Physical and Chemical Properties of Acacia mangium Lignin Isolated from Pulp Mill Byproduct for Potential Application in Wood Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Lignin Isolation
2.3. Chemical Features Measurement: Chemical Component, Total Phenolic Hydroxyl, and Solubility
2.4. Morphological Assessment by SEM
2.5. Functional Group Analysis by UATR-FTIR
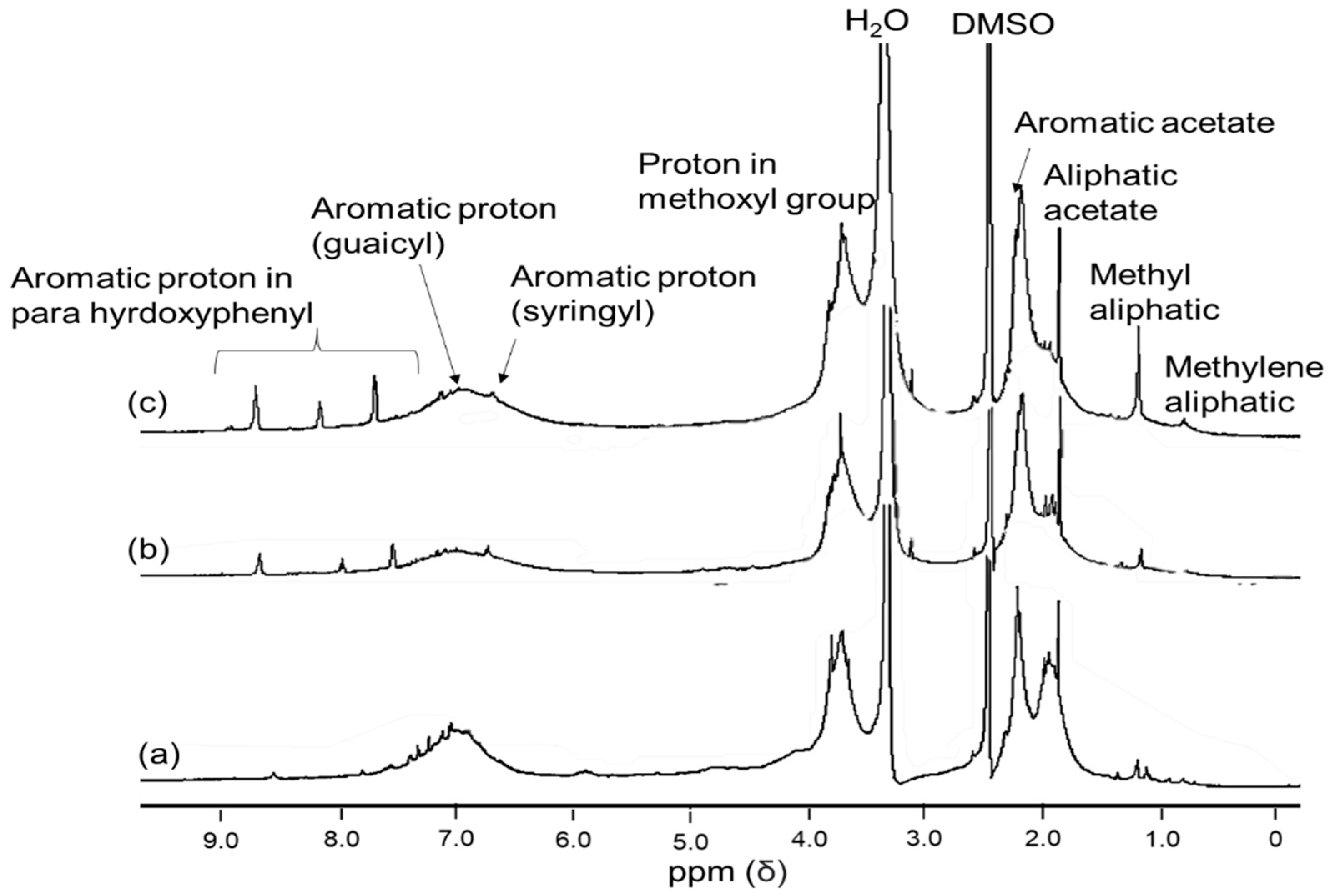
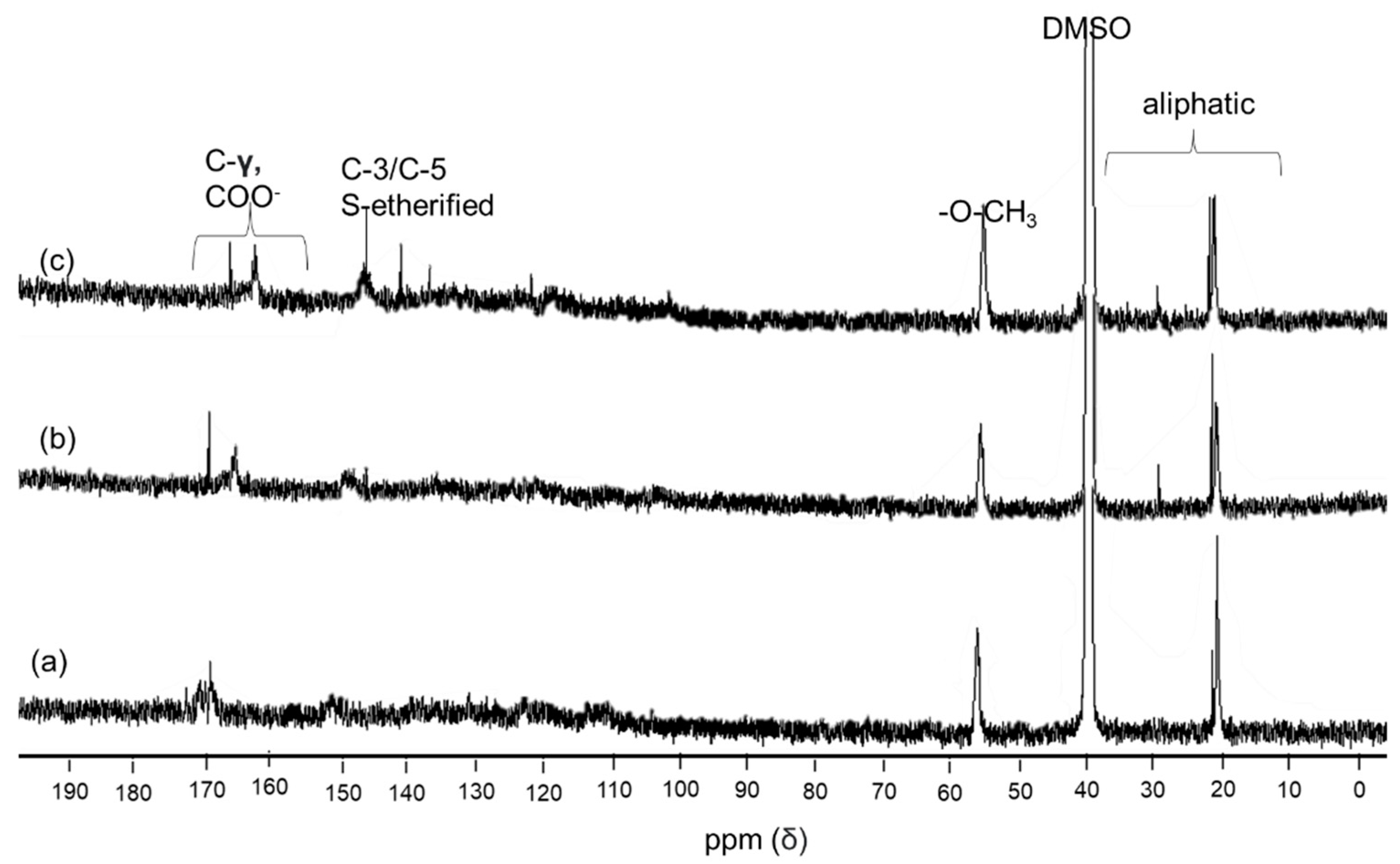
2.6. Fingerprint Observation by 1H and 1C NMR
2.7. Thermal Investigation by TGA and DSC
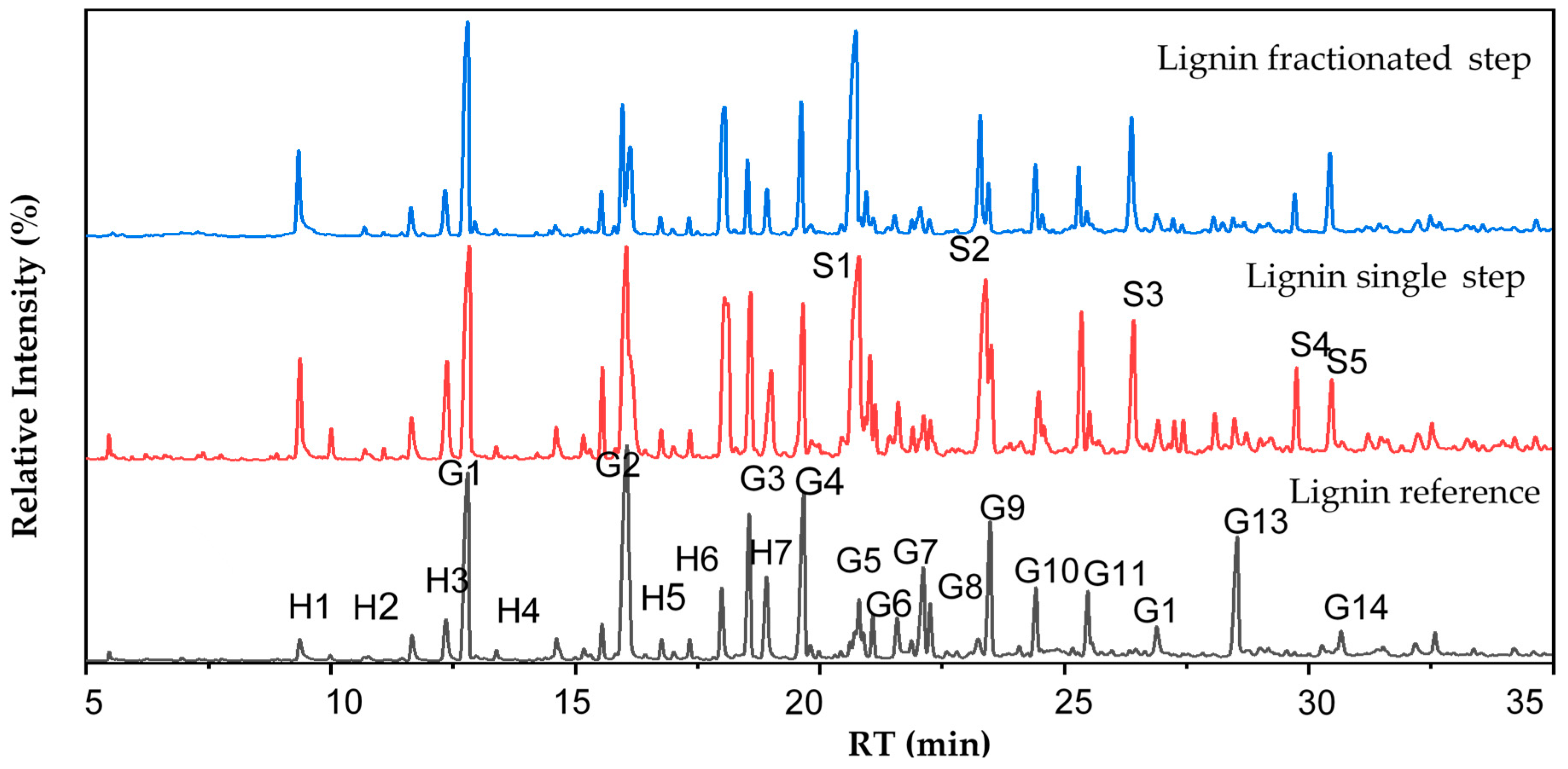
2.8. Chemical Elucidation by Py-GCMS
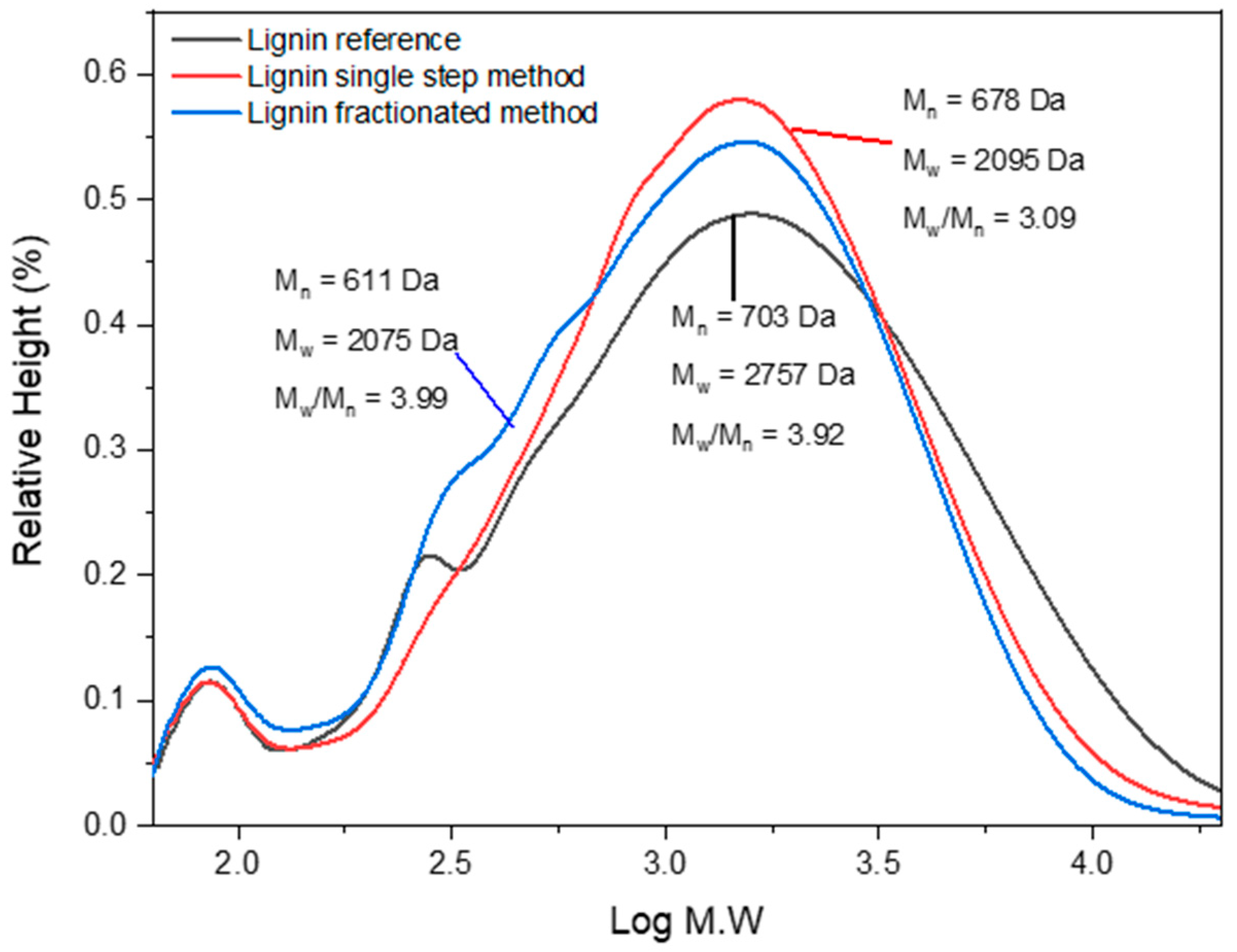
2.9. Molecular Weight by GPC
3. Result and Discussion
3.1. Chemical Composition and Lignin Solubility
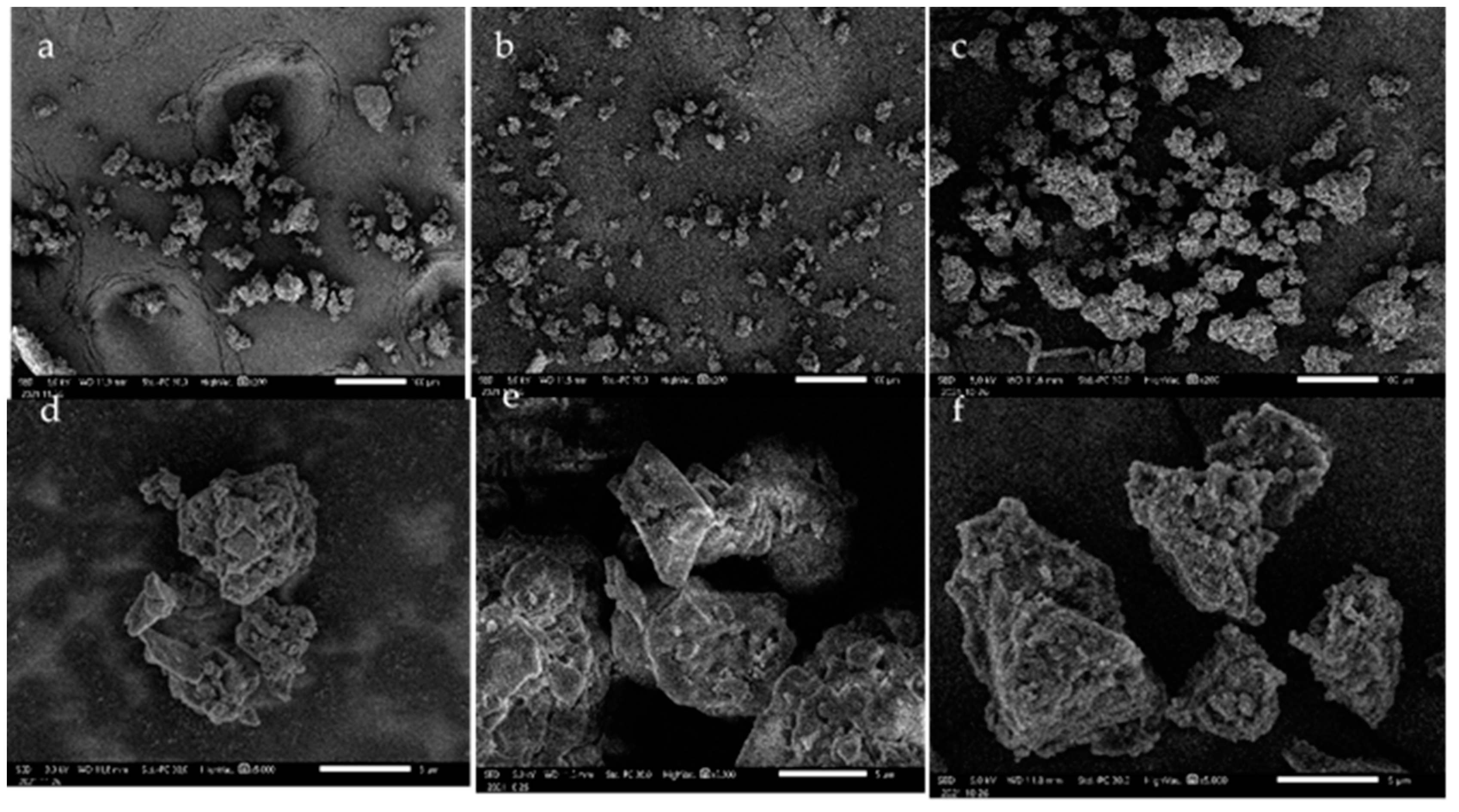
3.2. SEM Micrograph of Lignin
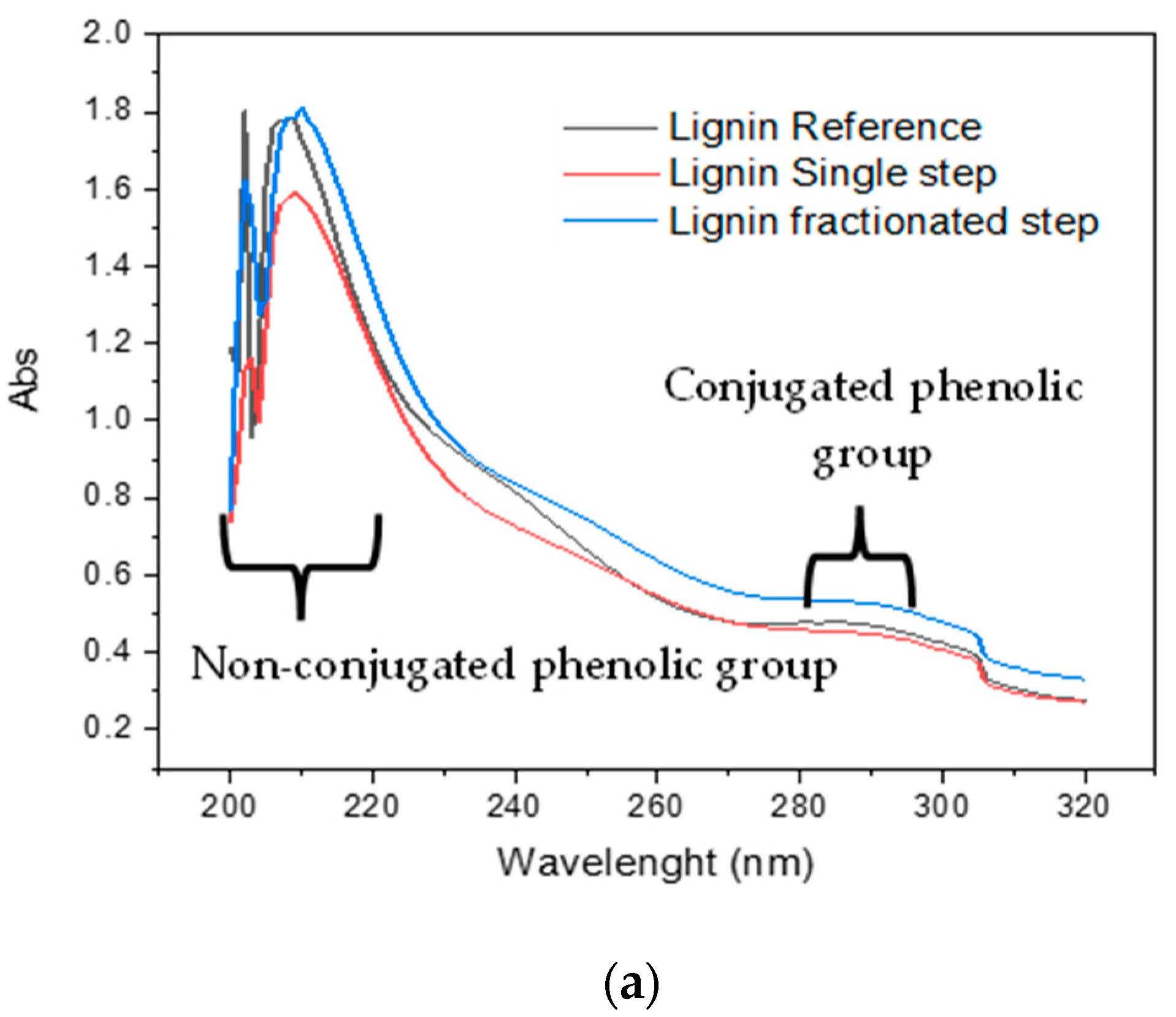
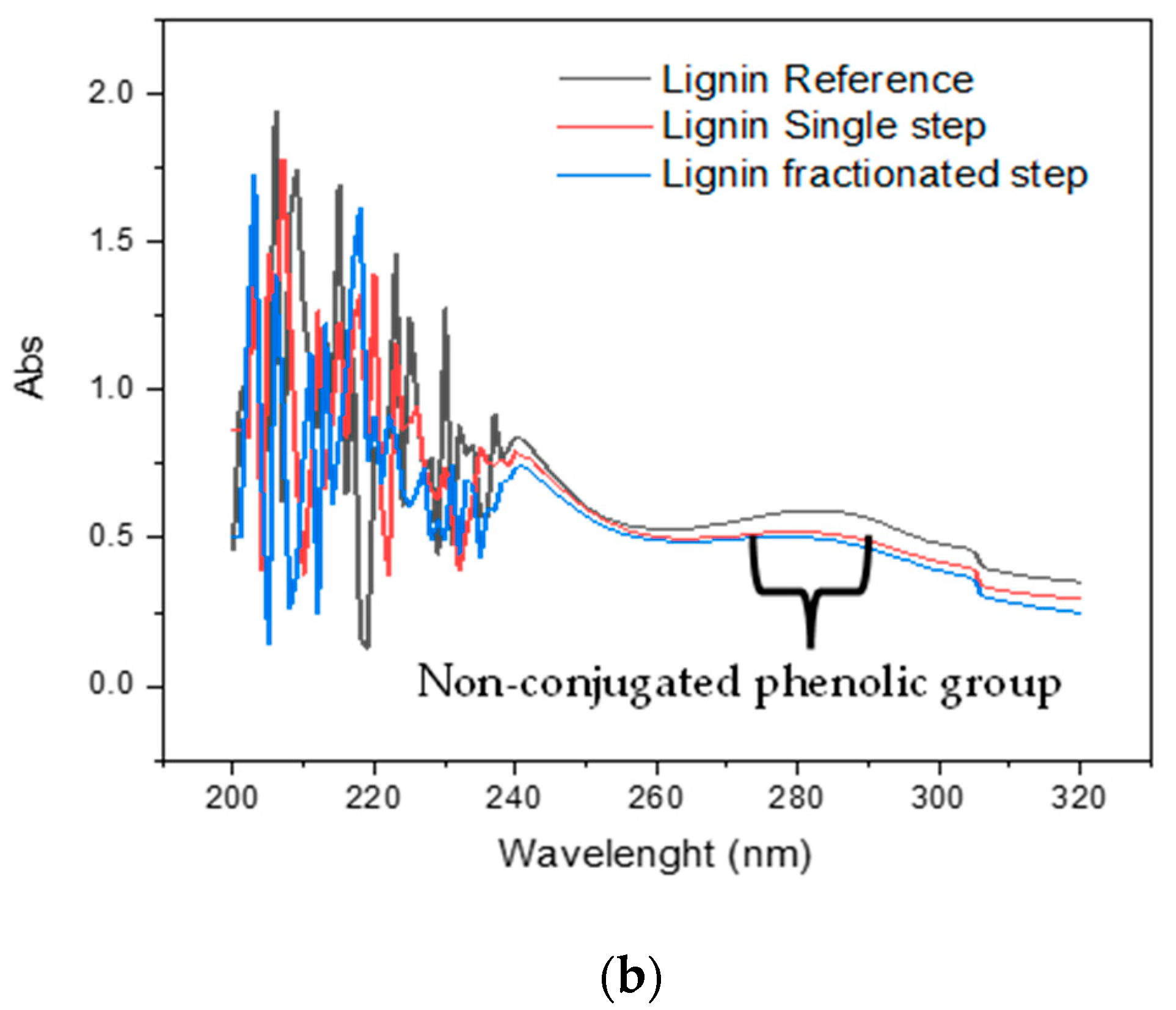
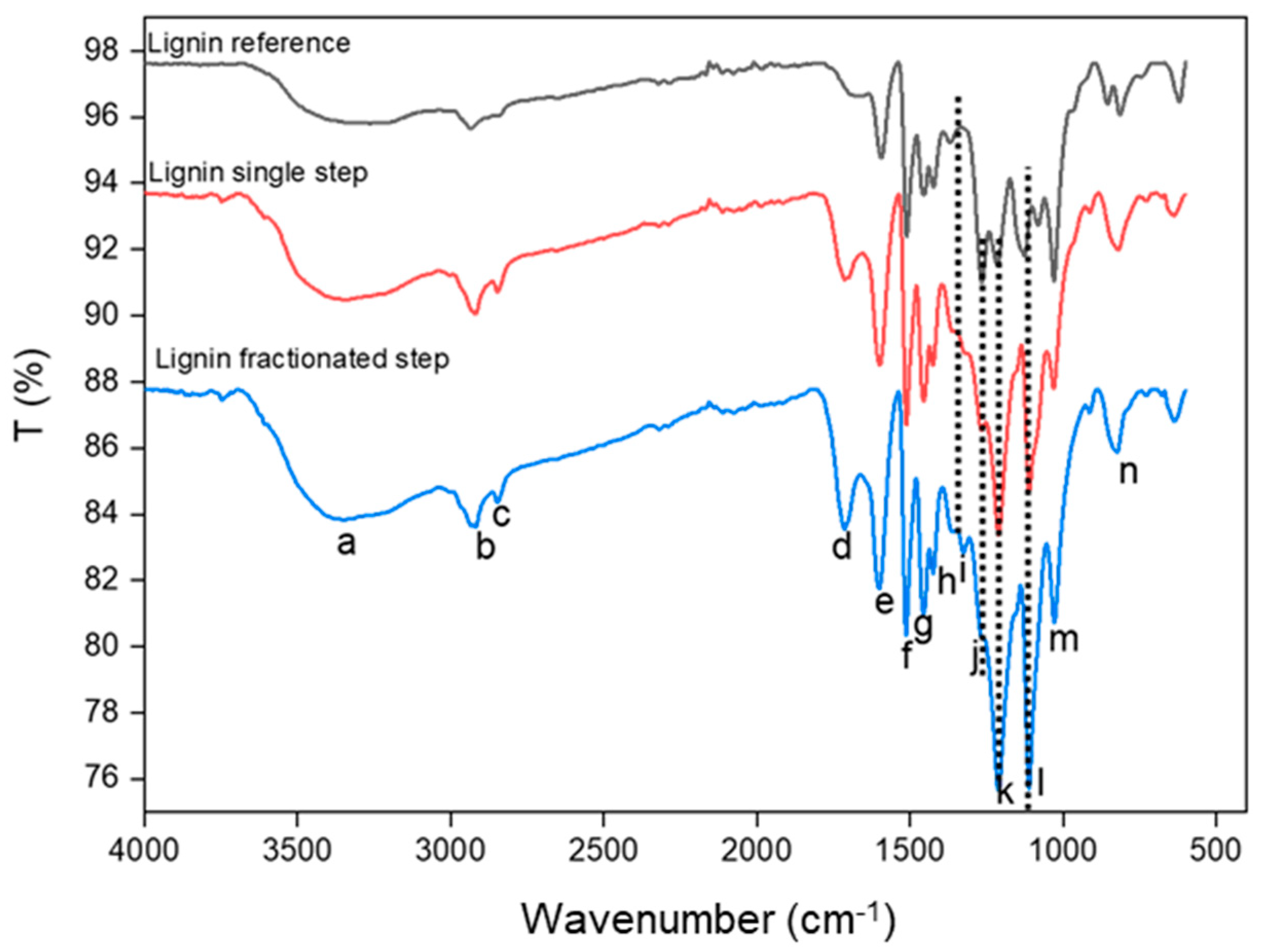
3.3. Functional Group of Lignin
3.4. 1H and 13C NMR
3.5. Thermal Behavior of Lignin
3.6. Chemical Elucidation by Mass Spectrometry
3.7. Molecular Weights
3.8. Future Potential of A. mangium Lignin from BL in Adhesive Applications for Wood-Based Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDNFinancial. Indonesia’s Pulp and Paper Are in the Top 10 in the World. Available online: https://www.idnfinancials.com/archive/news/22291/Indonesias-pulp-and-paper-are-in-the-top-10-in-the-world (accessed on 25 October 2021).
- Bajpai, P. Chapter 12—Pulping Fundamentals. In Biermann’s Handbook of Pulp and Paper, 3rd ed.; Bajpai, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 295–351. [Google Scholar]
- Speight, J.G. Chapter 13—Upgrading by Gasification. In Heavy Oil Recovery and Upgrading; Speight, J.G., Ed.; Gulf Professional Publishing: Houston, TX, USA, 2019; pp. 559–614. [Google Scholar]
- Melro, E.; Filipe, A.; Sousa, D.; Medronho, B.; Romano, A. Revisiting lignin: A tour through its structural features, characterization methods and applications. New J. Chem. 2021, 45, 6986–7013. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioRes 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Solihat, N.N.; Sari, F.P.; Falah, F.; Ismayati, M.; Lubis, M.A.R.; Fatriasari, W.; Santoso, E.B.; Syafii, W. Lignin as an Active Biomaterial: A Review. J. Sylva Lestari 2021, 9, 1–22. [Google Scholar] [CrossRef]
- Vahabi, H.; Brosse, N.; Latif, N.A.; Fatriasari, W.; Solihat, N.; Hashimd, R.; Hussin, M.; Laoutid, F.; Saeb, M. Chapter 24-Nanolignin in materials science and technology-does flame retardancy matter? In Biopolymeric Nanomaterials: Fundamental and Applications; Kanwar, S., Kumar, A., Nguyen, T.A., Sharma, S., Slimani, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, pp. 515–550. [Google Scholar]
- Sameni, J.; Krigstin, S.; Sain, M. Characterization of lignins isolated from industrial residues and their beneficial uses. BioRes 2016, 11, 8435–8456. [Google Scholar] [CrossRef] [Green Version]
- Ház, A.; Jablonský, M.; Šurina, I.; Kačík, F.; Bubeníková, T.; Ďurkovič, J. Chemical Composition and Thermal Behavior of Kraft Lignins. Forests 2019, 10, 483. [Google Scholar] [CrossRef] [Green Version]
- Haz, A.; Strizincova, P.; Majova, V.; Sskulcova, A.; Surina, I.; Jablonsky, M. Content of Phenolic Hydroxyl Groups In Lignin: Characterisation of 23 Isolated Non-Wood Lignin With Various Acids. Int. J. Recent Sci. Res. 2016, 7, 11547–11551. [Google Scholar]
- Handika, S.O.; Lubis, M.A.R.; Sari, R.K.; Laksana, R.P.B.; Antov, P.; Savov, V.; Gajtanska, M.; Iswanto, A.H. Enhancing Thermal and Mechanical Properties of Ramie Fiber via Impregnation by Lignin-Based Polyurethane Resin. Materials 2021, 14, 6850. [Google Scholar] [CrossRef]
- Ang, A.F.; Ashaari, Z.; Lee, S.H.; Md Tahir, P.; Halis, R. Lignin-based copolymer adhesives for composite wood panels—A review. Int. J. Adhes. Adhes. 2019, 95, 102408. [Google Scholar] [CrossRef]
- Çetin, N.S.; Özmen, N. Use of organosolv lignin in phenol-formaldehyde resins for particleboard production II. Particleboard production and properties. Int. J. Adhes. Adhes. 2002, 22, 477–480. [Google Scholar] [CrossRef]
- Donmez Cavdar, A.; Kalaycioglu, H.; Hiziroglu, S. Some of the properties of oriented strandboard manufactured using kraft lignin phenolic resin. J. Mater. Processing Technol. 2008, 202, 559–563. [Google Scholar] [CrossRef]
- Madyaratri, E.W.; Ridho, M.R.; Aristri, M.A.; Lubis, M.A.R.; Iswanto, A.H.; Nawawi, D.S.; Antov, P.; Kristak, L.; Majlingová, A.; Fatriasari, W. Recent Advances in the Development of Fire-Resistant Biocomposites—A Review. Polymers 2022, 14, 362. [Google Scholar] [CrossRef]
- Ahmad Saffian, H.; Hyun-Joong, K.; Md Tahir, P.; Ibrahim, N.A.; Lee, S.H.; Lee, C.H. Effect of Lignin Modification on Properties of Kenaf Core Fiber Reinforced Poly(Butylene Succinate) Biocomposites. Materials 2019, 12, 4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad Saffian, H.; Talib, M.A.; Lee, S.H.; Md Tahir, P.; Lee, C.H.; Ariffin, H.; Asa’ari, A.Z.M. Mechanical Strength, Thermal Conductivity and Electrical Breakdown of Kenaf Core Fiber/Lignin/Polypropylene Biocomposite. Polymers 2020, 12, 1833. [Google Scholar] [CrossRef]
- Tejado, A.; Pena, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Zinovyev, G.; Sumerskii, I.; Korntner, P.; Sulaeva, I.; Rosenau, T.; Potthast, A. Molar mass-dependent profiles of functional groups and carbohydrates in kraft lignin. J. Wood Chem. Technol. 2016, 37, 171–183. [Google Scholar] [CrossRef]
- Hermiati, E.; Risanto, L.; Lubis, M.A.R.; Laksana, R.P.B.; Dewi, A.R. Chemical characterization of lignin from kraft pulping black liquor of Acacia mangium. AIP Conf. Proc. 2017, 020005. [Google Scholar] [CrossRef]
- TAPPI. TAPPI Test Method T 264 cm-97 Preparation of Wood for Chemical Analysis. 1997. Available online: https://tappi.micronexx.com/CD/TESTMETHODS/T264.pdf (accessed on 26 December 2021).
- TAPPI. TAPPI Test Method T 211 om-02 Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 °C. 2002. Available online: https://www.tappi.org/content/sarg/t211.pdf (accessed on 26 December 2021).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass—Laboratory Analytical Procedure (LAP); Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Serrano, L.; Esakkimuthu, E.S.; Marlin, N.; Brochier-Salon, M.-C.; Mortha, G.; Bertaud, F. Fast, Easy, and Economical Quantification of Lignin Phenolic Hydroxyl Groups: Comparison with Classical Techniques. Energy Fuels 2018, 32, 5969–5977. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Xue, B.-L.; Sun, R.-C. Quantitative structural characterization of the lignins from the stem and pith of bamboo (Phyllostachys pubescens). Holzforschung 2013, 67, 613–627. [Google Scholar] [CrossRef]
- Hamzah, M.H.; Bowra, S.; Cox, P. Effects of Ethanol Concentration on Organosolv Lignin Precipitation and Aggregation from Miscanthus x giganteus. Processes 2020, 8, 845. [Google Scholar] [CrossRef]
- Ponnuchamy, V.; Gordobil, O.; Diaz, R.H.; Sandak, A.; Sandak, J. Fractionation of lignin using organic solvents: A combined experimental and theoretical study. Int. J. Biol. Macromol. 2021, 168, 792–805. [Google Scholar] [CrossRef]
- Gordobil, O.; Herrera, R.; Yahyaoui, M.; İlk, S.; Kaya, M.; Labidi, J. Potential use of kraft and organosolv lignins as a natural additive for healthcare products. RSC Adv. 2018, 8, 24525–24533. [Google Scholar] [CrossRef] [Green Version]
- Sadeghifar, H.; Argyropoulos, D.S. Correlations of the Antioxidant Properties of Softwood Kraft Lignin Fractions with the Thermal Stability of Its Blends with Polyethylene. ACS Sustain. Chem. Eng. 2015, 3, 349–356. [Google Scholar] [CrossRef]
- Alzagameem, A.; Khaldi-Hansen, B.E.; Buchner, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M. Lignocellulosic Biomass as Source for Lignin-Based Environmentally Benign Antioxidants. Molecules 2018, 23, 2664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, M.; Mechi, N.; Slimi, H.; Elaloui, E. Isolation and Purification of Alfa Grass Kraft Lignin from Industrial Waste. Curr. Trends Biomed. Eng. Biosci. 2017, 6, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Xu, F.; Sun, R. Molecular characteristics of Kraft-AQ pulping lignin fractionated by sequential organic solvent extraction. Int. J. Mol. Sci. 2010, 11, 2988–3001. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Hulteberg, C.P. Physicochemical Characterisation of Technical Lignins for Their Potential Valorisation. Waste Biomass Valorization 2016, 8, 859–869. [Google Scholar] [CrossRef] [Green Version]
- She, D.; Xu, F.; Geng, Z.; Sun, R.; Jones, G.L.; Baird, M.S. Physicochemical characterization of extracted lignin from sweet sorghum stem. Ind. Crops Prod. 2010, 32, 21–28. [Google Scholar] [CrossRef]
- Sameni, J.; Krigstin, S.; Santos Rosa, D.D.; Leao, A.; Sain, M. Thermal Characteristics of Lignin Residue from Industrial Processes. BioResources 2013, 9, 725–737. [Google Scholar] [CrossRef] [Green Version]
- Solihat, N.N.; Sari, F.P.; Risanto, L.; Anita, S.H.; Fitria, F.; Fatriasari, W.; Hermiati, E. Disruption of Oil Palm Empty Fruit Bunches by Microwave-assisted Oxalic Acid Pretreatment. J. Math. Fund. Sci. 2017, 49, 244–257. [Google Scholar] [CrossRef]
- Solihat, N.N.; Fajriutami, T.; Adi, D.T.N.; Fatriasari, W.; Hermiati, E. Reducing sugar production of sweet sorghum bagasse kraft pulp. AIP Conf. Proc. 2017, 1803, 020012. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.-C.; Hu, H.-Q.; Xie, F.-J.; Wei, X.-Y.; Fan, X. Structural Characterization of Lignin and Its Degradation Products with Spectroscopic Methods. J. Spectrosc. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Zhang, Y.; Guo, T.; Zhao, H.; Jin, Y. Structural Characterization of Lignin and Lignin-Carbohydrate Complex (LCC) from Ginkgo Shells (Ginkgo biloba L.) by Comprehensive NMR Spectroscopy. Polymers 2018, 10, 736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Recent Advances in Characterization of Lignin Polymer by Solution-State Nuclear Magnetic Resonance (NMR) Methodology. Materials 2013, 6, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, B.; Kusch, P.; Schulze, M.; Kamm, B. Qualitative and Quantitative Analysis of Lignin Produced from Beech Wood by Different Conditions of the Organosolv Process. J. Polym. Environ. 2016, 24, 85–97. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A Review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Ramezani, N.; Sain, M. Thermal and Physiochemical Characterization of Lignin Extracted from Wheat Straw by Organosolv Process. J. Polym. Environ. 2018, 26, 3109–3116. [Google Scholar] [CrossRef]
- Liu, S.-M.; Huang, J.-Y.; Jiang, Z.-J.; Zhang, C.; Zhao, J.-Q.; Chen, J. Flame retardance and mechanical properties of a polyamide 6/polyethylene/surface-modified metal hydroxide ternary composite via a master-batch method. J. Appl. Polym. Sci. 2010, 3370–3378. [Google Scholar] [CrossRef]
- Pourjafar, S. An Investigation of the Thermal Degradation of Lignin. Ph.D. Thesis, University of North Dakota, Grand Forks, ND, USA, 2017. [Google Scholar]
- Hu, J.; Shen, D.; Xiao, R.; Wu, S.; Zhang, H. Free-Radical Analysis on Thermochemical Transformation of Lignin to Phenolic Compounds. Energy Fuels 2013, 27, 285–293. [Google Scholar] [CrossRef]
- Aristri, M.A.; Lubis, M.A.R.; Laksana, R.P.B.; Falah, F.; Fatriasari, W.; Ismayati, M.; Wulandari, A.P.; Nurindah, N.; Ridho, M.R. Bio-Polyurethane Resins Derived from Liquid Fractions of Lignin for the Modification of Ramie Fibers. J. Sylva Lestari 2021, 9, 223–238. [Google Scholar] [CrossRef]
- Erdtman, H. Lignins: Occurrence, formation, structure and reactions, K.V. Sarkanen and C.H. Ludwig, Eds., John Wiley & Sons, Inc., New York, 1971. 916 pp. $35.00. J. Polym. Sci. Part B Polym. Lett. 1972, 10, 228–230. [Google Scholar] [CrossRef]
- Naseem, A.; Tabasum, S.; Zia, K.M.; Zuber, M.; Ali, M.; Noreen, A. Lignin-derivatives based polymers, blends and composites: A review. Int. J. Biol. Macromol. 2016, 93, 296–313. [Google Scholar] [CrossRef] [PubMed]
- Lora, J.H.; Glasser, W.G. Recent Industrial Applications of Lignin A Sustainable Alternative to Nonrenewable Materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Dastpak, A.; Lourenҫon, T.V.; Balakshin, M.; Farhan Hashmi, S.; Lundström, M.; Wilson, B.P. Solubility study of lignin in industrial organic solvents and investigation of electrochemical properties of spray-coated solutions. Ind. Crops Prod. 2020, 148, 112310. [Google Scholar] [CrossRef]
- Pang, T.; Wang, G.; Sun, H.; Sui, W.; Si, C. Lignin fractionation: Effective strategy to reduce molecule weight dependent heterogeneity for upgraded lignin valorization. Ind. Crops Prod. 2021, 165, 113442. [Google Scholar] [CrossRef]
- Gregorova, A. Application of Differential Scanning Calorimetry to the Characterization of Biopolymers; IntechOpen: London, UK, 2013. [Google Scholar]
- Sathawong, S.; Sridach, W.; Techato, K.-a. Lignin: Isolation and preparing the lignin based hydrogel. J. Environ. Chem. Eng. 2018, 6, 5879–5888. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Jardim, J.M.; Hart, P.W.; Lucia, L.; Jameel, H. Insights into the Potential of Hardwood Kraft Lignin to Be a Green Platform Material for Emergence of the Biorefinery. Polymers 2020, 12, 1795. [Google Scholar] [CrossRef]
- Heitner, C.; Dimmel, D.; Schmidt, J. Lignin and Lignans: Advances in Chemistry, 1st ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2010. [Google Scholar]
- Poletto, M. Assessment of the thermal behavior of lignins from softwood and hardwood species. Maderas. Cienc. y Tecnol. 2017, 19, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Torrezan, T. Avaliação do Comportamento Reológico, Térmico e Mecânico de Misturas de PBAT com Elevados Teores de Lignina; Universidade Federal de São Carlos: São Paulo, Brazil, 2019; Available online: https://repositorio.ufscar.br/handle/ufscar/12413 (accessed on 16 November 2021).
- Kadla, J.F.; Kubo, S. Lignin-based polymer blends: Analysis of intermolecular interactions in lignin–synthetic polymer blends. Compos. Part A Appl. Sci. Manuf. 2004, 35, 395–400. [Google Scholar] [CrossRef]
- Ropponen, J.; Räsänen, L.; Rovio, S.; Ohra-aho, T.; Liitiä, T.; Mikkonen, H.; van de Pas, D.; Tamminen, T. Solvent extraction as a means of preparing homogeneous lignin fractions. Holzforschung 2011, 65, 543–549. [Google Scholar] [CrossRef]
- Ponomarenko, J.; Dizhbite, T.; Lauberts, M.; Viksna, A.; Dobele, G.; Bikovens, O.; Telysheva, G. Characterization of Softwood and Hardwood LignoBoost Kraft Lignins with Emphasis on their Antioxidant Activity. BioResources 2014, 9, 2051–2068. [Google Scholar] [CrossRef]
- Haz, A.; Jablonský, M.; Orságová, A.; Surina, I. Characterization of lignins by Py-GC/MS. In Proceedings of the 4nd International Conference Renewable Energy Sources 2013, High Tatras, Slovak Republic, 21–23 May 2013. [Google Scholar]
- Nawawi, D.; Rahayu, I.; Wistara, N.; Sari, R.; Syafii, W. Distribusi sel pori pada kayu tarik dan korelasinya dengan komposisi lignin. J. Ilmu Kehutan. 2019, 13, 70–76. [Google Scholar]
- Nawawi, D.S.; Syafii, W.; Tomoda, I.; Uchida, Y.; Akiyama, T.; Yokoyama, T.; Matsumoto, Y. Characteristics and Reactivity of Lignin in Acacia and Eucalyptus Woods. J. Wood Chem. Technol. 2017, 37, 273–282. [Google Scholar] [CrossRef]
- González Martínez, M.; Ohra-aho, T.; da Silva Perez, D.; Tamminen, T.; Dupont, C. Influence of step duration in fractionated Py-GC/MS of lignocellulosic biomass. J. Anal. Appl. Pyrolysis 2019, 137, 195–202. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Janus, R.; Lewandowski, M.; Magdziarz, A. On mechanism of lignin decomposition—Investigation using microscale techniques: Py-GC-MS, Py-FT-IR and TGA. Renew. Energy 2021, 177, 942–952. [Google Scholar] [CrossRef]
- Chang, G.; Huang, Y.; Xie, J.; Yang, H.; Liu, H.; Yin, X.; Wu, C. The lignin pyrolysis composition and pyrolysis products of palm kernel shell, wheat straw, and pine sawdust. Energy Convers. Manag. 2016, 124, 587–597. [Google Scholar] [CrossRef]
- Baumberger, S.; Abaecherli, A.; Fasching, M.; Gellerstedt, G.; Gosselink, R.; Hortling, B.; Li, J.; Saake, B.; Jong, E.d. Molar mass determination of lignins by size-exclusion chromatography: Towards standardisation of the method. Holzforschung 2007, 61, 459–468. [Google Scholar] [CrossRef]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and analysis of the molecular weight of lignin for biorefining studies. Biofuels Bioprod. Biorefining 2014, 8, 836–856. [Google Scholar] [CrossRef]
- Stiefel, S.; Marks, C.; Schmidt, T.; Hanisch, S.; Spalding, G.; Wessling, M. Overcoming lignin heterogeneity: Reliably characterizing the cleavage of technical lignin. Green Chem. 2016, 18, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, Z.; Dajani, W.W.A.; Paleologou, M.; Xu, C.C. Sustainable Process for the Depolymerization/Oxidation of Softwood and Hardwood Kraft Lignins Using Hydrogen Peroxide under Ambient Conditions. Molecules 2020, 25, 2329. [Google Scholar] [CrossRef] [PubMed]
- Abd Latif, N.H.; Brosse, N.; Ziegler-Devin, I.; Chrusiel, L.; Hashim, R.; Hussin, M.H. A Comparison of Alkaline and Organosolv Lignin Extraction Methods from Coconut Husks as an Alternative Material for Green Application. BioRes 2022, 17, 469–491. [Google Scholar] [CrossRef]

| Water Content (%) | Ash Content (%) | AIL (%) | ASL (%) | Total phOH (mmol/g) | |
|---|---|---|---|---|---|
| Lignin reference | 2.60 ± 0.27 | 2.44 ± 0.00 | 96.02 ± 0.50 | 1.54 ± 0.06 | 6.00 ± 0.50 |
| Lignin single method | 5.65 ± 1.14 | 0.53 ± 0.07 | 77.45 ± 0.48 | 22.02 ± 0.83 | 7.40 ± 0.71 |
| Lignin fraction method | 15.79 ± 0.74 | 1.94 ± 0.08 | 69.94 ± 5.55 | 28.12 ± 0.94 | 7.31 ± 0.78 |
| Code | Wavelength (cm−1) | Functional Group |
|---|---|---|
| a | 3359 | Hydroxyl group stretching (O-H) from aliphatic and aromatic [36] |
| b | 2918 | C-H stretching in methylene [33] |
| c | 2854 | C-H stretching in methoxy [33] |
| d | 1710 | Carbonyl (C=O) stretching in unconjugated aldehyde and ketone [34] |
| e | 1590 | C=C (aromatic rings) [34] |
| f | 1511 | C=C (aromatic rings) [34] |
| g | 1470 | Aromatic ring vibration with C-O [34] |
| h | 1430 | Deformation C-H in methyl group [4] |
| i | 1326 | C-O breathing (syringyl) [37] |
| j | 1266 | C-O(H) (phenolic OH guaiacyl) [36] |
| k | 1213 | C-O(Ar) in guaicyl ring [35] |
| l | 1111 | Deformation Ar-CH in syringyl ring [35] |
| m | 1030 | Unconjugated C-O in guaicyl [35] |
| n | 855 | CH out of plane bending in guaicyl [35] |
| Unit | Pyrolysis Product | Relative Abundance (%) | Fragmentation (m/z) | ||
|---|---|---|---|---|---|
| Reference Lignin | Single-Step Lignin | Fractionated-Step Lignin | |||
| H1 | Phenol | 1.02 | 2.76 | 3.58 | 94, 66, 45 |
| H2 | Phenol, 2-methyl- | 1.18 | 1.39 | 1.32 | 108, 90, 79 |
| H3 | Phenol, 3 + 4-methyll | 2.24 | 3.25 | 2.26 | 107, 90, 79 |
| H4 | Phenol, 2,4-dimethyl- | 1.00 | 0.40 | 0.00 | 122, 107, 77 |
| H5 | Phenol, 4-vinyl | 0.20 | 0.48 | 0.66 | 120, 91, 65, 40 |
| H6 | Catechol, 3-methyl | 3.63 | 9.70 | 9.66 | 124, 78 |
| H7 | Catechol, 4-methyl | 3.90 | 3.81 | 1.99 | 124, 78 |
| Total relative abundance of H unit | 13.17 | 21.80 | 19.47 | ||
| G1 | Guaiacol | 12.36 | 9.99 | 15.23 | 124, 109, 81 |
| G2 | Guaiacol-4-methyl- | 18.62 | 13.49 | 5.96 | 138, 123, 95 |
| G3 | Guaiacol, 4-ethyl | 7.02 | 5.19 | 3.13 | 152, 137 |
| G4 | Guaiacol, 4-vinyl | 10.59 | 4.87 | 6.22 | 150, 135, 107, 77 |
| G5 | Guaiacol, 4-propyl | 1.70 | 3.45 | 0.00 | 166, 137 |
| G6 | Eugenol | 1.08 | 1.25 | 0.33 | 164, 149, 77 |
| G7 | Vanillin | 5.57 | 1.36 | 1.80 | 151, 123, 109 |
| G8 | Isoeugenol (cis) | 2.33 | 0.50 | 2.50 | 164, 149 |
| G9 | Isoeugenol (trans) | 7.08 | 3.39 | 1.98 | 164, 149 |
| G10 | Acetoguaiacone | 3.15 | 2.06 | 3.39 | 166, 151, 123 |
| G11 | Guaiacyl acetone | 3.47 | 0.62 | 0.48 | 180, 137 |
| G12 | Propioguaiacone | 1.70 | 0.00 | 1.23 | 180, 151, 123 |
| G13 | Dihydroconiferyl alcohol | 7.30 | 0.00 | 0.00 | 182, 137 |
| G14 | Coniferyl alcohol | 1.95 | 0.00 | 0.00 | 180, 137, 124, 91 |
| Total relative abundance of G unit | 83.93 | 46.17 | 42.26 | ||
| S1 | Syringol | 1.70 | 13.70 | 19.41 | 154, 139, 111, 96 |
| S2 | Syringol, 4-methyl | 0.96 | 9.47 | 6.45 | 168, 153, 125 |
| S3 | Syringol, 4-vinyl | 0.14 | 4.79 | 6.68 | 180, 165, 137 |
| S4 | Syringol, 4-propenyl (trans) | 0.11 | 2.32 | 1.62 | 194, 179, 91 |
| S5 | Acetosyringone | 0.00 | 2.24 | 4.12 | 196, 181, 153 |
| Total relative abundance of S unit | 2.90 | 32.52 | 34.15 | ||
| S/G ratio | 0.03 | 0.70 | 0.81 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solihat, N.N.; Santoso, E.B.; Karimah, A.; Madyaratri, E.W.; Sari, F.P.; Falah, F.; Iswanto, A.H.; Ismayati, M.; Lubis, M.A.R.; Fatriasari, W.; et al. Physical and Chemical Properties of Acacia mangium Lignin Isolated from Pulp Mill Byproduct for Potential Application in Wood Composites. Polymers 2022, 14, 491. https://doi.org/10.3390/polym14030491
Solihat NN, Santoso EB, Karimah A, Madyaratri EW, Sari FP, Falah F, Iswanto AH, Ismayati M, Lubis MAR, Fatriasari W, et al. Physical and Chemical Properties of Acacia mangium Lignin Isolated from Pulp Mill Byproduct for Potential Application in Wood Composites. Polymers. 2022; 14(3):491. https://doi.org/10.3390/polym14030491
Chicago/Turabian StyleSolihat, Nissa Nurfajrin, Eko Budi Santoso, Azizatul Karimah, Elvara Windra Madyaratri, Fahriya Puspita Sari, Faizatul Falah, Apri Heri Iswanto, Maya Ismayati, Muhammad Adly Rahandi Lubis, Widya Fatriasari, and et al. 2022. "Physical and Chemical Properties of Acacia mangium Lignin Isolated from Pulp Mill Byproduct for Potential Application in Wood Composites" Polymers 14, no. 3: 491. https://doi.org/10.3390/polym14030491










