Metronidazole Topically Immobilized Electrospun Nanofibrous Scaffold: Novel Secondary Intention Wound Healing Accelerator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal and Ethical Approval
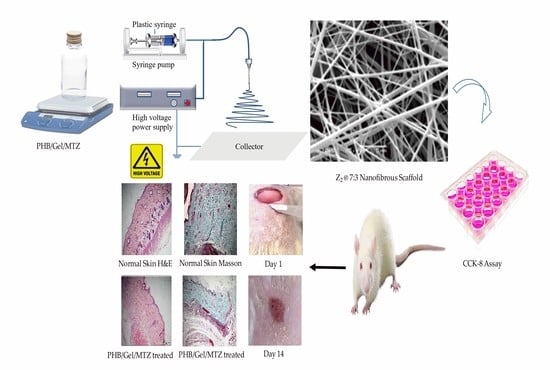
2.3. Fabrication of Nanofibrous Scaffolds
2.4. Physical Characterization of Nanofibrous Scaffolds
2.5. Mechanical Properties of Nanofibrous Scaffolds
2.6. Water Uptake, Porosity and Contact Angles of Nanofibrous Scaffolds
2.7. Anti Bacterial Activity of Electrospun Nanofibrous Scaffolds
2.8. Release of MTZ from Electrospun Nanofibrous Scaffolds
2.9. Cell Seeding
2.10. Cytocompatibility of Nanofibrous Scaffolds
2.11. In Vivo Wound Healing Study
2.12. Gene Expression of Interleukin-6 (IL-6) and Transforming Growth Factor-β1 (TGF-β1)
2.13. Statistical Analysis
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boer, M.; Duchnik, E.; Maleszka, R.; Marchlewicz, M. Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Adv. Dermatol. Allergol. 2016, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hussain, Z.; Ali, S.; Qamar, Z.; Imran, M.; Hafeez, F.Y. Fabrication of Electrospun Probiotic Functionalized Nanocomposite Scaffolds for Infection Control and Dermal Burn Healing in a Mice Model. ACS Biomater. Sci. Eng. 2019, 5, 6109–6116. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Ma, L. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Sarhan, W.A.; Azzazy, H.M.E.; El-Sherbiny, I.M. Honey/Chitosan Nanofiber Wound Dressing Enriched with Allium sativum and Cleome droserifolia: Enhanced Antimicrobial and Wound Healing Activity. ACS Appl. Mater. Interfaces 2016, 8, 6379–6390. [Google Scholar] [CrossRef] [PubMed]
- Kandhasamy, S.; Perumal, S.; Madhan, B.; Umamaheswari, N.; Banday, J.A.; Perumal, P.T.; Santhanakrishnan, V.P. Synthesis and Fabrication of Collagen-Coated Ostholamide Electrospun Nanofiber Scaffold for Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 8556–8568. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.H.; Khalil, I.A.; El-Sherbiny, I.M. Single-Dose Electrospun Nanoparticles-in-Nanofibers Wound Dressings with Enhanced Epithelialization, Collagen Deposition, and Granulation Properties. ACS Appl. Mater. Interfaces 2016, 8, 14453–14469. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Yang, B.; Sun, Y.; Zu, Y.; Deng, Y. A Berberine-Loaded Electrospun Poly-(ε-caprolactone) Nanofibrous Membrane with Hemostatic Potential and Antimicrobial Property for Wound Dressing. J. Biomed. Nanotechnol. 2013, 9, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen-wen, L.; Qing, W.; Min, H.; San-jun, S.; Zi-wei, L.; Guo-lin, W.; Huan-huan, C.; Yuan-yuan, L.; Qian, Z.; et al. Silver nanoparticles/chitosan oligosaccharide/poly(vinyl alcohol) nanofiber promotes wound healing by activating TGFβ1/Smad signaling pathway. Int. J. Nanomed. 2016, 11, 373. [Google Scholar] [CrossRef] [Green Version]
- Westling, K.; Farra, A.; Cars, B.; Ekblom, A.; Sandstedt, K.; Settergren, B.; Wretlind, B.; Jorup, C. Cat bite wound infections: A prospective clinical and microbiological study at three emergency wards in Stockholm, Sweden. J. Infect. 2006, 53, 403–407. [Google Scholar] [CrossRef]
- Radhakumary, C.; Antonty, M.; Sreenivasan, K. Drug loaded thermoresponsive and cytocompatible chitosan based hydrogel as a potential wound dressing. Carbohydr. Polym. 2011, 83, 705–713. [Google Scholar] [CrossRef]
- Hwang, M.-R.; Kim, J.O.; Lee, J.H.; Kim, Y.I.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.A.; Lyoo, W.S.; Han, S.S.; et al. Gentamicin-Loaded Wound Dressing With Polyvinyl Alcohol/Dextran Hydrogel: Gel Characterization and In Vivo Healing Evaluation. AAPS PharmSciTech 2010, 11, 1092–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, F.; Mushtaq, B.; Butt, F.A.; Zafar, M.S.; Ahmad, S.; Afzal, A.; Nawab, Y.; Rasheed, A.; Ulker, Z. Synthesis and Characterization of Nonwoven Cotton-Reinforced Cellulose Hydrogel for Wound Dressings. Polymers 2021, 13, 4098. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.J.; Miller, M.J.; Yasko, A.W.; Yaszemski, M.J.; Mikos, A.G. Polymer concepts in tissue engineering. J. Biomed. Mater. Res. 1998, 43, 422–427. [Google Scholar] [CrossRef]
- Ramier, J.; Grande, D.; Bouderlique, T.; Stoilova, O.; Manolova, N.; Rashkov, I.; Langlois, V.; Albanese, P.; Renard, E. From design of bio-based biocomposite electrospun scaffolds to osteogenic differentiation of human mesenchymal stromal cells. J. Mater. Sci. Mater. Med. 2014, 25, 1563–1575. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.-O.; Jafari, S.-H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2009, 21, 77–95. [Google Scholar] [CrossRef]
- Ladd, M.R.; Hill, T.K.; Yoo, J.J.; Lee, S.J. Electrospun nanofibers in tissue engineering. Nanofibers-Prod. Prop. Funct. Appl. 2011, 14, 347–373. [Google Scholar]
- Yang, Y.; Zhu, X.; Cui, W.; Li, X.; Jin, Y. Electrospun composite mats of poly [(D, L-lactide)-co-glycolide] and collagen with high porosity as potential scaffolds for skin tissue engineering. Macromol. Mater. Eng. 2009, 294, 611–619. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 2008, 29, 4100–4107. [Google Scholar] [CrossRef] [Green Version]
- Qasim, S.; Zafar, M.; Najeeb, S.; Khurshid, Z.; Shah, A.; Husain, S.; Rehman, I. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef] [Green Version]
- Sarhan, W.A.; Azzazy, H.M.E. High concentration honey chitosan electrospun nanofibers: Biocompatibility and antibacterial effects. Carbohydr. Polym. 2015, 122, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Powell, H.M.; Boyce, S.T. Engineered Human Skin Fabricated Using Electrospun Collagen–PCL Blends: Morphogenesis and Mechanical Properties. Tissue Eng. Part A 2009, 15, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Dong, C.; Yuan, X.; Yao, K. Electrospinning of chitosan solutions in acetic acid with poly(ethylene oxide). J. Biomater. Sci. Polym. Ed. 2004, 15, 797–811. [Google Scholar] [CrossRef]
- Kost, B.; Svyntkivska, M.; Brzeziński, M.; Makowski, T.; Piorkowska, E.; Rajkowska, K.; Kunicka-Styczyńska, A.; Biela, T. PLA/β-CD-based fibres loaded with quercetin as potential antibacterial dressing materials. Colloids Surf. B Biointerfaces 2020, 190, 110949. [Google Scholar] [CrossRef]
- Wu, G.; Ma, X.; Fan, L.; Gao, Y.; Deng, H.; Wang, Y. Accelerating dermal wound healing and mitigating excessive scar formation using LBL modified nanofibrous mats. Mater. Des. 2020, 185, 108265. [Google Scholar] [CrossRef]
- Liao, N.; Unnithan, A.R.; Joshi, M.K.; Tiwari, A.P.; Hong, S.T.; Park, C.-H.; Kim, C.S. Electrospun bioactive poly (ɛ-caprolactone)–cellulose acetate–dextran antibacterial composite mats for wound dressing applications. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 194–201. [Google Scholar] [CrossRef]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of Electrospun Nanofibers for Biomedical and Dental Applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef]
- Asran, A.S.; Razghandi, K.; Aggarwal, N.; Michler, G.H.; Groth, T. Nanofibers from Blends of Polyvinyl Alcohol and Polyhydroxy Butyrate As Potential Scaffold Material for Tissue Engineering of Skin. Biomacromolecules 2010, 11, 3413–3421. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef]
- Kuppan, P.; Vasanthan, K.S.; Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Development of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Fibers for Skin Tissue Engineering: Effects of Topography, Mechanical, and Chemical Stimuli. Biomacromolecules 2011, 12, 3156–3165. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piras, A.M.; Chiellini, F.; Chiellini, E.; Nikkola, L.; Ashammakhi, N. New Multicomponent Bioerodible Electrospun Nanofibers for Dual-controlled Drug Release. J. Bioact. Compat. Polym. 2008, 23, 423–443. [Google Scholar] [CrossRef]
- Nagiah, N.; Madhavi, L.; Anitha, R.; Srinivasan, N.T.; Sivagnanam, U.T. Electrospinning of poly (3-hydroxybutyric acid) and gelatin blended thin films: Fabrication, characterization, and application in skin regeneration. Polym. Bull. 2013, 70, 2337–2358. [Google Scholar] [CrossRef]
- da Silva, M.A.C.; Oliveira, R.N.; Mendonça, R.H.; Lourenço, T.G.B.; Colombo, A.P.V.; Tanaka, M.N.; Tude, E.M.O.; da Costa, M.F.; Thiré, R.M.S.M. Evaluation of metronidazole-loaded poly(3-hydroxybutyrate) membranes to potential application in periodontitis treatment. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Rennukka, M.; Sipaut, C.S.; Amirul, A.A. Synthesis of poly(3-hydroxybutyrate-co-4-hydroxybutyrate)/chitosan/silver nanocomposite material with enhanced antimicrobial activity. Biotechnol. Prog. 2014, 30, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-T.; Zhang, Y.; Chen, G.-Q. Nanofibrous polyhydroxyalkanoate matrices as cell growth supporting materials. Biomaterials 2008, 29, 3720–3728. [Google Scholar] [CrossRef] [PubMed]
- Paşcu, E.I.; Stokes, J.; McGuinness, G.B. Electrospun composites of PHBV, silk fibroin and nano-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C 2013, 33, 4905–4916. [Google Scholar] [CrossRef]
- Sadeghi, D.; Karbasi, S.; Razavi, S.; Mohammadi, S.; Shokrgozar, M.A.; Bonakdar, S. Electrospun poly(hydroxybutyrate)/chitosan blend fibrous scaffolds for cartilage tissue engineering. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Müller, T.; Schubert, D.W.; Boccaccini, A.R.; Yao, Q.; Roether, J.A. Electrospun Polyhydroxybutyrate/Poly(ε-caprolactone)/58S Sol–Gel Bioactive Glass Hybrid Scaffolds with Highly Improved Osteogenic Potential for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 17098–17108. [Google Scholar] [CrossRef]
- Nagiah, N.; Madhavi, L.; Anitha, R.; Anandan, C.; Srinivasan, N.T.; Sivagnanam, U.T. Development and characterization of coaxially electrospun gelatin coated poly (3-hydroxybutyric acid) thin films as potential scaffolds for skin regeneration. Mater. Sci. Eng. C 2013, 33, 4444–4452. [Google Scholar] [CrossRef]
- Zhan, J.; Morsi, Y.; EI-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Preparation and characterization of electrospun in-situ cross-linked gelatin-graphite oxide nanofibers. J. Biomater. Sci. Polym. Ed. 2016, 27, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K.; Chhabra, H.; Soni, V.P.; Bellare, J.R. Enhanced mechanical strength and biocompatibility of electrospun polycaprolactone-gelatin scaffold with surface deposited nano-hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 2376–2385. [Google Scholar] [CrossRef] [PubMed]
- Hofman, K.; Tucker, N.; Stanger, J.; Staiger, M.; Marshall, S.; Hall, B. Effects of the molecular format of collagen on characteristics of electrospun fibres. J. Mater. Sci. 2012, 47, 1148–1155. [Google Scholar] [CrossRef]
- Alvarez Perez, M.A.; Guarino, V.; Cirillo, V.; Ambrosio, L. In vitro mineralization and bone osteogenesis in poly(ε-caprolactone)/gelatin nanofibers. J. Biomed. Mater. Res. Part A 2012, 100, 3008–3019. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Pérez-Mateos, M.; Gómez-Estaca, J.; López-Caballero, E.; Giménez, B.; Montero, P. Fish gelatin: A renewable material for developing active biodegradable films. Trends Food Sci. Technol. 2009, 20, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Zhang, X.; Hu, X.; Kaplan, D.L. Green Process to Prepare Silk Fibroin/Gelatin Biomaterial Scaffolds. Macromol. Biosci. 2010, 10, 289–298. [Google Scholar] [CrossRef]
- Sisson, K.; Zhang, C.; Farach-Carson, M.C.; Chase, D.B.; Rabolt, J.F. Fiber diameters control osteoblastic cell migration and differentiation in electrospun gelatin. J. Biomed. Mater. Res. Part A 2010, 94, 1312–1320. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Y.; Fang, Z.; Xiao, R.; Yuan, W.; Khan, M. Fabrication and characterization of electrospun gelatin-heparin nanofibers as vascular tissue engineering. Macromol. Res. 2013, 21, 860–869. [Google Scholar] [CrossRef]
- Meng, Z.X.; Li, H.F.; Sun, Z.Z.; Zheng, W.; Zheng, Y.F. Fabrication of mineralized electrospun PLGA and PLGA/gelatin nanofibers and their potential in bone tissue engineering. Mater. Sci. Eng. C 2013, 33, 699–706. [Google Scholar] [CrossRef]
- Li, D.; Sun, H.; Jiang, L.; Zhang, K.; Liu, W.; Zhu, Y.; Fangteng, J.; Shi, C.; Zhao, L.; Sun, H.; et al. Enhanced Biocompatibility of PLGA Nanofibers with Gelatin/Nano-Hydroxyapatite Bone Biomimetics Incorporation. ACS Appl. Mater. Interfaces 2014, 6, 9402–9410. [Google Scholar] [CrossRef]
- Tan, L.; Hu, J.; Huang, H.; Han, J.; Hu, H. Study of multi-functional electrospun composite nanofibrous mats for smart wound healing. Int. J. Biol. Macromol. 2015, 79, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Hu, J.; Zhao, H. Design of bilayered nanofibrous mats for wound dressing using an electrospinning technique. Mater. Lett. 2015, 156, 46–49. [Google Scholar] [CrossRef]
- Merkle, V.M.; Martin, D.; Hutchinson, M.; Tran, P.L.; Behrens, A.; Hossainy, S.; Sheriff, J.; Bluestein, D.; Wu, X.; Slepian, M.J. Hemocompatibility of Poly(vinyl alcohol)–Gelatin Core–Shell Electrospun Nanofibers: A Scaffold for Modulating Platelet Deposition and Activation. ACS Appl. Mater. Interfaces 2015, 7, 8302–8312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, X.; Salick, M.R.; Cordie, T.; Mi, H.-Y.; Peng, X.-F.; Turng, L.-S. Electrospinning Homogeneous Nanofibrous Poly(propylene carbonate)/Gelatin Composite Scaffolds for Tissue Engineering. Ind. Eng. Chem. Res. 2014, 53, 9391–9400. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Ognibene, G.; Gangemi, C.M.A.; Spitaleri, L.; Gulino, A.; Purrello, R.; Cicala, G.; Fragalà, M.E. Role of the surface composition of the polyethersulfone–TiiP–H2T4 fibers on lead removal: From electrostatic to coordinative binding. J. Mater. Sci. 2019, 54, 8023–8033. [Google Scholar] [CrossRef]
- Abutaleb, A. Catalytic and Photocatalytic Electrospun Nanofibers for Hydrogen Generation from Ammonia Borane Complex: A Review. Polymers 2021, 13, 2290. [Google Scholar] [CrossRef]
- Gangemi, C.M.A.; Iudici, M.; Spitaleri, L.; Randazzo, R.; Gaeta, M.; D’Urso, A.; Gulino, A.; Purrello, R.; Fragalà, M.E. Polyethersulfone Mats Functionalized with Porphyrin for Removal of Para-nitroaniline from Aqueous Solution. Molecules 2019, 24, 3344. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Cáceres, R.; Cabeza, L.; Perazzoli, G.; Díaz, A.; López-Romero, J.M.; Melguizo, C.; Prados, J. Electrospun Nanofibers: Recent Applications in Drug Delivery and Cancer Therapy. Nanomaterials 2019, 9, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmeri, M.; Ognibene, G.; Saitta, L.; Lombardo, C.; Genovese, C.; Barcellona, M.; D’Urso, A.; Spitaleri, L.; Blanco, I.; Cicala, G.; et al. Optimization of ZnO Nanorods Growth on Polyetheresulfone Electrospun Mats to Promote Antibacterial Properties. Molecules 2020, 25, 1696. [Google Scholar] [CrossRef] [Green Version]
- Heunis, T.D.J.; Dicks, L.M.T. Nanofibers Offer Alternative Ways to the Treatment of Skin Infections. J. Biomed. Biotechnol. 2010, 2010, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, D.; EI-Shanshory, A.; El-Newehy, M.; EI-Hamshary, H.A.; Al-Deyab, S.S.; He, C.; Mo, X. Dexamethasone loaded core-shell SF/PEO nanofibers via green electrospinning reduced endothelial cells inflammatory damage. Colloids Surf. B Biointerfaces 2015, 126. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R. A review of the evidence for the use of topical antimicrobial agents in wound care. World Wide Wounds 2004, 1, 1–8. [Google Scholar]
- El-Newehy, M.H.; Al-Deyab, S.S.; Kenawy, E.-R.; Abdel-Megeed, A. Fabrication of electrospun antimicrobial nanofibers containing metronidazole using nanospider technology. Fibers Polym. 2012, 13, 709–717. [Google Scholar] [CrossRef]
- Saffaj, T.; Charrouf, M.; Abourriche, A.; Aboud, Y.; Bennamara, A.; Berrada, M. Spectrophotometric determination of Metronidazole and Secnidazole in pharmaceutical preparations based on the formation of dyes. Dye. Pigment. 2006, 70, 259–262. [Google Scholar] [CrossRef]
- Yolanda, L. Toro Suarez Martindale: The Extra Pharmacopoeia, 28th ed.; Reynolds, J.E.F., Ed.; The Pharmaceutical Press: London, UK, 2015; pp. 1–27. ISBN 9781787284395. [Google Scholar]
- Desai, N.C.; Maheta, A.S.; Rajpara, K.M.; Joshi, V.V.; Vaghani, H.V.; Satodiya, H.M. Green synthesis of novel quinoline based imidazole derivatives and evaluation of their antimicrobial activity. J. Saudi Chem. Soc. 2014, 18, 963–971. [Google Scholar] [CrossRef] [Green Version]
- Katsandri, A.; Avlamis, A.; Pantazatou, A.; Petrikkos, G.L.; Legakis, N.J.; Papaparaskevas, J. In vitro activities of tigecycline against recently isolated Gram-negative anaerobic bacteria in Greece, including metronidazole-resistant strains. Diagn. Microbiol. Infect. Dis. 2006, 55, 231–236. [Google Scholar] [CrossRef]
- Trindade, L.C.T.; Biondo-Simões, M.D.L.P.; Sampaio, C.P.P.; Farias, R.E.; Pierin, R.J.; Chomiski Netto, M. Evaluation of topical metronidazole in the healing wounds process: An experimental study. Rev. Col. Bras. Cir. 2010, 37, 358–362. [Google Scholar] [CrossRef]
- Grove, D.I.; Mahmoud, A.A.F.; Warren, K.S. Suppression of Cell-Mediated Immunity by Metronidazole. Int. Arch. Allergy Immunol. 1977, 54, 422–427. [Google Scholar] [CrossRef]
- Trindade, L.C.T.; Matias, J.E.F.; Sampaio, C.P.P.; Farias, R.E.; Biondo-Simões, M.D.L.P. Diferenciação de miofibroblastos em feridas após uso tópico do metronidazol: Estudo experimental. Rev. Col. Bras. Cir. 2019, 46, e2015. [Google Scholar] [CrossRef]
- Xue, J.; He, M.; Liu, H.; Niu, Y.; Crawford, A.; Coates, P.D.; Chen, D.; Shi, R.; Zhang, L. Drug loaded homogeneous electrospun PCL/gelatin hybrid nanofiber structures for anti-infective tissue regeneration membranes. Biomaterials 2014, 35, 9395–9405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, J.; Niu, Y.; Gong, M.; Shi, R.; Chen, D.; Zhang, L.; Lvov, Y. Electrospun Microfiber Membranes Embedded with Drug-Loaded Clay Nanotubes for Sustained Antimicrobial Protection. ACS Nano 2015, 9, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Shi, R.; Niu, Y.; Gong, M.; Coates, P.; Crawford, A.; Chen, D.; Tian, W.; Zhang, L. Fabrication of drug-loaded anti-infective guided tissue regeneration membrane with adjustable biodegradation property. Colloids Surf. B Biointerfaces 2015, 135, 846–854. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhong, Q.; Ge, Y.; Guo, Z.; Tian, J.; Zhou, Y.; Ding, S.; Li, H.; Zhou, C. Dual drug loaded coaxial electrospun PLGA/PVP fiber for guided tissue regeneration under control of infection. Mater. Sci. Eng. C 2018, 90, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Passos, P.C.; Moro, J.; Barcelos, R.C.S.; Da Rosa, H.Z.; Vey, L.T.; Bürguer, M.E.; Maciel, R.M.; Danesi, C.C.; Edwards, P.C.; Bottino, M.C.; et al. Nanofibrous antibiotic-eluting matrices: Biocompatibility studies in a rat model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 306–315. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Zhang, Y.; Wen, S.; Wang, Y.; Zhang, H. Dual functional electrospun core-shell nanofibers for anti-infective guided bone regeneration membranes. Mater. Sci. Eng. C 2019, 98, 134–139. [Google Scholar] [CrossRef]
- Zupančič, Š.; Preem, L.; Kristl, J.; Putrinš, M.; Tenson, T.; Kocbek, P.; Kogermann, K. Impact of PCL nanofiber mat structural properties on hydrophilic drug release and antibacterial activity on periodontal pathogens. Eur. J. Pharm. Sci. 2018, 122, 347–358. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Tong, Y.; Jiang, Z.; Wang, C. Nitrofurazone-loaded electrospun PLLA/sericin-based dual-layer fiber mats for wound dressing applications. RSC Adv. 2015, 5, 16940–16949. [Google Scholar] [CrossRef]
- Biemer, J.J. Antimicrobial susceptibility testing by the Kirby-Bauer disc diffusion method. Ann. Clin. Lab. Sci. 1973, 3, 135–140. [Google Scholar]
- El-Aassar, M.R.; Ibrahim, O.M.; Fouda, M.M.G.; El-Beheri, N.G.; Agwa, M.M. Wound healing of nanofiber comprising Polygalacturonic/Hyaluronic acid embedded silver nanoparticles: In-vitro and in-vivo studies. Carbohydr. Polym. 2020, 238, 116175. [Google Scholar] [CrossRef]
- Shalaby, M.; Agwa, M.; Saeed, H.; Khedr, S.M.; Morsy, O.; El-Demellawy, M.A. Fish Scale Collagen Preparation, Characterization and Its Application in Wound Healing. J. Polym. Environ. 2020, 28, 166–178. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; El-Beheri, N.G.; Agwa, M.M.; Eltaher, H.M.; Alseqely, M.; Sadik, W.S.; El-Khordagui, L. Antibiotic-free combinational hyaluronic acid blend nanofibers for wound healing enhancement. Int. J. Biol. Macromol. 2021, 167, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- El-Lakany, S.A.; Abd-Elhamid, A.I.; Kamoun, E.A.; El-Fakharany, E.M.; Samy, W.M.; Elgindy, N.A. α-Bisabolol-Loaded Cross-Linked Zein Nanofibrous 3D-Scaffolds For Accelerating Wound Healing And Tissue Regeneration In Rats. Int. J. Nanomed. 2019, 14, 8251–8270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fayez, M.S.; Hakim, T.A.; Agwa, M.M.; Abdelmoteleb, M.; Aly, R.G.; Montaser, N.N.; Abdelsattar, A.S.; Rezk, N.; El-Shibiny, A. Topically Applied Bacteriophage to Control Multi-Drug Resistant Klebsiella pneumoniae Infected Wound in a Rat Model. Antibiotics 2021, 10, 1048. [Google Scholar] [CrossRef]
- Omer, A.M.; Tamer, T.M.; Khalifa, R.E.; Eltaweil, A.S.; Agwa, M.M.; Sabra, S.; Abd-Elmonem, M.S.; Mohy-Eldin, M.S.; Ziora, Z.M. Formulation and Antibacterial Activity Evaluation of Quaternized Aminochitosan Membrane for Wound Dressing Applications. Polymers 2021, 13, 2428. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.; Swielam, E.M.; Atwa, N.A.; Agwa, M.M. Novel design of bandages using cotton pads, doped with chitosan, glycogen and ZnO nanoparticles, having enhanced antimicrobial and wounds healing effects. Int. J. Biol. Macromol. 2022, 197, 121–130. [Google Scholar] [CrossRef]
- Eslami, A.; Gallant-Behm, C.L.; Hart, D.A.; Wiebe, C.; Honardoust, D.; Gardner, H.; Häkkinen, L.; Larjava, H.S. Expression of Integrin αvβ6 and TGF-β in Scarless vs Scar-forming Wound Healing. J. Histochem. Cytochem. 2009, 57, 543–557. [Google Scholar] [CrossRef] [Green Version]
- Muyonga, J.; Cole, C.G.; Duodu, K. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Sanhueza, C.; Hermosilla, J.; Bugallo-Casal, A.; Da Silva-Candal, A.; Taboada, C.; Millán, R.; Concheiro, A.; Alvarez-Lorenzo, C.; Acevedo, F. One-step electrospun scaffold of dual-sized gelatin/poly-3-hydroxybutyrate nano/microfibers for skin regeneration in diabetic wound. Mater. Sci. Eng. C 2021, 119, 111602. [Google Scholar] [CrossRef]
- Chong, E.; Phan, T.; Lim, I.; Zhang, Y.; Bay, B.; Ramakrishna, S.; Lim, C. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef]
- Shan, Y.-H.; Peng, L.-H.; Liu, X.; Chen, X.; Xiong, J.; Gao, J.-Q. Silk fibroin/gelatin electrospun nanofibrous dressing functionalized with astragaloside IV induces healing and anti-scar effects on burn wound. Int. J. Pharm. 2015, 479, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Felciya, S.J.G.; Devi, M.V.; Ramanathan, G.; Poornima, V.; Sivagnanam, U.T. Fabrication of polyhydroxy butyric acid–Gelatin blended nanofibrous matrix integrated with silver sulfadiazine as an alternate wound dressing for treating burns. Mater. Lett. 2021, 282, 128541. [Google Scholar] [CrossRef]
- Hu, J.; Prabhakaran, M.P.; Tian, L.; Ding, X.; Ramakrishna, S. Drug-loaded emulsion electrospun nanofibers: Characterization, drug release and in vitro biocompatibility. RSC Adv. 2015, 5, 100256–100267. [Google Scholar] [CrossRef]
- Naderi, P.; Zarei, M.; Karbasi, S.; Salehi, H. Evaluation of the effects of keratin on physical, mechanical and biological properties of poly (3-hydroxybutyrate) electrospun scaffold: Potential application in bone tissue engineering. Eur. Polym. J. 2020, 124, 109502. [Google Scholar] [CrossRef]
- Zupančič, Š.; Potrč, T.; Baumgartner, S.; Kocbek, P.; Kristl, J. Formulation and evaluation of chitosan/polyethylene oxide nanofibers loaded with metronidazole for local infections. Eur. J. Pharm. Sci. 2016, 95, 152–160. [Google Scholar] [CrossRef]
- Sabra, S.; Ragab, D.M.; Agwa, M.M.; Rohani, S. Recent advances in electrospun nanofibers for some biomedical applications. Eur. J. Pharm. Sci. 2020, 144, 105224. [Google Scholar] [CrossRef]
- Gharibi, R.; Yeganeh, H.; Rezapour-Lactoee, A.; Hassan, Z.M. Stimulation of Wound Healing by Electroactive, Antibacterial, and Antioxidant Polyurethane/Siloxane Dressing Membranes: In Vitro and in Vivo Evaluations. ACS Appl. Mater. Interfaces 2015, 7, 24296–24311. [Google Scholar] [CrossRef]
- Ramanathan, G.; Singaravelu, S.; Raja, M.D.; Nagiah, N.; Padmapriya, P.; Ruban, K.; Kaveri, K.; Natarajan, T.S.; Sivagnanam, U.T.; Perumal, P.T. Fabrication and characterization of a collagen coated electrospun poly(3-hydroxybutyric acid)–gelatin nanofibrous scaffold as a soft bio-mimetic material for skin tissue engineering applications. RSC Adv. 2016, 6, 7914–7922. [Google Scholar] [CrossRef]
- Ousey, K. The role of topical metronidazole in management of infected wounds. Wounds UK 2018, 14, 105–109. [Google Scholar]
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Qin, W.; Zhou, Y.; Chen, B.; Zhao, X.; Zhao, H.; Mi, E.; Mi, E.; Wang, Q.; Ning, J. Transforming growth factor β plays an important role in enhancing wound healing by topical application of Povidone-iodine. Sci. Rep. 2017, 7, 991. [Google Scholar] [CrossRef]
- Alves, C.C.; Torrinhas, R.S.; Giorgi, R.; Brentani, M.M.; Logullo, A.F.; Waitzberg, D.L. TGF-β1 expression in wound healing is acutely affected by experimental malnutrition and early enteral feeding. Int. Wound J. 2014, 11, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, C.P.P.; Biondo-Simões, M.D.L.P.; Trindade, L.C.T.; Farias, R.E.; Pierin, R.J.; Martins, R.C. Alterações inflamatórias provocadas pelo metronidazol em feridas: Estudo experimental em ratos. J. Vasc. Bras. 2009, 8, 232–237. [Google Scholar] [CrossRef] [Green Version]
- Claros-Chacaltana, F.D.Y.; Aldrovani, M.; Kobashigawa, K.K.; Padua, I.R.M.; Valdetaro, G.P.; de Barros Sobrinho, A.A.F.; Abreu, T.G.M.; Laus, J.L. Effect of metronidazole ophthalmic solution on corneal neovascularization in a rat model. Int. Ophthalmol. 2019, 39, 1123–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalska, M.; Palatyńska-Ulatowska, A.; Palatyński, A.; Mirowski, M.; Kaplińska, K.; Nawrot-Modranka, J.; Lazarenkow, A. Influence of antibiotic therapy on the level of selected angiogenic factors in patients with benign gynecologic tumors—Preliminary report. Pharmazie 2011, 66, 619–622. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [Green Version]
- Banks, W.A.; Kastin, A.J.; Gutierrez, E.G. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci. Lett. 1994, 179, 53–56. [Google Scholar] [CrossRef]
- Mallikarjuna Rao, C.; Ghosh, A.; Raghothama, C.; Bairy, K. Does metronidazole reduce lipid peroxidation in burn injuries to promote healing? Burns 2002, 28, 427–429. [Google Scholar] [CrossRef]
- Ypsilantis, E.; Carapeti, E.; Chan, S. The use of topical 10% metronidazole in the treatment of non-healing pilonidal sinus wounds after surgery. Int. J. Colorectal Dis. 2016, 31, 765–767. [Google Scholar] [CrossRef] [PubMed]
- Nishimuta, K.; Ito, Y. Effects of metronidazole and tinidazole ointments on models for inflammatory dermatitis in mice. Arch. Dermatol. Res. 2003, 294, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, H.; Oguchi, M.; Nishijima, S.; Asada, Y.; Takahashi, M.; Ushijima, T.; Niwa, Y. The inhibition of free radical generation by human neutrophils through the synergistic effects of metronidazole with palmitoleic acid: A possible mechanism of action of metronidazole in rosacea and acne. Arch. Dermatol. Res. 1990, 282, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, Y.; Imamura, S.; Niwa, Y. Anti-oxidant action of metronidazole: A possible mechanism of action in rosacea. Br. J. Dermatol. 1986, 114, 231–234. [Google Scholar] [CrossRef]













| Thermal Characters | Polymer Matrices (PHB/GEL) without Drug | Drug Loaded Polymer Matrices (Z@PHB/GEL) | |||||
|---|---|---|---|---|---|---|---|
| 10:0 | 8:2 | 7:3 | Z1@8:2 | Z1@7:3 | Z2@ 8:2 | Z2@7:3 | |
| Td Tonset (°C) | 235.83 | 246.11 | 247.91 | 243.36 | 231.36 | 223.75 | 209.27 |
| Tmax 50% Weight Loss Temp. (°C) | 257.67 | 276.18 | 282.14 | 277.76 | 276.97 | 273.59 | 272.56 |
| 1st DS Temp. range (°C) | 25.84–217.93 | 24.2–69.06 | 27.56–73.36 | 18.12–63.34 | 25.72–77.76 | 26.31–72.96 | 16.06–89.68 |
| 1st DS Weight loss (%) | 0.384 | 1.647 | 2.41 | 1.548 | 2.354 | 1.342 | 2.868 |
| 2nd DS Temp. range (°C) | 217.93–272.27 | 69.06–225.10 | 73.36–229.87 | 63.34–227.96 | 77.76–205.58 | 72.96–193.42 | 89.68–199.33 |
| 2nd DS Weight loss (%) | 82.635 | 1.250 | 1.175 | 2.221 | 1.406 | 1.393 | 1.781 |
| 3rd DS Temp. range (°C) | 273.03–375.96 | 225.10–289.89 | 230.63–294 | 227.96–290.94 | 205.58–288.62 | 193.42–285.96 | 199.33–283.4 |
| 3rd DS Weight loss (%) | 10.104 | 78.6 | 69.012 | 75.496 | 68.819 | 77.752 | 66.46 |
| 4th DS Temp. range (°C) | 375.96–799.94 | 289.89–433.28 | 294–445.16 | 290.94–432.77 | 288.62–452.03 | 285.96–426.3 | 283.40–473.99 |
| 4th DS Weight loss (%) | 6.685 | 11.678 | 17.46 | 11.141 | 16.808 | 10.52 | 15.711 |
| 5th DS Temp. range (°C) | ------ | 433.28–799.81 | 444.4–799.79 | 432.54–799.1 | 452.03–799.89 | 427.07–799.91 | 473.99–799.1 |
| 5th DS Weight loss (%) | ------ | 5.633 | 7.201 | 8.297 | 6.72 | 6.409 | 11.117 |
| Sample Name | Mean Tensile Strength (MPa) | Mean Elongation at Break (%) | (%) Porosity | Water Uptake (%) | Contact Angle (°) |
|---|---|---|---|---|---|
| PHB | 6.5 ± 0.3 | 11.5 ± 0.4 | 80 ± 0.32 | 180.2 ± 0.3 | 102.8 ± 0.6 |
| 8:2 | 6.61 ± 0.05 | 15.6 ± 0.3 | 71.2 ± 1 | 320.1 ± 0.2 | 52.73 ± 1.1 |
| 7:3 | 8.5 ± 0.03 | 10.5 ± 0.2 | 56.2 ± 1.07 | 315.2 ± 0.1 | 44.79 ± 1.2 |
| Z1@8:2 | 2.5 ± 0.08 | 2.4 ±1.2 | 60.1 ± 0.1 | 320 ± 0.1 | 129.34 ± 0.7 |
| Z1@7:3 | 4.1 ± 0.05 | 7.2 ± 0.5 | 65.2 ± 0.3 | 330 ± 0.2 | 62.12 ± 08 |
| Z2@8:2 | 2.7 ± 0.06 | 2.8 ± 1.1 | 68.1 ± 1 | 335 ± 0.1 | 48.88 ± 1.2 |
| Z2@7:3 | 2.6 ± 0.03 | 3.9 ±1.4 | 72.4 ± 0.2 | 350 ± 0.2 | 35.2 ± 0.9 |
| Wound Diameter (mm) (Mean ± SDM) and% of Wound Closure (W.C.) | Observation Day | |||||
|---|---|---|---|---|---|---|
| Zero Day | Day 3 | Day 7 | Day 10 | Day 14 | ||
| Positive Control (sterile gauze) | Diameter | 15.55 ± 0.39 | 14.1 ± 0.56 | 12.08 ± 0.28 | 8.56 ± 0.38 | 5.5 ± 0.44 |
| % W.C | 0 | 17.39 | 39.41 | 69.53 | 87.39 | |
| 7:3 | Diameter | 15.56 ± 0.25 | 13.86 ± 0.30 | 11.05 ± 0.13 | 9.15 ± 0.45 | 0.5 ± 0.58 |
| % W.C | 0 | 22.24 | 49.82 | 65.54 | 99.79 | |
| Z2@7:3 | Diameter | 15.46 ± 0.19 | 13.58 ± 13.58 | 9.85 ± 0.78 | 6.025 ± 0.32 | 0.4 ± 0.55 |
| % W.C | 0 | 24.18 | 60.56 | 85.28 | 99.83 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Shanshory, A.A.; Agwa, M.M.; Abd-Elhamid, A.I.; Soliman, H.M.A.; Mo, X.; Kenawy, E.-R. Metronidazole Topically Immobilized Electrospun Nanofibrous Scaffold: Novel Secondary Intention Wound Healing Accelerator. Polymers 2022, 14, 454. https://doi.org/10.3390/polym14030454
El-Shanshory AA, Agwa MM, Abd-Elhamid AI, Soliman HMA, Mo X, Kenawy E-R. Metronidazole Topically Immobilized Electrospun Nanofibrous Scaffold: Novel Secondary Intention Wound Healing Accelerator. Polymers. 2022; 14(3):454. https://doi.org/10.3390/polym14030454
Chicago/Turabian StyleEl-Shanshory, Ahmed A., Mona M. Agwa, Ahmed I. Abd-Elhamid, Hesham M. A. Soliman, Xiumei Mo, and El-Refaie Kenawy. 2022. "Metronidazole Topically Immobilized Electrospun Nanofibrous Scaffold: Novel Secondary Intention Wound Healing Accelerator" Polymers 14, no. 3: 454. https://doi.org/10.3390/polym14030454
APA StyleEl-Shanshory, A. A., Agwa, M. M., Abd-Elhamid, A. I., Soliman, H. M. A., Mo, X., & Kenawy, E.-R. (2022). Metronidazole Topically Immobilized Electrospun Nanofibrous Scaffold: Novel Secondary Intention Wound Healing Accelerator. Polymers, 14(3), 454. https://doi.org/10.3390/polym14030454