RAFT Copolymerization of Vinyl Acetate and Acrylic Acid in the Selective Solvent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Polymer Synthesis
2.2. Instrumentation
3. Results and Discussion
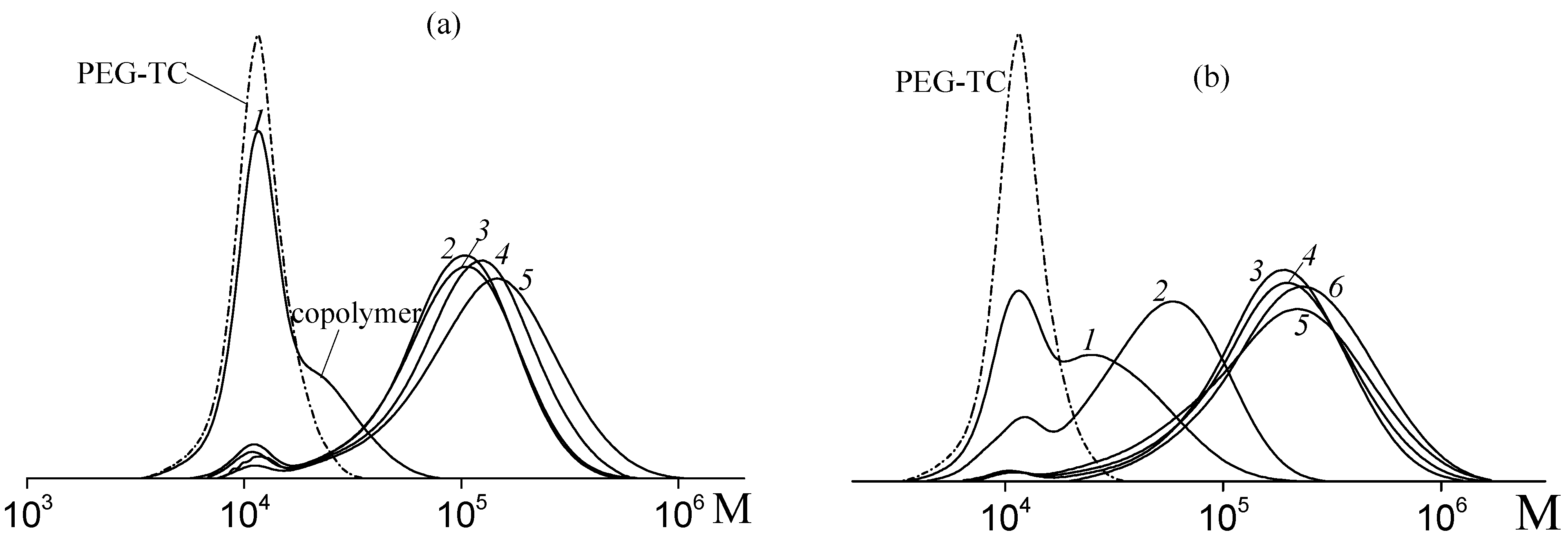
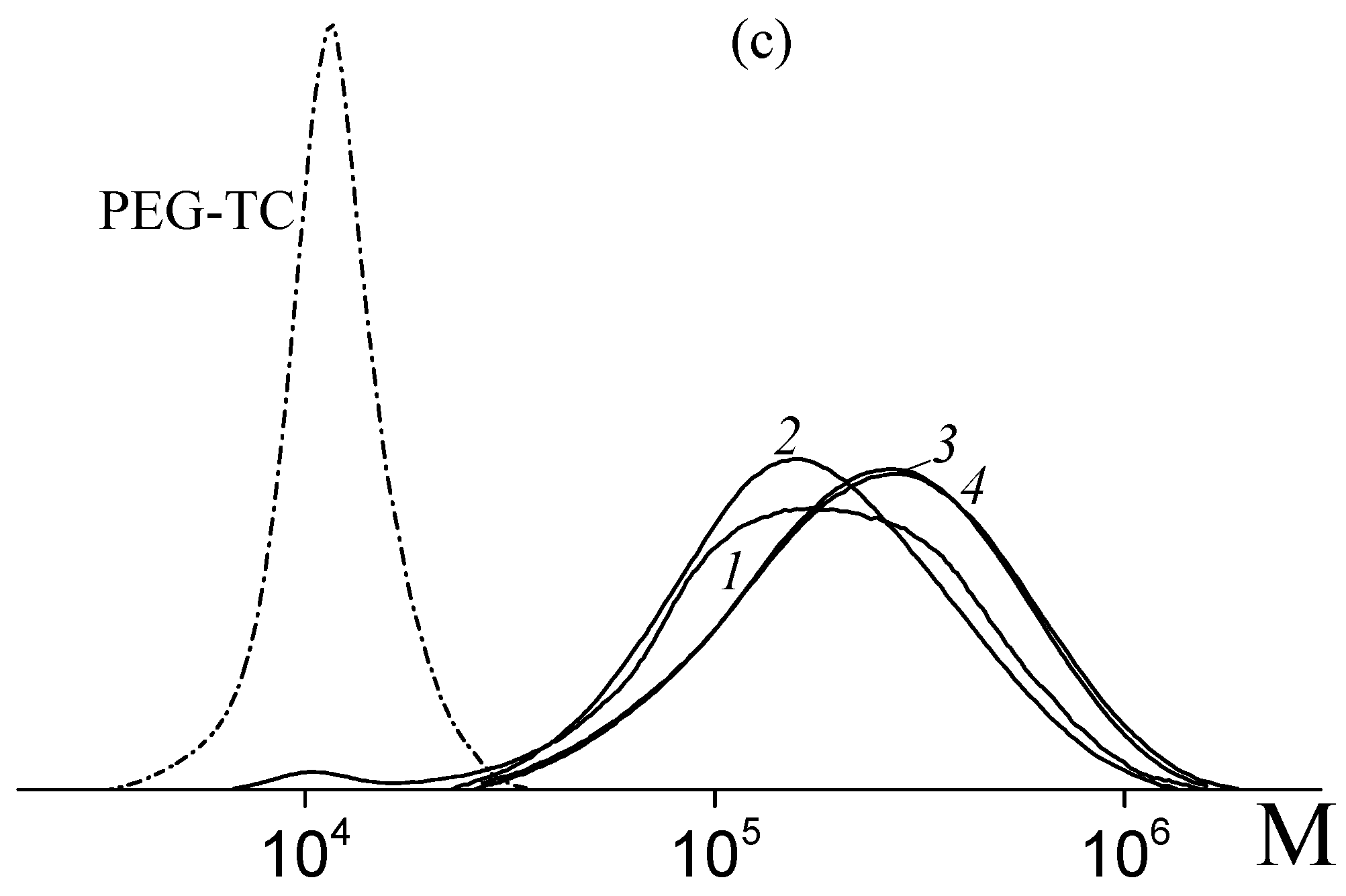
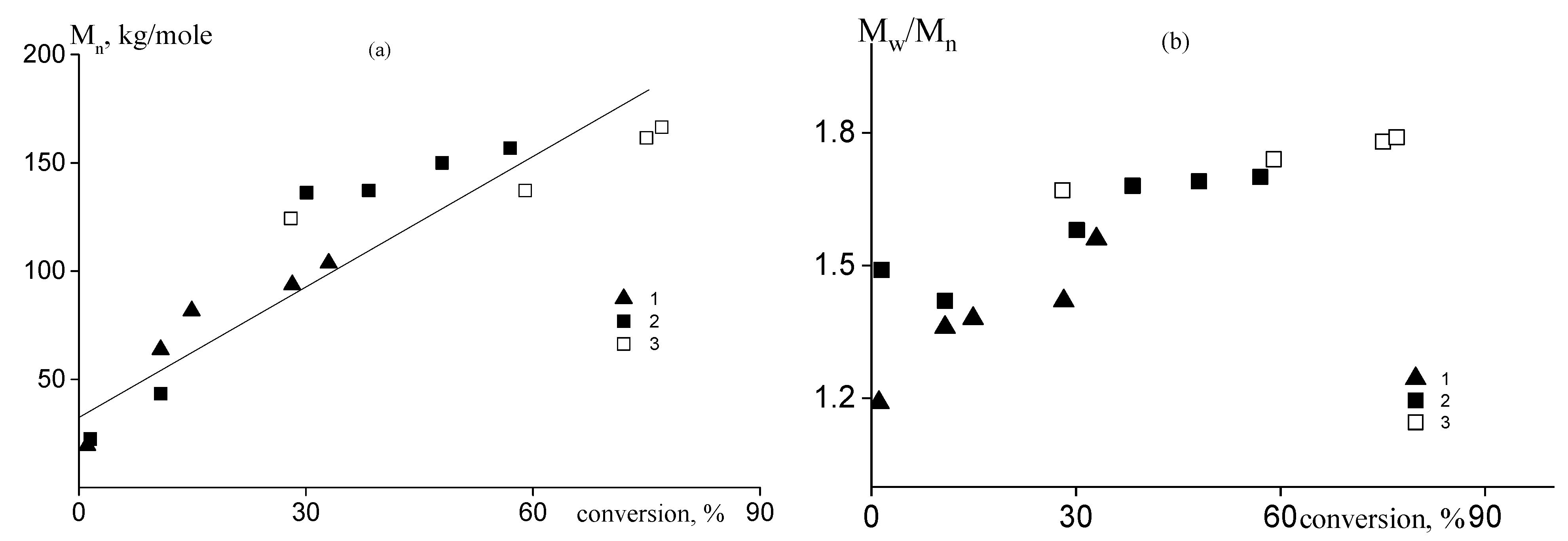
3.1. Copolymerization of Vinyl Acetate and Acrylic Acid
3.2. Self-Assembly
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Derry, M.J.; Fielding, L.A.; Armes, S.P. Industrially-Relevant Polymerization-Induced Self-Assembly Formulations in Non-Polar Solvents: RAFT Dispersion Polymerization of Benzyl Methacrylate. Polym. Chem. 2015, 6, 3054–3062. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Cao, M.; Duan, W.; Ying, T.; Zhang, W. Synthesis of Diblock Copolymer Nano-Assemblies: Comparison Between PISA and Micellization. Polymer 2018, 150, 204–213. [Google Scholar] [CrossRef]
- Varlas, S.; Foster, J.C.; O’Reilly, R.K. Ring-Opening Metathesis Polymerization-Induced Self-Assembly (ROM-PISA). Chem. Commun. 2019, 55, 9066–9071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, D.; Keller, D.; Delaittre, G. Reactive and Functional Nanoobjects by Polymerization-Induced Self-Assembly. Macromol. Rapid Commun. 2019, 40, 1800551. [Google Scholar] [CrossRef] [PubMed]
- Penfold, N.J.W.; Yeow, J.; Boyer, C.; Armes, S.P. Emerging Trends in Polymerization-Induced Self-Assembly. ACS Macro Lett. 2019, 8, 1029–1054. [Google Scholar] [CrossRef] [Green Version]
- Mane, S.R. Trending Methods Employed for Polymerization Induced Self-Assembly. New J. Chem. 2020, 44, 6690–6698. [Google Scholar] [CrossRef]
- D’Agosto, F.; Rieger, J.; Lansalot, M. RAFT-Mediated Polymerization-Induced Self-Assembly. Angew. Chem. Int. Ed. 2020, 59, 8368–8392. [Google Scholar] [CrossRef]
- Liu, C.; Hong, C.Y.; Pan, C.Y. Polymerization Techniques in Polymerization-Induced Self-Assembly (PISA). Polym. Chem. 2020, 11, 3673–3689. [Google Scholar] [CrossRef]
- Cornel, E.J.; Jiang, J.; Chen, S.; Du, J. Polymerization-Induced Self-Assembly with Various Polymerization Techniques. CCS Chem. 2020, 2, 2104–2125. [Google Scholar]
- Delaittre, G.; Nicolas, J.; Lefay, C.; Save, M.; Charleux, B. Aqueous Suspension of Amphiphilic Diblock Copolymer Nanoparticles Prepared In Situ from a Water-Soluble Poly(Sodium Acrylate) Alkoxyamine Macroinitiator. Soft Matter. 2006, 2, 223–231. [Google Scholar] [CrossRef]
- Zhang, X.; Rieger, J.; Charleux, B. Effect of the solvent composition on the morphology of nano-objects synthesized via RAFT polymerization of benzyl methacrylate in dispersed systems. Polym. Chem. 2012, 3, 1502–1509. [Google Scholar] [CrossRef]
- Hatton, F.L.; Lovett, J.R.; Armes, S.P. Synthesis of Well-Defined Epoxy-Functional Spherical Nanoparticles by RAFT Aqueous Emulsion Polymerization. Polym. Chem. 2017, 8, 4856–4868. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Wang, Y.; Zhang, W. Synthesis of Diblock Copolymer Nano-Assemblies by PISA Under Dispersion Polymerization: Comparison Between ATRP and RAFT. Polym. Chem. 2017, 8, 6407–6415. [Google Scholar] [CrossRef]
- Sugihara, S.; Armes, S.P.; Lewis, A.L. One-Pot Synthesis of Biomimetic Shell Cross-Linked Micelles and Nanocages by ATRP in Alcohol/Water Mixtures. Angew. Chem. Int. Ed. 2010, 49, 3500–3503. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, S.; Sugihara, K.; Armes, S.P.; Ahmad, H.; Lewis, A.L. Synthesis of Biomimetic Poly(2-(Methacryloyloxy)Ethyl Phosphorylcholine) Nanolatexes via Atom Transfer Radical Dispersion Polymerization in Alcohol/Water Mixtures. Macromolecules 2010, 43, 6321–6329. [Google Scholar] [CrossRef]
- Yañez-Macias, R.; Kulai, I.; Ulbrich, J.; Yildirim, T.; Sungur, P.; Hoeppener, S.; Guerrero-Santos, R.; Schubert, U.S.; Destarac, M.; Guerrero-Sanchez, C.; et al. Thermosensitive spontaneous gradient copolymers with block- and gradient-like features. Polym. Chem. 2017, 8, 5023–5032. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, T.; Kuchel, R.P.; Yeow, J.; Boyer, C. Gradient Polymerization–Induced Self-Assembly: A One-Step Approach. Macromol. Rapid Commun. 2019, 41, 1900493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Corrigan, N.; Boyer, C. Forced gradient copolymerisation: A simplified approach for polymerization-induced self-assembly. Polym. Chem. 2021, 12, 57–68. [Google Scholar] [CrossRef]
- Zhang, J.; Farias-Mancilla, B.; Destarac, M.; Schubert, U.S.; Keddie, D.J.; Guerrero-Sanchez, C.; Harrisson, S. Asymmetric Copolymers: Synthesis, properties and applications of gradient and other partially segregated copolymers. Macromol. Rapid Commun. 2018, 39, 1800357. [Google Scholar] [CrossRef]
- Kozhunova, E.Y.; Gavrilov, A.A.; Zaremski, M.Y.; Chertovich, A.V. Copolymerization on Selective Substrates: Experimental Test and Computer Simulations. Langmuir 2017, 33, 3548–3555. [Google Scholar] [CrossRef]
- Madruga, E.L. From classical to living/controlled statistical free-radical copolymerization. Prog. Polym. Sci. 2002, 27, 1879–1924. [Google Scholar] [CrossRef]
- Moskowitz, J.D.; Wiggins, J.S. Semibatch RAFT copolymerization of acrylonitrile and Nisopropylacrylamide: Effect of comonomer distribution on cyclization and thermal stability. Polymer 2016, 84, 311–318. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Zaitsev, S.D.; Plutalova, A.V.; Mineeva, K.O.; Zotova, O.S.; Vishnevetsky, D.V. Control over the relative reactivities of monomers in RAFT copolymerization of styrene and acrylic acid. RSC Adv. 2018, 8, 14300–14310. [Google Scholar] [CrossRef] [Green Version]
- Mineeva, K.O.; Osipova, N.I.; Zaitsev, S.D.; Plutalova, A.V.; Medentseva, E.I.; Serkhacheva, N.S.; Lysenko, E.A.; Chernikova, E.V. Synthesis of Amphiphilic Copolymers of Acrylic Acid and Styrene with the Desired Microstructure and Their Properties. Polym. Sci. Ser. B 2020, 62, 630–640. [Google Scholar] [CrossRef]
- Zaremski, M.Y.; Kozhunova, E.Y.; Abramchuk, S.S.; Glavatskaya, M.E.; Chertovich, A.V. Polymerization-induced phase separation in gradient copolymers. Mendeleev Commun. 2021, 31, 277–279. [Google Scholar] [CrossRef]
- Zaldivar, C.; del Sol, O.; Iglesias, G.D. On the preparation of acrylic acid/vinyl acetate copolymers with constant composition—1. Copolymerization reactivity ratios. Polymer 1997, 39, 245–246. [Google Scholar] [CrossRef]
- Wang, W.; Xie, W.-Y.; Wang, G.-X.; Xu, W.; Liang, E. PET-RAFT copolymerization of vinyl acetate and acrylic acid. Iran. Polym. J. 2021, 30, 1–7. [Google Scholar] [CrossRef]
- Provencher, S.W. CONTIN: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Yulusov, V.V.; Mineeva, K.O.; Golubev, V.B.; Garina, E.S. Pseudoliving Polymerization of Vinyl Acetate Mediated by Reversible Addition-Fragmentation Chain-Transfer Agents. Polym. Sci. Ser. B 2011, 53, 438–448. [Google Scholar] [CrossRef]
- Egorova, E.A.; Zubov, V.P.; Bakeeva, I.V.; Chernikova, E.V.; Litmanovich, E.A. Controlled Synthesis of Oligomeric Poly(acrylic acid) and Its Behavior in Aqueous Solutions. Polym. Sci. Ser. A 2013, 55, 519–525. [Google Scholar] [CrossRef]
- Kargin, V.A. Encyclopedia of Polymers. In Soviet Encyclopedia; Sovetskaya Entsiklopediya: Moscow, Russia, 1972. (In Russian) [Google Scholar]
- Brandrup, J.; Immergut, E.H.; Crulue, E.A. (Eds.) Polymer Handbook; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Lindeman, M.K. The Mechanism of Vinyl Acetate Polymerization. In Vinyl Polymerization, V. 1, Part I; Ham, G.E., Ed.; Marcel Dekker: New York, NY, USA, 1967; Chapter 4. [Google Scholar]
- Lee, S.B.; Russell, A.J.; Matyjaszewski, K. ATRP Synthesis of Amphiphilic Random, Gradient, and Block Copolymers of 2-(Dimethylamino)ethyl Methacrylate and n-Butyl Methacrylate in Aqueous Media. Biomacromolecules 2003, 4, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Seno, K.I.; Tsujimoto, I.; Kanaoka, S.; Aoshima, S. Synthesis of Various Stimuli-Responsive Gradient Copolymers by Living Cationic Polymerization and their Thermally or Solvent Induced Association Behavior. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6444–6454. [Google Scholar] [CrossRef]
- Kravchenko, V.S.; Abetz, V.; Potemkin, I.I. Self-assembly of gradient copolymers in a selective solvent. New structures and comparison with diblock and statistical copolymers. Polymer 2021, 235, 124288. [Google Scholar]
- Kravchenko, V.S.; Potemkin, I.I. Micelles of Gradient vs Diblock Copolymers. Difference in the Internal Structure and Properties. J. Phys. Chem. B 2016, 120, 12211–12217. [Google Scholar] [CrossRef]





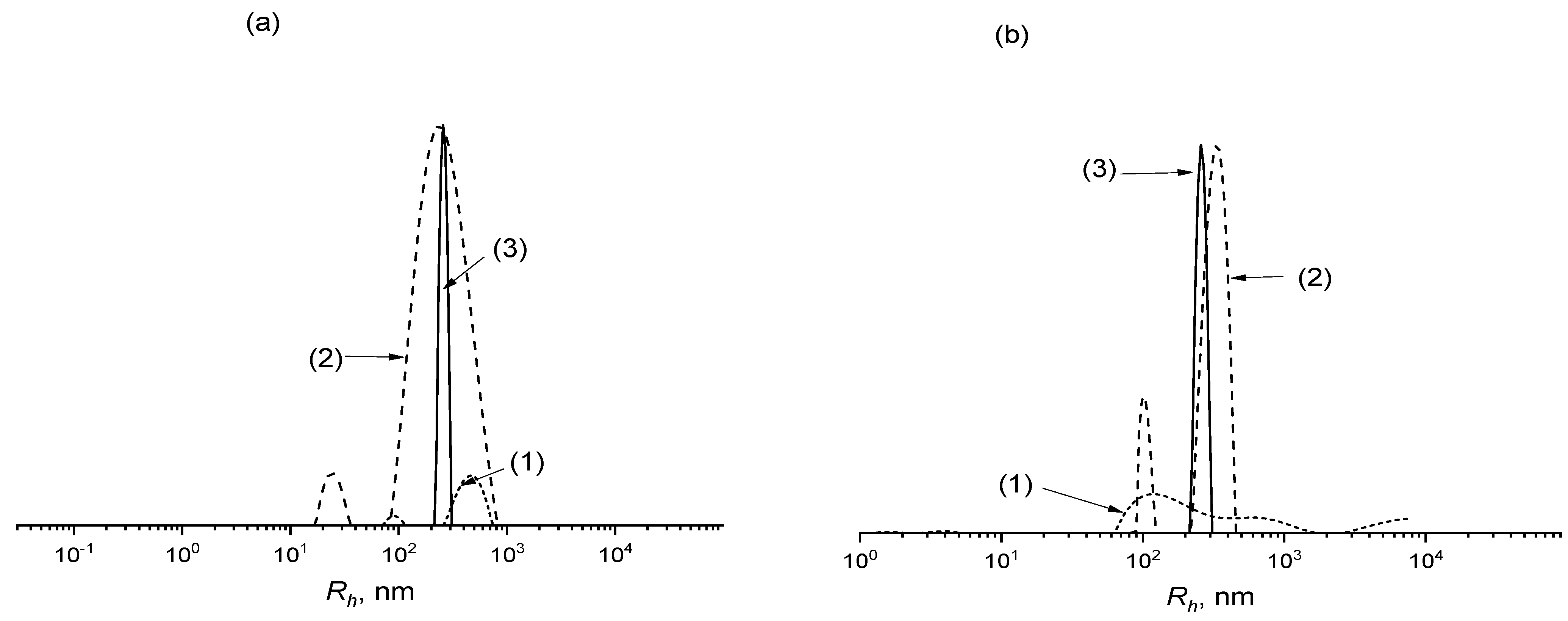
| VAc/AA, mole/mole | VAc | AA | 1,4-Dioxane | Water | PSK | PEG-TC | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| mL | mole | mL | Mole | mL | mL | mg | mole | mg | Mole | |
| 43/57 | 6.0 | 0.065 | 6.0 | 0.087 | 10 | 14 | 6.6 | 2.4 × 10−5 | 129 | 2.4 × 10−5 |
| 55/45 | 7.4 | 0.080 | 4.6 | 0.067 | 10 | 14 | 6.6 | 2.4 × 10−5 | 129 | 2.4 × 10−5 |
| 70/30 | 9.1 | 0.099 | 2.9 | 0.042 | 10 | 14 | 6.6 | 2.4 × 10−5 | 129 | 2.4 × 10−5 |
| fAA, mole% | Conversion,% | <FAA> *, mole% |
|---|---|---|
| 57 | 28.0 59.0 77.0 | 89 82 80 |
| 45 | 57.0 | 75 |
| 30 | 33.7 | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozhunova, E.Y.; Plutalova, A.V.; Chernikova, E.V. RAFT Copolymerization of Vinyl Acetate and Acrylic Acid in the Selective Solvent. Polymers 2022, 14, 555. https://doi.org/10.3390/polym14030555
Kozhunova EY, Plutalova AV, Chernikova EV. RAFT Copolymerization of Vinyl Acetate and Acrylic Acid in the Selective Solvent. Polymers. 2022; 14(3):555. https://doi.org/10.3390/polym14030555
Chicago/Turabian StyleKozhunova, Elena Yu., Anna V. Plutalova, and Elena V. Chernikova. 2022. "RAFT Copolymerization of Vinyl Acetate and Acrylic Acid in the Selective Solvent" Polymers 14, no. 3: 555. https://doi.org/10.3390/polym14030555






