Structural Changes in Resin-Based Composites in Saliva of Patients with Leukemia before Starting Chemotherapeutic Regimen
Abstract
:1. Introduction
2. Material and Methods
2.1. Preparation Saliva Sampling and Dental Composite Systems
2.2. Atomic Force Microscopy and Scanning Electron Microscopy Analysis
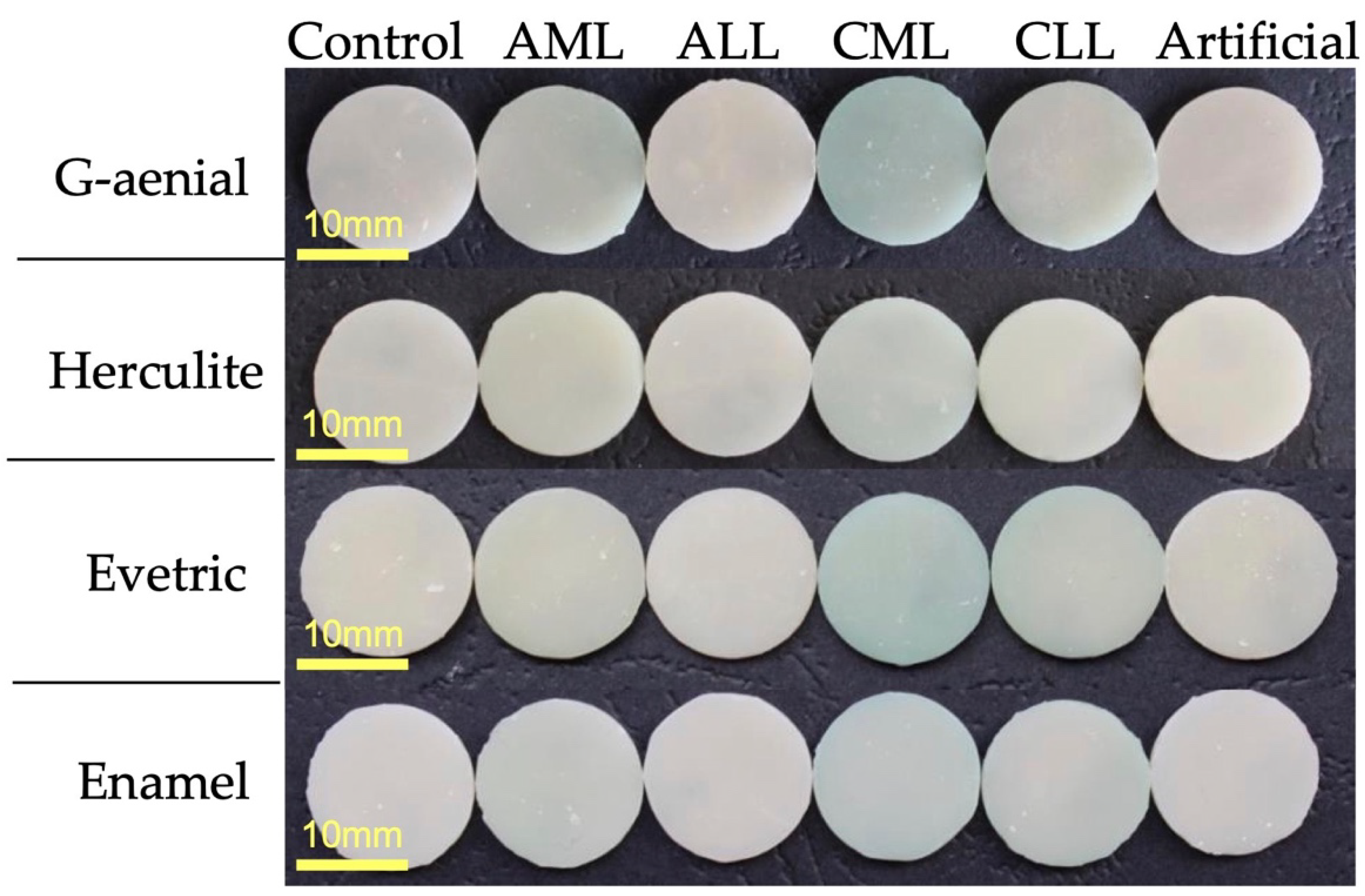
2.3. Color Stability
2.4. Saliva Elements Analysis
2.5. Statistical Analysis
3. Results
3.1. AFM and SEM Analysis
3.2. Color Analysis
3.3. Saliva Elements Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mester, A.; Irimie, A.I.; Tanase, A.; Tranca, S.; Campian, R.S.; Tomuleasa, C.; Dima, D.; Piciu, A.; Lucaciu, O. Periodontal disease might be a risk factor for graft versus host disease. A systematic review. Crit. Rev. Oncol. Hematol. 2020, 147, 102878. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Mealey, B.L.; Mariotti, A.; Chapple, I.L.C. Dental plaque-induced gingival conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 2), S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.A.; Ship, I.I. Initial oral manifestations of leukemia. J. Am. Dent. Assoc. 1967, 75, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Mester, A.; Irimie, A.; Oprita, L.; Dima, D.; Petrushev, B.; Lucaciu, O.; Campian, R.-S.; Tanase, A. Oral manifestations in stem cell transplantation for acute myeloid leukemia. Med. Hypotheses 2018, 121, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Angst, P.D.M.; Maier, J.; Dos Santos Nogueira, R.; Manso, I.S.; Tedesco, T.K. Oral health status of patients with leukemia: A systematic review with meta-analysis. Arch. Oral Biol. 2020, 120, 104948. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, X.; Yang, X.; Que, J.; Du, Q.; Zhang, Q.; Zou, J. Oral Health, Caries Risk Profiles, and Oral Microbiome of Pediatric Patients with Leukemia Submitted to Chemotherapy. Biomed Res. Int. 2021, 2021, 6637503. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.H.L.; Napenas, J.J.; Hodgson, B.D.; Stokman, M.A.; Mathers-Stauffer, V.; Elting, L.S.; Spijkervet, F.K.L.; Brennan, M.T. A systematic review of dental disease in patients undergoing cancer therapy. Support. Care Cancer 2010, 18, 1007–1021. [Google Scholar] [CrossRef]
- Hong, C.H.L.; Hu, S.; Haverman, T.; Stokman, M.; Napeñas, J.J.; Braber, J.B.; Gerber, E.; Geuke, M.; Vardas, E.; Waltimo, T.; et al. A systematic review of dental disease management in cancer patients. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2018, 26, 155–174. [Google Scholar] [CrossRef]
- Hansen, H.J.; Estilo, C.; Owosho, A.; Solano, A.K.; Randazzo, J.; Huryn, J.; Yom, S.K. Dental status and risk of odontogenic complication in patients undergoing hematopoietic stem cell transplant. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2021, 29, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, M.; Akashi, M.; Tsuji, K.; Kusumoto, J.; Furudoi, S.; Shibuya, Y.; Inui, Y.; Yakushijin, K.; Kawamoto, S.; Okamura, A.; et al. Intensity and duration of neutropenia relates to the development of oral mucositis but not odontogenic infection during chemotherapy for hematological malignancy. PLoS ONE 2017, 12, e0182021. [Google Scholar] [CrossRef]
- Tsuji, K.; Shibuya, Y.; Akashi, M.; Furudoi, S.; Yakushijin, K.; Kawamoto, S.; Okamura, A.; Matsuoka, H.; Komori, T. Prospective study of dental intervention for hematopoietic malignancy. J. Dent. Res. 2015, 94, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Ertas, E.T.; Kurnaz, F.; Zorba, Y.O.; Kocyigit, I.; Sisman, Y.; Kaynar, L.; Sekerci, A.E.; Ertas, H.; Cetin, M. Comparison of chemotherapy and hematopoietic stem cell transplantation pre and postterm DMFT scores: A preliminary study. Niger. J. Clin. Pract. 2014, 17, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Dobr, T.; Passweg, J.; Weber, C.; Tichelli, A.; Heim, D.; Meyer, J.; Gratwohl, A.; Waltimo, T. Oral health risks associated with HLA-types of patients undergoing hematopoietic stem cell transplantation. Eur. J. Haematol. 2007, 78, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Raber-Durlacher, J.E.; Brennan, M.T.; Saunders, D.P.; Mank, A.P.; Zadik, Y.; Quinn, B.; Epstein, J.B.; Blijlevens, N.M.A.; Waltimo, T.; et al. Basic oral care for hematology-oncology patients and hematopoietic stem cell transplantation recipients: A position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC). Support. Care Cancer 2015, 23, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Münchow, E.A.; Ferreira, A.C.A.; Machado, R.M.M.; Ramos, T.S.; Rodrigues-Junior, S.A.; Zanchi, C.H. Effect of acidic solutions on the surface degradation of a micro-hybrid composite resin. Braz. Dent. J. 2014, 25, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-C.; Lin, P.-Y.; Kirankumar, R.; Chuang, Z.-W.; Wu, I.-H.; Hsieh, S. Surface degradation effects of carbonated soft drink on a resin based dental compound. Heliyon 2021, 7, e06400. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Sokolowski, J.; Kleczewska, J.; Bociong, K. Ageing of Dental Composites Based on Methacrylate Resins-A Critical Review of the Causes and Method of Assessment. Polymers 2020, 12, 882. [Google Scholar] [CrossRef]
- Cheaib, Z.; Lussi, A. Impact of acquired enamel pellicle modification on initial dental erosion. Caries Res. 2011, 45, 107–112. [Google Scholar] [CrossRef]
- Berge, T.L.L.; Lygre, G.B.; Lie, S.A.; Lindh, C.H.; Björkman, L. Bisphenol A in human saliva and urine before and after treatment with dental polymer-based restorative materials. Eur. J. Oral Sci. 2019, 127, 435–444. [Google Scholar] [CrossRef]
- Agrawal, V.; Kapoor, S. Color and shade management in esthetic dentistry. Univers. Res. J. Dent. 2013, 3, 120. [Google Scholar] [CrossRef]
- Pröfrock, D.; Prange, A. Inductively coupled plasma-mass spectrometry (ICP-MS) for quantitative analysis in environmental and life sciences: A review of challenges, solutions, and trends. Appl. Spectrosc. 2012, 66, 843–868. [Google Scholar] [CrossRef] [PubMed]
- Ashok, N.G.; Jayalakshmi, S. Factors that Influence the Color Stability of Composite Restorations Factors aFFecting color stability oF composite restorations. Int. J. Orofac. Biol. 2017, 1, 1–3. [Google Scholar]
- Shamszadeh, S.; Sheikh-Al-Eslamian, S.M.; Hasani, E.; Abrandabadi, A.N.; Panahandeh, N. Color Stability of the Bulk-Fill Composite Resins with Different Thickness in Response to Coffee/Water Immersion. Int. J. Dent. 2016, 2016, 7186140. [Google Scholar] [CrossRef]
- Imamura, S.; Takahashi, H.; Hayakawa, I.; Loyaga-Rendon, P.G.; Minakuchi, S. Effect of filler type and polishing on the discoloration of composite resin artificial teeth. Dent. Mater. J. 2008, 27, 802–808. [Google Scholar] [CrossRef]
- Selow, M.L.C.; Vieira, I.; Tommasi, M.H.; Ritt, A.C.; Maistro, A.E.; Guerreiro, A.L.; Lange, F.; Souza, J.; Andrade, J. Use of zinc in health from early childhood to late age. Alimentos e Nutrição Araraquara 2008, 18, 457–469. [Google Scholar]
- Selow, M.L.C.; Lunelli, F.; Vieira, I.; Sotta, M.D.; Martins, W.D.B.; Ignacio, S.A.; Brancher, J.A.; De Oliveira Ribas, M. Analysis of zinc concentration in the saliva of individuals at different age ranges. Rev. Odonto Cienc. 2016, 31, 12–15. [Google Scholar] [CrossRef]
- Goto, T.; Komai, M.; Suzuki, H.; Furukawa, Y. Long-term zinc deficiency decreases taste sensitivity in rats. J. Nutr. 2001, 131, 305–310. [Google Scholar] [CrossRef]
- Romano, F.; Castiblanco, A.; Spadotto, F.; Di Scipio, F.; Malandrino, M.; Berta, G.N.; Aimetti, M. ICP-Mass-Spectrometry Ionic Profile of Whole Saliva in Patients with Untreated and Treated Periodontitis. Biomedicines 2020, 8, 354. [Google Scholar] [CrossRef]
- Baumann, T.; Bereiter, R.; Lussi, A.; Carvalho, T.S. The effect of different salivary calcium concentrations on the erosion protection conferred by the salivary pellicle. Sci. Rep. 2017, 7, 12999. [Google Scholar] [CrossRef]
- De Medeiros, J.A.G.; Zamboni, C.B.; Kovacs, L.; Lewgoy, H.R. Investigation of Fe and Ca in non-stimulated human saliva using NAA. J. Phys. Conf. Ser. 2015, 630, 012006. [Google Scholar] [CrossRef]
- Monaci, F.; Bargagli, E.; Bravi, F.; Rottoli, P. Concentrations of major elements and mercury in unstimulated human saliva. Biol. Trace Elem. Res. 2002, 89, 193–203. [Google Scholar] [CrossRef]
- Dos Reis, F.D.; Pereira Júnior, O.D.S.; De Sousa, R.A. Direct analysis of Na, K, Mg and Ca in human saliva and correlations with physiological conditions. Anal. Methods 2020, 12, 1702–1710. [Google Scholar] [CrossRef]
- Schützemberger, M.E.; Souza, R.T.; Petruccix, R.E.; Machado, M.N.; Papalexiou, V.; Brancher, J.A. Análise bioquímica do fluido salivar de indivíduos portadores de doença periodontal (Biochemical analysis of saliva of subjects with periodontal disease). Endereço para correspondência. RSBO Revista Sul-Brasileira de Odontologia 2007, 4, 46–52. [Google Scholar]
- Lewgoy, H.R.; Zamboni, C.B.; Metairon, S.; Medeiros, I.M.M.A.; De Medeiros, J.A.G. Quantitative study of non-stimulated human whole saliva using NAA. J. Radioanal. Nucl. Chem. 2013, 296, 573–577. [Google Scholar] [CrossRef]
- Zamboni, C.B.; Metairon, S.; Medeiros, I.M.M.A.; Lewgoy, H.R. Investigation of saliva of patients with periodontal disease using NAA. AIP Conf. Proc. 2013, 1529, 70–72. [Google Scholar]
- Burch, R.E.; Hahn, H.K.; Sullivan, J.F. Newer aspects of the roles of zinc, manganese, and copper in human nutrition. Clin. Chem. 1975, 21, 501–520. [Google Scholar] [CrossRef]
- Gaur, S.; Agnihotri, R. Trace Mineral Micronutrients and Chronic Periodontitis—A Review. Biol. Trace Elem. Res. 2017, 176, 225–238. [Google Scholar] [CrossRef]
- Yu, B.S.; Yuan, Q.G.; Nie, L.H.; Yao, S.Z. Ion chromatographic determination of calcium and magnesium cations in human saliva and urine with a piezoelectric detector. J. Pharm. Biomed. Anal. 2001, 25, 1027–1032. [Google Scholar] [CrossRef]
- Olmez, I.; Gulovali, M.C.; Gordon, G.E.; Henkin, R.I. Trace elements in human parotid saliva. Biol. Trace Elem. Res. 1988, 17, 259–270. [Google Scholar] [CrossRef]
- Mester, A.; Moldovan, M.; Cuc, S.; Tomuleasa, C.; Pasca, S.; Filip, M.; Piciu, A.; Onisor, F. Characteristics of Dental Resin-Based Composites in Leukemia Saliva: An In Vitro Analysis. Biomedicines 2021, 9, 1618. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, A.F.; Neves, C.B.; de Almeida, M.S.; Pinheiro, L.M.; e Oliveira, S.A.; Lopes, L.P.; Castro, M.F. Biodegradation of acrylic based resins: A review. Dent. Mater. 2010, 26, e171–e180. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Nomura, Y.; Tanaka, N.; Teshima, W.; Okazaki, M.; Shintani, H. Leachability of plasticizer and residual monomer from commercial temporary restorative resins. J. Dent. 2004, 32, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Faltermeier, A.; Rosentritt, M.; Müssig, D. Acrylic removable appliances: Comparative evaluation of different postpolymerization methods. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 301.e16–301.e22. [Google Scholar] [CrossRef] [PubMed]



| Material | Composition | Manufacturer |
|---|---|---|
| G-aenial Anterior A2, |
| GC corporation, Tokyo, Japan |
| Herculite XRV Ultra A2; |
| Kerr, Bolzano, Italy |
| Evetric filling material A2, |
| Ivoclar Vivadent, Schaan, Liechtenstein |
| Enamel Plus HRi, UD2, |
| Micerium, Avegno, Italy |
| Saliva | ENAMEL | EVETRIC | G-AENIAL | HERCULITE | ||||
|---|---|---|---|---|---|---|---|---|
| Ra, nm | Rq, nm | Ra, nm | Rq, nm | Ra, nm | Rq, nm | Ra, nm | Rq, nm | |
| Unexposed | 5.28 ± 0.21 | 7.42 ± 0.83 | 6.82 ± 0.66 | 9.08 ± 1.07 | 6.38 ± 0.39 | 8.38 ± 0.28 | 7.37 ± 2.82 | 10.20 ± 3.18 |
| Healthy | 6.21 ± 2.23 | 8.42 ± 2.32 | 7.05 ± 2.15 | 8.85 ± 2.54 | 15.38 ± 2.23 | 19.40 ± 2.64 | 7.01 ± 2.67 | 9.08 ± 3.36 |
| Artificial | 6.76 ± 0.33 | 8.73 ± 0.51 | 10.0 ± 3.30 | 14.43 ± 6.35 | 15.68 ± 0.93 | 19.98 ± 1.02 | 5.92 ± 2.52 | 7.76 ± 3.22 |
| AML | 8.78 ± 3.55 | 11.4 ± 3.96 | 10.91 ± 2.35 | 15.6 ± 3.10 | 13.80 ± 3.55 | 17.58 ± 4.25 | 6.23 ± 3.59 | 9.36 ± 4.44 |
| CML | 6.24 ± 1.06 | 8.30 ± 1.43 | 8.97 ± 1.45 | 12.37 ± 2.58 | 13.53 ± 4.29 | 19.19 ± 6.61 | 5.91 ± 0.47 | 7.76 ± 0.84 |
| ALL | 10.32 ± 2.20 | 13.30 ± 2.57 | 9.02 ± 0.69 | 11.46 ± 1.05 | 11.17 ± 1.86 | 14.42 ± 2.33 | 6.15 ± 0.54 | 8.50 ± 1.15 |
| CLL | 7.56 ± 1.04 | 9.74 ± 1.53 | 8.66 ± 1.07 | 10.85 ± 1.05 | 16.42 ± 2.90 | 21.04 ± 4.03 | 17.28 ± 6.30 | 22.08 ± 9.40 |
| p value | 0.00369 | 6.19194 × 10−4 | 0.02203 | 0.01198 | 5.46568 × 10−4 | 0.00506 | 4.31649 × 10−5 | 3.99809 × 10−4 |
| Dental Composite | ΔE ± SD Initial | ΔE ± SD after 7 Days of Immersion | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| Control | Artificial | ALL | AML | CLL | CML | |||
| G-aenial | 23.15 ± 0.26 | 22.04 ± 1.04 | 20.74 ± 0.80 | 21.27 ± 1.06 | 20.68 ± 1.04 | 18.25 ± 1.81 | 15.28 ± 1.43 | 2.28931 × 10−16 |
| Evetric | 21.27 ± 0.55 | 18.98 ± 1.36 | 19.91 ± 1.68 | 19.67 ± 0.70 | 18.30 ± 0.85 | 17.29 ± 1.28 | 17.57 ± 2.48 | 2.05817 × 10−4 |
| Herculite | 22.55 ± 0.39 | 23.34 ± 1.45 | 21.63 ± 1.37 | 21.99 ± 1.30 | 19.46 ± 1.70 | 19.50 ± 1.04 | 18.85 ± 1.20 | 2.71886 × 10−9 |
| Enamel | 20.04 ± 0.05 | 18.97 ± 0.41 | 18.96 ± 0.64 | 18.65 ± 1.08 | 17.72 ± 0.96 | 16.88 ± 1.47 | 13.88 ± 1.19 | 4.549 × 10−9 |
| Saliva | Zn (mg/L) | Ca (mg/L) | P (mg/L) | Fe (mg/L) | Cu (mg/L) | K (mg/L) | Mg (mg/L) | Na (mg/L) |
|---|---|---|---|---|---|---|---|---|
| Control | 8.4246 | 47.8872 | 149.2041 | 3.7689 | 4.434 | 528.7545 | 7.5378 | 160.0541 |
| Artificial | 4.002524 | 19.54173 | 87.82008 | 4.002524 | 4.002524 | 375.5309 | 1.883541 | 152.3314 |
| ALL | 8.460541 | 28.80184 | 52.02333 | 3.060196 | 3.60023 | 140.949 | 5.220334 | 40.18635 |
| AML | 9.075908 | 32.34323 | 126.0726 | 2.970297 | 3.79538 | 492.5743 | 5.445545 | 66.3663 |
| CLL | 2.993056 | 21.74954 | 59.06297 | 2.993056 | 3.990741 | 171.2028 | 3.591667 | 41.15093 |
| CML | 3.506879 | 31.29215 | 72.56542 | 4.855678 | 5.125438 | 265.4438 | 4.855678 | 103.4985 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mester, A.; Moldovan, M.; Cuc, S.; Petean, I.; Tomuleasa, C.; Piciu, A.; Dinu, C.; Bran, S.; Onisor, F. Structural Changes in Resin-Based Composites in Saliva of Patients with Leukemia before Starting Chemotherapeutic Regimen. Polymers 2022, 14, 569. https://doi.org/10.3390/polym14030569
Mester A, Moldovan M, Cuc S, Petean I, Tomuleasa C, Piciu A, Dinu C, Bran S, Onisor F. Structural Changes in Resin-Based Composites in Saliva of Patients with Leukemia before Starting Chemotherapeutic Regimen. Polymers. 2022; 14(3):569. https://doi.org/10.3390/polym14030569
Chicago/Turabian StyleMester, Alexandru, Marioara Moldovan, Stanca Cuc, Ioan Petean, Ciprian Tomuleasa, Andra Piciu, Cristian Dinu, Simion Bran, and Florin Onisor. 2022. "Structural Changes in Resin-Based Composites in Saliva of Patients with Leukemia before Starting Chemotherapeutic Regimen" Polymers 14, no. 3: 569. https://doi.org/10.3390/polym14030569







