Polyelectrolyte Multilayered Capsules as Biomedical Tools
Abstract
:1. Introduction
2. Assembly Methodologies for the Fabrication of PEMUCs
2.1. Immersive Assembly: Dip-Coating Deposition on Flat Surfaces
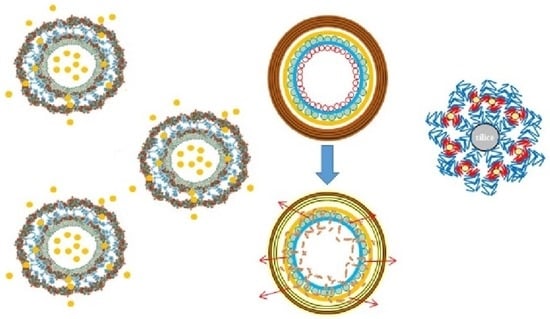
2.2. Towards the Fabrication of Hollow Capsules: Immersive Assembly on Colloidal Templates
2.3. Deposition Assisted by Magnetic Fields
2.4. Deposition on Immobilized Colloids
2.5. Towards the Scalability of the Fabrication of PEMUCs
2.6. New Avenues on the Fabrication of PEMUCs: Microfluidic Approaches
3. A Brief Introduction to the Physico-Chemical Aspects Driving the Formation of LbL Polyelectrolyte Multilayers
3.1. Growth of Polyelectrolyte Multilayers
3.2. Charge Balance in Polyelectrolyte Multilayers: Inversion and Compensation
4. Encapsulation in LbL Materials
4.1. Encapsulation Approaches
4.1.1. Post-Loading
4.1.2. Pre-Loading
4.2. Controlling the Release of Encapsulated Molecules
4.2.1. Environmental Induced Release
4.2.2. Physically Induced Release
4.2.3. Redox Induced Release
4.2.4. Biochemical Induced Release
5. Layer-by-Layer Capsules for Antibiotic Release
6. Layer-by-Layer Capsules for Anticancer Therapy
7. Layer-by-Layer Capsules for Gene Delivery
8. Layer-by-Layer Capsules for Diabetes
9. Layer-by-Layer Capsules for Theranostic Purposes
10. Layer-by-Layer Capsules for Hyperthermia Treatments
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caruso, F.; Caruso, R.A.; Möhwald, H. Nanoengineering of Inorganic and Hybrid Hollow Spheres by Colloidal Templating. Science 1998, 282, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel Hollow Polymer Shells by Colloid-Templated Assembly of Polyelectrolytes. Angew. Chem. Int. Ed. 1998, 37, 2201–2205. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Donath, E.; Lichtenfeld, H.; Knippel, E.; Knippel, M.; Budde, A.; Möhwald, H. Layer-by-layer self assembly of polyelectrolytes on colloidal particles. Colloids Surf. A 1998, 137, 253–266. [Google Scholar] [CrossRef]
- Caruso, F.; Lichtenfeld, H.; Giersig, M.; Mohwald, H. Electrostatic self-assembly of silica nanoparticle—Polyelectrolyte multilayers on polystyrene latex particles. J. Am. Chem. Soc. 1998, 120, 8523–8524. [Google Scholar] [CrossRef]
- Musin, E.V.; Kim, A.L.; Dubrovskii, A.V.; Tikhonenko, S.A. New sight at the organization of layers of multilayer polyelectrolyte microcapsules. Sci. Rep. 2021, 11, 14040. [Google Scholar] [CrossRef]
- Ariga, K.; Lvov, Y.; Decher, G. There is still plenty of room for layer-by-layer assembly for constructing nanoarchitectonics-based materials and devices. Phys. Chem. Chem. Phys. 2022, 24. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Zhao, S.; Caruso, F.; Dähne, L.; Decher, G.; De Geest, B.G.; Fan, J.; Feliu, N.; Gogotsi, Y.; Hammond, P.T.; Hersam, M.C.; et al. The Future of Layer-by-Layer Assembly: A Tribute to ACS Nano Associate Editor Helmuth Möhwald. ACS Nano 2019, 13, 6151–6169. [Google Scholar] [CrossRef] [Green Version]
- Schlenoff, J.B. Retrospective on the Future of Polyelectrolyte Multilayers. Langmuir 2009, 25, 14007–14010. [Google Scholar] [CrossRef]
- Lavalle, P.; Voegel, J.-C.; Vautier, D.; Senger, B.; Schaaf, P.; Ball, V. Dynamic Aspects of Films Prepared by a Sequential Deposition of Species: Perspectives for Smart and Responsive Materials. Adv. Mater. 2011, 23, 1191–1221. [Google Scholar] [CrossRef]
- Guzmán, E.; Mateos-Maroto, A.; Ruano, M.; Ortega, F.; Rubio, R.G. Layer-by-Layer polyelectrolyte assemblies for encapsulation and release of active compounds. Adv. Colloid Interface Sci. 2017, 249, 290–307. [Google Scholar] [CrossRef] [PubMed]
- del Mercato, L.L.; Rivera-Gil, P.; Abbasi, A.Z.; Ochs, M.; Ganas, C.; Zins, I.; Sönnichsen, C.; Parak, W.J. LbL multilayer capsules: Recent progress and future outlook for their use in life sciences. Nanoscale 2010, 2, 458–467. [Google Scholar] [CrossRef]
- del Mercato, L.L.; Ferraro, M.M.; Baldassarre, F.; Mancarella, S.; Greco, V.; Rinaldi, R.; Leporatti, S. Biological applications of LbL multilayer capsules: From drug delivery to sensing. Adv. Colloid Interface Sci. 2014, 207, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Rubio, R.G.; Ortega, F. A closer physico-chemical look to the Layer-by-Layer electrostatic self-assembly of polyelectrolyte multilayers. Adv. Colloid Interface Sci. 2020, 282, 102197. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.R.; Mano, J.F. Polyelectrolyte multilayered assemblies in biomedical technologies. Chem. Soc. Rev. 2014, 43, 3453–3479. [Google Scholar] [CrossRef]
- Hammond, P.T. Building biomedical materials layer-by-layer. Mater. Today 2012, 15, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Kurapati, R.; Groth, T.W.; Raichur, A.M. Recent Developments in Layer-by-Layer Technique for Drug Delivery Applications. ACS Appl. Bio Mater. 2019, 2, 5512–5527. [Google Scholar] [CrossRef]
- Song, X.; Li, H.; Tong, W.; Gao, C. Fabrication of triple-labeled polyelectrolyte microcapsules for localized ratiometric pH sensing. J. Colloid Interface Sci. 2014, 416, 252–257. [Google Scholar] [CrossRef]
- Popov, A.L.; Popova, N.R.; Tarakina, N.V.; Ivanova, O.S.; Ermakov, A.M.; Ivanov, V.K.; Sukhorukov, G.B. Intracellular Delivery of Antioxidant CeO2 Nanoparticles via Polyelectrolyte Microcapsules. ACS Biomater. Sci. Eng. 2018, 4, 2453–2462. [Google Scholar] [CrossRef]
- Gao, H.; Wen, D.; Sukhorukov, G.B. Composite silica nanoparticle/polyelectrolyte microcapsules with reduced permeability and enhanced ultrasound sensitivity. J. Mater. Chem. B 2015, 3, 1888–1897. [Google Scholar] [CrossRef]
- She, Z.; Wang, C.; Li, J.; Sukhorukov, G.B.; Antipina, M.N. Encapsulation of Basic Fibroblast Growth Factor by Polyelectrolyte Multilayer Microcapsules and Its Controlled Release for Enhancing Cell Proliferation. Biomacromolecules 2012, 13, 2174–2180. [Google Scholar] [CrossRef] [PubMed]
- Bukreeva, T.V.; Parakhonsky, B.V.; Skirtach, A.G.; Susha, A.S.; Sukhorukov, G.B. Preparation of polyelectrolyte microcapsules with silver and gold nanoparticles in a shell and the remote destruction of microcapsules under laser irradiation. Crystallogr. Rep. 2006, 51, 863–869. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Shutava, T.; Shchukina, E.; Sukhorukov, G.B.; Lvov, Y.M. Modified Polyelectrolyte Microcapsules as Smart Defense Systems. Chem. Mater. 2004, 16, 3446–3451. [Google Scholar] [CrossRef]
- Reshetilov, A.; Plekhanova, Y.; Tarasov, S.; Tikhonenko, S.; Dubrovsky, A.; Kim, A.; Kashin, V.; Machulin, A.; Wang, G.-J.; Kolesov, V.; et al. Bioelectrochemical Properties of Enzyme-Containing Multilayer Polyelectrolyte Microcapsules Modified with Multiwalled Carbon Nanotubes. Membranes 2019, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volodkin, D.V.; Larionova, N.I.; Sukhorukov, G.B. Protein Encapsulation via Porous CaCO3 Microparticles Templating. Biomacromolecules 2004, 5, 1962–1972. [Google Scholar] [CrossRef]
- Lucia, A.; Toloza, A.C.; Guzmán, E.; Ortega, F.; Rubio, R.G. Novel polymeric micelles for insect pest control: Encapsulation of essential oil monoterpenes inside a triblock copolymer shell for head lice control. PeerJ 2017, 5, e3171. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.L.; Johnston, A.P.R.; Caruso, F. Layer-By-Layer-Assembled Capsules and Films for Therapeutic Delivery. Small 2010, 6, 1836–1852. [Google Scholar] [CrossRef] [PubMed]
- Anandhakumar, S.; Debapriya, M.; Nagaraja, V.; Raichur, A.M. Polyelectrolyte microcapsules for sustained delivery of water-soluble drugs. Mater. Sci. Eng. C 2011, 31, 342–349. [Google Scholar] [CrossRef]
- Borodina, T.; Yurina, D.; Sokovikov, A.; Karimov, D.; Bukreeva, T.; Khaydukov, E.; Shchukin, D. A microwave-triggered opening of the multifunctional polyelectrolyte capsules with nanodiamonds in the shell composition. Polymer 2021, 212, 123299. [Google Scholar] [CrossRef]
- She, S.; Xu, C.; Yin, X.; Tong, W.; Gao, C. Shape Deformation and Recovery of Multilayer Microcapsules after Being Squeezed through a Microchannel. Langmuir 2012, 28, 5010–5016. [Google Scholar] [CrossRef]
- Borvinskaya, E.; Gurkov, A.; Shchapova, E.; Mutin, A.; Timofeyev, M. Histopathological analysis of zebrafish after introduction of non-biodegradable polyelectrolyte microcapsules into the circulatory system. PeerJ 2021, 9, e11337. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, T.A.; Kiragosyan, G.; Le, T.H.N.; Springer, S.; Winterhalter, M. Protein A Functionalized Polyelectrolyte Microcapsules as a Universal Platform for Enhanced Targeting of Cell Surface Receptors. ACS Appl. Mater. Interfaces 2017, 9, 11506–11517. [Google Scholar] [CrossRef] [PubMed]
- Simioni, A.R.; de Jesus, P.C.C.; Tedesco, A.C. Layer-by-layer hollow photosensitizer microcapsule design via a manganese carbonate hard template for photodynamic therapy in cells. Photodiagn. Photodyn. Ther. 2018, 22, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Novoselova, M.V.; Voronin, D.V.; Abakumova, T.O.; Demina, P.A.; Petrov, A.V.; Petrov, V.V.; Zatsepin, T.S.; Sukhorukov, G.B.; Gorin, D.A. Focused ultrasound-mediated fluorescence of composite microcapsules loaded with magnetite nanoparticles: In vitro and in vivo study. Colloids Surf. B 2019, 181, 680–687. [Google Scholar] [CrossRef]
- Pechenkin, M.A.; Möhwald, H.; Volodkin, D.V. pH- and salt-mediated response of layer-by-layer assembled PSS/PAH microcapsules: Fusion and polymer exchange. Soft Matter 2012, 8, 8659–8665. [Google Scholar] [CrossRef]
- Kalenichenko, D.; Nifontova, G.; Karaulov, A.; Sukhanova, A.; Nabiev, I. Designing Functionalized Polyelectrolyte Microcapsules for Cancer Treatment. Nanomaterials 2021, 11, 3055. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Yashchenok, A.M.; Möhwald, H. Encapsulation, release and applications of LbL polyelectrolyte multilayer capsules. Chem. Commun. 2011, 47, 12736–12746. [Google Scholar] [CrossRef]
- De Geest, B.G.; Sukhorukov, G.B.; Möhwald, H. The pros and cons of polyelectrolyte capsules in drug delivery. Exp. Opin. Drug Deliv. 2009, 6, 613–624. [Google Scholar] [CrossRef]
- Boehnke, N.; Correa, S.; Hao, L.; Wang, W.; Straehla, J.P.; Bhatia, S.N.; Hammond, P.T. Theranostic Layer-by-Layer Nanoparticles for Simultaneous Tumor Detection and Gene Silencing. Angew. Chem. Int. Ed. 2020, 59, 2776–2783. [Google Scholar] [CrossRef]
- Hong, X.; Li, J.; Wang, M.; Xu, J.; Guo, W.; Li, J.; Bai, Y.; Li, T. Fabrication of Magnetic Luminescent Nanocomposites by a Layer-by-Layer Self-assembly Approach. Chem. Mater. 2004, 16, 4022–4027. [Google Scholar] [CrossRef]
- Sousa, C.F.V.; Fernandez-Megia, E.; Borges, J.; Mano, J.F. Supramolecular dendrimer-containing layer-by-layer nanoassemblies for bioapplications: Current status and future prospects. Polym. Chem. 2021, 12, 5902–5930. [Google Scholar] [CrossRef]
- Mateos-Maroto, A.; Abelenda-Núñez, I.; Ortega, F.; Rubio, R.G.; Guzmán, E. Polyelectrolyte Multilayers on Soft Colloidal Nanosurfaces: A New Life for the Layer-By-Layer Method. Polymers 2021, 13, 1221. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.J.; Cui, J.; Björnmalm, M.; Braunger, J.A.; Ejima, H.; Caruso, F. Innovation in Layer-by-Layer Assembly. Chem. Rev. 2016, 116, 14828–14867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decher, G.; Hong, J.-D. Buildup of ultrathin multilayer films by a self-assembly process, 1 consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Makromol. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D. Buildup of Ultrathin Multilayer Films by a Self-Assembly Process: II. Consecutive Adsorption of Anionic and Cationic Bipolar Amphiphiles and Polyelectrolytes on Charged Surfaces. Ber. Bunsenges. Physik. Chem. 1991, 95, 1430–1434. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 1992, 210–211, 831–835. [Google Scholar] [CrossRef]
- Iler, R.K. Multilayers of colloidal particles. J. Colloid Interface Sci. 1966, 21, 569–594. [Google Scholar] [CrossRef]
- Decher, G.; Schmitt, J. Fine-Tuning of the film thickness of ultrathin multilayer film composed of consecutively alternating layers of anionic and cationic polyelectrolytes. Prog. Colloid Polym. Sci. 1992, 89, 160–164. [Google Scholar] [CrossRef]
- Xiang, Y.; Lu, S.; Jiang, S.P. Layer-by-layer self-assembly in the development of electrochemical energy conversion and storage devices from fuel cells to supercapacitors. Chem. Soc. Rev. 2012, 41, 7291–7321. [Google Scholar] [CrossRef]
- Michel, M.; Toniazzo, V.; Ruch, D.; Ball, V. Deposition mechanisms in layer-by-layer or step-by-step deposition methods: From elastic and impermeable films to soft membranes with ion exchange properties. ISRN Mater. Sci. 2012, 2012, 701695. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, P.; Jonas, A.; Laschewsky, A.; Legras, R. Ultrathin polymer coatings by complexation of polyelectrolytes at interfaces: Suitable materials, structure and properties. Macromol. Rapid Commun. 2000, 21, 319–348. [Google Scholar] [CrossRef]
- Yan, Y.; Björnmalm, M.; Caruso, F. Assembly of Layer-by-Layer Particles and Their Interactions with Biological Systems. Chem. Mater. 2014, 26, 452–460. [Google Scholar] [CrossRef]
- Donath, E.; Walther, D.; Shilov, V.N.; Knippel, E.; Budde, A.; Lowack, K.; Helm, C.A.; Möhwald, H. Nonlinear Hairy Layer Theory of Electrophoretic Fingerprinting Applied to Consecutive Layer by Layer Polyelectrolyte Adsorption onto Charged Polystyrene Latex Particles. Langmuir 1997, 13, 5294–5305. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Donath, E.; Davis, S.; Lichtenfeld, H.; Caruso, F.; Popov, V.I.; Möhwald, H. Stepwise polyelectrolyte assembly on particle surfaces: A novel approach to colloid design. Polym. Adv. Technol. 1998, 9, 759–767. [Google Scholar] [CrossRef]
- Bagaria, H.G.; Wong, M.S. Polyamine-salt aggregate assembly of capsules as responsive drug delivery vehicles. J. Mater. Chem. 2011, 21, 9454–9466. [Google Scholar] [CrossRef]
- Tong, W.; Gao, C. Multilayer microcapsules with tailored structures for bio-related applications. J. Mater. Chem. 2008, 18, 3799–3812. [Google Scholar] [CrossRef]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef] [Green Version]
- Decher, G.; Schlenoff, J.B. (Eds.) Multilayer Thin Films-Sequential Assembly of Nanocomposite Materials; Wiley-VCH Verlag: Berlin, Germany, 2003. [Google Scholar]
- Voigt, A.; Lichtenfeld, H.; Sukhorukov, G.B.; Zastrow, H.; Donath, E.; Baumler, H.; Mohwald, H. Membrane Filtration for Microencapsulation and Microcapsules Fabrication by Layer-by-Layer Polyelectrolyte Adsorption. Ind. Eng. Chem. Res. 1999, 38, 4037–4043. [Google Scholar] [CrossRef]
- Hong, J.; Char, K.; Kim, B.-S. Hollow Capsules of Reduced Graphene Oxide Nanosheets Assembled on a Sacrificial Colloidal Particle. J. Phys. Chem. Lett. 2010, 1, 3442–3445. [Google Scholar] [CrossRef]
- Szczepanowicz, K.; Hoel, H.J.; Szyk-Warszynska, L.; Bielańska, E.; Bouzga, A.M.; Gaudernack, G.; Simon, C.; Warszynski, P. Formation of Biocompatible Nanocapsules with Emulsion Core and Pegylated Shell by Polyelectrolyte Multilayer Adsorption. Langmuir 2010, 26, 12592–12597. [Google Scholar] [CrossRef]
- Hoogeveen, N.G.; Cohen Stuart, M.A.; Fleer, G.J.; Böhmer, M.R. Formation and Stability of Multilayers of Polyelectrolytes. Langmuir 1996, 12, 3675–3681. [Google Scholar] [CrossRef]
- Bantchev, G.; Lu, Z.; Lvov, Y. Layer-by-layer nanoshell assembly on colloids through simplified washless process. J. Nanosci. Nanotechnol. 2009, 9, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowicz, K.; Dronka-Góra, D.; Para, G.; Warszyński, P. Encapsulation of liquid cores by layer-by-layer adsorption of polyelectrolytes. J. Microencapsul. 2010, 27, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Grigoriev, D.O.; Bukreeva, T.; Möhwald, H.; Shchukin, D.G. New Method for Fabrication of Loaded Micro- and Nanocontainers: Emulsion Encapsulation by Polyelectrolyte Layer-by-Layer Deposition on the Liquid Core. Langmuir 2008, 24, 999–1004. [Google Scholar] [CrossRef]
- Thanasukarn, P.; Pongsawatmanit, R.; McClements, D. Utilization of layer-by-layer interfacial deposition technique to improve freeze–thaw stability of oil-in-water emulsions. Food Res. Int. 2006, 39, 721–729. [Google Scholar] [CrossRef]
- Li, J.; Stöver, H.D.H. Pickering Emulsion Templated Layer-by-Layer Assembly for Making Microcapsules. Langmuir 2010, 26, 15554–15560. [Google Scholar] [CrossRef] [PubMed]
- Rossier-Miranda, F.J.; Schroën, K.; Boom, R. Microcapsule production by an hybrid colloidosome-layer-by-layer technique. Food Hydrocoll. 2012, 27, 119–125. [Google Scholar] [CrossRef]
- Liu, H.; Gu, X.; Hu, M.; Hu, Y.; Wang, C. Facile fabrication of nanocomposite microcapsules by combining layer-by-layer self-assembly and Pickering emulsion templating. RSC Adv. 2014, 4, 16751–16758. [Google Scholar] [CrossRef]
- Guzmán, E.; Ruano, M.; Ortega, F.; Rubio, R.G. Stratified Interpolyelectrolyte Complexes: Fabrication, Structure and Properties. In Polyelectrolytes; Visakh, P.M., Bayraktar, O., Picó, G.A., Eds.; Springer: Cham, Switzerland, 2014; pp. 299–347. [Google Scholar] [CrossRef]
- Ruano, M.; Mateos-Maroto, A.; Ortega, F.; Ritacco, H.; Rubio, J.E.F.; Guzmán, E.; Rubio, R.G. Fabrication of Robust Capsules by Sequential Assembly of Polyelectrolytes onto Charged Liposomes. Langmuir 2021, 37, 6189–6200. [Google Scholar] [CrossRef]
- Mu, B.; Liu, P.; Du, P.; Dong, Y.; Lu, C. Magnetic-targeted pH-responsive drug delivery system via layer-by-layer self-assembly of polyelectrolytes onto drug-containing emulsion droplets and its controlled release. J. Polym. Sci. A Polym. Chem. 2011, 49, 1969–1976. [Google Scholar] [CrossRef]
- Wilson, R.; Spiller, D.G.; Prior, I.A.; Bhatt, R.; Hutchinson, A. Magnetic microspheres encoded with photoluminescent quantum dots for multiplexed detection. J. Mater. Chem. 2007, 17, 4400–4406. [Google Scholar] [CrossRef]
- Richardson, J.J.; Liang, K.; Kempe, K.; Ejima, H.; Cui, J.; Caruso, F. Immersive Polymer Assembly on Immobilized Particles for Automated Capsule Preparation. Adv. Mater. 2013, 25, 6874–6878. [Google Scholar] [CrossRef] [PubMed]
- Jaklenec, A.; Anselmo, A.C.; Hong, J.; Vegas, A.J.; Kozminsky, M.; Langer, R.; Hammond, P.T.; Anderson, D.G. High Throughput Layer-by-Layer Films for Extracting Film Forming Parameters and Modulating Film Interactions with Cells. ACS Appl. Mater. Interfaces 2016, 8, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Alkekhia, D.; Hammond, P.T.; Shukla, A. Layer-by-Layer Biomaterials for Drug Delivery. Ann. Rev. Biomed. Eng. 2020, 22, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Peiffre, D.G.; Corley, T.J.; Halpern, G.M.; Brinker, B.A. Utilization of polymeric materials in laser fusion target fabrication. Polymer 1981, 22, 450–460. [Google Scholar] [CrossRef]
- Fan, R.; Li, J.; Fei, J.; Zhao, J.; Wang, A.; Sun, B.; Li, J. Automatic Assembly of Ultra-Multilayered Nanotube–Nanoparticle Composites. Chem. Asian J. 2016, 11, 2667–2670. [Google Scholar] [CrossRef]
- Nagaraja, A.T.; You, Y.-H.; Choi, J.-W.; Hwang, J.-H.; Meissner, K.E.; McShane, M.J. Layer-by-layer modification of high surface curvature nanoparticles with weak polyelectrolytes using a multiphase solvent precipitation process. J. Colloid Interface Sci. 2016, 466, 432–441. [Google Scholar] [CrossRef] [Green Version]
- Elizarova, I.S.; Luckham, P.F. Fabrication of polyelectrolyte multilayered nano-capsules using a continuous layer-by-layer approach. J. Colloid Interface Sci. 2016, 470, 92–99. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Guo, X.; Hu, Q.; Qin, C.; Liu, H.; Dong, M.; Chen, Y. Layer-by-layer assembled biopolymer microcapsule with separate layer cavities generated by gas-liquid microfluidic approach. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 13–19. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Cheng, Y.; Kim, E.; Rubloff, G.W.; Bentley, W.E.; Payne, G.F. Coupling Electrodeposition with Layer-by-Layer Assembly to Address Proteins within Microfluidic Channels. Adv. Mater. 2011, 23, 5817–5821. [Google Scholar] [CrossRef]
- Lee, U.N.; Day, J.H.; Haack, A.J.; Bretherton, R.C.; Lu, W.; DeForest, C.A.; Theberge, A.B.; Berthier, E. Layer-by-layer fabrication of 3D hydrogel structures using open microfluidics. Lab Chip 2020, 20, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Björnmalm, M.; Yan, Y.; Caruso, F. Engineering and evaluating drug delivery particles in microfluidic devices. J. Control. Release 2014, 190, 139–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priest, C.; Quinn, A.; Postma, A.; Zelikin, A.N.; Ralston, J.; Caruso, F. Microfluidic polymer multilayer adsorption on liquid crystal droplets for microcapsule synthesis. Lab Chip 2008, 8, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Matosevic, S.; Paegel, B.M. Layer-by-layer cell membrane assembly. Nat. Chem. 2013, 5, 958–963. [Google Scholar] [CrossRef] [Green Version]
- Mets, J.M.; Wilson, J.T.; Cui, W.; Chaikof, E.L. An automated process for layer-by-layer assembly of polyelectrolyte multilayer thin films on viable cell aggregates. Adv. Healthc. Mater. 2013, 2, 266–270. [Google Scholar] [CrossRef]
- Kantak, C.; Beyer, S.; Yobas, L.; Bansal, T.; Trau, D. A ‘microfluidic pinball’ for on-chip generation of Layer-by-Layer polyelectrolyte microcapsules. Lab Chip 2011, 11, 1030–1035. [Google Scholar] [CrossRef]
- Raman, N.; Lee, M.-R.; Palecek, S.P.; Lynn, D.M. Polymer multilayers loaded with antifungal β-peptides kill planktonic Candida albicans and reduce formation of fungal biofilms on the surfaces of flexible catheter tubes. J. Control. Release 2014, 191, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Madaboosi, N.; Uhlig, K.; Jäger, M.S.; Möhwald, H.; Duschl, C.; Volodkin, D.V. Microfluidics as A Tool to Understand the Build-Up Mechanism of Exponential-Like Growing Films. Macromol. Rapid Commun. 2012, 33, 1775–1779. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, K.; Kumar, S.; Kim, J. Dynamic Sequential Layer-by-Layer Deposition Method for Fast and Region-Selective Multilayer Thin Film Fabrication. Langmuir 2005, 21, 8532–8538. [Google Scholar] [CrossRef]
- Richardson, J.J.; Teng, D.; Björnmalm, M.; Gunawan, S.T.; Guo, J.; Cui, J.; Franks, G.V.; Caruso, F. Fluidized Bed Layer-by-Layer Microcapsule Formation. Langmuir 2014, 30, 10028–10034. [Google Scholar] [CrossRef]
- Noi, K.F.; Roozmand, A.; Björnmalm, M.; Richardson, J.J.; Franks, G.V.; Caruso, F. Assembly-Controlled Permeability of Layer-by-Layer Polymeric Microcapsules Using a Tapered Fluidized Bed. ACS Appl. Mater. Interfaces 2015, 7, 27940–27947. [Google Scholar] [CrossRef] [Green Version]
- von Klitzing, R. Internal Structure of polyelectrolyte multilayer assemblies. Phys. Chem. Chem. Phys. 2006, 8, 5012–5033. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, V.A.; Zezin, A.B. Soluble interpolymeric complexes as a new class of synthetic polyelectrolytes. Pure Appl. Chem. 1984, 56, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Philipp, B.; Dautzenberg, H.; Linow, K.J.; Kötz, J.; Dawydoff, W. Polyelectrolyte complexes—Recent developments and open problems. Prog. Polym. Sci. 1989, 14, 91–172. [Google Scholar] [CrossRef]
- van der Gucht, J.; Spruijt, E.; Lemmers, M.; Cohen Stuart, M.A. Polyelectrolyte complexes: Bulk phases and colloidal systems. J. Colloid Interface Sci. 2011, 361, 407–422. [Google Scholar] [CrossRef]
- Guzmán, E.; Ritacco, H.; Ortega, F.; Rubio, R.G. Evidence of the influence of adsorption kinetics on the internal reorganization of polyelectrolyte multilayers. Colloids Surf. A 2011, 384, 274–281. [Google Scholar] [CrossRef]
- Guzmán, E.; Ritacco, H.A.; Ortega, F.; Rubio, R.G. Growth of Polyelectrolyte Layers Formed by Poly (4-styrenesulfonate sodium salt) and Two Different Polycations: New Insights from Study of Adsorption Kinetics. J. Phys. Chem. C 2012, 116, 15474–15483. [Google Scholar] [CrossRef]
- Johansson, E.; Blomberg, E.; Lingström, R.; Wägberg, L. Adhesive Interaction between Polyelectrolyte Multilayers of Polyallylamine Hydrochloride and Polyacrylic Acid Studied Using Atomic Force Microscopy and Surface Force Apparatus. Langmuir 2009, 25, 2887–2894. [Google Scholar] [CrossRef]
- Picart, C.; Lavalle, P.; Hubert, P.; Cuisinier, F.J.G.; Decher, G.; Schaaf, P.; Voegel, J.C. Buildup Mechanism for Poly(l-lysine)/Hyaluronic Acid Films onto a Solid Surface. Langmuir 2001, 17, 7414–7424. [Google Scholar] [CrossRef]
- Lavalle, P.; Gergely, C.; Cuisinier, F.J.G.; Decher, G.; Schaaf, P.; Voegel, J.C.; Picart, C. Comparison of the Structure of Polyelectrolyte Multilayer Films Exhibiting a Linear and an Exponential Growth Regime: An in Situ Atomic Force Microscopy Study. Macromolecules 2002, 35, 4458–4465. [Google Scholar] [CrossRef]
- Schneider, A.; Richert, L.; Francius, G.; Voegel, J.-C.; Picart, C. Elasticity, biodegradability and cell adhesive properties of chitosan/hyaluronan multilayer films. Biomed. Mater. 2007, 2, S45–S51. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Cavallo, J.A.; Chuliá-Jordán, R.; Gómez, C.; Strumia, M.C.; Ortega, F.; Rubio, R.G. pH-Induced Changes in the Fabrication of Multilayers of Poly (acrylic acid) and Chitosan: Fabrication, Properties, and Tests as a Drug Storage and Delivery System. Langmuir 2011, 27, 6836–6845. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Chuliá-Jordán, R.; Ortega, F.; Rubio, R.G. Influence of the percentage of acetylation on the assembly of LbL multilayers of poly (acrylic acid) and chitosan. Phys. Chem. Chem. Phys. 2011, 13, 18200–18207. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Maestro, A.; Llamas, S.; Álvarez-Rodríguez, J.; Ortega, F.; Maroto-Valiente, Á.; Rubio, R.G. 3D solid supported inter-polyelectrolyte complexes obtained by the alternate deposition of poly (diallyldimethylammonium chloride) and poly (sodium 4-styrenesulfonate). Beilstein J. Nanotechnol. 2016, 7, 197–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzmán, E.; Ritacco, H.; Rubio, J.E.F.; Rubio, R.G.; Ortega, F. Salt-induced changes in the growth of polyelectrolyte layers of poly (diallyl-dimethylammonium chloride) and poly (4-styrene sulfonate of sodium). Soft Matter 2009, 5, 2130–2142. [Google Scholar] [CrossRef]
- Schlenoff, J.B.; Dubas, S.T. Mechanism of Polyelectrolyte Multilayer Growth: Charge Overcompensation and Distribution. Macromolecules 2001, 34, 592–598. [Google Scholar] [CrossRef]
- Dubas, S.T.; Schlenoff, J.B. Factors Controlling the Growth of Polyelectrolyte Multilayers. Macromolecules 1999, 32, 8153–8160. [Google Scholar] [CrossRef]
- Cini, N.; Tulun, T.; Decher, G.; Ball, V. Step-by-Step Assembly of Self-Patterning Polyelectrolyte Films Violating (Almost) All Rules of Layer-by-Layer Deposition. J. Am. Chem. Soc. 2010, 132, 8264–8265. [Google Scholar] [CrossRef]
- Cini, N.; Tulun, T.; Blanck, C.; Toniazzo, V.; Ruch, D.; Decher, G.; Ball, V. Slow complexation dynamics between linear short polyphosphates and polyallylamines: Analogies with “layer-by-layer” deposition. Phys. Chem. Chem. Phys. 2012, 14, 3048–3056. [Google Scholar] [CrossRef]
- Joanny, J.F. Polyelectrolyte adsorption and charge inversion. Eur. Phys. J. B 1999, 9, 117–122. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Comment on “Formation of polyelectrolyte multilayers: Ionic strengths and growth regimes” by K. Tang and A. M. Besseling, Soft Matter, 2016, 12, 1032. Soft Matter 2016, 12, 8460–8463. [Google Scholar] [CrossRef] [PubMed]
- Berndt, P.; Kurihara, K.; Kunitake, T. Adsorption of poly (styrenesulfonate) onto an ammonium monolayer on mica: A surface forces study. Langmuir 1992, 8, 2486–2490. [Google Scholar] [CrossRef]
- Caruso, F.; Donath, E.; Möhwald, H. Influence of Polyelectrolyte Multilayer Coatings on Förster Resonance Energy Transfer between 6-Carboxyfluorescein and Rhodamine B-Labeled Particles in Aqueous Solution. J. Phys. Chem. B 1998, 102, 2011–2016. [Google Scholar] [CrossRef]
- Schwarz, S.; Eichhorn, K.J.; Wischerhoff, E.; Laschewsky, A. Polyelectrolyte adsorption onto planar surfaces: A study by streaming potential and ellipsometry measurements. Colloids Surf. A 1999, 159, 491–501. [Google Scholar] [CrossRef]
- Ringwald, C.; Ball, V. Shear induced changes in the streaming potential of polyelectrolyte multilayer films. Colloids Surf. A 2015, 464, 41–45. [Google Scholar] [CrossRef]
- Ladam, G.; Schaad, P.; Voegel, J.C.; Schaaf, P.; Decher, G.; Cuisinier, F. In Situ Determination of the Structural Properties of Initially Deposited Polyelectrolyte Multilayers. Langmuir 2000, 16, 1249–1255. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Zembala, M.; Kolasińska, M.; Warszyński, P. Characterization of polyelectrolyte multilayers on mica and oxidized titanium by streaming potential and wetting angle measurements. Colloids Surf. A 2007, 302, 455–460. [Google Scholar] [CrossRef]
- Ferriz-Mañas, M.; Schlenoff, J.B. Zeta Potential of Polyelectrolyte Multilayers Using the Spinning Disk Method. Langmuir 2014, 30, 8776–8783. [Google Scholar] [CrossRef]
- Ghostine, R.A.; Markarian, M.Z.; Schlenoff, J.B. Asymmetric Growth in Polyelectrolyte Multilayers. J. Am. Chem. Soc. 2013, 135, 7636–7646. [Google Scholar] [CrossRef]
- Fares, H.M.; Schlenoff, J.B. Equilibrium Overcompensation in Polyelectrolyte Complexes. Macromolecules 2017, 50, 3968–3978. [Google Scholar] [CrossRef]
- von Klitzing, R.; Moehwald, H. Proton Concentration Profile in Ultrathin Polyelectrolyte Films. Langmuir 1995, 11, 3554–3559. [Google Scholar] [CrossRef]
- Fernández-Peña, L.; Guzmán, E.; Ortega, F.; Bureau, L.; Leonforte, F.; Velasco, D.; Rubio, R.G.; Luengo, G.S. Physico-chemical study of polymer mixtures formed by a polycation and a zwitterionic copolymer in aqueous solution and upon adsorption onto negatively charged surfaces. Polymer 2021, 217, 123442. [Google Scholar] [CrossRef]
- Rmaile, H.H.; Schlenoff, J.B. “Internal pKa’s” in Polyelectrolyte Multilayers: Coupling Protons and Salt. Langmuir 2002, 18, 8263–8265. [Google Scholar] [CrossRef]
- Lourenço, J.M.C.; Ribeiro, P.A.; do Rego, A.M.B.; Raposo, M. Counterions in layer-by-layer films—Influence of the drying process. J. Colloid Interface Sci. 2007, 313, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, J.M.C.; Ribeiro, P.A.; do Rego, A.M.B.; Braz Fernandes, F.M.; Moutinho, A.M.C.; Raposo, M. Counterions in Poly (allylamine hydrochloride) and Poly (styrene sulfonate) Layer-by-Layer Films. Langmuir 2004, 20, 8103–8109. [Google Scholar] [CrossRef]
- Llamas, S.; Guzmán, E.; Ortega, F.; Rubio, R.G. Adsorption of Mixtures of a Pegylated Lipid with Anionic and Zwitterionic Surfactants at Solid/Liquid. Colloids Interfaces 2020, 4, 47. [Google Scholar] [CrossRef]
- Guzmán, E.; San Miguel, V.; Peinado, C.; Ortega, F.; Rubio, R.G. Polyelectrolyte Multilayers Containing Triblock Copolymers of Different Charge Ratio. Langmuir 2010, 26, 11494–11502. [Google Scholar] [CrossRef] [Green Version]
- Ghoussoub, Y.E.; Zerball, M.; Fares, H.M.; Ankner, J.F.; von Klitzing, R.; Schlenoff, J.B. Ion distribution in dry polyelectrolyte multilayers: A neutron reflectometry study. Soft Matter 2018, 14, 1699–1708. [Google Scholar] [CrossRef]
- Lehaf, A.M.; Hariri, H.H.; Schlenoff, J.B. Homogeneity, Modulus, and Viscoelasticity of Polyelectrolyte Multilayers by Nanoindentation: Refining the Buildup Mechanism. Langmuir 2012, 28, 6348–6355. [Google Scholar] [CrossRef] [Green Version]
- Ghostine, R.A.; Jisr, R.M.; Lehaf, A.; Schlenoff, J.B. Roughness and Salt Annealing in a Polyelectrolyte Multilayer. Langmuir 2013, 29, 11742–11750. [Google Scholar] [CrossRef]
- Volodkin, D.; von Klitzing, R. Competing mechanisms in polyelectrolyte multilayer formation and swelling: Polycation–polyanion pairing vs. polyelectrolyte–ion pairing. Curr. Opin. Colloid Interface Sci. 2014, 19, 25–31. [Google Scholar] [CrossRef]
- Laugel, N.; Betscha, C.; Winterhalter, M.; Voegel, J.-C.; Schaaf, P.; Ball, V. Relationship between the Growth Regime of Polyelectrolyte Multilayers and the Polyanion/Polycation Complexation Enthalpy. J. Phys. Chem. B 2006, 110, 19443–19449. [Google Scholar] [CrossRef] [PubMed]
- Haynie, D.T.; Cho, E.; Waduge, P. “In and Out Diffusion” Hypothesis of Exponential Multilayer Film Buildup Revisited. Langmuir 2011, 27, 5700–5704. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, D.B.; Sukhorukov, G.B. Engineered microcrystals for direct surface modification with layer-by-layer technique for optimized dissolution. Eur. J. Pharm. Biopharm. 2004, 58, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Villiers, M.M.d.; Otto, D.P.; Strydom, S.J.; Lvov, Y.M. Introduction to nanocoatings produced by layer-by-layer (LbL) self-assembly. Adv. Drug Deliv. Rev. 2011, 63, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Leporatti, S.; Donath, E.; Möhwald, H. Studies on the Drug Release Properties of Polysaccharide Multilayers Encapsulated Ibuprofen Microparticles. Langmuir 2001, 17, 5375–5380. [Google Scholar] [CrossRef]
- Hirsjärvi, S.; Qiao, Y.; Royere, A.; Bibette, J.; Benoit, J.-P. Layer-by-layer surface modification of lipid nanocapsules. Eur. J. Pharm. Biopharm. 2010, 76, 200–207. [Google Scholar] [CrossRef]
- Veerabadran, N.G.; Goli, P.L.; Stewart-Clark, S.S.; Lvov, Y.M.; Mills, D.K. Nanoencapsulation of stem cells within polyelectrolyte multilayer shells. Macromol. Biosci. 2007, 7, 877–882. [Google Scholar] [CrossRef]
- Gao, C.; Leporatti, S.; Moya, S.; Donath, E.; Möhwald, H. Swelling and Shrinking of Polyelectrolyte Microcapsules in Response to Changes in Temperature and Ionic Strength. Chem. Eur. J. 2003, 9, 915–920. [Google Scholar] [CrossRef]
- Antipov, A.A.; Sukhorukov, G.B.; Leporatti, S.; Radtchenko, I.L.; Donath, E.; Mohwald, H. Polyelectrolyte multilayer capsule permeability control. Colloids Surf. A 2002, 198–200, 535–541. [Google Scholar] [CrossRef]
- Petrov, A.I.; Gavryushkin, A.V.; Sukhorukov, G.B. Effect of Temperature, pH and Shell Thickness on the Rate of Mg2+ and Ox2- Release from Multilayered Polyelectrolyte Shells Deposited onto Microcrystals of Magnesium Oxalate. J. Phys. Chem. B 2003, 107, 868–875. [Google Scholar] [CrossRef]
- Liu, X.Q.; Picart, C. Layer-by-Layer Assemblies for Cancer Treatment and Diagnosis. Adv. Mater. 2016, 28, 1295–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koker, S.D.; Hoogenboom, R.; Geest, B.G.D. Polymeric multilayer capsules for drug delivery. Chem. Soc. Rev. 2012, 41, 2867–2884. [Google Scholar] [CrossRef] [PubMed]
- Skorb, E.V.; Möhwald, H. 25th anniversary article: Dynamic interfaces for responsive encapsulation systems. Adv. Mater. 2013, 25, 5029–5043. [Google Scholar] [CrossRef] [PubMed]
- Podgórna, K.; Szczepanowicz, K. Synthesis of polyelectrolyte nanocapsules with iron oxide (Fe3O4) nanoparticles for magnetic targeting. Colloids Surf. A 2016, 505, 132–137. [Google Scholar] [CrossRef]
- Yi, Q.; Sukhorukov, G.B. UV light stimulated encapsulation and release by polyelectrolyte microcapsules. Adv. Colloid Interface Sci. 2014, 207, 280–289. [Google Scholar] [CrossRef]
- Städler, B.; Chandrawati, R.; Price, A.D.; Chong, S.-F.; Breheney, K.; Postma, A.; Connal, L.A.; Zelikin, A.N.; Caruso, F. A Microreactor with Thousands of Subcompartments: Enzyme-Loaded Liposomes within Polymer Capsules. Angew. Chem. Int. Ed. 2009, 48, 4359–4362. [Google Scholar] [CrossRef]
- Städler, B.; Chandrawati, R.; Goldie, K.; Caruso, F. Capsosomes: Subcompartmentalizing Polyelectrolyte Capsules Using Liposomes. Langmuir 2009, 25, 6725–6732. [Google Scholar] [CrossRef]
- Hosta-Rigau, L.; Städler, B.; Yan, Y.; Nice, E.C.; Heath, J.K.; Albericio, F.; Caruso, F. Capsosomes with Multilayered Subcompartments: Assembly and Loading with Hydrophobic Cargo. Adv. Funct. Mater. 2010, 20, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Stadler, B.; Price, A.D.; Chandrawati, R.; Hosta-Rigau, L.; Zelikin, A.N.; Caruso, F. Polymer hydrogel capsules: En route toward synthetic cellular systems. Nanoscale 2009, 1, 68–73. [Google Scholar] [CrossRef]
- Omidvar, M.; Zdarta, J.; Sigurdardóttir, S.B.; Pinelo, M. Mimicking natural strategies to create multi-environment enzymatic reactors: From natural cell compartments to artificial polyelectrolyte reactors. Biotechnol. Adv. 2022, 54, 107798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-N.; Gu, J.; Jia, S.; Guan, Y.; Zhang, Y. Zero-order release of polyphenolic drugs from dynamic, hydrogen-bonded LBL films. Soft Matter 2016, 12, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Shan, G.; Bao, Y.; Wu, Z.L.; Pan, P. Thermoresponsive physical hydrogels of poly (lactic acid)/poly (ethylene glycol) stereoblock copolymers tuned by stereostructure and hydrophobic block sequence. Soft Matter 2016, 12, 4628–4637. [Google Scholar] [CrossRef] [PubMed]
- Poon, Z.; Lee, J.B.; Morton, S.W.; Hammond, P.T. Controlling in vivo stability and biodistribution in electrostatically assembled nanoparticles for systemic delivery. Nano Lett. 2011, 11, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- Gil, P.R.; del Mercato, L.L.; del_Pino, P.; Muñoz_Javier, A.; Parak, W.J. Nanoparticle-modified polyelectrolyte capsules. Nano Today 2008, 3, 12–21. [Google Scholar] [CrossRef]
- Ibarz, G.; Dähne, L.; Donath, E.; Möhwald, H. Smart Micro- and Nanocontainers for Storage, Transport, and Release. Adv. Mater. 2001, 13, 1324–1327. [Google Scholar] [CrossRef]
- De Cock, L.J.; De Koker, S.; De Geest, B.G.; Grooten, J.; Vervaet, C.; Remon, J.P.; Sukhorukov, G.B.; Antipina, M.N. Polymeric Multilayer Capsules in Drug Delivery. Angew. Chem. Int. Ed. 2010, 49, 6954–6973. [Google Scholar] [CrossRef]
- Skorb, E.V.; Möhwald, H. “Smart” Surface Capsules for Delivery Devices. Adv. Mater. Interfaces 2014, 1, 1400237. [Google Scholar] [CrossRef]
- Gao, C.Y.; Donath, E.; Mohwald, H.; Shen, J.C. Spontaneous deposition of water-soluble substances into microcapsules: Phenomenon, mechanism, and application Angew. Chem. Int. Ed. 2002, 41, 3789–3793. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Chen, J.; Zavgorodnya, O.; Hasan, M.B.; Kharlampieva, E. Multilayer Hydrogel Capsules of Interpenetrated Network for Encapsulation of Small Molecules. Langmuir 2018, 34, 11832–11842. [Google Scholar] [CrossRef]
- Ermakov, A.V.; Verkhovskii, R.A.; Babushkina, I.V.; Trushina, D.B.; Inozemtseva, O.A.; Lukyanets, E.A.; Ulyanov, V.J.; Gorin, D.A.; Belyakov, S.; Antipina, M.N. In Vitro Bioeffects of Polyelectrolyte Multilayer Microcapsules Post-Loaded with Water-Soluble Cationic Photosensitizer. Pharmaceutics 2020, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- She, Z.; Antipina, M.N.; Li, J.; Sukhorukov, G.B. Mechanism of Protein Release from Polyelectrolyte Multilayer Microcapsules. Biomacromolecules 2010, 11, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, A.V.; Inozemtseva, O.A.; Gorin, D.A.; Sukhorukov, G.B.; Belyakov, S.; Antipina, M.N. Influence of Heat Treatment on Loading of Polymeric Multilayer Microcapsules with Rhodamine B. Macromol. Rapid Commun. 2019, 40, 1800200. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, L.; Bell, M.; Ashwell, R.; Volodkin, D.; Vikulina, A.S. Internal Structure of Matrix-Type Multilayer Capsules Templated on Porous Vaterite CaCO3 Crystals as Probed by Staining with a Fluorescence Dye. Micromachines 2018, 9, 547. [Google Scholar] [CrossRef] [Green Version]
- Parakhonskiy, B.V.; Yashchenok, A.M.; Möhwald, H.; Volodkin, D.; Skirtach, A.G. Release from Polyelectrolyte Multilayer Capsules in Solution and on Polymeric Surfaces. Adv. Mater. Interfaces 2017, 4, 1600273. [Google Scholar] [CrossRef] [Green Version]
- Petrov, A.I.; Volodkin, D.V.; Sukhorukov, G.B. Protein-calcium carbonate coprecipitation: A tool for protein encapsulation. Biotechnol. Progr. 2005, 21, 918–925. [Google Scholar] [CrossRef]
- Vergaro, V.; Scarlino, F.; Bellomo, C.; Rinaldi, R.; Vergara, D.; Maffia, M.; Baldassarre, F.; Giannelli, G.; Zhang, X.; Lvov, Y.M.; et al. Drug-loaded polyelectrolyte microcapsules for sustained targeting of cancer cells. Adv. Drug Deliv. Rev. 2011, 63, 847–864. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; de Guerenu, A.V.L.; Feoktistova, N.A.; Skirtach, A.G.; Volodkin, D. Protein-Containing Multilayer Capsules by Templating on Mesoporous CaCO3 Particles: POST- and PRE-Loading Approaches. Macromol. Biosci. 2016, 16, 95–105. [Google Scholar] [CrossRef]
- De Temmerman, M.L.; Demeester, J.; De Vos, F.; De Smedt, S.C. Encapsulation performance of layer-by-layer microcapsules for proteins. Biomacromolecules 2011, 12, 1283–1289. [Google Scholar] [CrossRef] [Green Version]
- Radtchenko, I.L.; Sukhorukov, G.B.; Möhwald, H. Incorporation of macromolecules into polyelectrolyte micro- and nanocapsules via surface controlled precipitation on colloidal particles. Colloids Surf. A 2002, 202, 127–133. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yu, A.M.; Caruso, F. Nanoporous Polyelectrolyte Spheres Prepared by Sequentially Coating Sacrificial Mesoporous Silica Spheres. Angew. Chem. Int. Ed. 2005, 44, 2888–2892. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.M.; Wang, Y.J.; Barlow, E.; Caruso, F. Mesoporous silica particles as templates for preparing enzyme-loaded biocompatible microcapsules. Adv. Mater. 2005, 17, 1737–1743. [Google Scholar] [CrossRef]
- Lvov, Y.; Antipov, A.A.; Mamedov, A.; Mohwald, H.; Sukhorukov, G. Urease encapsulation in nano-organized microshells. Nano Lett. 2001, 1, 125–128. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Srivastava, S.; Brown, J.Q.; Zhu, H.; McShane, M.J. Stable encapsulation of active enzyme by application of multilayer nanofilm coatings to alginate microspheres. Macromol. Biosci. 2005, 5, 717–727. [Google Scholar] [CrossRef]
- Mak, W.C.; Bai, J.; Chang, X.Y.; Trau, D. Matrix-Assisted Colloidosome Reverse-Phase Layer-by-Layer Encapsulating Biomolecules in Hydrogel Microcapsules with Extremely High Efficiency and Retention Stability. Langmuir 2009, 25, 769–775. [Google Scholar] [CrossRef]
- De Koker, S.; Cock, L.J.D.; Rivera-Gil, P.; Parak, W.J.; Velty, R.A.; Vervaet, C.; Remon, J.P.; Grooten, J.; Geest, B.G.D. Polymeric multilayer capsules delivering biotherapeutics. Adv. Drug Deliv. Rev. 2011, 63, 748–761. [Google Scholar] [CrossRef] [Green Version]
- Beyer, S.; Mak, W.C.; Trau, D. Reverse-Phase LbLEncapsulation of Highly Water Soluble Materials by Layer-by-Layer Polyelectrolyte Self-Assembly. Langmuir 2007, 23, 8827–8832. [Google Scholar] [CrossRef]
- Pan, H.M.; Yu, H.; Guigas, G.; Fery, A.; Weiss, M.; Patzel, V.; Trau, D. Engineering and Design of Polymeric Shells: Inwards Interweaving Polymers as Multilayer Nanofilm, Immobilization Matrix, or Chromatography Resins. ACS Appl. Mater. Interface 2017, 9, 5447–5456. [Google Scholar] [CrossRef]
- Pan, H.M.; Subramanian, A.; Ochs, C.J.; Dewavrin, J.-Y.; Beyer, S.; Trau, D.W. Edible polyelectrolyte microcapsules with water-soluble cargo assembled in organic phase. RSC Adv. 2014, 4, 35163–35166. [Google Scholar] [CrossRef]
- Mak, W.C.; Cheung, K.Y.; Trau, D. Diffusion Controlled and Temperature Stable Microcapsule Reaction Compartments for High-Throughput Microcapsule-PCR. Adv. Funct. Mater. 2008, 18, 2930–2937. [Google Scholar] [CrossRef]
- Bazylinska, U.; Drozdek, S.; Nyk, M.; Kulbacka, J.; Samoc, M.; Wilk, K.A. Core/shell quantum dots encapsulated in biocompatible oil-core nanocarriers as two-photon fluorescent markers for bioimaging. Langmuir 2014, 30, 14931–14943. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowicz, K.; Bazylińska, U.; Pietkiewicz, J.; Szyk-Warszyńska, L.; Wilk, K.A.; Warszyński, P. Biocompatible long-sustained release oil-core polyelectrolyte nanocarriers: From controlling physical state and stability to biological impact. Adv. Colloid Interface Sci. 2015, 222, 678–691. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Jing, J.; Boudou, T.; Pignot-Paintrand, I.; Koker, S.D.; Geest, B.G.D.; Picart, C.; Velty, R.A. Hydrophobic Shell Loading of Biopolyelectrolyte Capsules. Adv. Mater. 2011, 23, H200–H204. [Google Scholar] [CrossRef]
- Boudou, T.; Kharkar, P.; Jing, J.; Guillot, R.; Pignot-Paintrand, I.; Velty, R.A.; Picart, C. Polyelectrolyte multilayer nanoshells with hydrophobic nanodomains for delivery of Paclitaxel. J. Control. Release 2012, 159, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Bazylińska, U.; Saczko, J. Nanoemulsion-templated polylelectrolyte multifunctional nanocapsules for DNA entrapment and bioimaging. Colloids Surf. B 2016, 137, 191–202. [Google Scholar] [CrossRef]
- Jain, S.; Patil, S.R.; Swarnakar, N.K.; Agrawal, A.K. Oral Delivery of Doxorubicin Using Novel Polyelectrolyte-Stabilized Liposomes (Layersomes). Mol. Pharm. 2012, 9, 2626–2635. [Google Scholar] [CrossRef]
- Ibraheem, D.; Elaissari, A.; Fessi, H. Gene therapy and DNA delivery systems. Int. J. Pharm. 2014, 459, 70–83. [Google Scholar] [CrossRef]
- Dubas, S.T.; Schlenoff, J.B. Swelling and Smoothing of Polyelectrolyte Multilayers by Salt. Langmuir 2001, 17, 7725–7727. [Google Scholar] [CrossRef]
- Sukhishvili, S.A.; Granick, S. Layered, Erasable Polymer Multilayers Formed by Hydrogen-Bonded Sequential Self-Assembly. Macromolecules 2002, 35, 301–310. [Google Scholar] [CrossRef]
- Sui, Z.; Schlenoff, J.B. Phase Separations in pH-Responsive Polyelectrolyte Multilayers: Charge Extrusion versus Charge Expulsion. Langmuir 2004, 20, 6026–6031. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Kessler, B.; Adler, H.J.; Lunkwitz, K. Reversible Switching of Protein Uptake and Release at Polyelectrolyte Multilayers Detected by ATR-FTIR Spectroscopy. Macromol. Symp. 2004, 210, 157–164. [Google Scholar] [CrossRef]
- Sharma, V.; Sundaramurthy, A. Multilayer capsules made of weak polyelectrolytes: A review on the preparation, functionalization and applications in drug delivery. Beilstein J. Nanotechnol. 2020, 11, 508–532. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Liu, P. pH-sensitive fluorescent hepatocyte-targeting multilayer polyelectrolyte hollow microspheres as a smart drug delivery system. Mol. Pharm. 2014, 11, 1599–1610. [Google Scholar] [CrossRef]
- Luo, G.F.; Xu, X.D.; Zhang, J.; Yang, J.; Gong, Y.H.; Lei, Q.; Jia, H.Z.; Li, C.; Zhuo, R.X.; Zhang, X.Z. Encapsulation of an Adamantane-Doxorubicin Prodrug in pH-Responsive Polysaccharide Capsules for Controlled Release. ACS Appl. Mater. Interfaces 2012, 4, 5317–5324. [Google Scholar] [CrossRef]
- Li, Q.L.; Sun, Y.; Sun, Y.L.; Wen, J.; Zhou, Y.; Bing, Q.M.; Isaacs, L.D.; Jin, Y.; Gao, H.; Yang, Y.W. Mesoporous Silica Nanoparticles Coated by Layer-by-Layer Self-assembly Using Cucurbit [7] uril for in Vitro and in Vivo Anticancer Drug Release. Chem. Mater. 2014, 26, 6418–6431. [Google Scholar] [CrossRef] [Green Version]
- Han, U.; Seo, Y.; Hong, J. Effect of pH on the structure and drug release profiles of layer-by-layer assembled films containing polyelectrolyte, micelles, and graphene oxide. Sci. Rep. 2016, 6, 24158. [Google Scholar] [CrossRef]
- Lvov, Y.; Caruso, F. Biocolloids with Ordered Urease Multilayer Shells as Enzymatic Reactors. Anal. Chem. 2001, 73, 4212–4217. [Google Scholar] [CrossRef]
- Kohler, K.; Sukhorukov, G.B. Heat treatment of polyelectrolyte multilayer capsules: A versatile method for encapsulation. Adv. Funct. Mater. 2007, 17, 2053–2061. [Google Scholar] [CrossRef]
- Quinn, J.F.; Caruso, F. Facile Tailoring of Film Morphology and Release Properties Using Layer-by-Layer Assembly of Thermoresponsive Materials. Langmuir 2004, 20, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Nakamura, S.; Sakai, K.; Aoyagi, T.; Kikuchi, A.; Sakurai, Y.; Okano, T. Rapid Deswelling Response of Poly (N-isopropylacrylamide) Hydrogels by the Formation of Water Release Channels Using Poly (ethylene oxide) Graft Chains. Macromolecules 1998, 31, 6099–6105. [Google Scholar] [CrossRef]
- Zhu, Z.; Gao, N.; Wang, H.; Sukhishvili, S.A. Temperature-triggered on-demand drug release enabled by hydrogen-bonded multilayers of block copolymer micelles. J. Control. Release 2013, 171, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Pishko, M.V.; Lutkenhaus, J.L. Thermoresponsive layer-by-layer assemblies for nanoparticle-based drug delivery. Langmuir 2014, 30, 5903–5910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lengert, E.V.; Koltsov, S.I.; Li, J.; Ermakov, A.V.; Parakhonskiy, B.V.; Skorb, E.V.; Skirtach, A.G. Nanoparticles in Polyelectrolyte Multilayer Layer-by-Layer (LbL) Films and Capsules—Key Enabling Components of Hybrid Coatings. Coatings 2020, 10, 1131. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Javier, A.M.; Kreft, O.; Köhler, K.; Alberola, A.P.; Möhwald, H.; Parak, W.J.; Sukhorukov, G.B. Laser-induced release of encapsulated materials inside living cells. Angew. Chem. Int. Ed. 2006, 45, 4612–4617. [Google Scholar] [CrossRef]
- Brueckner, M.; Hollenbach-Latzko, S.; Reibetanz, U. Dual Transport of Active Substances with a Layer-by-Layer-Based Drug Delivery System to Terminate Inflammatory Processes. Macromol. Biosci. 2020, 20, 2000097. [Google Scholar] [CrossRef]
- Brkovic, N.; Zhang, L.; Peters, J.N.; Kleine-Doepke, S.; Parak, W.J.; Zhu, D. Quantitative Assessment of Endosomal Escape of Various Endocytosed Polymer-Encapsulated Molecular Cargos upon Photothermal Heating. Small 2020, 16, 2003639. [Google Scholar] [CrossRef]
- Zograf, G.P.; Timin, A.S.; Muslimov, A.R.; Shishkin, I.I.; Nominé, A.; Ghanbaja, J.; Ghosh, P.; Li, Q.; Zyuzin, M.V.; Makarov, S.V. All-Optical Nanoscale Heating and Thermometry with Resonant Dielectric Nanoparticles for Controllable Drug Release in Living Cells. Laser Photonics Rev. 2020, 14, 1900082. [Google Scholar] [CrossRef]
- Wu, Z.; Lin, X.; Zou, X.; Sun, J.; He, Q. Biodegradable Protein-Based Rockets for Drug Transportation and Light-Triggered Release. ACS Appl. Mater. Interfaces 2015, 7, 250–255. [Google Scholar] [CrossRef]
- Javier, A.M.; del Pino, P.; Bedard, M.F.; Ho, D.; Skirtach, A.G.; Sukhorukov, G.B.; Plank, C.; Parak, W.J. Photoactivated release of cargo from the cavity of polyelectrolyte capsules to the cytosol of cells. Langmuir 2008, 24, 12517–12520. [Google Scholar] [CrossRef] [PubMed]
- Radt, B.; Smith, T.A.; Caruso, F. Optically Addressable Nanostructured Capsules. Adv. Mater. 2004, 16, 2184–2189. [Google Scholar] [CrossRef]
- Gupta, M.; Sivakumar, S. Light responsive Gold NPs-polymer hybrid LBL capsules for the Lysosomal Storage Disorder. Int. J. Commun. Sci. Technol. 2021, 4, 1–15. [Google Scholar]
- Kurapati, R.; Raichur, A.M. Near-infrared light-responsive graphene oxide composite multilayer capsules: A novel route for remote controlled drug delivery. Chem. Commun. 2013, 49, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.W.; Desai, N.K.; Maru, B.S.; Mohanty, D.K. Photohydrolysis of Substituted Benzyl Esters in Multilayered Polyelectrolyte Films. Macromolecules 2004, 37, 4196–4200. [Google Scholar] [CrossRef]
- Wang, K.; He, Q.; Yan, X.; Cui, Y.; Qi, W.; Duan, L.; Li, J. Encapsulated photosensitive drugs by biodegradable microcapsules to incapacitate cancer cells. J. Mater. Chem. 2007, 17, 4018–4021. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Gorin, D.A.; Mohwald, H. Ultrasonically induced opening of polyelectrolyte microcontainers. Langmuir 2006, 22, 7400–7404. [Google Scholar] [CrossRef]
- Kolesnikova, T.A.; Gorin, D.A.; Fernandes, P.; Kessel, S.; Khomutov, G.B.; Fery, A.; Shchukin, D.C.; Möhwald, H. Nanocomposite microcontainers with high ultrasound sensitivity. Adv. Funct. Mater. 2010, 20, 1189–1195. [Google Scholar] [CrossRef]
- Stavarache, C.E.; Paniwnyk, L. Controlled rupture of magnetic LbL polyelectrolyte capsules and subsequent release of contents employing high intensity focused ultrasound. J. Drug Deliv. Sci. Technol. 2018, 45, 60–69. [Google Scholar] [CrossRef]
- Lu, Z.H.; Prouty, M.D.; Guo, Z.H.; Golub, V.O.; Kummar, S.S.R.; Lvov, Y.M. Magnetic switch of permeability for polyelectrolyte microcapsules embedded with Co@Au nanoparticles. Langmuir 2005, 21, 2042–2050. [Google Scholar] [CrossRef]
- Kummar, P.; Bohidar, H.B. Interaction of soot derived multi-carbon nanoparticles with lung surfactant and their possible internalization inside alveolar cavity. Indian J. Exp. Biol. 2010, 48, 1037–1042. [Google Scholar]
- Burmistrov, I.A.; Veselov, M.M.; Mikheev, A.V.; Borodina, T.N.; Bukreeva, T.V.; Chuev, M.A.; Starchikov, S.S.; Lyubutin, I.S.; Artemov, V.V.; Khmelenin, D.N.; et al. Permeability of the Composite Magnetic Microcapsules Triggered by a Non-Heating Low-Frequency Magnetic Field. Pharmaceutics 2022, 14, 65. [Google Scholar] [CrossRef]
- Luo, D.; Poston, R.N.; Gould, D.J.; Sukhorukov, G.B. Magnetically targetable microcapsules display subtle changes in permeability and drug release in response to a biologically compatible low frequency alternating magnetic field. Mater. Sci. Eng. C 2019, 94, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Temmerman, M.-L.D.; Demeester, J.; Smedt, S.C.D.; Rejman, J. Tailoring layer-by-layer capsules for biomedical applications. Nanomedicine 2012, 7, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Yuan, J.; Tao, L.; Wei, Y. Redox-responsive polymers for drug delivery: From molecular design to applications. Polym. Chem. 2014, 5, 1519–1528. [Google Scholar] [CrossRef]
- Yan, Y.; Johnston, A.P.R.; Dodds, S.J.; Kamphuis, M.M.J.; Ferguson, C.; Parton, R.G.; Nice, E.C.; Heath, J.K.; Caruso, F. Uptake and intracellular fate of disulfide-bonded polymer hydrogel capsules for doxorubicin delivery to colorectal cancer cells. ACS Nano 2010, 4, 2928–2936. [Google Scholar] [CrossRef] [Green Version]
- Shu, S.J.; Zhang, X.G.; Wu, Z.M.; Wang, Z.H.; Li, C.X. Gradient cross-linked biodegradable polyelectrolyte nanocapsules for intracellular protein drug delivery. Biomaterials 2010, 31, 6039–6049. [Google Scholar] [CrossRef]
- Seras-Franzoso, J.; Sanchez-Chardi, A.; Garcia-Fruitos, E.; Vazquez, E.; Villaverde, A. Cellular uptake and intracellular fate of protein releasing bacterial amyloids in mammalian cells. Soft Matter 2016, 12, 3451–3460. [Google Scholar] [CrossRef]
- Zelikin, A.N. Drug Releasing Polymer Thin Films: New Era of Surface-Mediated Drug Delivery. ACS Nano 2010, 4, 2494–2509. [Google Scholar] [CrossRef]
- Thierry, B.; Kujawa, P.; Tkaczyk, C.; Winnik, F.o.M.; Bilodeau, L.; Tabrizian, M. Delivery Platform for Hydrophobic Drugs: Prodrug Approach Combined with Self-Assembled Multilayers. J. Am. Chem. Soc. 2005, 127, 1626–1627. [Google Scholar] [CrossRef]
- Serizawa, T.; Takeshita, H.; Akashi, M. Electrostatic Adsorption of Polystyrene Nanospheres onto the Surface of an Ultrathin Polymer Film Prepared by Using an Alternate Adsorption Technique. Langmuir 1998, 14, 4088–4094. [Google Scholar] [CrossRef]
- Orozco, V.H.; Kozlovskaya, V.; Kharlampieva, E.; López, B.L.; Tsukruk, V.V. Biodegradable self-reporting nanocomposite films of poly (lactic acid) nanoparticles engineered by layer-by-layer assembly. Polymer 2010, 51, 4127–4139. [Google Scholar] [CrossRef]
- Itoh, Y.; Matsusaki, M.; Kida, T.; Akashi, M. Enzyme-responsive release of encapsulated proteins from biodegradable hollow capsules. Biomacromolecules 2006, 7, 2715–2718. [Google Scholar] [CrossRef] [PubMed]
- De Koker, S.; De Geest, B.G.; Singh, S.K.; De Rycket, R.; Nessens, T.; Van Kooyt, Y.; Demeester, J.; De Smedt, S.C.; Grooten, J. Polyelectrolyte microcapsules as antigen delivery vehicles to dendritic cells: Uptake, processing, and cross-presentation of encapsulated antigens. Angew. Chem. Int. Ed. 2009, 48, 8485–8489. [Google Scholar] [CrossRef]
- Borodina, T.; Markvicheva, E.; Kunizhev, S.; Mohwald, H.; Sukhorukov, G.B.; Kreft, O. Controlled release of DNA from self-degrading microcapsules. Macromol. Rapid Commun. 2007, 28, 1894–1899. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Khashab, N.M. Stimuli responsive nanomaterials for controlled release applications. Nanotechnol. Rev. 2012, 1, 493–513. [Google Scholar] [CrossRef]
- Al Thaher, Y. Tailored gentamicin release from silica nanocarriers coated with polyelectrolyte multilayers. Colloids Surf. A 2021, 614, 126210. [Google Scholar] [CrossRef]
- Craig, M.; Altskär, A.; Nordstierna, L.; Holmberg, K. Bacteria-triggered degradation of nanofilm shells for release of antimicrobial agents. J. Mater. Chem. B 2016, 4, 672–682. [Google Scholar] [CrossRef] [Green Version]
- Zhuk, I.; Jariwala, F.; Attygalle, A.B.; Wu, Y.; Libera, M.R.; Sukhishvili, S.A. Self-Defensive Layer-by-Layer Films with Bacteria-Triggered Antibiotic Release. ACS Nano 2014, 8, 7733–7745. [Google Scholar] [CrossRef]
- Pawlak, A.; Michely, L.; Belbekhouche, S. Multilayer dextran derivative based capsules fighting bacteria resistant to Antibiotic: Case of Kanamycin-Resistant Escherichia coli. Int. J. Biol. Macromol. 2022, 200, 242–246. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, T.; Ma, Q. Layer-by-Layer assembled nano-drug delivery systems for cancer treatment. Drug Deliv. 2021, 28, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Szarpak-Jankowska, A.; Guillot, R.; Pignot-Paintrand, I.; Picart, C.; Auzély-Velty, R. Cyclodextrin/Paclitaxel Complex in Biodegradable Capsules for Breast Cancer Treatment. Chem. Mater. 2013, 25, 3867–3873. [Google Scholar] [CrossRef]
- Vergaro, V.; Papadia, P.; Leporatti, S.; De Pascali, S.A.; Fanizzi, F.P.; Ciccarella, G. Synthesis of biocompatible polymeric nano-capsules based on calcium carbonate: A potential cisplatin delivery system. J. Inorg. Biochem. 2015, 153, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Li, F.; Wang, D.; Yang, Z.; Yao, C.; Ye, Y.; Wang, X. Chitosan-alginate BSA-gel-capsules for local chemotherapy against drug-resistant breast cancer. Drug Des. Dev. Ther. 2018, 12, 921–934. [Google Scholar] [CrossRef] [Green Version]
- Trushina, D.; Akasov, R.; Khovankina, A.; Borodina, T.; Bukreeva, T.; Markvicheva, E. Doxorubicin-loaded biodegradable capsules: Temperature induced shrinking and study of cytotoxicity in vitro. J. Mol. Liq. 2019, 284, 215–224. [Google Scholar] [CrossRef]
- Liu, F.; Kozlovskaya, V.; Zavgorodnya, O.; Martinez-Lopez, C.; Catledge, S.; Kharlampieva, E. Encapsulation of anticancer drug by hydrogen-bonded multilayers of tannic acid. Soft Matter 2014, 10, 9237–9247. [Google Scholar] [CrossRef]
- Yang, X.; Han, X.; Zhu, Y. (PAH/PSS) 5 microcapsules templated on silica core: Encapsulation of anticancer drug DOX and controlled release study. Colloids Surf. A 2005, 264, 49–54. [Google Scholar] [CrossRef]
- Zhou, D.; Xiao, H.; Meng, F.; Zhou, S.; Guo, J.; Li, X.; Jing, X.; Huang, Y. Layer-by-Layer Assembled Polypeptide Capsules for Platinum-Based Pro-Drug Delivery. Bioconjugate Chem. 2012, 23, 2335–2343. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Thomas, M.B.; Pulakkat, S.; Gnanadhas, D.P.; Chakravortty, D.; Raichur, A.M. Stimuli-responsive protamine-based biodegradable nanocapsules for enhanced bioavailability and intracellular delivery of anticancer agents. J. Nanopart. Res. 2015, 17, 341. [Google Scholar] [CrossRef]
- Xue, B.; Wang, W.; Qin, J.-J.; Nijampatnam, B.; Murugesan, S.; Kozlovskaya, V.; Zhang, R.; Velu, S.E.; Kharlampieva, E. Highly efficient delivery of potent anticancer iminoquinone derivative by multilayer hydrogel cubes. Acta Biomater. 2017, 58, 386–398. [Google Scholar] [CrossRef]
- Kittitheeranun, P.; Sajomsang, W.; Phanpee, S.; Treetong, A.; Wutikhun, T.; Suktham, K.; Puttipipatkhachorn, S.; Ruktanonchai, U.R. Layer-by-layer engineered nanocapsules of curcumin with improved cell activity. Int. J. Pharm. 2015, 492, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Vijay, J.; Ganesh, M.R.; Sundaramurthy, A. Multilayer capsules encapsulating nimbin and doxorubicin for cancer chemo-photothermal therapy. Int. J. Pharm. 2020, 582, 119350. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, T.; Haidar, Z.S.; Tran, T.H.; Choi, J.Y.; Jeong, J.-H.; Shin, B.S.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Layer-by-layer assembly of liposomal nanoparticles with PEGylated polyelectrolytes enhances systemic delivery of multiple anticancer drugs. Acta Biomater. 2014, 10, 5116–5127. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Andalib, F.; Mohammadikish, M.; Divsalar, A.; Sahebi, U. Hollow microcapsule with pH-sensitive chitosan/polymer shell for in vitro delivery of curcumin and gemcitabine. Eur. Polym. J. 2022, 162, 110887. [Google Scholar] [CrossRef]
- Shao, J.; Xuan, M.; Si, T.; Dai, L.; He, Q. Biointerfacing polymeric microcapsules for in vivo near-infrared light-triggered drug release. Nanoscale 2015, 7, 19092–19098. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Simanek, E.E. Nonviral vectors for gene delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef]
- Santos, J.L.; Pandita, D.; Rodrigues, J.; Pêgo, A.P.; Granja, P.L.; Tomás, H. Non-viral gene delivery to mesenchymal stem cells: Methods, strategies and application in bone tissue engineering and regeneration. Curr. Gene Ther. 2011, 11, 46–57. [Google Scholar] [CrossRef]
- Gupta, V.; Lourenço, S.P.; Hidalgo, I.J. Development of Gene Therapy Vectors: Remaining Challenges. J. Pharm. Sci. 2021, 110, 1915–1920. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Green, J.J.; Chan, J.M.; Langer, R.; Anderson, D.G. Polymeric Materials for Gene Delivery and DNA Vaccination. Adv. Mater. 2009, 21, 847–867. [Google Scholar] [CrossRef]
- Li, S.D.; Huang, L. Non-viral is superior to viral gene delivery. J. Control. Release 2007, 123, 181–183. [Google Scholar] [CrossRef]
- Mastrobattista, E.; Bravo, S.A.; van der Aa, M.; Crommelin, D.J. Nonviral gene delivery systems: From simple transfection agents to artificial viruses. Drug Discov. Today Technol. 2005, 2, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Linnik, D.S.; Tarakanchikova, Y.V.; Zyuzin, M.V.; Lepik, K.V.; Aerts, J.L.; Sukhorukov, G.; Timin, A.S. Layer-by-Layer technique as a versatile tool for gene delivery applications. Exp. Opin. Drug Deliv. 2021, 18, 1047–1066. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, A.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Beigi, V.; Mousavi, S.M.; Hashemi, S.A.R.; Zade, A.K.; Amani, A.M.; Savardashtaki, A.; Mirzaei, E.; et al. Polyethylenimine-based nanocarriers in co-delivery of drug and gene: A developing horizon. Nano Rev. Exp. 2018, 9, 1488497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, D.; Chen, S.; Guo, W. Liposome Crosslinked Polyacrylamide/DNA Hydrogel: A Smart Controlled-Release System for Small Molecular Payloads. Small 2018, 14, 1704039. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Patel, A.A.; Sukhorukov, G.B.; Lvov, Y.M. Nanoassembly of biodegradable microcapsules for DNA encasing. J. Am. Chem. Soc. 2004, 126, 3374–3375. [Google Scholar] [CrossRef]
- Santos, J.L.; Nouri, A.; Fernandes, T.; Rodrigues, J.; Tomás, H. Gene delivery using biodegradable polyelectrolyte microcapsules prepared through the layer-by-layer technique. Biotechnol. Progr. 2012, 28, 1088–1094. [Google Scholar] [CrossRef]
- Selina, O.E.; Belov, S.Y.; Vlasova, N.N.; Balysheva, V.I.; Churin, A.I.; Bartkoviak, A.; Sukhorukov, G.B.; Markvicheva, E.A. Biodegradable microcapsules with entrapped DNA for development of new DNA vaccines. Russ. J. Bioorg. Chem. 2009, 35, 103–110. [Google Scholar] [CrossRef]
- Reibetanz, U.; Claus, C.; Typlt, E.; Hofmann, J.; Donath, E. Defoliation and Plasmid Delivery with Layer-by-Layer Coated Colloids. Macromol. Biosci. 2006, 6, 153–160. [Google Scholar] [CrossRef]
- Tarakanchikova, Y.V.; Muslimov, A.R.; Zyuzin, M.V.; Nazarenko, I.; Timin, A.S.; Sukhorukov, G.B.; Lepik, K.V. Layer-by-Layer-Assembled Capsule Size Affects the Efficiency of Packaging and Delivery of Different Genetic Cargo. Part. Part. Syst. Charact. 2021, 38, 2000228. [Google Scholar] [CrossRef]
- Kakran, M.; Muratani, M.; Tng, W.J.; Liang, H.; Trushina, D.B.; Sukhorukov, G.B.; Ng, H.H.; Antipina, M.N. Layered polymeric capsules inhibiting the activity of RNases for intracellular delivery of messenger RNA. J. Mater. Chem. B 2015, 3, 5842–5848. [Google Scholar] [CrossRef]
- Xie, L.; Ding, X.; Budry, R.; Mao, G. Layer-by-layer DNA films incorporating highly transfecting bioreducible poly (amido amine) and polyethylenimine for sequential gene delivery. Int. J. Nanomed. 2018, 13, 4943–4960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, O.; Neumann, B.; Schlensak, C.; Wendel, H.P.; Nolte, A. Hyaluronic acid/poly (ethylenimine) polyelectrolyte multilayer coatings for siRNA-mediated local gene silencing. PLoS ONE 2019, 14, e0212584. [Google Scholar] [CrossRef] [PubMed]
- Tarakanchikova, Y.V.; Linnik, D.S.; Mashel, T.; Muslimov, A.R.; Pavlov, S.; Lepik, K.V.; Zyuzin, M.V.; Sukhorukov, G.B.; Timin, A.S. Boosting transfection efficiency: A systematic study using layer-by-layer based gene delivery platform. Mater. Sci. Eng. C 2021, 126, 112161. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Arbit, E.; Kidron, M. Oral insulin: The rationale for this approach and current developments. J. Diabetes Sci. Technol. 2009, 3, 562–567. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xiong, G.M.; Ali, Y.; Boehm, B.O.; Huang, Y.Y.; Venkatraman, S. Layer-by-layer coated nanoliposomes for oral delivery of insulin. Nanoscale 2021, 13, 776–789. [Google Scholar] [CrossRef]
- Wu, J.-Z.; Williams, G.R.; Li, H.-Y.; Wang, D.-X.; Li, S.-D.; Zhu, L.-M. Insulin-loaded PLGA microspheres for glucose-responsive release. Drug Deliv. 2017, 24, 1513–1525. [Google Scholar] [CrossRef] [Green Version]
- Balabushevich, N.G.; Pechenkin, M.A.; Shibanova, E.D.; Volodkin, D.V.; Mikhalchik, E.V. Multifunctional Polyelectrolyte Microparticles for Oral Insulin Delivery. Macromol. Biosci. 2013, 13, 1379–1388. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, S.; Gupta, P.K.; Singh, A.; Teja, B.V.; Dwivedi, P.; Gupta, G.K.; Trivedi, R.; Mishra, P.R. Vitamin B12 functionalized layer by layer calcium phosphate nanoparticles: A mucoadhesive and pH responsive carrier for improved oral delivery of insulin. Acta Biomater. 2016, 31, 288–300. [Google Scholar] [CrossRef]
- Song, L.; Zhi, Z.-L.; Pickup, J.C. Nanolayer encapsulation of insulin-chitosan complexes improves efficiency of oral insulin delivery. Int. J. Nanomed. 2014, 9, 2127–2136. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Kankala, R.K.; Fan, J.; Long, R.; Liu, Y.; Wang, S. Poly-l-ornithine/fucoidan-coated calcium carbonate microparticles by layer-by-layer self-assembly technique for cancer theranostics. J. Mater. Sci. Mater. Med. 2018, 29, 68. [Google Scholar] [CrossRef] [PubMed]
- Rosch, J.G.; DuRoss, A.N.; Landry, M.R.; Sun, C. Formulation of Folate-Modified Raltitrexed-Loaded Nanoparticles for Colorectal Cancer Theranostics. Pharmaceutics 2020, 12, 133. [Google Scholar] [CrossRef] [Green Version]
- Svenskaya, Y.; Garello, F.; Lengert, E.; Kozlova, A.; Verkhovskii, R.; Bitonto, V.; Ruggiero, M.R.; German, S.; Gorin, D.; Terreno, E. Biodegradable polyelectrolyte/magnetite capsules for MR imaging and magnetic targeting of tumors. Nanotheranostics 2021, 5, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowicz, K.; Piechota, P.; Węglarz, W.P.; Warszyński, P. Polyelectrolyte nanocapsules containing iron oxide nanoparticles as MRI detectable drug delivery system. Colloids Surf. A 2017, 532, 351–356. [Google Scholar] [CrossRef]
- Hanafy, N.A.N. Optimally designed theranostic system based folic acids and chitosan as a promising mucoadhesive delivery system for encapsulating curcumin LbL nano-template against invasiveness of breast cancer. Int. J. Biol. Mol. 2021, 182, 1981–1993. [Google Scholar] [CrossRef]
- Chen, J.; Ratnayaka, S.; Alford, A.; Kozlovskaya, V.; Liu, F.; Xue, B.; Hoyt, K.; Kharlampieva, E. Theranostic Multilayer Capsules for Ultrasound Imaging and Guided Drug Delivery. ACS Nano 2017, 11, 3135–3146. [Google Scholar] [CrossRef] [Green Version]
- Alford, A.; Rich, M.; Kozlovskaya, V.; Chen, J.; Sherwood, J.; Bolding, M.; Warram, J.; Bao, Y.; Kharlampieva, E. Ultrasound-Triggered Delivery of Anticancer Therapeutics from MRI-Visible Multilayer Microcapsules. Adv. Ther. 2018, 1, 1800051. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Alford, A.; Dolmat, M.; Ducharme, M.; Caviedes, R.; Radford, L.; Lapi, S.E.; Kharlampieva, E. Multilayer Microcapsules with Shell-Chelated 89Zr for PET Imaging and Controlled Delivery. ACS Appl. Mater. Interfaces 2020, 12, 56792–56804. [Google Scholar] [CrossRef]
- Muslimov, A.R.; Antuganov, D.O.; Tarakanchikova, Y.V.; Zhukov, M.V.; Nadporojskii, M.A.; Zyuzin, M.V.; Timin, A.S. Calcium Carbonate Core–Shell Particles for Incorporation of 225Ac and Their Application in Local α-Radionuclide Therapy. ACS Appl. Mater. Interfaces 2021, 13, 25599–25610. [Google Scholar] [CrossRef]
- Muslimov, A.R.; Antuganov, D.; Tarakanchikova, Y.V.; Karpov, T.E.; Zhukov, M.V.; Zyuzin, M.V.; Timin, A.S. An investigation of calcium carbonate core-shell particles for incorporation of 225Ac and sequester of daughter radionuclides: In vitro and in vivo studies. J. Control. Release 2021, 330, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Novoselova, M.V.; German, S.V.; Abakumova, T.O.; Perevoschikov, S.V.; Sergeeva, O.V.; Nesterchuk, M.V.; Efimova, O.I.; Petrov, K.S.; Chernyshev, V.S.; Zatsepin, T.S.; et al. Multifunctional nanostructured drug delivery carriers for cancer therapy: Multimodal imaging and ultrasound-induced drug release. Colloids Surf. B 2021, 200, 111576. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zharkov, M.N.; Brodovskaya, E.P.; Kulikov, O.A.; Gromova, E.V.; Ageev, V.P.; Atanova, A.V.; Kozyreva, Z.V.; Tishin, A.M.; Pyatakov, A.P.; Pyataev, N.A.; et al. Enhanced cytotoxicity caused by AC magnetic field for polymer microcapsules containing packed magnetic nanoparticles. Colloids Surf. B 2021, 199, 111548. [Google Scholar] [CrossRef]
- Zyuzin, M.V.; Cassani, M.; Barthel, M.J.; Gavilan, H.; Silvestri, N.; Escudero, A.; Scarpellini, A.; Lucchesi, F.; Teran, F.J.; Parak, W.J.; et al. Confining Iron Oxide Nanocubes inside Submicrometric Cavities as a Key Strategy To Preserve Magnetic Heat Losses in an Intracellular Environment. ACS Appl. Mater. Interfaces 2019, 11, 41957–41971. [Google Scholar] [CrossRef]
- Cristofolini, L.; Szczepanowicz, K.; Orsi, D.; Rimoldi, T.; Albertini, F.; Warszynski, P. Hybrid Polyelectrolyte/Fe3O4 Nanocapsules for Hyperthermia Applications. ACS Appl. Mater. Interfaces 2016, 8, 25043–25050. [Google Scholar] [CrossRef]












| Multilayer Components | Encapsulated Drug | Loading | Release | Reference |
|---|---|---|---|---|
| PAH and PSS | gentamicin | Pre-loading (trapping in silicon dioxide templates) | pH-induced | Al Thaher [239] |
| PLL and HA | vancomycin Polyhexamethylene biguanide | Post-loading (direct incubation) | pH-induced (bacteria protease-triggered) | Craig et al. [240] |
| branched polyethyleneimine and tannic acid | tobramycin gentamicin polymyxin B | Pre-loading (combined with tannic acid layers) | pH-induced | Zhuk et al. [241] |
| diethylaminoethyl-dextran hydrochloride and dextran sulphate | kamayicin | Post-loading (direct incubation) | pH-induced | Pawlak et al. [242] |
| Multilayer Components | Encapsulated Drug | Loading | Release | Type of Tumor | Reference |
|---|---|---|---|---|---|
| HA modified with β-cyclodextrin and PLL | paclitaxel | Pre-loading (host-guest interactions with β-cyclodextrin) | environmentally triggered (hyaluronidase enzyme action) | breast cancer (MDA-MB-231 cells) | Jing et al. [244] |
| alginic acid and protamine | cisplatin | Post-loading (direct incubation) | pH-induced | human breast cancer (MCF-7 cells) human cervical cancer (HeLa cells) human ovarian cancer (SKOV-3 cells) human epithelial colorectal adenocarcinoma (CACO-2 cells) | Vergaro et al. [245] |
| chitosan and alginate | doxorubicin | Post-loading (direct incubation with pH changes) | pH-induced (acidic conditions) | human breast cancer (MCF-7 and MCF-7/ADR cells) | Shen et al. [246] |
| poly(l-arginine) and dextran sulphate | doxorubicin | Post-loading (direct incubation with temperature changes) | pH-induced | human breast cancer (MCF-7 and MCF-7/ADR cells) | Trushina et al. [247] |
| PAH and PSS | doxorubicin | Post-loading (direct incubation with pH changes) | pH-induced (acidic conditions) | without tests | Yang et al. [249] |
| PLL and PGA | cisplatin | Pre-loading (combined with PLL layers) | pH-induced (acidic conditions) redox induced (reductive conditions) | colon cancer (CT-26 cells) | Zhou et al. [250] |
| protamine and heparin | doxorubicin | Post-loading (direct incubation with pH changes) | pH-induced (erosion in acid medium) | human breast cancer (MCF-7 cells) | Radhakrishnan et al. [251] |
| poly(N-vinylpyrrolidone) and poly(methacrylic acid) | iminoquinone | Pre-loading (included within the template) | redox induced (reductive conditions induced by glutathione) | hepatocellular carcinoma (HepG2 and Huh7cells) | Xue et al. [252] |
| PDADMAC and PSS | doxorubicin | Post-loading (direct incubation) | pH-induced | human cervical cancer (HeLa cells) | Kittitheeranun et al. [253] |
| Multilayer Components | Genetic Material | Loading | Release | Reference |
|---|---|---|---|---|
| PAH and PSS dextran sulphate and poly-(l-arginine) | DNA plasmid | Pre-loading (trapping in CaCO3 templates) | environmentally triggered (trip sine action) | Santos et al. [258] |
| PAH, protamine sulfate and dextrane sulfate | DNA plasmid | Pre-loaded (incorporated as a layer) | environmentally triggered (erosion process) | Reibetanz et al. [270] |
| poly(l-arginine) and dextran sulphate | mRNA siRNA | Pre-loading (trapping in CaCO3 templates) | environmentally triggered | Tarakanchikova et al. [271] |
| poly(l-arginine) and dextran sulphate | mRNA | Pre-loading (trapping in CaCO3 templates) | environmentally triggered | Kakran et al. [272] |
| poly(ethyleneimine) or poly(amino pentanol) and DNA | DNA | Pre-loaded (incorporated as a layer) | environmentally triggered | Xie et al. [273] |
| poly(ethyleneimine) and HA | siRNA | Pre-loaded (incorporated as a layer) | environmentally triggered | Koenig et al. [274] |
| Multilayer Components | Loading | Release | Reference |
|---|---|---|---|
| chitosan and insulin | Pre-loading (insulin incorporated as a layer) | pH-induced (acidic condition) | Zhang et al. [278] |
| poly(vinyl alcohol) and poly(acrylamide phenyl boronic acid-co-N-vinylcaprolactam) | Pre-loading (trapping in poly(lactic-co-glycolic acid) templates) | environmentally triggered (glucose levels) | Wu et al. [279] |
| chitosan and dextran sulphate | Pre-loading (incorporated as core) | pH-induced | Balabushevich et al. [280] |
| vitamin B12 grafted chitosan and sodium alginate | Pre-loading (incorporated in the template) | pH-induced | Verma et al. [281] |
| chitosan and heparin | Pre-loading (as hybrid core with chitosan) | pH-induced | Song et al. [282] |
| Multilayer Components | Drug | Diagnostic Probe | Purpose | Reference |
|---|---|---|---|---|
| poly-(l-ornithine) and fucoidan | doxorubicin (pre-loaded combined with fucoidan layers) | fluorescent probe | anticancer | Wang et al. [284] |
| PLL and folic acid (or mixture of folic acid and folate antimetabolites) | folic acid (or mixture of folic acid and folate antimetabolites) included as layers | fluorescent probe | anticancer | Rosch et al. [285]. |
| poly-l-arginine and dextran sulfate | magnetite particles (pre-loaded within the template or adsorbed as layers) | magnetite particles (NMR imaging) | anticancer | Svenskaya et al. [286] |
| PLL and poly-l-glutamic acid | doxorubicin (preloaded in the oil core of the emulsion droplets used as template) | Fe2O3 nanoparticles (NMR imaging) | anticancer | Szczepanowicz et al. [287] |
| dextran, protamine, chitosan, and folic acid | curcumin (post-loaded by direct incubation) | fluorescent probe (dextran marked with rhodamine as layer) | anticancer | Hanafy [288] |
| PAH and PSS | doxorubicin (pre-loaded in CaCO3 templates) | quantum dots (fluorescence imaging) | anticancer | Kalinechenko et al. [36] |
| tannic acid and poly(N-vinylpyrrolidone) | doxorubicin (pre-loaded in the template) | capsule itself ultrasound imaging | anticancer | Chen et al. [289] |
| tannic acid and poly(N-vinylpyrrolidone) | doxorubicin (pre-loaded in silicon dioxide templates) | iron nanoparticles (NMR imaging) | anticancer | Alford et al. [290] |
| tannic acid and poly(N-vinylpyrrolidone) | doxorubicin (post-loaded by direct incubation) | 89Zr (PET imaging) | anticancer | Kozlovskaya et al. [291] |
| tannic acid and bovine serum albumin | 225Ac (pre-loading by radiolabeling) | 89Zr (PET imaging) | anticancer | Muslimov et al. [292] |
| poly-l-arginine and dextran sulfate | doxorubicin (pre-loaded in CaCO3 templates) | fluorescent probes and magnetic nanoparticles (fluorescent tomography and optoacoustic signal) | anticancer | Novoselova et al. [294] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateos-Maroto, A.; Fernández-Peña, L.; Abelenda-Núñez, I.; Ortega, F.; Rubio, R.G.; Guzmán, E. Polyelectrolyte Multilayered Capsules as Biomedical Tools. Polymers 2022, 14, 479. https://doi.org/10.3390/polym14030479
Mateos-Maroto A, Fernández-Peña L, Abelenda-Núñez I, Ortega F, Rubio RG, Guzmán E. Polyelectrolyte Multilayered Capsules as Biomedical Tools. Polymers. 2022; 14(3):479. https://doi.org/10.3390/polym14030479
Chicago/Turabian StyleMateos-Maroto, Ana, Laura Fernández-Peña, Irene Abelenda-Núñez, Francisco Ortega, Ramón G. Rubio, and Eduardo Guzmán. 2022. "Polyelectrolyte Multilayered Capsules as Biomedical Tools" Polymers 14, no. 3: 479. https://doi.org/10.3390/polym14030479