1. Introduction
Urethane-containing elastomers (UCEs) constitute a unique series of block copolymers with the structure and properties that can be modified within a wide range [
1]. Owing to a high mechanical strength, elasticity, damping ability, abrasion and corrosion resistance, such elastomers are widely used as polymer sealants, foams, coatings, and lining materials [
2,
3,
4,
5].
Segmented polyurethanes (SPU) and polyurethane ureas (SPUM) are block copolymers with macromolecular chains consist of alternating flexible soft and hard segments, SS and HS respectively [
6,
7]. These polymers are usually synthesized via a two-step route. At the first step, oligodiol reacts with a double excess of diisocyanate to form an isocyanate terminal compound-oligodiisocyanate. At the second step, these products react with chain extenders, amines or alcohols, to form polyurethane ureas (SPUM) or polyurethanes (SPU). The structure of soft segments is defined by oligodiols used for SPU synthesis [
8]. Hard segments are formed by the reaction of isocyanate terminated compounds, oligodiisocyanates, with low molecular weight chain extenders diamines or diols [
9].
Undoubtedly, SPUs and SPUMs have several advantages over other polymers. However, the final properties of these elastomers depend on moisture, as the isocyanate group of oligodiisocyanates can react with moisture. To solve this problem and to reduce the toxicity of isocyanate terminated compounds, shielding of the isocyanate groups of oligodiisocyanates with an epoxy alcohol is the most efficient approach. For example, 2,3-epoxy-1-propanol can be used for this purpose [
10]. In this case, the isocyanate groups of the oligodiisocyanate and the hydroxyl group of 2,3-epoxy-1-propanol react to give epoxyurethane oligomer (EUO). The deformation and strength properties of the elastomers, based on these oligomers, are only slightly dependent on the presence of moisture. Amines, dicarboxylic acid anhydrides, carboxyl-containing compounds, and imidazoles are used for curing of epoxyurethane oligomers [
11,
12,
13,
14,
15,
16].
Elastomers based on epoxyurethane oligomers are characterized by good dielectric properties and mechanical characteristics. In addition, adhesion characteristics of these elastomers are higher than that of SPUs and SPUMs. So, they are widely used as adhesives, polymer matrices of casting low-modulus compounds for different purposes, and biomedical materials.
Elastomers based on epoxyurethane oligomers consist of alternating soft and urethane hydroxyl hard segments. The polarity difference of these structural units leads to microphase separation, and a separate phase, or domains, is formed. The domains play the role of a reinforcing nanodispersed filler, or nodes of a specific physical network, which is important for achievement of high strength characteristics of the developed materials. In the domains hydrogen bonds play a crucial role in stabilization of the hard phase structure. In this case, the structure of the hard segments can affect the structural organization of the polymer phase.
The use of a polyester as a precursor of an epoxyurethane oligomer makes it possible to obtain polymer coatings with improved oil and petrol resistance [
17]. In addition, when using polyester oligomer, biocompatible and biodegradable polymers can be obtained [
18,
19]. For this purpose, polycaprolactone diols of different molecular weight are used as an oligodiol. Biodegradable and biocompatible polyester, oligo(1,4-butylene adipate) (OBA) characterized by semi-crystalline structure and hydrophobicity [
18], can be also used for preparation of biodegradable polymers [
18,
19].
The present study was aimed at the synthesis and characterization of a number of biodegradable polyurethane elastomers with high oil and petrol resistance. Three polyesters of different molecular weights were synthesized from adipic acid and ethylene glycol. These polyesters were used for preparation of six epoxy terminated oligomers. The oligomer curing takes place due to epoxy ring opening with amines, extra hydroxyl moieties in the polymer backbone being formed. The presence of these groups, as well as oligodiol hydroxy groups, was considered to be the main condition of high hydrophilicity and hydrolysis rate of resulting elastomers.
3. Results
3.1. NMR Spectra of Epoxyurethanes
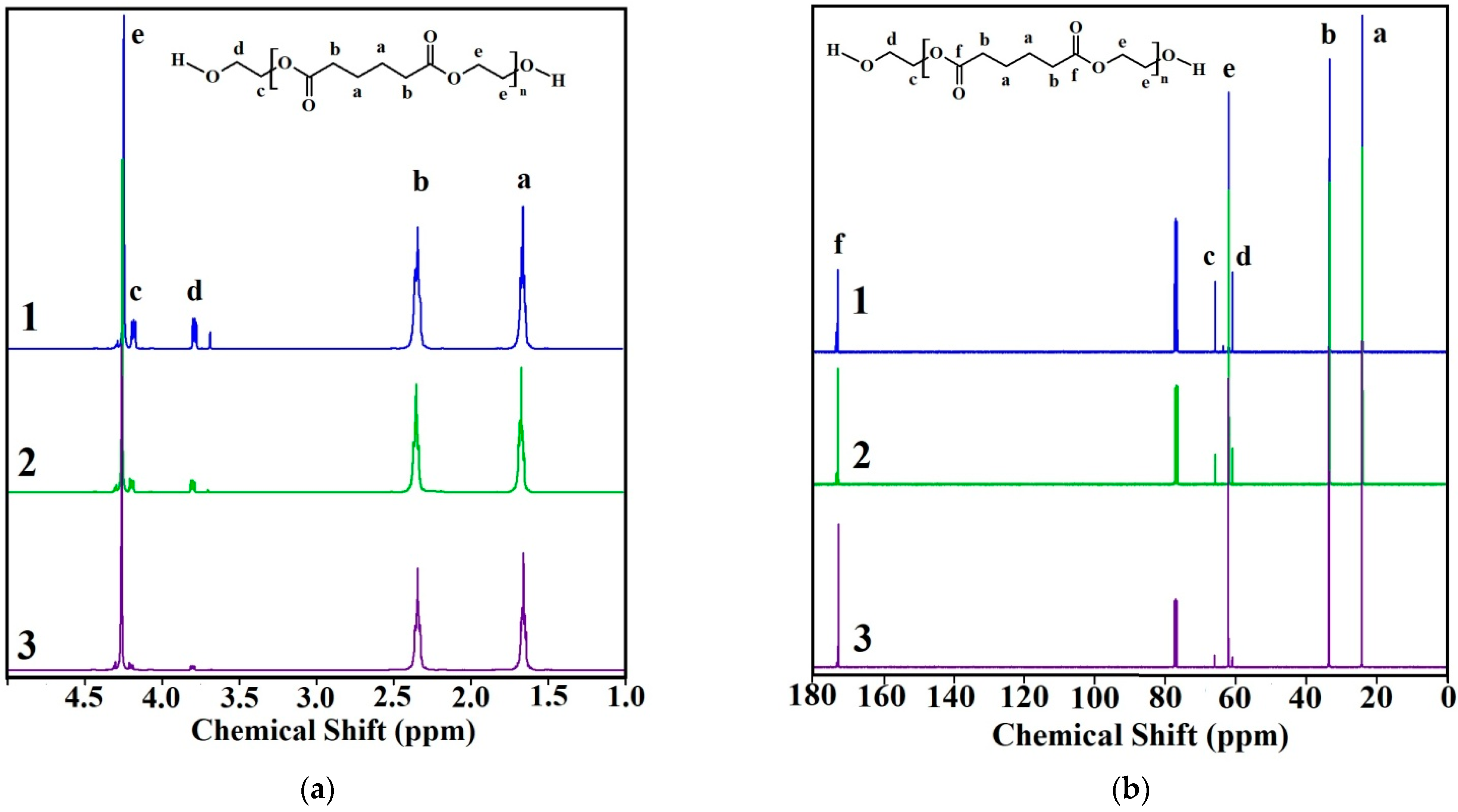
The structure of the synthesized oligoethylene adipates
1–
3 was confirmed by NMR
1H (
Figure 4a) and
13C (
Figure 4b) data.
In the
1H spectrum, there are two peaks attributed to protons of methylene groups of the adipate fragment- at 1.65 (C(O)-CH
2-C
H2-C
H2-CH
2-C(O) (a)) and 2.33 (C(O)-C
H2-CH
2-CH
2-C
H2-C(O) (b)) ppm, respectively. Triplet signals at 3.78 (d) and 4.18 (c) ppm correspond to the protons of methylene groups of the terminal ethylene glycol fragment. The signal at 4.25 ppm (e) corresponds to the remaining protons of the ethylene glycol fragment. This NMR signal interpretation corresponds to the one reported in [
26,
27]. At the same time, integration of the
1H-spectra peaks showed the molecular weights of oligoethylene glycol adipates 1–3 to be different.
In the 13C spectrum, the signals of carbon atoms of the carbonyl bond in ester groups are observed at 172.9 ppm (C = O (f)). The peaks at 24.2 (a) and 33.6 (b) ppm refer to the CH2 groups of the adipate fragment. The signals of the methylene carbon atoms in the terminal ethylene glycol fragment are observed at 60.9 (-CH2-CH2-OH (d)) and 65.9 (-CH2-CH2-OH (c)) ppm. The peak at 62.1 ppm (e) corresponds to the remaining CH2 groups of the ethylene glycol fragment.
3.2. Differential Scanning Calorimetry Data
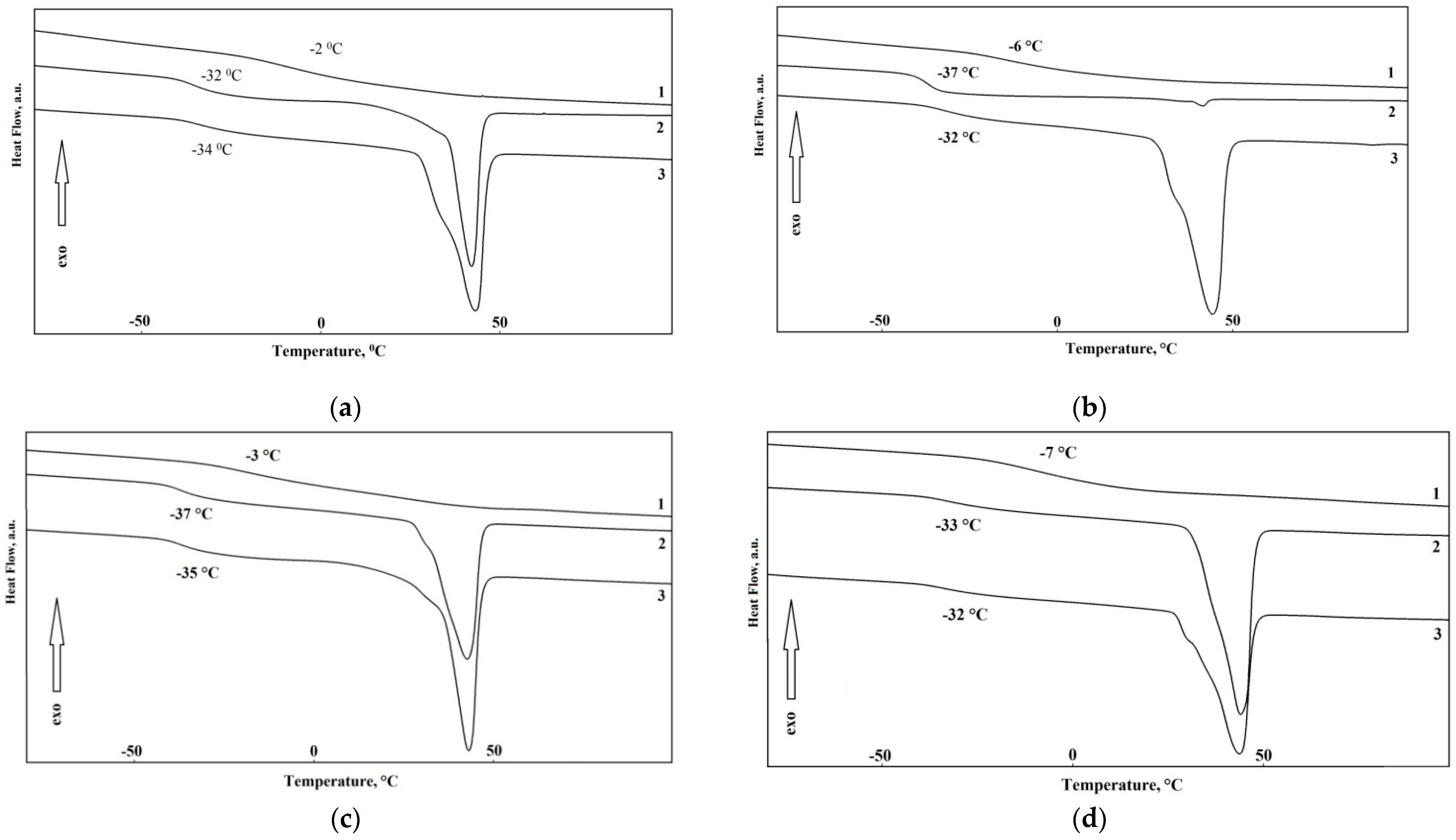
The thermal properties of the synthesized elastomers were studied by differential scanning calorimetry. First the samples were heated to 150 °C, then cooled to 100 °C below zero, kept for 30 min, and heated at a heating rate of 5 K/min. In
Figure 5a–d the reheating thermograms of the samples K1–12 are shown. The thermophysical properties of the synthesized elastomers are shown in
Table 4.
From DSC thermograms, it can be seen that the glass transition temperature of the soft phase decreases with the increase in the soft segment molecular weight from 991 to 3430. At higher molecular weights this tendency is leveled. This is associated with lower segmental mobility of polymer chains due to the increased degree of crystallinity. In this case, when aminoethylpiperazine is used a hardener, a lower glass transition temperature of elastomers with a soft segment molecular weight of 991 can be achieved. The soft phase crystallizes at the soft segment molecular weight of 3437. In this case, the melting point of the soft phase is 41–42 °C. No noticeable effect of the hard block type or molecular weight of polyester on melting temperature was observed. It is worth noting that the higher molecular weight of initial polyester, the higher are ΔHm values of the elastomer samples. This is due to the presence of longer soft segments which are able to crystallize more easily.
3.3. FTIR Data
The FTIR spectra of the synthesized elastomers are shown in
Figure 6a,b. The NH band of urethane can be found at 3350 cm
−1 as a broad absorption. A broad band with the center at 2950 cm
−1 and the one at 2860 cm
−1 were assigned to the CH asymmetric stretching and the symmetric one in the CH
2 groups. The absorption bands at 1542, 1454, and 1412 cm
−1 were assigned to the amide−NH stretching. It should be noted that important differences in structure between the synthesized elastomers are reflected on the carbonyl absorption region at 1600–1760 cm
−1. These are the differences determining mechanical behavior of the materials. So, this FTIR spectra region was examined in more detail.
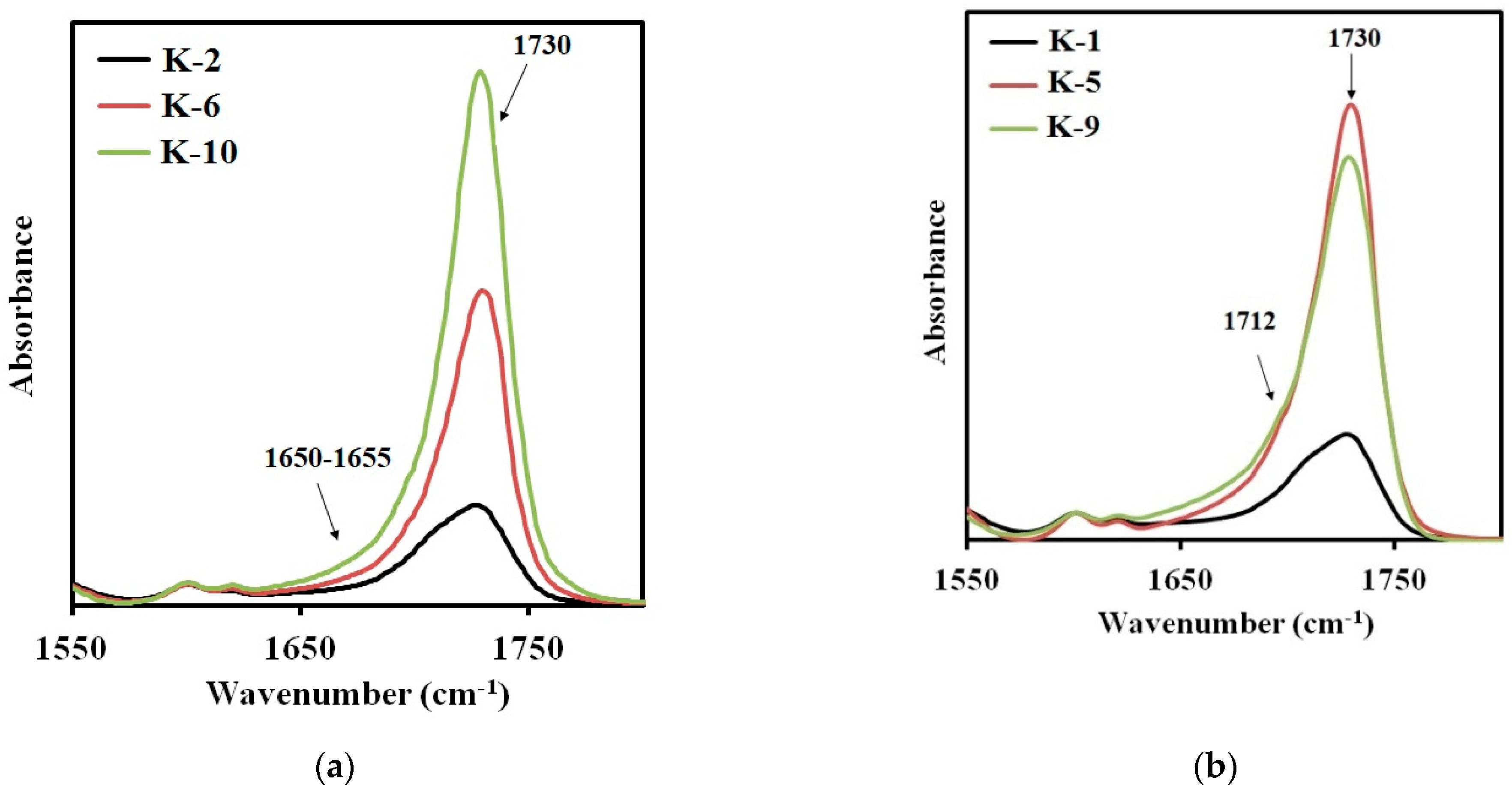
The band at 1730 cm
−1 appears in the spectra of the samples, synthesized from 2,4-toluene diisocyanate and aminoethylpiperazine (
Figure 7a), due to the absorption of carbonyl in free urethane groups. It can be assigned to the hard segments dissolved in the soft phase [
28]. As expected, the intensity of this absorption band increases with the molecular weight of the polyester soft segment. The intensity of the band at 1650 cm
−1 characterizing the microphase separation in elastomers containing this type of the hard segments, is negligibly low. Therefore, the degree of microphase separation in the elastomer decreases with an increase in the molecular weight of the polyester used in the synthesis.
When using aminoethylpiperazine instead of isophorone diamine, a more complex picture appears. The increase in the intensity of the absorption band at 1730 cm
−1 (
Figure 7b) is not additive. The intensity of this band for the elastomer with a soft segment molecular length of 3437, was found to be the highest. Hence, this elastomer is characterized by the lowest degree of microphase separation. This contributes to manifestation of the soft segment crystallization in case of the sample K-5, in contrast to K-6 (
Figure 7a,b). In addition, for the samples containing the hard segments of the second type, an absorption band at 1712 cm
−1 appears. It is this band that characterizes the microphase separation for the hard segments of the second type.
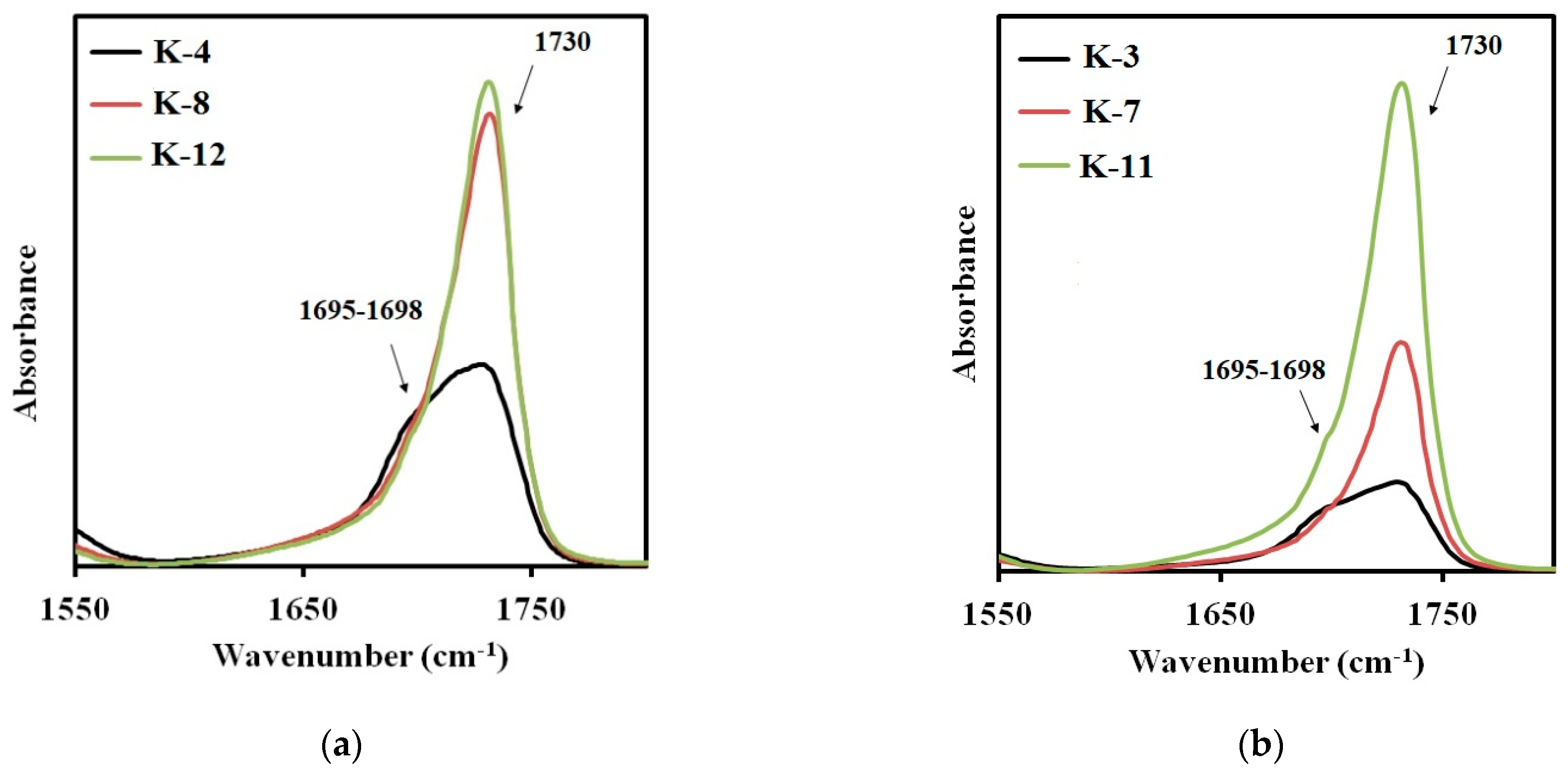
For the elastomers with hard urethane hydroxyl segments based on isophorone diisocyanate, the absorption band characteristic for the microphase separation is observed in the range of 1695–1698 cm
−1 [
28]. In
Figure 8a,b the FTIR spectra sections are presented for the elastomers prepared using isophorone diisocyanate and cured with aminoethylpiperazine (
Figure 8a) or isophorone diamine (
Figure 8b). In all spectra, the two absorption bands can be easily distinguished: at 1730 cm
−1 and at 1695–1698 cm
−1. When using aminoethylpiperazine, the highest degree of SS-HS microphase separation is achieved at the soft segment molecular weight of 991. Further increase in the soft segment molecular weight results in a lower degree of microphase separation. At the same time, structural phase organization in the elastomers K-8 and K-12 is similar.
For elastomers K-3, K-7, and K-11, the picture is somewhat different. The number of the hard segments, dissolved in a soft polyester matrix, increases with the soft segment molecular weight.
In general, for all elastomers, the degree of microphase separation decreases inversely to the soft segment molecular weight.
3.4. Deformation and Strength Characteristics
According to the data obtained at mechanical testing (
Figure 9a–d,
Table 5), deformation properties of the elastomers depend on the nature of a curing agent. Deformation properties of isophorone diamine-cured elastomers are higher. It should be noted that mechanical properties of the elastomers prepared using isophorone diisocyanate over than two times exceed the values obtained for 2,4-toluene diisocyanate-based elastomers.
3.5. The Study of Elastomer Hydrofilicity
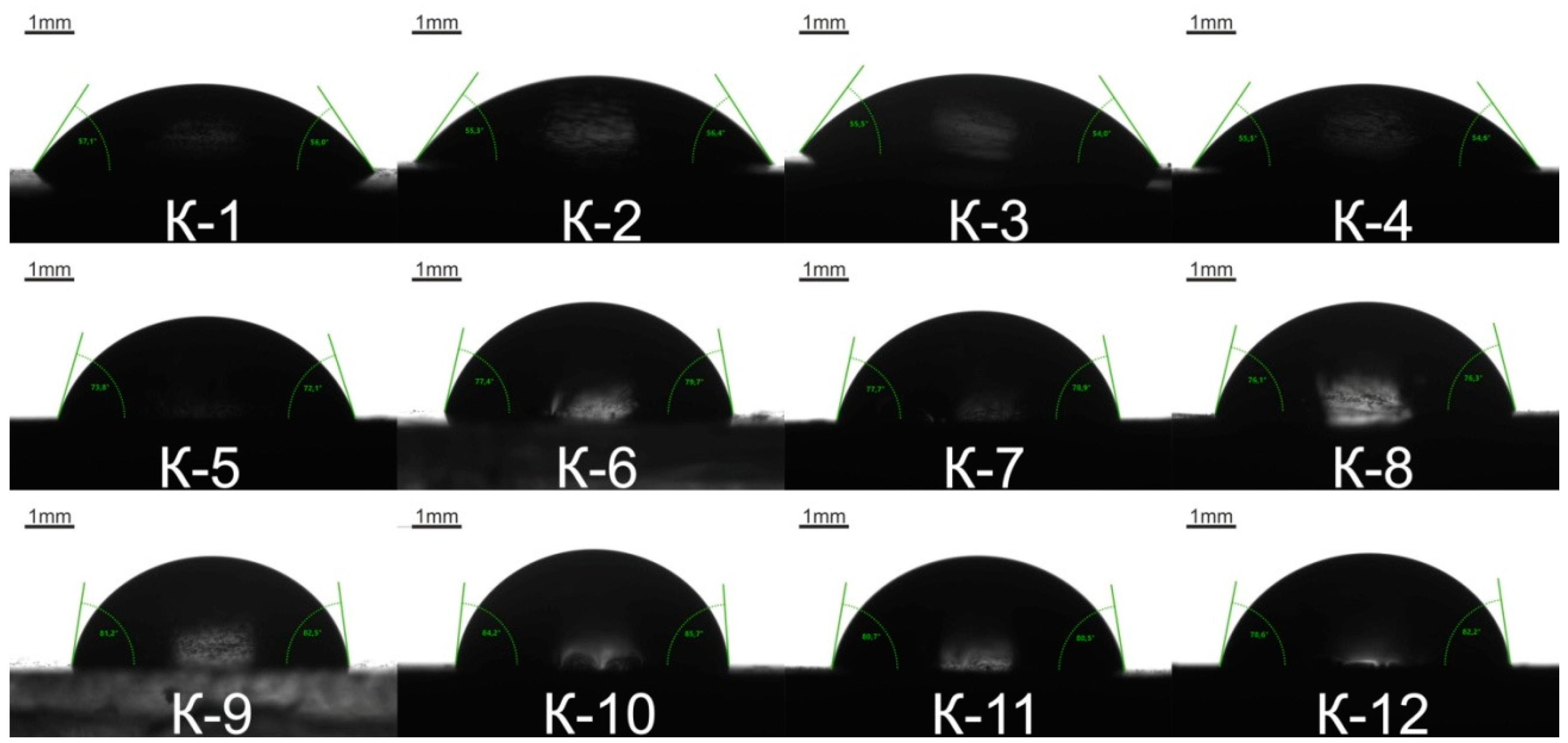
The surface hydrophilicity of the synthesized samples, characterized by the statistical contact angle of water, is illustrated in
Figure 10, and the contact angle values are given in
Table 6. The higher is molecular weight of oligoethylene adipate used in the EUO synthesis, the higher the soft segment length is. Epoxyurethane oligomer reacts with an amine to form additional hydroxyl groups due to oxirane rings opening. Hence, an elastomer with longer soft segments contains fewer hydroxyl and its surface becomes less hydrophilic. This tendency was demonstrated by measuring the contact angle of water.
The surface hydrophilicity of the synthesized samples, characterized by the statistical contact angle of water, is illustrated in
Figure 10, and the contact angle values are given in
Table 6. The surface hydrophilicity is inversely proportional to the molecular weight of the OEA used in the EUO synthesis, i.e., to the length soft segment chain. As was expected, a gradual increase in the contact angle of water was observed. In addition, hydrophilicity decreases with lowering the content of hydroxyl groups which are formed as a result of the oxirane ring opening at epoxyurethane oligomer–amine interaction. There is no considerable increase in the water amount absorbed at different time intervals. This means that crosslinking of the polymer network has no impact on its ability to take up water. The OEA molecular weight is the main factor that can control the absorbed water amount. The water uptake of the samples decreases as the polyol molecular weight increases.
3.6. Determination of the Equilibrium Swelling Degree of the Synthesized Elastomers in Industrial Oil and Gasoline
The data on the equilibrium swelling of the synthesized elastomers in industrial oil I-50 A and in gasoline are shown in
Table 7. It is seen that the swelling degree slightly increases with the molecular weight of the polyester soft block. Nevertheless, as the data on swelling are rather low, the elastomers can be considered to be oil/gasoline-resistant.
4. Conclusions
Three polyesters of different molecular weights were synthesized from adipic acid and ethylene glycol. The polyester structure was identified by NMR spectroscopy. Six epoxyurethane oligomers were prepared using the synthesized polyesters, isophorone diisocyanate, 2.4-toluene diisocyanate, and epoxy alcohol- glycidol. Twelve oligomer-based elastomers with urethane hydroxyl hard segments were prepared using isophorone diamine and aminoethylpiperazine.
The glass transition temperature of the synthesized elastomers was found to be decreased inversely to the soft segment molecular weight taken in the range from 991 to 3437. No effect on the glass transition temperature was observed at a further increase in the soft segment molecular weight. This phenomenon can be explained by decreased segmental mobility of the polymer chains due to the polymer crystallization.
The interaction with an amine or reaction of oxirane ring opening resulted in an increase in the hydroxyl group number in the polymer chain. The contact angle of water on the elastomer surface was shown to be decreased inversely to hydroxyl group content. This means that the material became more hydrophilic.