Metal Complexes of the Porphyrin-Functionalized Polybenzoxazine
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
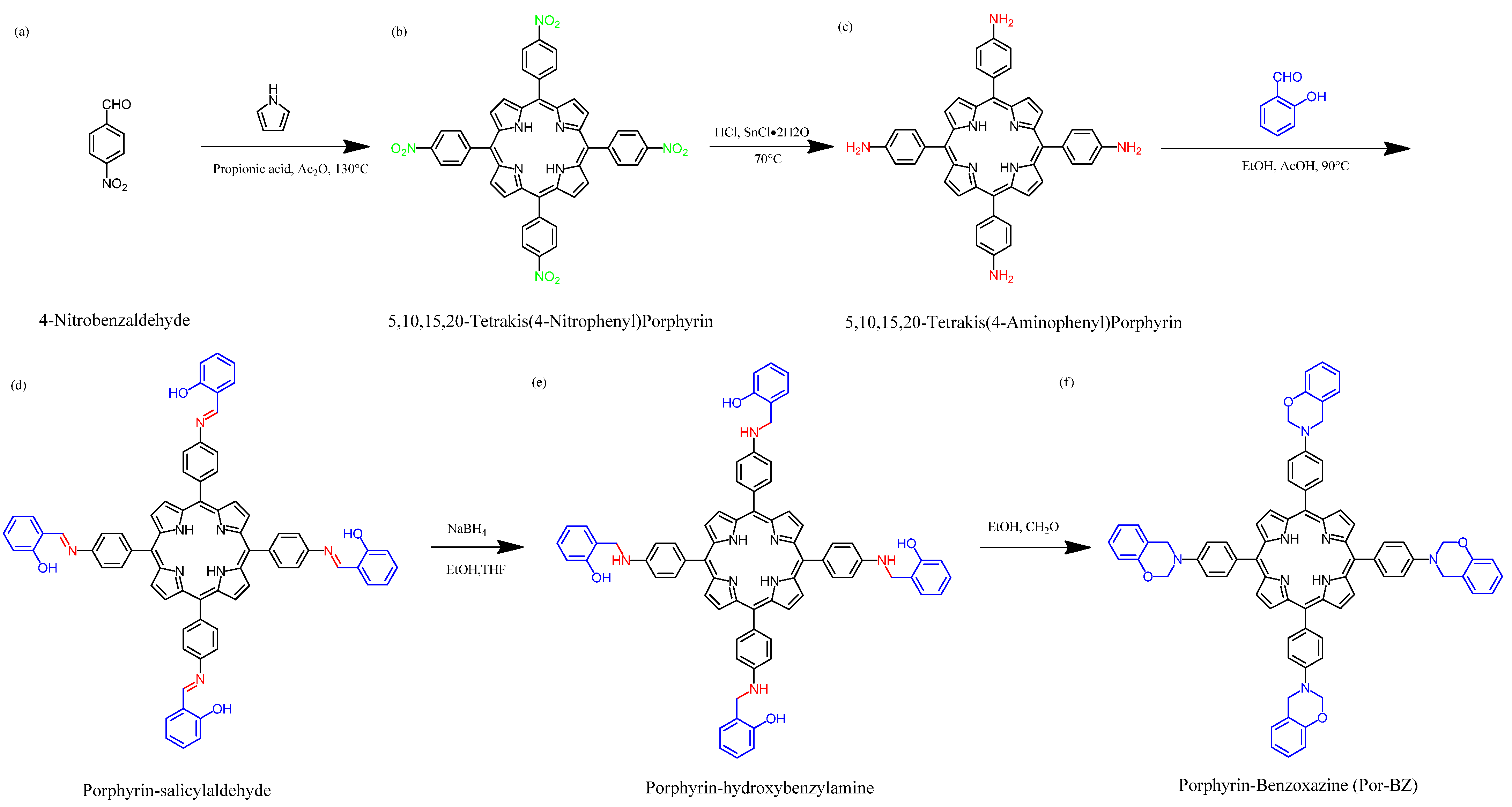
2.2. 5,10,15,20-Tetrakis(4-Aminophenyl)Porphyrin (TAPP)
2.3. Porphyrin-Salicylaldehyde (Por-Sa)
2.4. Porphyrin-Hydroxybenzylamine (Por-Hy)
2.5. Porphyrin-Salicylaldehyde-Benzoxazine (Por-BZ)
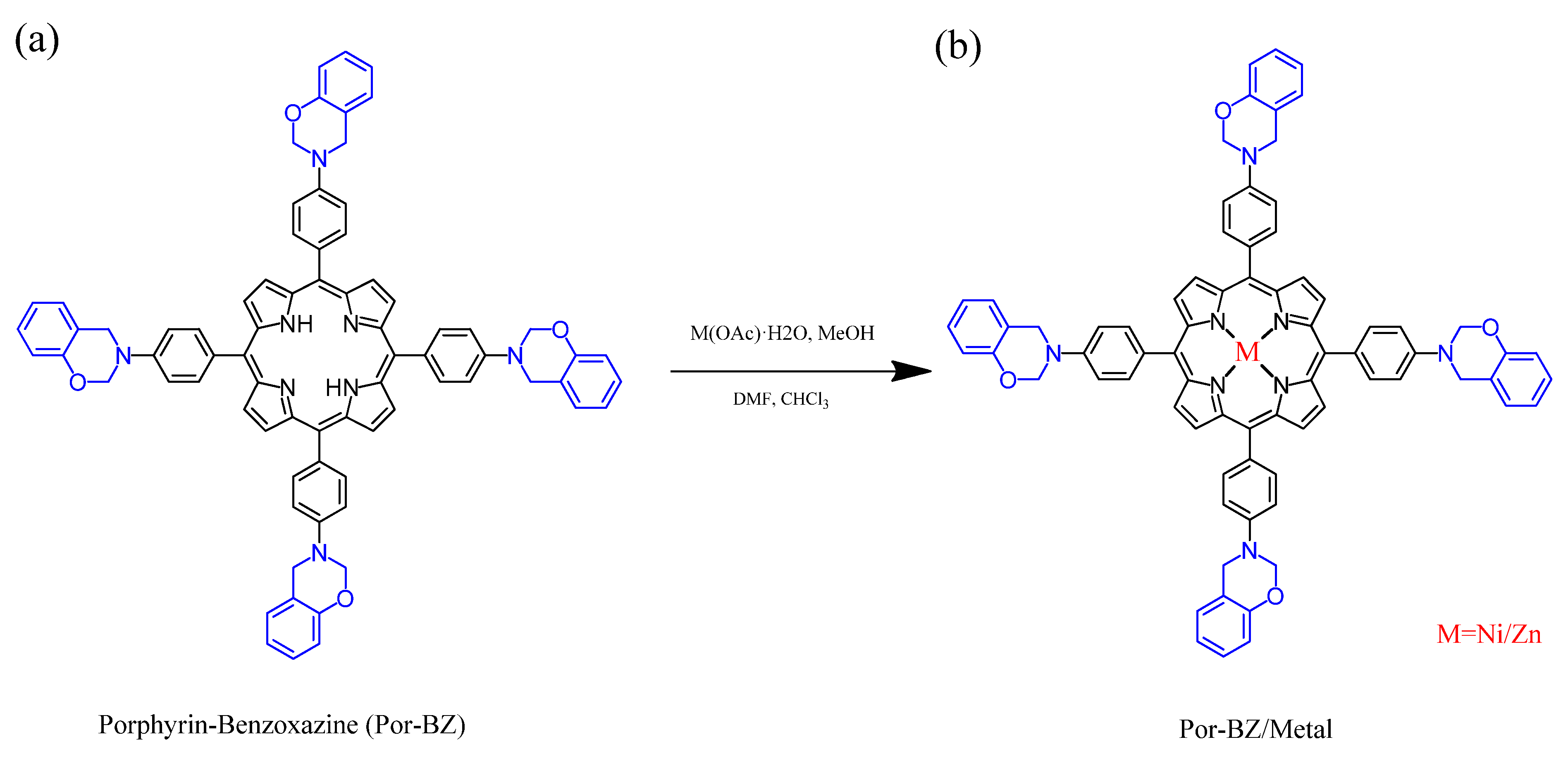
2.6. Por-Benzoxazine–Nickel(II) and –Zinc(II) Complexes (Por-BZ/Ni and Por-BZ/Zn)
2.7. Polymerization of BZ Monomer
3. Results and Discussion
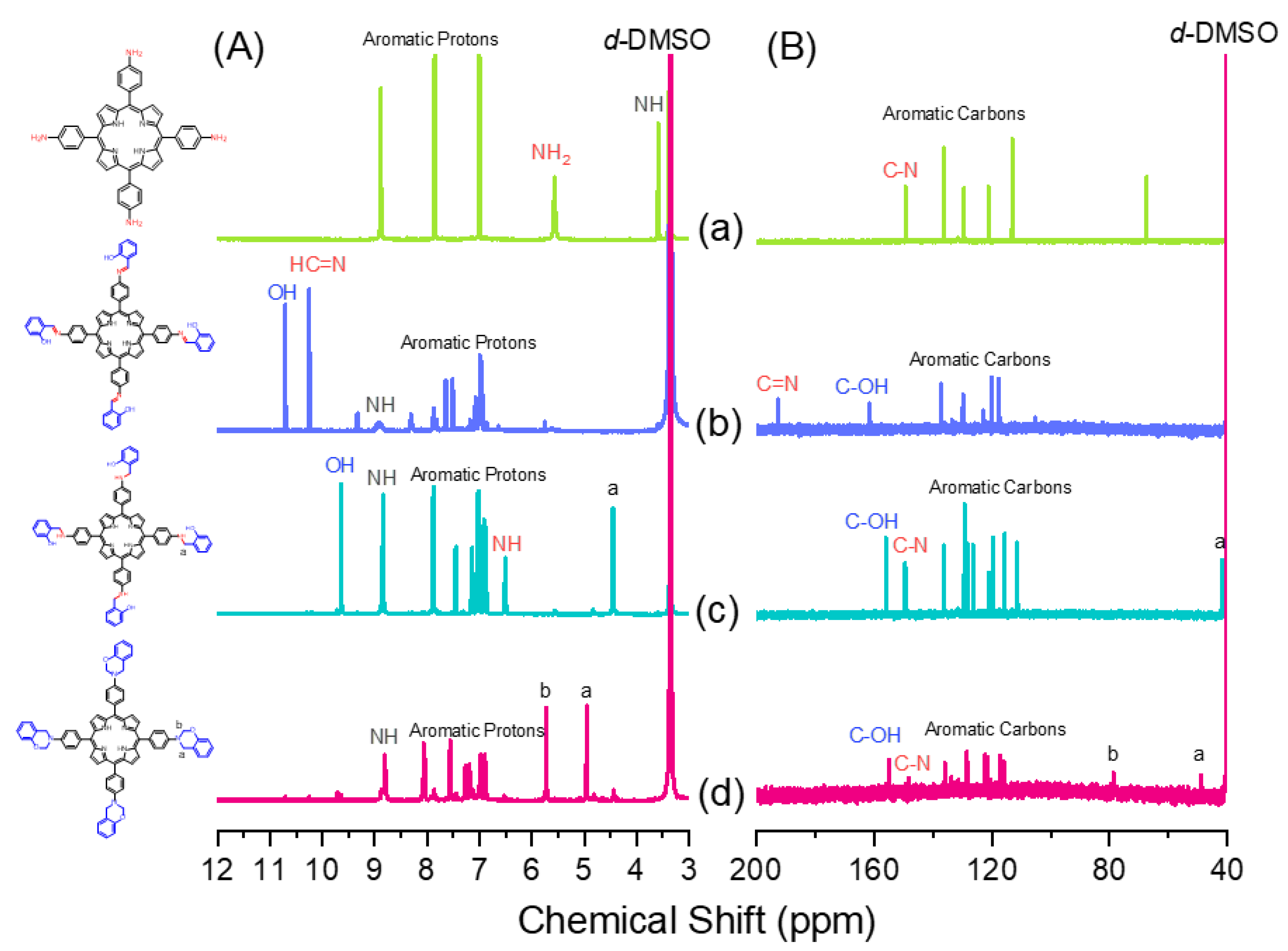
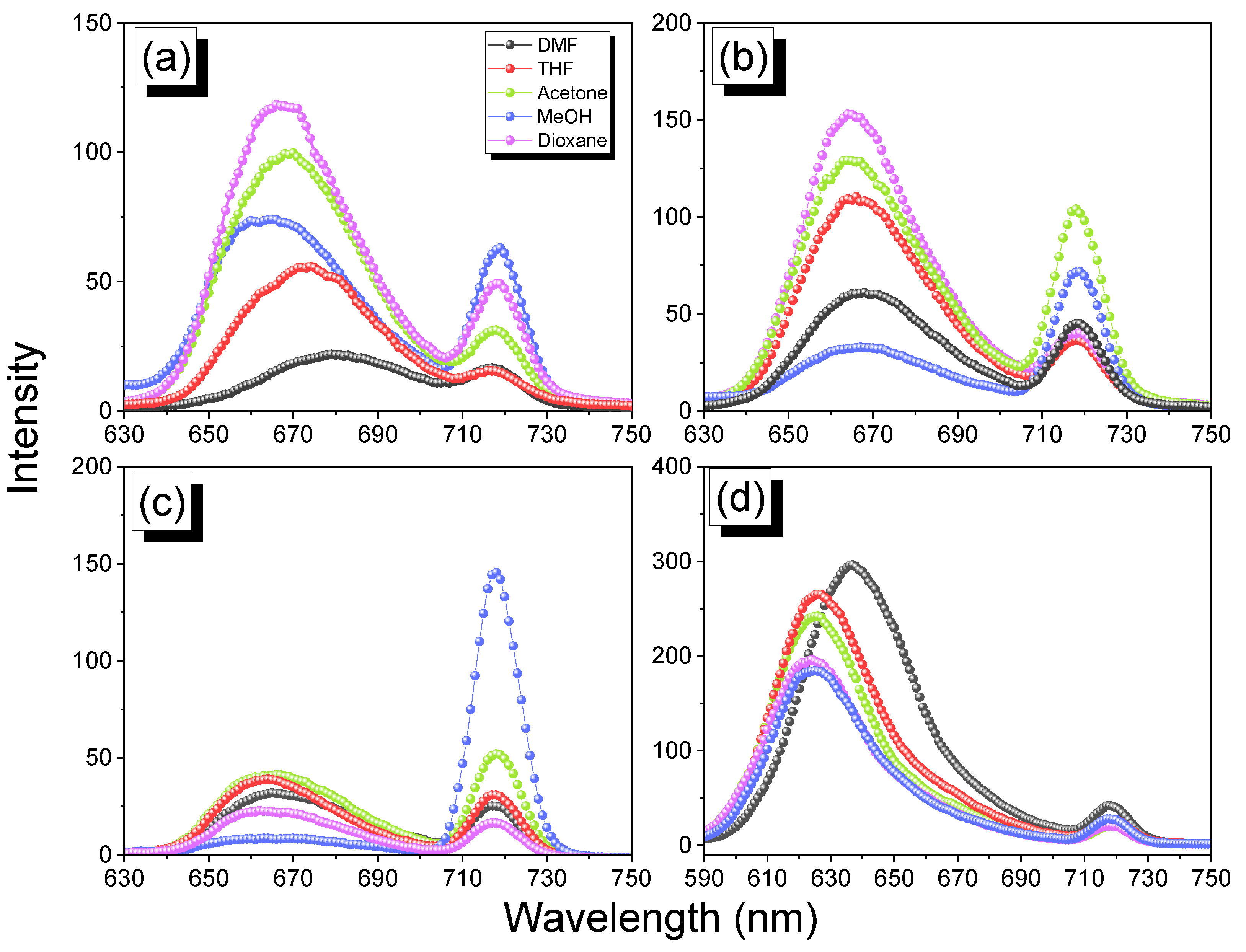
3.1. Synthesis of TAPP
3.2. Synthesis of Por-BZ
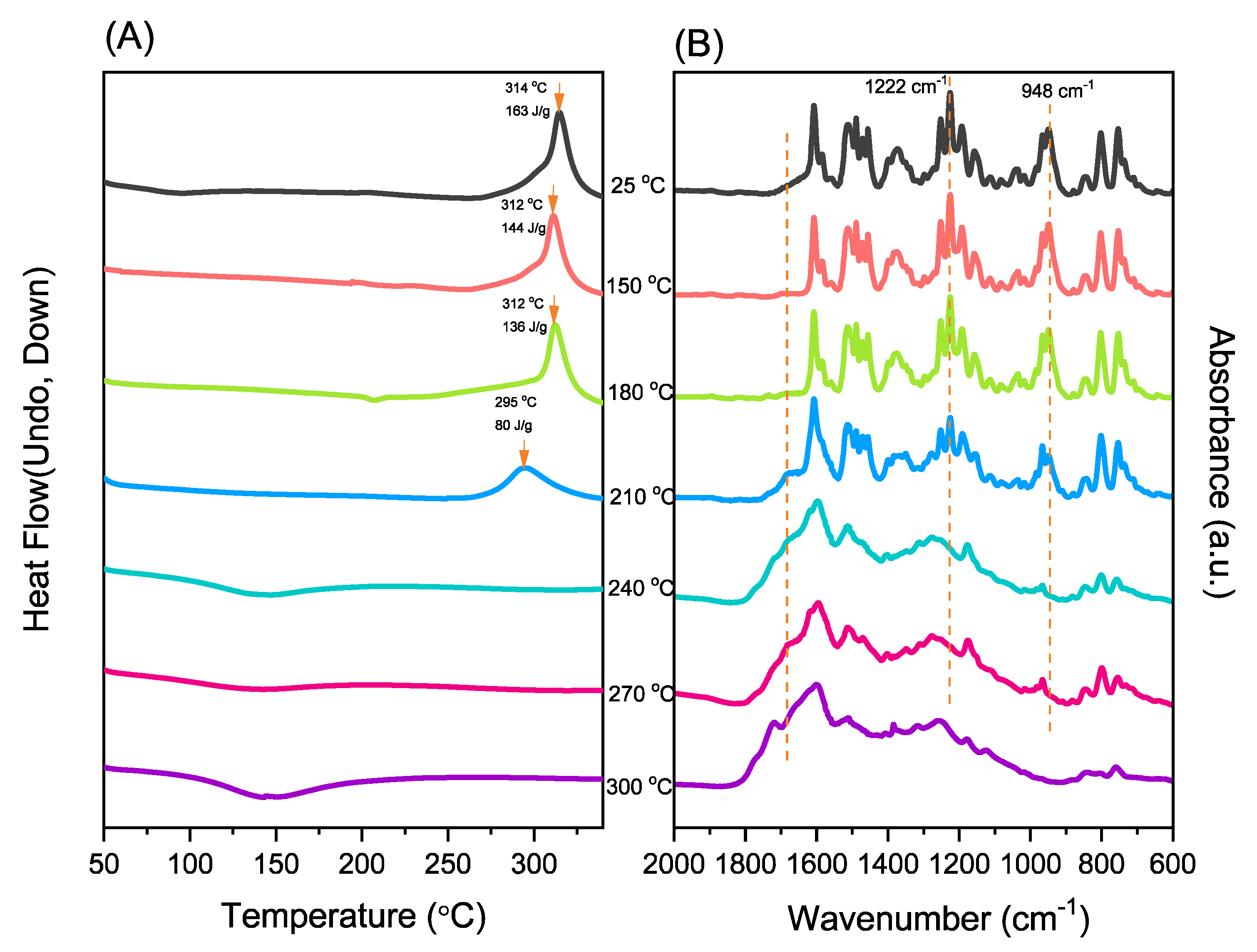
3.3. Thermal Curing Polymerization of Por-BZ Monomer
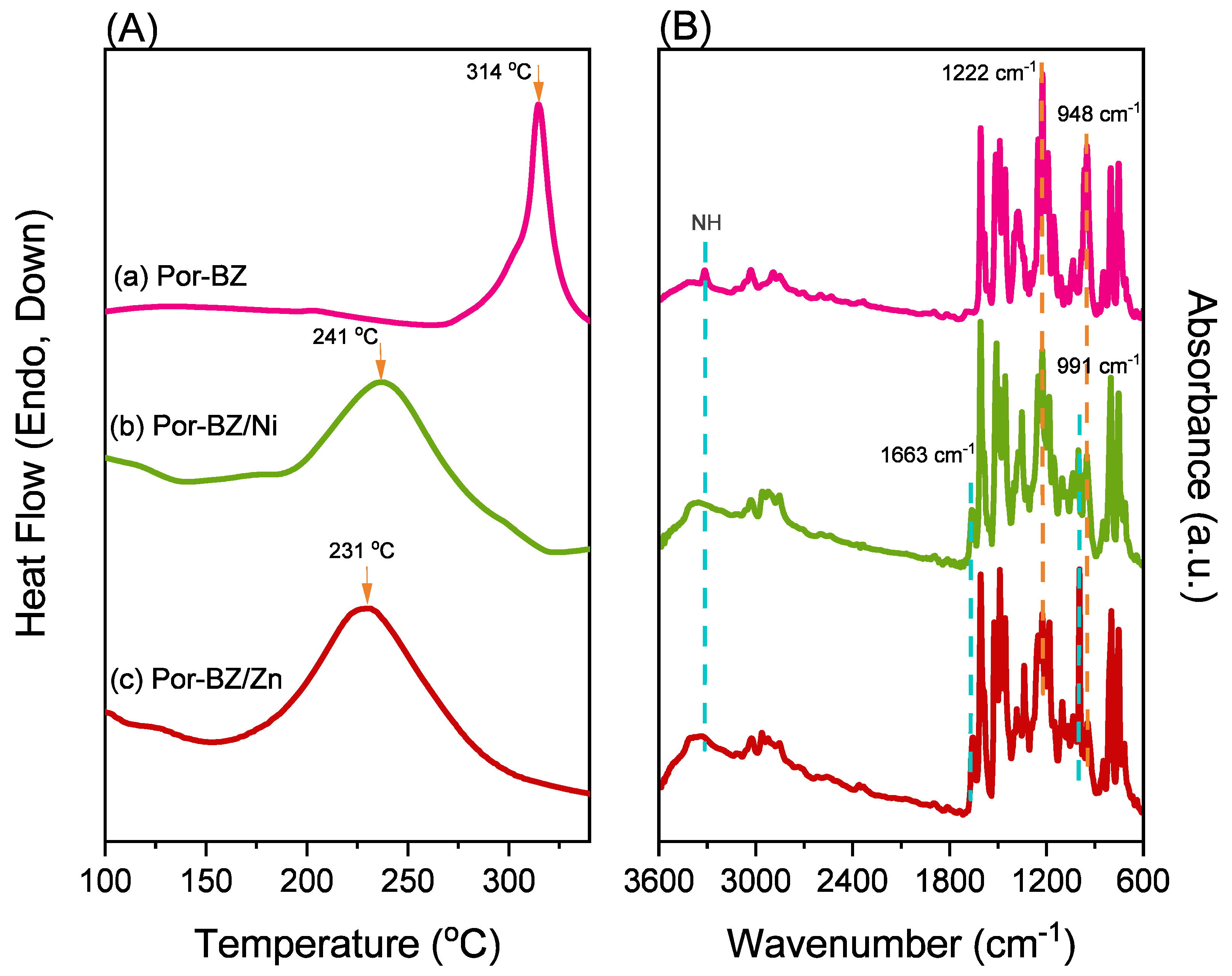
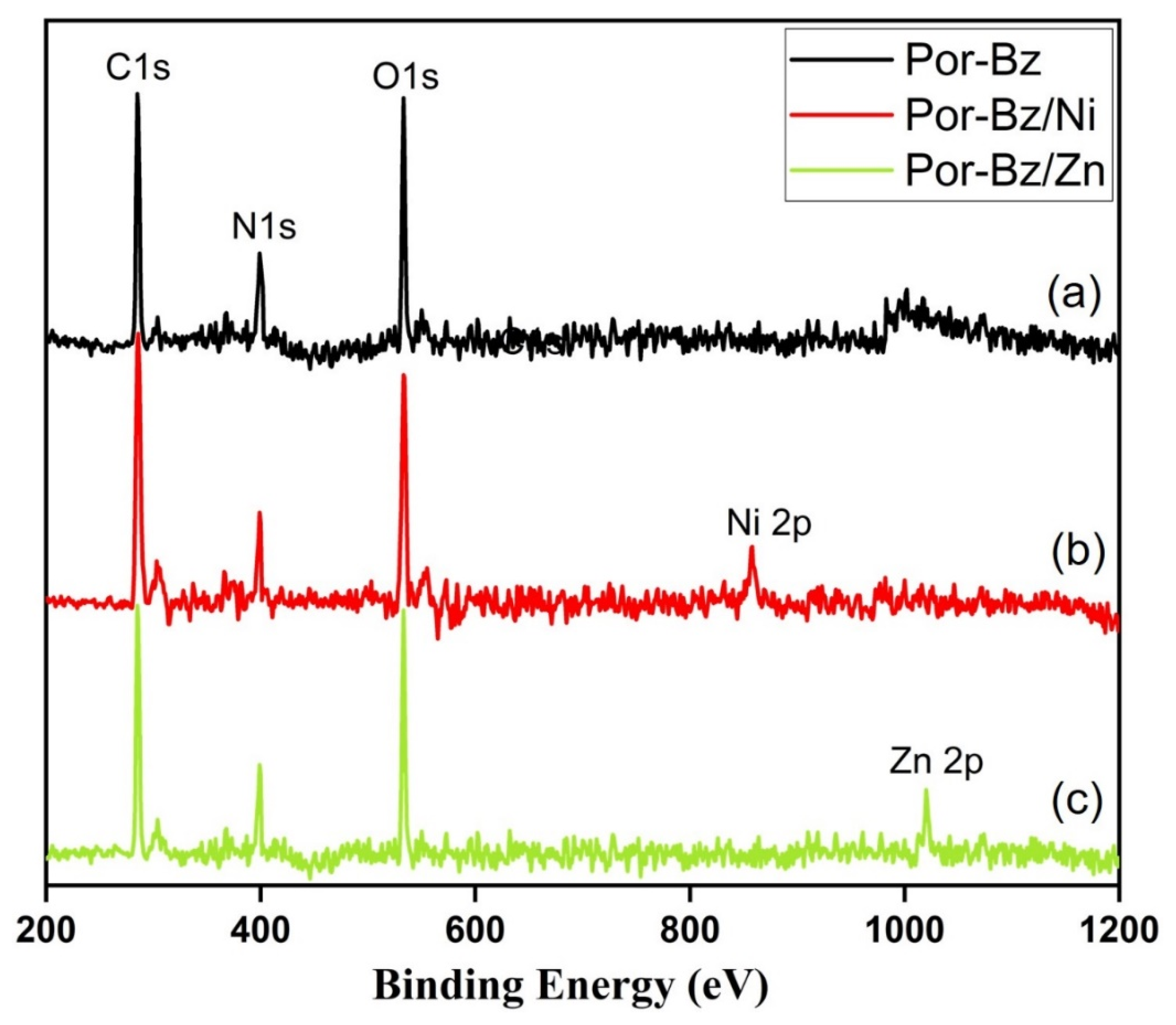
3.4. Characterization of Por-BZ/Metal Complex
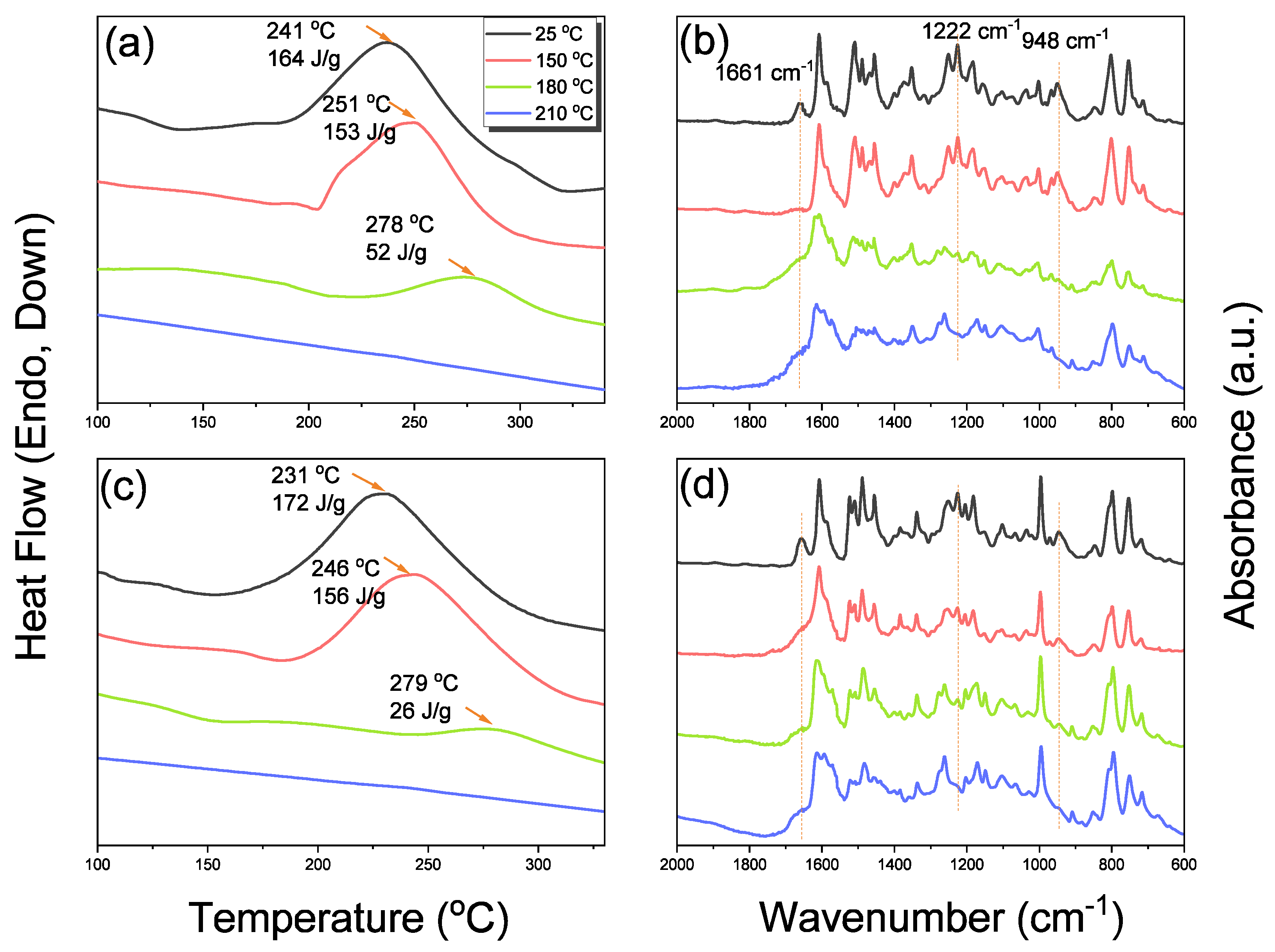
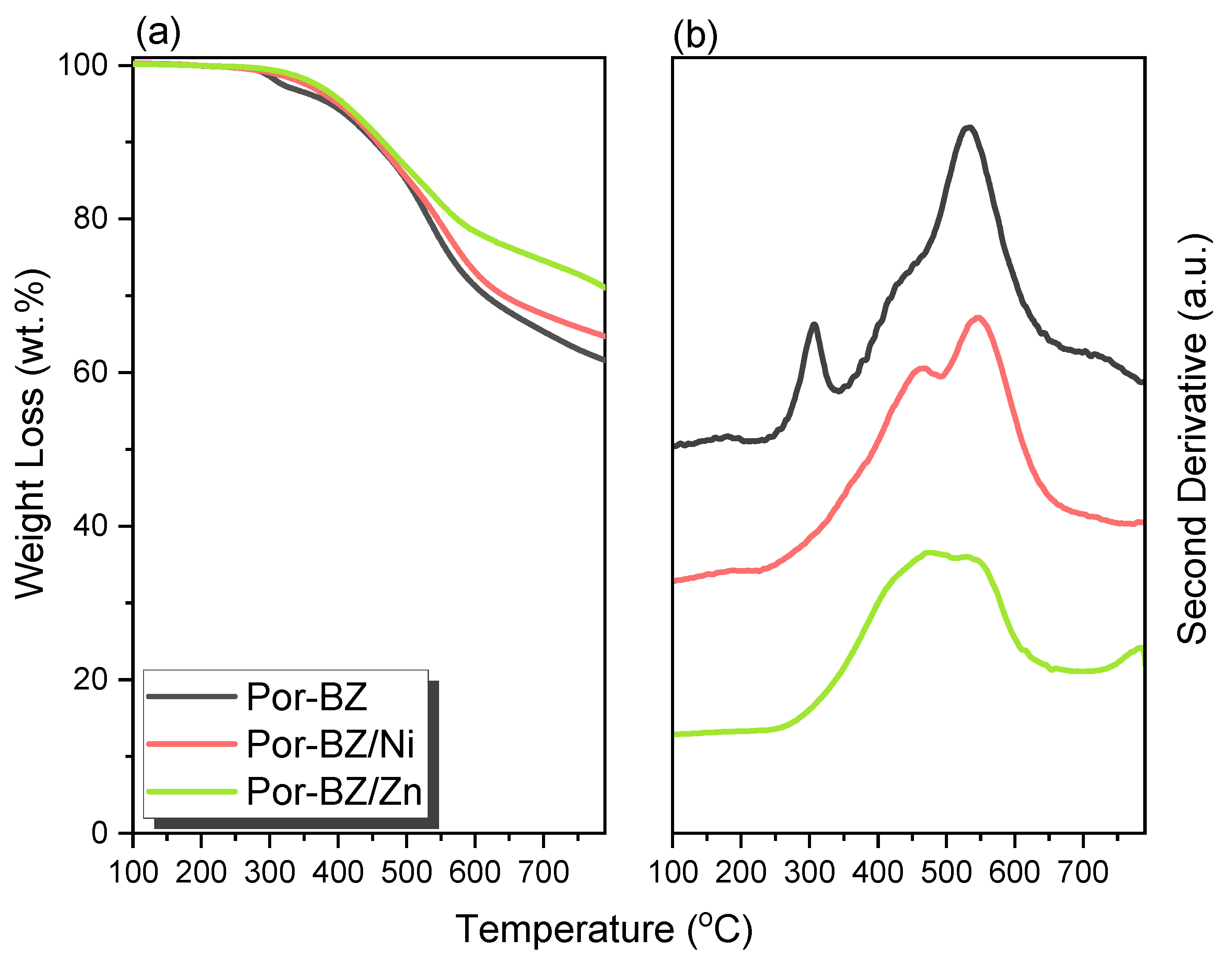
3.5. Thermal ROP of Por-BZ/Metal Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, N.; Monisha, M.; Niranjan, R.; Dubey, A.; Patil, S.; Priyadarshini, R.; Lochab, B. Antibacterial performance of fully biobased chitosan-grafted-polybenzoxazine films: Elaboration and properties of released material. Carbohydr. Polym. 2021, 254, 117296. [Google Scholar] [CrossRef]
- Lyu, Y.; Rachita, E.; Pogharian, N.; Froimowicz, P.; Ishida, H. Electronic effects of asymmetric and meta-alkoxy substituents on the polymerization behavior of bis-benzoxazines. Polym. Chem. 2020, 11, 800–809. [Google Scholar] [CrossRef]
- Zhang, X.; Mohamed, M.G.; Xin, Z.; Kuo, S.W. A tetraphenylethylene-functionalized benzoxazine and copper(II) acetylacetonate form a high-performance polybenzoxazine. Polymer 2020, 201, 122552. [Google Scholar] [CrossRef]
- Wu, J.J.; Zhao, C.X.; Li, Y.T.; Li, H.; Xiang, D.; Sun, Z.M.; Li, X. Properties of bio-based thermosetting composites synthesized from epoxidized soybean oil and azo-cardanol benzoxazine. J. Polym. Res. 2021, 28, 77. [Google Scholar] [CrossRef]
- Ohashi, S.; Rachita, E.; Baxley, S.; Zhou, J.; Erlichman, A.; Ishida, H. The first observation on polymerization of 1,3-benzothiazines: Synthesis of mono- and bis-thiazine monomers and thermal properties of their polymers. Polym. Chem. 2021, 12, 379–388. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, X.; Kuo, S.W. Outstanding dielectric and thermal properties of main chain-type poly(benzoxazine-co-imide-co-siloxane)-based cross-linked networks. Polym. Chem. 2019, 10, 2387–2396. [Google Scholar] [CrossRef]
- Abuzeid, H.R.; EL-Mahdy, A.F.M.; Ahmed, M.M.M.; Kuo, S.W. Triazine-functionalized covalent benzoxazine framework for direct synthesis of N-doped microporous carbon. Polym. Chem. 2019, 10, 6010–6020. [Google Scholar] [CrossRef]
- Kolanadiyil, S.N.; Minami, M.; Endo, T. Implementation of meta-Positioning in Tetrafunctional Benzoxazines: Synthesis, Properties, and Differences in the Polymerized Structure. Macromolecules 2020, 53, 6866–6886. [Google Scholar] [CrossRef]
- Hao, B.; Han, L.; Liu, Y.; Zhang, K. An apigenin-based bio-benzoxazine with three polymerizable functionalities: Sustainable synthesis, thermal latent polymerization, and excellent thermal properties of its thermosets. Polym. Chem. 2020, 11, 5800–5809. [Google Scholar] [CrossRef]
- Deliballi, Z.; Kiskan, B.; Yagci, Y. Advanced Polymers from Simple Benzoxazines and Phenols by Ring-Opening Addition Reactions. Macromolecules 2020, 53, 2354–2361. [Google Scholar] [CrossRef]
- El-Mahdy, A.F.; Lin, F.W.; Su, W.H.; Chen, T.; Kuo, S.W. Photoresponsive azobenzene materials based on pyridine-functionalized benzoxazines as surface relief gratings. ACS Appl. Polym. Mater. 2019, 2, 791–804. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Chen, H.; Li, H. Benzoxazine monomers containing triphenylimidazole: Polymerization of monomers and properties of polybenzoxazines. Eur. Polym. J. 2019, 121, 109347. [Google Scholar] [CrossRef]
- Tavernier, R.; Granado, L.; Foyer, G.; David, G.; Caillol, S. Formaldehyde-Free Polybenzoxazines for High Performance Thermosets. Macromolecules 2020, 53, 2557–2567. [Google Scholar] [CrossRef]
- Wang, C.F.; Su, Y.C.; Kuo, S.W.; Huang, C.F.; Sheen, Y.C.; Chang, F.C. Low-Surface-Free-Energy Materials Based on Polybenzoxazines. Angew. Chem. Int. Ed. 2006, 118, 2306–2309. [Google Scholar] [CrossRef]
- Sawaryn, C.; Landfester, K.; Taden, A. Benzoxazine Miniemulsions Stabilized with Polymerizable Nonionic Benzoxazine Surfactants. Macromolecules 2010, 43, 8933–8941. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Agag, T.; Ishida, H.; Qutubuddin, S. Chitosan Biobased Polybenzoxazine Cross-Linked Films: Preparation in Aqueous Media and Synergistic Improvements in Thermal and Mechanical Properties. Biomacromolecules 2013, 14, 1806–1815. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Y.; Lu, X.; Yao, H.; Xin, Z. Copolymer of eugenol-based and pyrogallol-based benzoxazines: Low curing temperature and enhanced corrosion resistance. Colloids Surf. A Physicochem. Eng. 2021, 609, 125605. [Google Scholar] [CrossRef]
- Yang, R.; Han, M.; Hao, B.; Zhang, K. Biobased high-performance tri-furan functional bis-benzoxazine resin derived from renewable guaiacol, furfural and furfurylamine. Eur. Polym. J. 2020, 131, 109706. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, K. Studies on the isomeric effect of nitrile functionality on the polymerization and thermal properties of ortho-norbornene-based benzoxazine resins. J. Polym. Res. 2020, 27, 130. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Chen, T.C.; Kuo, S.W. Solid-State Chemical Transformations to Enhance Gas Capture in Benzoxazine-Linked Conjugated Microporous Polymers. Macromolecules 2021, 54, 5866–5877. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, W.; Yin, R.; Zhang, K. Propargylamine: An attractive amine source for designing high-performance benzoxazine resins with low polymerization temperatures. Polym. Chem. 2021, 12, 6694–6704. [Google Scholar] [CrossRef]
- Abuzeid, H.R.; EL-Mahdy, A.F.M.; Kuo, S.W. Covalent Organic Frameworks: Design Principles, Synthetic Strategies, and Diverse Applications. Giant 2021, 6, 100054. [Google Scholar] [CrossRef]
- Samy, M.M.; Mohamed, M.G.; Mansoure, T.H.; Meng, T.S.; Khan, M.A.R.; Liaw, C.C.; Kuo, S.W. Solid state chemical transformations through ring-opening polymerization of ferrocene-based conjugated microporous polymers in host–guest complexes with benzoxazine-linked cyclodextrin. J. Taiwan Inst. Chem. Eng. 2022, 132, 104110. [Google Scholar] [CrossRef]
- Tavernier, R.; Granado, L.; Foyer, G.; David, G.; Caillol, S. Aromatic dialdehyde-based bisbenzoxazines: The influence of relative position of oxazine rings. Polymer 2021, 216, 123270. [Google Scholar] [CrossRef]
- Ohara, M.; Yoshimoto, K.; Kawauchi, T.; Takeichi, T. Synthesis of high-molecular-weight benzoxazines having azomethine linkages in the main-chain and the properties of their thermosetting resins. Polymer 2020, 202, 122668. [Google Scholar] [CrossRef]
- Salum, M.L.; Iguchi, D.; Arza, C.R.; Han, L.; Ishida, H.; Froimowicz, P. Making Benzoxazines Greener: Design, Synthesis, and Polymerization of a Biobased Benzoxazine Fulfilling Two Principles of Green Chemistry. ACS Sustain. Chem. Eng. 2018, 6, 13096–13106. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Crown Ether-Functionalized Polybenzoxazine for Metal Ion Adsorption. Macromolecules 2020, 53, 2420–2429. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Liu, T.E.; Kuo, S.W. Direct synthesis of nitrogen-doped mesoporous carbons from triazine-functionalized resol for CO2 uptake and highly efficient removal of dyes. J. Hazard. Mater. 2020, 391, 122163. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Yu, T.C.; Kuo, S.W. Synthesis of multiple heteroatom–doped mesoporous carbon/silica composites for supercapacitors. Chem. Eng. J. 2021, 414, 128796. [Google Scholar] [CrossRef]
- El-Mahdy, A.F.M.; Kuo, S.W. Direct synthesis of poly (benzoxazine imide) from an ortho-benzoxazine: Its thermal conversion to highly cross-linked polybenzoxazole and blending with poly (4-vinylphenol). Poly. Chem. 2018, 9, 1815–1826. [Google Scholar] [CrossRef]
- Coban, Z.G.; Yagci, Y.; Kiskan, B. Catalyzing the Ring-Opening Polymerization of 1,3-Benzoxazines via Thioamide from Renewable Sources. ACS Appl. Polym. Mater. 2021, 3, 4203–4212. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional Silica and Carbon Nanocomposites Based on Polybenzoxazines. Macromol. Chem. Phys. 2019, 220, 1800306. [Google Scholar] [CrossRef]
- Samy, M.M.; Mohamed, M.G.; Kuo, S.W. Pyrene-functionalized tetraphenylethylene polybenzoxazine for dispersing single-walled carbon nanotubes and energy storage. Compos. Sci. Technol. 2020, 199, 108360. [Google Scholar]
- Martinez, V.G.; Gude, M.R.; Calvo, S.; Urena, A. Enhancing an Aerospace Grade Benzoxazine Resin by Means of Graphene Nanoplatelets Addition. Polymers 2021, 13, 2544. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, R.; Kumar, A.V.; Ramaprabhu, S.; Subramanian, V. Absorption-enhanced EMI shielding using silver decorated three-dimensional porous architected reduced graphene oxide in polybenzoxazine composites. New J. Chem. 2021, 45, 16939–16948. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, W.B.; Whang, W.T.; Chen, C.H. Characteristics of Thermosetting Polymer Nanocomposites: Siloxane-Imide-Containing Benzoxazine with Silsesquioxane Epoxy Resins. Polymers 2020, 12, 2510. [Google Scholar] [CrossRef]
- Zhang, S.; Lan, T.; Ren, D.; Liu, X.; Ran, Q. Tuning the polymerization sequence of alkynyl-functionalized benzoxazine: Application as precursor for efficient magnetic EMI shielding materials. J. Mater. Sci. 2021, 56, 10691. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional Polyimide/Polyhedral Oligomeric Silsesquioxane Nanocomposites. Polymers 2019, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Prasomsin, W.; Parnklang, T.; Sapcharoenkun, C.; Tiptipakorn, S.; Rimdusit, S. Multiwalled Carbon Nanotube Reinforced Bio-Based Benzoxazine/Epoxy Composites with NIR-Laser Stimulated Shape Memory Effects. Nanomaterials 2019, 9, 881. [Google Scholar] [CrossRef] [Green Version]
- Ahn, D.; Choi, H.J.; Kim, H.D.; Yeo, S.Y. Properties of Conductive Polyacrylonitrile Fibers Prepared by Using Benzoxazine Modified Carbon Black. Polymers 2020, 12, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arivalagan, V.; Stephen, L.D.; Meera, M.; Gunasekaran, S.G. Carbazole terminal phenylene core imine skeletal nanosilica reinforced polybenzoxazine (nSiO2O2/PBZ) hybrid nanocomposites. J. Polym. Res. 2021, 28, 381. [Google Scholar] [CrossRef]
- Auwärter, W.; Seufert, K.; Bischoff, F.; Ecija, D.; Vijayaraghavan, S.; Joshi, S.; Klappenberger, F.; Samudrala, N.; Barth, J.V. A surface-anchored molecular four-level conductance switch based on single proton transfer. Nat. Nanotechnol. 2012, 7, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spitaleri, L.; Gangemi, C.M.A.; Purrello, R.; Nicotra, G.; Trusso Sfrazzetto, G.; Casella, G.; Casarin, M.; Gulino, A. Covalently Conjugated Gold–Porphyrin Nanostructures. Nanomaterials 2020, 10, 1644. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.Z.; Liu, Z.; Ma, D.L.; Liu, L.; Haung, T.; Zhang, P.; Tan, D.; Wang, F.; Jiang, G.F.; Wu, Y. Polyarylimide and porphyrin based polymer microspheres for zinc ion hybrid capacitors. Chem. Eng. J. 2021, 405, 127038. [Google Scholar] [CrossRef]
- Mukherjee, G.; Thote, J.; Aiyappa, H.B.; Kandambeth, S.; Banerjee, S.; Vanka, K.; Banerjee, R. A porous porphyrin organic polymer (PPOP) for visible light triggered hydrogen production. Chem. Commun. 2017, 53, 4461–4464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Z.; Mao, Y.; Zhang, Y.; Li, Y.; Lao, J.; Wang, L. Integrating Porphyrinic Metal-Organic Frameworks in Nanofibrous Carrier for Photodynamic Antimicrobial Application. Polymers 2021, 13, 3942. [Google Scholar] [CrossRef] [PubMed]
- Ognibene, G.; Gangemi, C.M.A.; Spitaleri, L.; Gulino, A.; Purrello, R.; Cicala, G.; Fragalà, M.E. Role of the surface composition of the polyethersulfone–TiiP–H2T4 fibers on lead removal: From electrostatic to coordinative binding. J. Mater. Sci. 2019, 54, 8023. [Google Scholar] [CrossRef]
- Li, D.; Fang, Y.; Zhang, X. Bacterial Detection and Elimination Using a Dual-Functional Porphyrin-Based Porous Organic Polymer with Peroxidase-Like and High Near-Infrared-Light-Enhanced Antibacterial Activity. ACS Appl. Mater. Interfaces 2020, 12, 8989–8999. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, Y.; Yu, J.; Gao, W.; Zhao, H.; Zhang, X. A silsesquioxane-porphyrin-based porous organic polymer as a highly efficient and recyclable absorbent for wastewater treatment. J. Hazard. Mater. 2021, 406, 124769. [Google Scholar] [CrossRef]
- Li, M.; Zhao, H.; Lu, Z.Y. Porphyrin-based porous organic polymer, Py-POP, as a multifunctional platform for efficient selective adsorption and photocatalytic degradation of cationic dyes. Microporous Mesoporous Mater. 2020, 292, 109774. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Zakaria, M.B.; Wang, H.X.; Chen, T.; Yamauchi, Y.; Kuo, S.W. Heteroporous bifluorenylidene-based covalent organic frameworks displaying exceptional dye adsorption behavior and high energy storage. J. Mater. Chem. A 2020, 8, 25148–25155. [Google Scholar] [CrossRef]
- Bain-Ackerman, M.J.; Lavallee, D.K. Kinetics of Metal-Ion Complexation with N-Methyltetraphenylporphyrin. Evidence Concerninga General Mechanism of Porphyrin Metalation. Inorg. Chem. 1979, 18, 3358–3364. [Google Scholar] [CrossRef]
- Angelis, F.D.; Fantacci, S.; Sgamellotti, A.; Pizzotti, M.; Tessore, F.; Biroli, A.O. Time-dependent and coupled-perturbed DFT and HF investigations on the absorption spectrum and non-linear optical properties of push–pull M(II)–porphyrin complexes (M = Zn, Cu, Ni). Chem. Phy. Lett. 2007, 447, 10–15. [Google Scholar] [CrossRef]
- Torre, G.; Vazquez, P.; Lopez, F.A.; Torres, T. Role of Structural Factors in the Nonlinear Optical Properties of Phthalocyanines and Related Compounds. Chem. Rev. 2004, 104, 3723–3750. [Google Scholar] [CrossRef] [PubMed]
- Uttamlal, M.; Holmes-Smith, A.S. The excitation wavelength dependent fluorescence of porphyrins. Chem. Phys. Lett. 2008, 54, 223. [Google Scholar] [CrossRef]
- Gangemi, C.M.A.; Iudici, M.; Spitaleri, L.; Randazzo, R.; Gaeta, M.; D’Urso, A.; Gulino, A.; Purrello, R.; Fragalà, M.E. Polyethersulfone Mats Functionalized with Porphyrin for Removal of Para-nitroaniline from Aqueous Solution. Molecules 2019, 24, 3344. [Google Scholar] [CrossRef] [Green Version]
- EL-Mahdy, A.F.M.; Lai, M.Y.; Kuo, S.W. Highly fluorescent covalent organic framework as hydrogen chloride sensor: Roles of schiff base bonding and π-stacking. J. Mater. Chem. C 2020, 8, 9520–9528. [Google Scholar] [CrossRef]
- Wu, J.H.; Chen, W.C.; Liou, G.S. Triphenylamine-based luminogens and fluorescent polyimides: Effects of functional groups and substituents on photophysical behaviors. Polym. Chem. 2016, 7, 1569–1576. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Elewa, A.M.; Hung, S.W.; Chou, H.H.; Kuo, S.W. Dual-Function Fluorescent Covalent Organic Frameworks: HCl Sensing and Photocatalytic H2 Evolution from Water. Adv. Opt. Mater. 2020, 8, 2000641. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Young, C.; Kim, J.; You, J.; Yamauchi, Y.; Kuo, S.W. Hollow Microspherical and Microtubular [3 + 3] Carbazole-Based Covalent Organic Frameworks and Their Gas and Energy Storage Applications. ACS Appl. Mater. Interface 2019, 11, 9343–9354. [Google Scholar] [CrossRef]
- Mohamed, M.G.; EL-Mahdy, A.F.M.; Kotp, M.G.; Kuo, S.W. Advances in porous organic polymers: Syntheses, structures, and diverse applications. Mater. Adv. 2022, 3, 707–733. [Google Scholar] [CrossRef]
- Annalinda, C.; Giuseppe, M.; Maria, E.F.; Luca, S.; Antonino, G. Conjugated Gold–Porphyrin Monolayers Assembled on Inorganic Surfaces. Chem. Eur. J. 2017, 23, 14937–14943. [Google Scholar] [CrossRef]





| Compound | Solvent | λabs (nm) (Soret Band) | λabs (nm) (Q Band) |
|---|---|---|---|
| TAPP | THF | 524, 566, 602, 662 | 432 |
| Dioxane | 524, 564, 600, 660 | 430 | |
| Acetone | 522, 564, 600, 660 | 428 | |
| MeOH | 522, 564, 596, 656 | 426 | |
| DMF | 528, 574, 608, 666 | 436 | |
| Por-BZ | THF | 522, 562, 598, 658 | 428 |
| Dioxane | 522, 562, 598, 656 | 428 | |
| Acetone | 520, 560, 596, 654 | 424 | |
| MeOH | 522, 564, 606, 662 | 430 | |
| DMF | 522, 564, 600, 658 | 428 | |
| Por-BZ/Ni | THF | 535, 574, 657.5 | 432 |
| Acetone | 533.5, 571, 656.5 | 428 | |
| DMF | 538, 579, 660.5 | 434 | |
| Por-BZ/Zn | THF | 564, 608 | 436 |
| Acetone | 562, 606 | 432 | |
| DMF | 572, 620 | 440 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; EL-Mahdy, A.F.M.; Ahmed, L.R.; Matsagar, B.M.; Al-Saeedi, S.; Kuo, S.-W.; Wu, K.C.-W. Metal Complexes of the Porphyrin-Functionalized Polybenzoxazine. Polymers 2022, 14, 449. https://doi.org/10.3390/polym14030449
Zhang G, EL-Mahdy AFM, Ahmed LR, Matsagar BM, Al-Saeedi S, Kuo S-W, Wu KC-W. Metal Complexes of the Porphyrin-Functionalized Polybenzoxazine. Polymers. 2022; 14(3):449. https://doi.org/10.3390/polym14030449
Chicago/Turabian StyleZhang, Guohu, Ahmed F. M. EL-Mahdy, Lamiaa Reda Ahmed, Babasaheb M. Matsagar, Sameerah Al-Saeedi, Shiao-Wei Kuo, and Kevin C.-W. Wu. 2022. "Metal Complexes of the Porphyrin-Functionalized Polybenzoxazine" Polymers 14, no. 3: 449. https://doi.org/10.3390/polym14030449







