 5 | 3 |
| 5 | 5 | 5 | 2/3 | |
| 10 | 5 | 5 | 2 | |
| PA6_P | 1 | 5 | 5 | 5 |
| 5 | 5 | 5 | 5 | |
| 10 | 5 | 5 | 5 | |
| Sample | Dry Rubbing | Wet Rubbing | ||
|---|---|---|---|---|
| Warp | Weft | Warp | Weft | |
| CO_P | 5 | 5 | 5 | 2/3 |
| PA6_P | 5 | 5 | 5 | 2/3 |
| Sample | L* | a* | b* | C*ab | hab (°) | ∆E*ab |
|---|---|---|---|---|---|---|
| CO_P | 78.69 | −6.73 | 23.34 | 24.30 | 106.10 | 17.36 |
| PA6_P | 78.99 | −16.21 | −6.41 | 17.44 | 201.49 | 31.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorjanc, M.; Gerl, A.; Kert, M. Screen Printing of pH-Responsive Dye to Textile. Polymers 2022, 14, 447. https://doi.org/10.3390/polym14030447
Gorjanc M, Gerl A, Kert M. Screen Printing of pH-Responsive Dye to Textile. Polymers. 2022; 14(3):447. https://doi.org/10.3390/polym14030447
Chicago/Turabian StyleGorjanc, Marija, Ana Gerl, and Mateja Kert. 2022. "Screen Printing of pH-Responsive Dye to Textile" Polymers 14, no. 3: 447. https://doi.org/10.3390/polym14030447
APA StyleGorjanc, M., Gerl, A., & Kert, M. (2022). Screen Printing of pH-Responsive Dye to Textile. Polymers, 14(3), 447. https://doi.org/10.3390/polym14030447







