Characterization of Polyester Coatings Intended for Food Contact by Different Analytical Techniques and Migration Testing by LC-MSn
Abstract
:1. Introduction
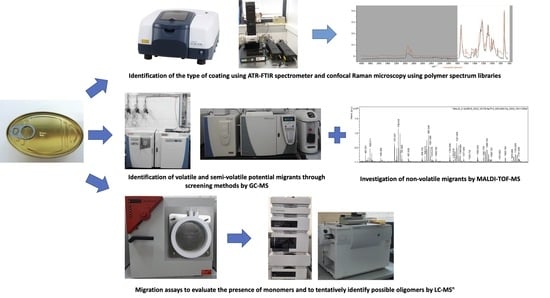
2. Materials and Methods
2.1. Reagents and Standards
2.2. Samples and Extraction Procedures
2.2.1. Volatile and Semi-Volatile Compounds Extraction Methods
2.2.2. Non-Volatile Compounds Extraction Method
2.3. Migration Tests
2.4. Equipment Instrumental Analysis
2.4.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4.2. Confocal Raman Microscopy
2.4.3. Gas Chromatography (GC)
2.4.4. Matrix-Assisted Laser Desorption coupled to Time-of-Flight Mass Spectrometry (MALDI-TOF MS)
2.4.5. Liquid Chromatography Coupled to Ion Trap Mass Spectrometry (LC-MSn)
3. Results and Discussion
3.1. FTIR-ATR and Confocal Raman Microscopy Analysis
3.2. GC Analysis
3.2.1. P&T GC-MS
3.2.2. Semi-Volatile Compounds by GC-MS
3.2.3. HS-SPME-GC-MS
3.3. MALDI-TOF MS
3.4. Analysis of Migration Tests by LC-MSn
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geueke, B. FPF Dossier: Can Coatings; Food Packaging Forum: Zurich, Switzerland, 2016. [Google Scholar]
- Zhang, N.; Scarsella, J.B.; Hartman, T.G. Identification and Quantitation Studies of Migrants from BPA Alternative Food-Contact Metal Can Coatings. Polymers 2020, 12, 2846. [Google Scholar] [CrossRef] [PubMed]
- European Commission Regulation (EC). No. 1895/2005 of 18 November 2005 on the restriction of use of certain epoxy derivatives in materials and articles intended to come into contact with food. J. Eur. Union L 2005, 302, 28–32. [Google Scholar]
- Forrest, M. Coatings and Inks for Food Contact Materials; Smithers Rapra Publishing: Shrewsbury, UK, 2007; Volume 16. [Google Scholar]
- Louise Bradley, E.; Driffield, M.; Guthrie, J.; Harmer, N.; Thomas Oldring, P.K.; Castle, L. Analytical approaches to identify potential migrants in polyester–polyurethane can coatings. Food Addit. Contam. 2009, 26, 1602–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paseiro-Cerrato, R.; Noonan, G.O.; Begley, T.H. Evaluation of long-term migration testing from can coatings into food simulants: Polyester coatings. J. Agric. Food Chem. 2016, 64, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Brenz, F.; Linke, S.; Simat, T. Qualitative and quantitative analysis of monomers in polyesters for food contact materials. Food Addit. Contam. Part A 2017, 34, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Paseiro-Cerrato, R.; MacMahon, S.; Ridge, C.D.; Noonan, G.O.; Begley, T.H. Identification of unknown compounds from polyester cans coatings that may potentially migrate into food or food simulants. J. Chromatogr. A 2016, 1444, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Paseiro-Cerrato, R.; DeJager, L.; Begley, T.H. Determining the migration of nadic acid, terephthalic acid, isophthalic acid and two oligomers from polyester food cans into food in the US market. Food Control 2019, 101, 69–76. [Google Scholar] [CrossRef]
- Eckardt, M.; Hetzel, L.; Brenz, F.; Simat, T.J. Release and migration of cyclic polyester oligomers from bisphenol A non-intent polyester–phenol-coatings into food simulants and infant food–a comprehensive study. Food Addit. Contam. Part A 2020, 37, 681–703. [Google Scholar] [CrossRef]
- Sánchez, B.; Coronado, J.M.; Candal, R.; Portela, R.; Tejedor, I.; Anderson, M.A.; Tompkins, D.; Lee, T. Preparation of TiO2 coatings on PET monoliths for the photocatalytic elimination of trichloroethylene in the gas phase. Appl. Catal. B: Environ. 2006, 66, 295–301. [Google Scholar] [CrossRef] [Green Version]
- LaKind, J.S. Can coatings for foods and beverages: Issues and options. Int. J. Technol. 2013, 13, 80–95. [Google Scholar] [CrossRef] [Green Version]
- Robertson, G.L. Food Packaging: Principles and Practice; CRC Press; Taylor & Francis Group: New York, NY, USA, 2016. [Google Scholar]
- Piergiovanni, L.; Limbo, S. Food Packaging Materials; Springer: Basel, Switzerland, 2016; pp. 33–49. [Google Scholar]
- Gramshaw, J.W.; Vandenburg, H.J.; Lakin, R.A. Identification of potential migrants from samples of dual-ovenable plastics. Food Addit. Contam. 1995, 12, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Cannella, W.J. Xylenes and Ethylbenzene; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Raj, K.J.A.; Meenakshi, M.S.; Vijayaraghavan, V.R. Ethylation and disproportionation of ethylbenzene over substituted AFI type molecular sieves. J. Mol. Catal. A-Chem. 2007, 270, 195–200. [Google Scholar] [CrossRef]
- Hirai, N.; Tatsukawa, Y.; Kameda, M.; Sakaguchi, S.; Ishii, Y. Aerobic oxidation of trimethylbenzenes catalyzed by N, N′, N ″-trihydroxyisocyanuric acid (THICA) as a key catalyst. Tetrahedron 2006, 62, 6695–6699. [Google Scholar] [CrossRef]
- Pan, D.; Li, G.; Su, Y.; Wei, H.; Luo, Z. Kinetic study for the oxidation of cyclohexanol and cyclohexanone with nitric acid to adipic acid. Chin. J. Chem. Eng. 2021, 29, 183–189. [Google Scholar] [CrossRef]
- Ilayaraja, N.; Radhakrishnan, S.; Renganathan, N.G. Electrochemical fluorination of dimethyl glutarate and its characterization. Ionics 2010, 16, 137–144. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No. 10/2011, on Plastic Materials and Articles Intended to Come into Contact with Food. Off. J. Eur. Union 2011, 12, 1–89. [Google Scholar]
- SCF (Scientific Committee on Food). Reports of the Scientific Committee for Food, 42nd Series. 1999. Available online: http://ec.europa.eu/food/fs/sc/scf/reports/scf_reports_42.pdf (accessed on 22 October 2021).
- Wu, Y.; Fei, M.; Qiu, R.; Liu, W.; Qiu, J. A Review on Styrene Substitutes in Thermosets and Their Composites. Polymers 2019, 11, 1815. [Google Scholar] [CrossRef] [Green Version]
- Nerín, C.; Alfaro, P.; Aznar, M.; Domeño, C. The challenge of identifying non-intentionally added substances from food packaging materials: A review. Anal. Chim. Acta 2013, 775, 14–24. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, X.; Zhang, D.; Lan, Z.; Zhang, Y.; Wan, J. Headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography-mass spectrometric (GC-MS) analysis of volatile profiles during the stir-frying process of malt. Anal. Methods 2016, 8, 1699–1704. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). Inert Reassessment—Dipropylene Glycol Monomethyl Ether (DPGME). Available online: https://www.epa.gov/sites/default/files/2015-04/documents/dipropylene.pdf (accessed on 22 October 2021).
- Oldring, P.K.; Nehring, U. Packaging Materials: Metal Packaging for Foodstuffs; ILSI Europe Report Series: Brussels, Belgium, 2007; ISBN 90-78637-06-6. [Google Scholar]
- Dutra, C.; Pezo, D.; de Alvarenga Freire, M.T.; Nerín, C.; Reyes, F.G.R. Determination of volatile organic compounds in recycled polyethylene terephthalate and high-density polyethylene by headspace solid phase microextraction gas chromatography mass spectrometry to evaluate the efficiency of recycling processes. J. Chromatogr. A 2011, 1218, 1319–1330. [Google Scholar] [CrossRef]
- Scarsella, J.B.; Zhang, N.; Hartman, T.G. Identification and migration studies of photolytic decomposition products of UV-photoinitiators in food packaging. Molecules 2019, 24, 3592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.M.; Sheehan, R.J. Phthalic Acids and Other Benzenepolycarboxylic Acids; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Nerín, C.; Su, Q.Z.; Vera, P.; Mendoza, N.; Ausejo, R. Influence of nonylphenol from multilayer plastic films on artificial insemination of sows. Anal. Bioanal. Chem. 2020, 412, 6519–6528. [Google Scholar] [CrossRef] [PubMed]
- Genta, M.; Iwaya, T.; Sasaki, M.; Goto, M.; Hirose, T. Depolymerization mechanism of poly (ethylene terephthalate) in supercritical methanol. Ind. Eng. Chem. Res. 2005, 44, 3894–3900. [Google Scholar] [CrossRef]
- Uyama, H.; Kobayashi, S. Enzymatic synthesis of polyesters via polycondensation. Enzym.-Catalyzed Synth. Polym. 2006, 194, 133–158. [Google Scholar] [CrossRef]
- Ash, M.; Ash, I. Handbook of Preservatives; Synapse Information Resources: Endicott, NY, USA, 2004. [Google Scholar]
- Schmitz, A.D.; Song, C. Shape-selective isopropylation of naphthalene. Reactivity of 2, 6-diisopropylnaphthalene on dealuminated mordenites. Catal. Today 1996, 31, 19–25. [Google Scholar] [CrossRef]
- Putzu, C. The Occurrence of Bisphenol A and Phthalates in Portuguese Wines and the Migration of Selected Substances from Coatings in Contact with a Wine Simulant; Universidade Católica Portuguesa: Lisbon, Portugal, 2016. [Google Scholar]
- Wicks, Z.W., Jr. Blocked isocyanates. Prog. Org. Coat. 1975, 3, 73–99. [Google Scholar] [CrossRef]
- Pietropaolo, E.; Albenga, R.; Gosetti, F.; Toson, V.; Koster, S.; Marin-Kuan, M.; Veyrand, J.; Patin, A.; Schilter, B.; Pistone, A.; et al. Synthesis, identification and quantification of oligomers from polyester coatings for metal packaging. J. Chromatogr. A 2018, 1578, 15–27. [Google Scholar] [CrossRef]
- Nardeli, J.V.; Fugivara, C.S.; Pinto, E.R.P.; Polito, W.L.; Messaddeq, Y.; José Lima Ribeiro, S.; Benedetti, A.V. Preparation of Polyurethane Monolithic Resins and Modification with a Condensed Tannin-Yielding Self-Healing Property. Polymers 2019, 11, 1890. [Google Scholar] [CrossRef] [Green Version]
- Van Haveren, J.; Oostveen, E.A.; Micciche, F.; Noordover, B.A.J.; Koning, C.E.; Van Benthem, R.A.T.M.; Frissen, A.E.; Weijnen, J.G.J. Resins and additives for powder coatings and alkyd paints, based on renewable resources. J. Coat. Technol. Res. 2007, 4, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Gay, M.; Gledhill, A. Chemical Analysis of Food Packaging Migrants and Other Chemical Contaminants in Infant Formula Using a TOF-Based Approach. Waters Application Note. Available online: https://www.gimitec.com/file/720003905en.pdf (accessed on 1 November 2021).
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Sendón, R.; Bustos, J.; Lomo, M.; Paseiro-Losada, P.; Rodríguez-Bernaldo de Quirós, A. Dietary Exposure Estimation to Chemicals Transferred from Milk and Dairy Products Packaging Materials in Spanish Child and Adolescent Population. Foods 2020, 9, 1554. [Google Scholar] [CrossRef] [PubMed]
- Domeño, C.; Aznar, M.; Nerín, C.; Isella, F.; Fedeli, M.; Bosetti, O. Safety by design of printed multilayer materials intended for food packaging. Food Addit. Contam. Part A 2017, 34, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- García-Ibarra, V.; Rodríguez-Bernaldo de Quirós, A.; Paseiro-Losada, P.; Sendón, R. Non-target analysis of intentionally and non intentionally added substances from plastic packaging materials and their migration into food simulants. Food Packag. Shelf Life 2019, 21, 100325. [Google Scholar] [CrossRef]
- Dupáková, Z.; Dobiáš, J.; Votavová, L.; Klaudisová, K.; Voldrich, M. Occurrence of extractable ink residuals in packaging materials used in the Czech Republic. Food Addit. Contam. 2010, 27, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demertzis, P.G.; Franz, R.; Welle, F. The effects of γ-irradiation on compositional changes in plastic packaging films. Packag. Technol. Sci. 1999, 12, 119–130. [Google Scholar] [CrossRef]
- Ohno, H.; Mutsuga, M.; Kawamura, Y. Identification and quantitation of volatile organic compounds in poly (methyl methacrylate) kitchen utensils by headspace gas chromatography/mass spectrometry. J. AOAC Int. 2014, 97, 1452–1458. [Google Scholar] [CrossRef]
- Aalto-Korte, K.; Pesonen, M.; Suuronen, K. Occupational allergic contact dermatitis caused by epoxy chemicals: Occupations, sensitizing products, and diagnosis. Contact Dermat. 2015, 73, 336–342. [Google Scholar] [CrossRef]
- Eckardt, M.; Simat, T.J. Bisphenol A and alternatives in thermal paper receipts-a German market analysis from 2015 to 2017. Chemosphere 2017, 186, 1016–1025. [Google Scholar] [CrossRef]
- Su, Q.Z.; Vera, P.; Nerín, C.; Lin, Q.B.; Zhong, H.N. Safety concerns of recycling postconsumer polyolefins for food contact uses: Regarding (semi-) volatile migrants untargetedly screened. Resour. Conserv. Recy. 2021, 167, 105365. [Google Scholar] [CrossRef]
- Bravo, A.; Hotchkiss, J.H. Identification of volatile compounds resulting from the thermal oxidation of polyethylene. J. Appl. Polym. Sci. 1993, 47, 1741–1748. [Google Scholar] [CrossRef]
- García-Ibarra, V.; Sendón, R.; Bustos, J.; Paseiro-Losada, P.; Rodríguez-Bernaldo de Quirós, A. Estimates of dietary exposure of Spanish population to packaging contaminants from cereal based foods contained in plastic materials. Food Chem. Toxicol. 2019, 128, 180–192. [Google Scholar] [CrossRef]
- Alam, M.; Akram, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable oil based eco-friendly coating materials: A review article. Arab. J. Chem. 2014, 7, 469–479. [Google Scholar] [CrossRef]
- Van Gorkum, R.; Bouwman, E. The oxidative drying of alkyd paint catalysed by metal complexes. Coord. Chem. Rev. 2005, 249, 1709–1728. [Google Scholar] [CrossRef]
- Crespo, E.A.; Costa, J.M.L.; Hanafiah, Z.B.M.A.; Kurnia, K.A.; Oliveira, M.B.; Llovell, F.; Vega, L.F.; Carvalho, P.J.; Coutinho, J.A. New measurements and modeling of high pressure thermodynamic properties of glycols. Fluid Phase Equilib. 2017, 436, 113–123. [Google Scholar] [CrossRef]
- Zhao, X.; Noro, J.; Fu, J.; Wang, H.; Silva, C.; Cavaco-Paulo, A. “In-situ” lipase-catalyzed cotton coating with polyesters from ethylene glycol and glycerol. Process Biochem. 2018, 66, 82–88. [Google Scholar] [CrossRef] [Green Version]
- European Chemical Agency (ECHA). 1-butoxypropan-2-ol. 2019. Available online: https://echa.europa.eu/es/substance-information/-/substanceinfo/100.023.526 (accessed on 27 October 2021).
- Clemente, I.; Aznar, M.; Nerín, C.; Bosetti, O. Migration from printing inks in multilayer food packaging materials by GC-MS analysis and pattern recognition with chemometrics. Food Addit. Contam. Part A 2016, 33, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Lestido-Cardama, A.; Loureiro, P.V.; Sendón, R.; Paseiro-Losada, P.; Rodríguez-Bernaldo de Quirós, A. Application of chromatographic analysis for detecting components from polymeric can coatings and further determination in beverage samples. J. Chromatogr. A 2021, 1638, 461886. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Luo, S.; Wang, C.; Wang, J.; Weinell, C.E.; Dam-Johansen, K.; Segura, J.J.; Kiil, S. Methanol degradation mechanisms and permeability phenomena in novolac epoxy and polyurethane coatings. J. Coat. Technol. Res. 2021, 18, 831–842. [Google Scholar] [CrossRef]
- Aznar, M.; Vera, P.; Canellas, E.; Nerín, C.; Mercea, P.; Störmer, A. Composition of the adhesives used in food packaging multilayer materials and migration studies from packaging to food. J. Mater. Chem. 2011, 21, 4358–4370. [Google Scholar] [CrossRef]
- Higgins, C.; Cahill, J.; Jolanki, R.; Nixon, R. Epoxy resins. In Kanerva’s Occupational Dermatology; Springer: Cham, Switzerland, 2020; pp. 757–788. [Google Scholar] [CrossRef]
- Arnould, M.A.; Wesdemiotis, C.; Geiger, R.J.; Park, M.E.; Buehner, R.W.; Vanderorst, D. Structural characterization of polyester copolymers by MALDI mass spectrometry. Prog. Org. Coat. 2002, 45, 305–312. [Google Scholar] [CrossRef]
- Omer, E.; Cariou, R.; Remaud, G.; Guitton, Y.; Germon, H.; Hill, P.; Dervilly-Pinel, G.; Le Bizec, B. Elucidation of non-intentionally added substances migrating from polyester-polyurethane lacquers using automated LC-HRMS data processing. Anal. Bioanal. Chem. 2018, 410, 5391–5403. [Google Scholar] [CrossRef] [PubMed]
- Toxtree v3.1.0—Toxic Hazard Estimation by Decision Tree Approach. Available online: http://toxtree.sourceforge.net/download.html (accessed on 27 October 2021).
- Úbeda, S.; Aznar, M.; Vera, P.; Nerín, C.; Henríquez, L.; Taborda, L.; Restrepo, C. Overall and specific migration from multilayer high barrier food contact materials–kinetic study of cyclic polyester oligomers migration. Food Addit. Contam. Part A 2017, 34, 1784–1794. [Google Scholar] [CrossRef] [PubMed]




| TR (min) | CAS Nº | Compound | m/z | CM1 | CM2 | CM3 |
|---|---|---|---|---|---|---|
| 6.38 | 123-72-8 | Butanal | 44, 72 | X | X | X |
| 6.57 | 78-93-3 | 2-butanone | 43, 72 | X | ||
| 7.69 | 78-83-1 | Isobutanol | 43, 74 | X | ||
| 8.66 | 71-36-3 | 1-butanol * | 41, 56 | X | X | X |
| 9.31 | 80-62-6 | Methyl methacrylate | 41, 69, 100 | X | ||
| 10.92 | 108-88-3 | Toluene * | 65, 91 | X | X | X |
| 12.24 | 66-25-1 | Hexanal * | 43, 56 | X | ||
| 13.59 | 100-41-4 | Ethyl benzene | 91, 106 | X | ||
| 13.85 | Xylene structure | 91, 106 | X | X | X | |
| 14.50 | Xylene structure | 91, 106 | X | X | X | |
| 15.28 | 111-76-2 | 2-butoxyethanol * | 57, 87 | X | X | X |
| 15.42 | 108-94-1 | Cyclohexanone | 55, 69, 98 | X | ||
| 16.45 | Trimethylbenzene | 105, 120 | X | |||
| 17.18 | Trimethylbenzene | 77, 105, 120 | X | X | X | |
| 17.61 | 124-13-0 | Octanal | 41, 56, 69 | X | ||
| 17.99 | Trimethylbenzene | 59, 105, 120 | X | |||
| 18.01 | 13429-07-7 | 1-(2-methoxypropoxy)-2-propanol | 59, 105, 120 | X | ||
| 18.28 | 104-76-7 | 2-ethyl-1-hexanol | 57, 70, 83 | X | ||
| 18.57 | 105-05-5 | 1,4-diethylbenzene | 105, 119, 134 | X | ||
| 18.77 | 108-95-2 | Phenol * | 66, 94 | X | ||
| 19.30 | Unknown (benzene structure) | 119, 134 | X | |||
| 19.32 | 112-07-2 | 2-butoxyethyl acetate | 43, 57, 87 | X | X | |
| 19.79 | 98-86-2 | Acetophenone | 70, 105, 120 | X | ||
| 20.00 | 124-19-6 | Nonanal * | 28, 32, 57, 70 | X | X | X |
| 20.26 | Unknown (benzene structure) | 119, 134 | X | |||
| 20.82 | 1119-40-0 | Dimethyl glutarate | 59, 100, 129 | X | X | |
| 22.20 | 112-31-2 | Decanal | 28, 41, 57, 70 | X | X | X |
| 23.44 | 95-16-9 | Benzothiazole | 69, 108, 135 | X | ||
| 24.79 | Unknown (benzene structure) | 119, 131, 147 | X | |||
| 25.00 | Unknown (naphthalene structure) | 117, 131, 160 | X |
| TR (min) | CAS Nº | Compound | m/z | SI | RSI | CM1 | CM2 | CM3 |
|---|---|---|---|---|---|---|---|---|
| 9.34 | 104-76-7 | 2-ethyl-1-hexanol | 43, 57, 79, 83 | 709 | 955 | X | ||
| 10.56 | 93-58-3 | Methyl benzoate | 77, 105, 136 | 750 | 854 | X | ||
| 11.03 | 1587-15-1 | Dimethyl maleate | 43, 71, 103 | 739 | 922 | X | ||
| 11.77 | 65-85-0 | Benzoic acid | 51, 77, 105, 122 | 805 | 956 | X | ||
| 12.17 | 112-41-4 | 1-dodecene | 43, 55, 69, 83 | 934 | 935 | X | ||
| 12.44 | 527-54-8 | 3,4,5-trimethylphenol | 91, 121, 136 | 760 | 885 | X | ||
| 12.95 | 627-93-0 | Dimethyl adipate | 101, 114, 143 | 700 | 818 | X | ||
| 13.01 | 100-97-0 | Hexamethylenetetramine * | 42, 112, 140 | 617 | 905 | X | ||
| 13.11 | 1014-60-4 | 1,3-di-tert-butylbenzene | 41, 57, 91, 175 | 744 | 832 | X | ||
| 13.32 | 105-60-2 | ε-caprolactam * | 55, 85, 113 | 766 | 849 | X | ||
| 13.38 | 112-05-0 | Nonanoic acid | 60, 73, 115 | 700 | 773 | X | ||
| 13.50 | 629-62-9 | Pentadecane | 43, 57, 71, 85 | 765 | 867 | X | ||
| 13.55 | 77-99-6 | Trimethylolpropane | 41, 57, 71, 86 | 903 | 916 | X | ||
| 13.75 | 627-91-8 | Methyl adipate | 55, 74, 100, 114, 129 | 713 | 882 | X | ||
| 14.03 | 487-68-3 | 2,4,6-trimethylbenzaldehyde | 91, 119, 147 | 859 | 946 | X | ||
| 14.20 | 85-44-9 88-99-3 | Phthalic acid pure or anhydride phthalic | 50, 76, 104, 148 | 776 | 880 | X | ||
| 14.56 | 2282-84-0 | Methyl 2,4,6-trimethylbenzoate | 91, 119, 147, 178 | 860 | 862 | X | ||
| 14.88 | 124-04-9 | Adipic acid | 41, 55, 87, 100 | 725 | 823 | X | ||
| 15.83 | 480-63-7 | 2,4,6-trimethylbenzoic acid | 119, 146, 164 | 900 | 902 | X | ||
| 15.95 | 6745-75-1 | 2,5-dimethyl-4-methoxybenzaldehyde | 77, 163, 164 | 744 | 765 | X | ||
| 16.09 | 7310-95-4 | 2-hydroxy-5-methylisophthalaldehyde | 77, 90, 107, 136, 164 | 825 | 831 | X | ||
| 16.44 | 7534-94-3 | Isobornyl methacrylate | 41, 69, 95, 136 | 927 | 928 | X | ||
| 16.51 | 2233-18-3 | 4-hydroxy-3,5-dimethylbenzaldehyde | 77, 91, 121, 149, 150 | 892 | 918 | X | ||
| 16.63 | 487-69-4 | 2,4-dihydroxy-6-methylbenzaldehyde | 106, 123, 151 | 714 | 745 | X | ||
| 16.95 | 96-76-4 | 2,4-di-tert-butylphenol * | 57, 191, 206 | 781 | 834 | X | X | X |
| 17.03 | 1459-93-4 | Dimethyl isophthalate * | 76, 135, 163, 194 | 860 | 924 | X | X | |
| 17.66 | 143-07-7 | Dodecanoic acid | 43, 60, 73 | 853 | 901 | X | ||
| 18.09 | 4098-71-9 | Isophorone diisocyanate | 81, 110, 123 | 703 | 791 | X | X | |
| 18.81 | Unknown (acrylate structure) | 55, 68 | X | |||||
| 19.18 | Unknown (alcohol structure) | 69, 83, 97 | 726 | 842 | X | X | ||
| 19.35 | 100-21-0 121-91-5 | Terephthalic acid or isophthalic acid | 65, 120, 149, 166 | 716 | 753 | X | ||
| 19.46 | Unknown (alkane structure) | 57, 71, 85 | X | X | ||||
| 19.82 | 24157-81-1 | 2,6-diisopropylnaphthalene | 155, 197, 212 | 793 | 834 | X | X | |
| 19.89 | Unknown (ester of adipic acid) | 55, 111, 143 | X | |||||
| 20.19 | 544-63-8 | Tetradecanoic acid | 43, 60, 73, 129 | 883 | 893 | X | X | |
| 20.67 | 26896-48-0 | Tricyclodecanedimethanol | 67, 79, 91, 119, 147 | 826 | 832 | X | ||
| 20.82 | 104-66-5 | Diphenyl glycol | 77, 121, 214 | 815 | 824 | X | ||
| 21.16 | 502-69-2 | 6,10,14-trimethylpentadecan-2-one | 43, 58, 71 | 908 | 939 | X | ||
| 21.20 | 3645-00-9 | Methyl-(2-hydroxyethyl) terephthalate | 76, 163, 181 | 832 | 907 | X | ||
| 21.35 | 1002-84-2 | Pentadecanoic acid | 60, 73, 129 | 700 | 767 | X | X | |
| 21.39 | 84-69-5 | Diisobutyl phthalate * | 41, 57, 149 | 858 | 932 | X | X | X |
| 21.62 | Unknown (alcohol structure) | 69, 83, 97 | 846 | 897 | X | |||
| 21.92 | 82304-66-3 | 7,9-di-tert-butyl-1-oxaspiro [4.5]deca-6,9-diene-2,8-dione | 41, 57, 175, 205 | 709 | 709 | X | ||
| 22.10 | 112-39-0 | Methyl palmitate * | 43, 74, 87 | 891 | 932 | X | X | X |
| 22.16 | Unknown (eicosene structure) | 43, 55, 70, 83 | X | |||||
| 22.27 | Unknown (acid structure) | 55, 69, 81, 96 | X | X | ||||
| 22.45 | 84-74-2 | Dibutyl phthalate * | 149 | 892 | 926 | X | ||
| 22.51 | 57-10-3 | Hexadecanoic acid | 43, 60, 73, 129 | 922 | 962 | X | X | |
| 22.58 | Unknown (alcohol structure) | 82, 95, 109 | X | |||||
| 23.27 | Unknown (phthalate structure) | 56, 76, 163, 181 | X | |||||
| 23.43 | 91-76-9 | Benzoguanamine | 43, 76, 103, 187 | 921 | 937 | X | X | |
| 23.46 | Unknown (phthalate structure) | 56, 163, 181 | X | |||||
| 23.72 | Unknown (phthalate structure) | 149 | 747 | 766 | X | |||
| 23.83 | Unknown (alcohol structure) | 43, 57, 69, 83, 97 | 851 | 939 | X | |||
| 23.98 | 112-62-9 | Methyl oleate | 55, 69, 74, 83, 97 | 888 | 907 | X | X | |
| 23.98 | 629-92-5 | Nonadecane | 43, 57, 71 | 754 | 855 | X | ||
| 24.24 | 112-61-8 | Methyl stearate | 74, 87, 143 | 771 | 898 | X | X | X |
| 24.39 | Unknown compound (octadecenoic acid structure) | 55, 69, 83, 97 | 888 | 888 | X | X | ||
| 24.64 | 57-11-4 | Stearic acid | 43, 57, 73 | 876 | 931 | X | X | |
| 24.78 | 607-58-9 | 1-(benzyloxy)naphthalene * | 91, 65, 115, 234 | 717 | 819 | X | ||
| 24.88 | 80-05-7 | Bisphenol A (BPA) * | 91, 119, 213 | 855 | 860 | X | X | |
| 25.00 | Unknown (alkane structure) | 43, 57, 71, 85 | X | |||||
| 25.42 | 77-90-7 | Acetyltributyl citrate (ATBC) * | 129, 185, 259 | 771 | 901 | X | ||
| 25.63 | Unknown (acetophenone structure) | 77, 91, 119, 147 | 861 | 906 | X | |||
| 25.72 | Unknown (benzoic acid structure) | 77, 105 | 735 | 892 | X | |||
| 25.97 | Unknown (isophthalic acid structure) | 82, 149, 167, 205 | X | |||||
| 26.15 | Unknown compound (octadecenoic acid structure) | 55, 69, 85, 97 | 779 | 790 | X | |||
| 26.17 | Unknown (phenol structure) | 121, 227, 256 | X | |||||
| 26.45 | 1235-74-1 | Methyl dehydroabietate | 239, 299 | 700 | 711 | X | ||
| 26.78 | 103-23-1 | Bis(2-ethylhexyl) adipate (DEHA) * | 57, 70, 111, 129 | 750 | 943 | X | ||
| 26.89 | Unknown (alkane structure) | 57, 71, 85 | X | |||||
| 26.96 | 115-86-6 | Triphenyl phosphate | 77, 94, 326 | 752 | 908 | X | ||
| 27.57 | 10546-70-0 | n-propylbenzamide | 77, 105, 163 | 809 | 955 | X | X | X |
| 27.64 | 120-55-8 | Di(ethylene glycol) dibenzoate | 77, 105, 149 | 888 | 967 | X | ||
| 27.78 | Unknown (alkane structure) | 43, 57, 71 | X | |||||
| 28.07 | 117-81-7 | Bis(2-ethylhexyl) phthalate (DEHP) * | 149, 167 | 841 | 916 | X | X | X |
| 28.25 | Unknown (isophthalic acid structure) | 82, 149, 231 | X | |||||
| 28.39 | Unknown (phthalate or benzoic acid structure) | 149, 167, 235, 253 | X | X | X | |||
| 28.87 | Unknown (phthalate or benzoic acid structure) | 149, 167, 235 | X | |||||
| 29.11 | 6197-30-4 | Octocrylene * | 178, 204, 248 | 613 | 840 | X | ||
| 29.75 | Unknown (phthalate structure) | 82, 104, 149, 383 | X | |||||
| 29.47 | Unknown (alkane structure) | 43, 57, 71 | X | |||||
| 30.20 | Unknown (phthalate structure) | 82, 104, 149, 383 | X | |||||
| 30.39 | 111-02-4 | Squalene * | 69, 81 | 677 | 787 | X | X | X |
| 35.76 | Unknown (ester of adipic acid) | 55, 83, 129 | X | |||||
| 36.70 | Unknown (phthalate structure) | 83, 149 | X | |||||
| 38.36 | Terephthalic acid ester of neopentyl glycol cyclic dimer (C26H28O8) | 76, 104, 132, 149, 338, 383, 468 | 773 | 961 | X | X | X |
| TR (min) | CAS Nº | Compound | m/z | SI | RSI | CM1 | CM2 | CM3 |
|---|---|---|---|---|---|---|---|---|
| 5.72 | Xylene structure | 91, 106 | 814 | 930 | X | X | X | |
| 5.94 | 140-88-5 | Ethyl acrylate | 55, 27, 99 | 700 | 818 | X | ||
| 6.1 | 108-94-1 | Cyclohexanone | 55, 42, 98, 69 | 893 | 913 | X | ||
| 6.3 | 111-71-7 | Heptanal | 43,70,55 | 811 | 876 | X | X | X |
| 6.4 | 111-76-2 | 2-butoxyethanol | 57,45,87 | 856 | 878 | X | X | X |
| 6.9 | 126-30-7 | Neophentyl glycol | 56,73,31 | 839 | 881 | X | X | X |
| 7.1 | 5131-66-8 | 1-butoxy-2-propanol | 45,57,87 | 823 | 878 | X | X | |
| 7.6 | Trimethylbenzene | 105,12 | X | |||||
| 8.1 | Trimethylbenzene | 105,12 | 730 | 924 | X | X | X | |
| 8.2 | 124-13-0 | Octanal | 41,57,84 | 749 | 944 | X | X | X |
| 8.5 | 584-03-2 | 1,2-butanediol | 59,43,73 | 785 | 810 | X | X | X |
| 8.6–9.6 | 56-81-5 | Glycerol | 61,43,31 | 735 | 804 | X | X | X |
| 8.63 | Trimethylbenzene | 105, 120 | 767 | 848 | X | |||
| 8.7 | 104-76-7 | 2-ethyl-1-hexanol | 57,41,70 | 764 | 877 | X | X | X |
| 8.8 | 106-65-0 | Dimethyl succinate | 115, 55, 87 | 789 | 943 | X | ||
| 8.84 | 100-51-6 | Benzyl alcohol | 79, 108 | 785 | 867 | X | ||
| 9.4 | 98-86-2 | Acetophenone | 105, 77, 120 | 704 | 827 | X | ||
| 9.5 | 103-09-3 | 2-ethylhexyl acetate | 43,56,70 | 759 | 775 | X | ||
| 9.5 | 111-87-5 | 1-octanol | 56, 70, 83 | 768 | 891 | X | X | |
| 9.6 | 3101-60-8 | 4-tert-butylphenyl glycidyl ether | 191, 135, 206 | 700 | 683 | X | ||
| 9.8 | Tetramethylbenzene | 119,91,134 | X | X | ||||
| 9.86 | 112-07-2 | 2-butoxyethyl acetate | 43,57,87 | 794 | 859 | X | X | X |
| 10.05 | 1120-21-4 | Undecane | 43,57,71,85 | 896 | 945 | X | X | |
| 10.1 | 124-19-6 | Nonanal | 41,57,29,70 | 948 | 948 | X | X | X |
| 10.4 | 488-23-3/527-53-7/934-74-7 | Tetramethylbenzene | 119,134 | 715 | 798 | X | X | X |
| 10.6 | 1119-40-0 | Dimethyl glutarate | 59,100,129 | 862 | 900 | X | X | X |
| 11.05 | 18829-56-6 | Trans-2-nonenal | 41, 55, 70 | 761 | 865 | X | X | |
| 11.4 | 124-07-2 | Octanoic acid | 60, 73, 43 | 836 | 894 | X | X | X |
| 11.55 | 112-34-5 | Diethylene glycol monobutyl ether | 45, 57, 29 | 717 | 917 | X | X | X |
| 11.6 | 112-41-4 | 1-codecene | 43, 55, 69, 83, 97 | 836 | 894 | X | ||
| 11.7 | 112-40-3 | Dodecane | 43, 57, 71, 85 | 932 | 933 | X | X | X |
| 11.8 | 112-31-2 | Decanal | 41, 57, 82, 95 | 948 | 951 | X | X | X |
| 11.99 | 87-61-6/120-82-1 | Trichlorobenzene | 180, 145, 109, 74 | 878 | 901 | X | X | |
| 12.07 | 122-99-6 | 2-phenoxyethanol | 94, 138, 77 | 911 | 912 | X | X | X |
| 12.2 | 95-16-9 | Benzothiazole | 135, 108, 69 | 855 | 937 | X | X | X |
| 12.4 | 627-93-0 | Dimethyl adipate | 59, 114, 143 | 811 | 895 | X | X | X |
| 12.7 | 3913-81-3 | 2-decenal | 41, 55, 70, 83 | 778 | 903 | X | X | X |
| 12.88 | 112-05-0 | Nonanoic acid | 55,41,73 | 739 | 847 | X | X | X |
| 13.1 | 7473-98-5 | 2-hydroxy-2-methylpropiophenone | 57,77,105 | 739 | 837 | X | ||
| 13.3 | Unknown (methyl-naphthalene structure) | 142, 115 | X | |||||
| 13.3 | 629-50-5 | Tridecane | 43,57,71 | 901 | 935 | X | ||
| 13.4 | Unknown (aldehyde structure) | 41, 57, 68, 82 | 738 | 901 | X | X | ||
| 13.44 | Unknown (benzaldehyde structure) | 147,119,91 | 898 | 913 | X | |||
| 13.8-13.9 | Unknown (naphthalene structure) | 131,160,145 | X | X | ||||
| 14.2 | 6846-50-0 | 2,2,4-trimethyl-1,3-pentanediol diisobutyrate | 71,43,56,83 | 814 | 840 | X | X | X |
| 14.3 | 2463-77-6 | 2-undecenal | 70,57,41 | 760 | 806 | X | X | X |
| 14.4 | 334-48-5 | Decanoic acid | 60,73,129,41 | 701 | 778 | X | X | X |
| 14.8 | 629-59-4 | Tetradecane | 57,43,71,85 | 865 | 932 | X | X | X |
| 14.95 | Unknown (aldehyde structure) | 41,57,82 | 917 | 959 | X | X | X | |
| 15.2 | Unknown (naphthalene structure) | 156,141 | X | X | ||||
| 15.87 | Unknown (alcohol structure) | 55,41,69,83 | X | X | ||||
| 16.28 | 4792-15-8 or 2615-15-8 | Pentaethylene glycol or hexaethylene glycol | 45, 89 | 700 | 794 | X | ||
| 16.38 | 56554-89-3 | 14-octadecenal | 82,57,41 | 715 | 742 | X | X | X |
| 16.45 | 96-76-4 | 2,4-di-tert-butylphenol | 191,206 | 884 | 906 | X | X | X |
| 16.8 | Unknown (alcohol structure) | 57,41,69,83 | X | X | ||||
| 16.9 | Unknown (ester of carboxylic acid) | 129, 111, 55, 83 | X | |||||
| 17.5 | 84-66-2 | Diethyl phthalate | 149,177 | 935 | 949 | X | X | X |
| 17.5 | Unknown (alkane structure) | 57,71,43,85 | 807 | 895 | X | X | X | |
| 17.74 | 124-25-4 | Tetradecanal | 57,41,82 | 775 | 912 | X | X | X |
| 18.5 | Unknown (alcohol structure) | 43,55,69,83 | 915 | 936 | X | X | X | |
| 18.7 | 24157-81-1 | 2,6-diisopropylnaphthalene | 197,155,212 | 737 | 877 | X | X | |
| 18.8 | 629-78-7 | Heptadecane | 57,43,71,85 | 757 | 885 | X | X | X |
| 20.04 | Unknown (alkane structure) | 43, 57, 71, 85 | X | X | X | |||
| 20.18 | 118-60-5 | 2-ethylhexyl salicylate | 120, 138, 250 | 738 | 890 | X | X | |
| 20.3 | 110-27-0 | Isopropyl myristate | 102, 228, 129, 185 | 720 | 758 | X | ||
| 20.8 | Unknown (phthalate structure) | 149, 223, 167 | X | X | X | |||
| 20.9 | Unknown (alcohol structure) | 69, 97, 55 | 789 | 847 | X | |||
| 21.46 | 82304-66-3 | 7,9-di-tert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione | 205, 175, 189 | 843 | 858 | X | X | X |
| TR (min) | m/z (Adduct) * | Product Ions | Proposed Compound | CT | Sample |
|---|---|---|---|---|---|
| 16.6, 17.4 | 584.3 (NH4+), 567.3 (H+), 589.3 (Na+), 605.3 (K+) | 2PA+2CHDM (L) | I | CM3 | |
| 18.8, 64.8 | 419.2 (H+), 436.2 (NH4+), 441.2 (Na+), 457.2 (K+) | 201, 149 | PA+2CHDM | I | CM2, CM3 |
| 28.4 | 636.3 (NH4+), 619.3 (H+), 641.3 (Na+), 657.3 (K+) | 3PA+NPG+2EG (C) | III | CM2, CM3 | |
| 29.5 | 423.2 (Na+), 401.2 (H+), 418.3 (NH4+), 439.2 (K+) | 2PA+NPG | I | CM2 | |
| 35.5 | 385.1 (H+), 407.1 (Na+), 423.0 (K+), 402.1 (NH4+) | 193, 149, 341, 359 | 2PA+2EG (C) | III | CM2, CM3 |
| 39.7 | 425.2 (Na+), 441.2 (K+), 420.2 (NH4+), 403.2 (H+) | 2PA+2EG (L) | I | CM2, CM3 | |
| 39.7 | 761.3 (H+), 778.4 (NH4+), 783.4 (Na+), 799.3 (K+) | 3PA+2NPG+CHDM (L) | I | CM2, CM3 | |
| 44.6 | 504.3 (NH4+), 509.3 (Na+), 525.2 (K+), 487.3 (H+) | 2PA+2NPG (L) | I | CM2 | |
| 51.6, 53.0 | 683.4 (Na+), 678.3 (NH4+), 699.3 (K+) | 3PA+3BD (C) | III | CM1 | |
| 52.3 | 427.1 (H+), 449.1 (Na+), 444.1 (NH4+), 465.1 (K+) | 217, 149, 341, 193, 359 | 2PA+NPG+EG (C) | III | CM2, CM3 |
| 54.5, 55.6 | 441.1 (H+), 463.1 (Na+), 458.1 (NH4+) | 149, 167 | 2PA+CHDM | I | CM2 |
| 54.9, 56.3, 56.8 | 469.2 (H+), 486.2 (NH4+), 507.1 (K+), 491.2 (Na+) | 383, 235, 149, 162, 217, 401 | 2PA+2NPG (C) | III | CM1, CM2, CM3 |
| 58.6, 59.6 | 483.2 (H+), 500.2 (NH4+), 505.2 (Na+), 521.1 (K+) | 415, 231, 149, 383, 397 | 2PA+NPG+HD (C) | III | CM1 |
| 60.3, 60.7 | 683.4 (Na+), 678.3 (NH4+), 699.3 (K+), 661.3 (H+) | 3PA+2NPG+EG (C) | III | CM2, CM3 | |
| 60.8, 61.9 | 509.4 (H+), 531.4 (Na+) | 491, 383, 235, 149, 257, 217 | 2PA+NPG+CHDM (C) | III | CM3 |
| 61.3 | 497.2 (H+), 514.2 (NH4+), 519.2 (Na+), 535.1 (K+) | 479, 415, 231, 149 | 2PA+2HD (C) | III | CM1 |
| 62.5, 62.8 | 725.3 (Na+), 720.3 (NH4+), 703.2 (H+), 741.2 (K+) | 235, 469, 401 | 3PA+3NPG (C) | III | CM1, CM2, CM3 |
| 62.7 | 781.5 (Na+), 797.4 (K+), 776.4 (NH4+) | 3PA+2CHDM+EG (L) | I | CM2 | |
| 63.4, 64.0 | 917.2 (Na+), 912.2 (NH4+), 933.2 (K+), 895.2 (H+) | 4PA+3NPG+EG (C) | III | CM3 | |
| 63.6, 63.9 | 739.3 (Na+), 734.3 (NH4+), 755.2 (K+), 717.3 (H+) | 3PA+2NPG+HD (C) | III | CM1 | |
| 64.4, 64.7 | 753.3 (Na+), 748.3 (NH4+), 731.3 (H+), 769.2 (K+) | 3PA+NPG+2HD (C) | III | CM1 | |
| 65.2, 65.4 | 767.3 (Na+), 762.3 (NH4+), 745.3 (H+), 783.2 (K+) | 3PA+3HD (C) | III | CM1 | |
| 63.9 | 612.5 (NH4+), 617.5 (Na+), 633.4 (K+) | 3PA+3EG (L) | I | CM2, CM3 | |
| 64.8 | 743.3 (H+), 760.3 (NH4+), 765.3 (Na+), 781.2 (K+) | 3PA+2NPG+CHDM (C) | III | CM3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lestido-Cardama, A.; Vázquez-Loureiro, P.; Sendón, R.; Bustos, J.; Santillana, M.I.; Paseiro Losada, P.; Rodríguez Bernaldo de Quirós, A. Characterization of Polyester Coatings Intended for Food Contact by Different Analytical Techniques and Migration Testing by LC-MSn. Polymers 2022, 14, 487. https://doi.org/10.3390/polym14030487
Lestido-Cardama A, Vázquez-Loureiro P, Sendón R, Bustos J, Santillana MI, Paseiro Losada P, Rodríguez Bernaldo de Quirós A. Characterization of Polyester Coatings Intended for Food Contact by Different Analytical Techniques and Migration Testing by LC-MSn. Polymers. 2022; 14(3):487. https://doi.org/10.3390/polym14030487
Chicago/Turabian StyleLestido-Cardama, Antía, Patricia Vázquez-Loureiro, Raquel Sendón, Juana Bustos, Mª Isabel Santillana, Perfecto Paseiro Losada, and Ana Rodríguez Bernaldo de Quirós. 2022. "Characterization of Polyester Coatings Intended for Food Contact by Different Analytical Techniques and Migration Testing by LC-MSn" Polymers 14, no. 3: 487. https://doi.org/10.3390/polym14030487
APA StyleLestido-Cardama, A., Vázquez-Loureiro, P., Sendón, R., Bustos, J., Santillana, M. I., Paseiro Losada, P., & Rodríguez Bernaldo de Quirós, A. (2022). Characterization of Polyester Coatings Intended for Food Contact by Different Analytical Techniques and Migration Testing by LC-MSn. Polymers, 14(3), 487. https://doi.org/10.3390/polym14030487