PVP/Highly Dispersed AgNPs Nanofibers Using Ultrasonic-Assisted Electrospinning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
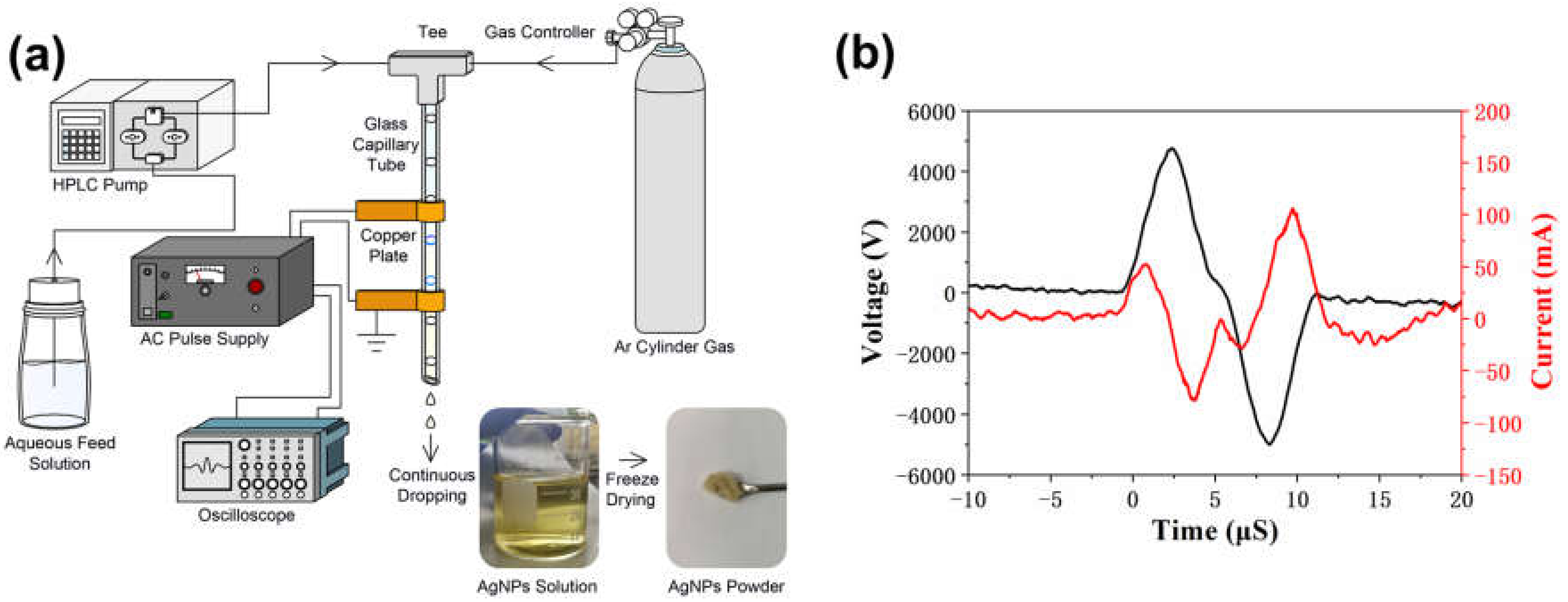
2.2.1. Synthesizing of AgNPs Using APDP
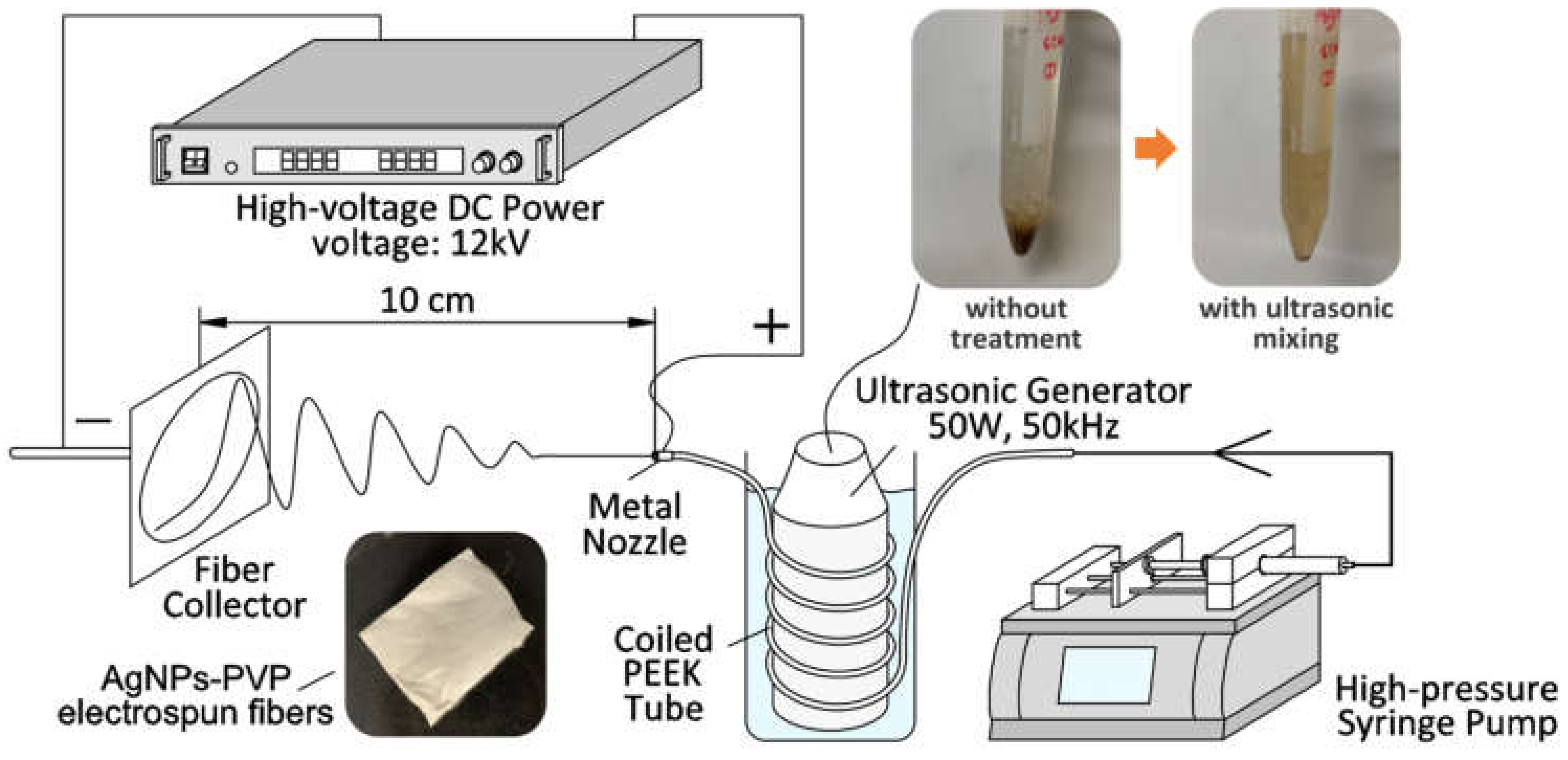
2.2.2. Fabrication of AgNPs-PVP Nanofibers by Ultrasonic-Assisted Electrospinning (US-ES)
2.3. Characterization
3. Results and Discussion
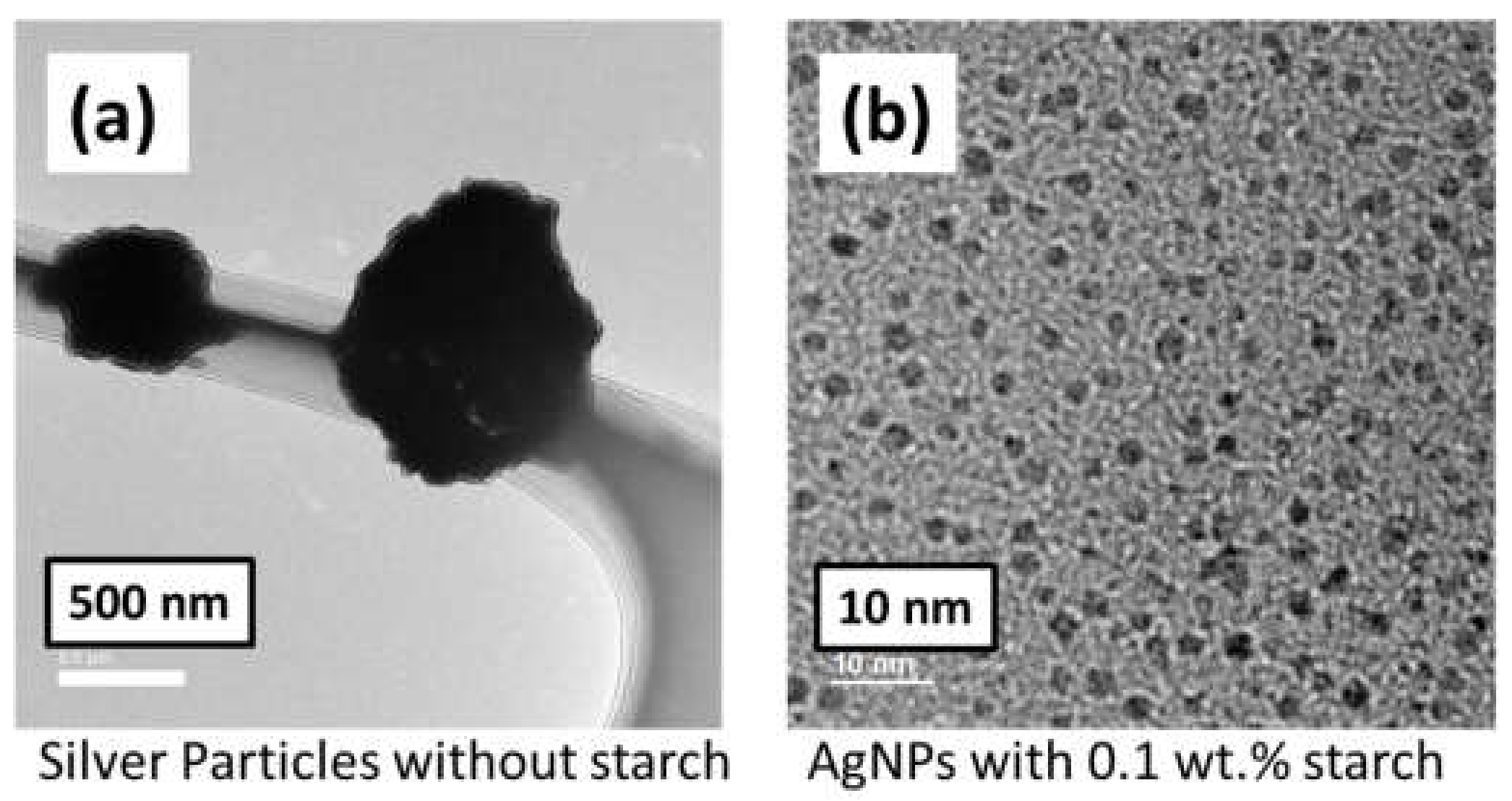
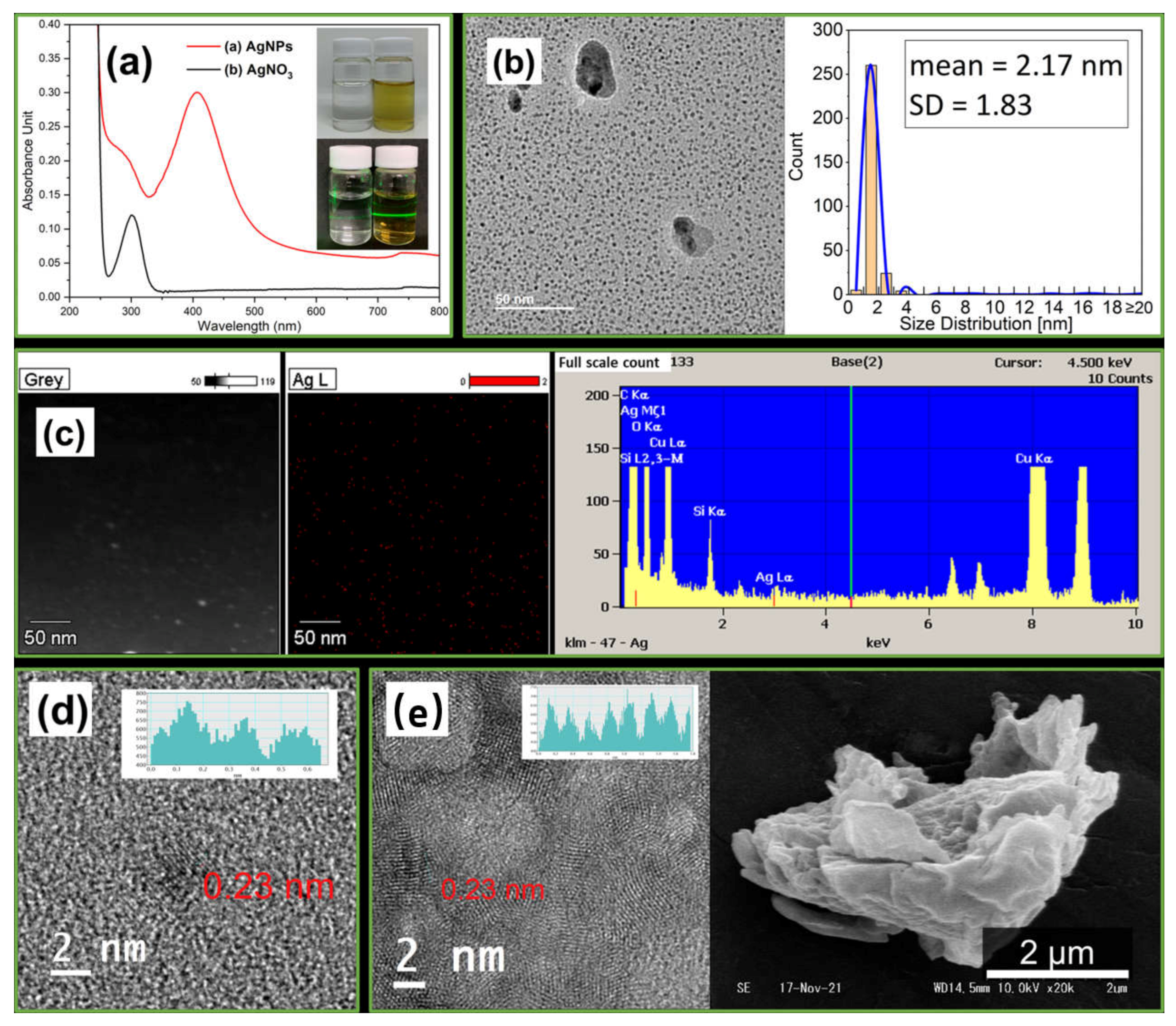
3.1. Synthesizing of AgNPs
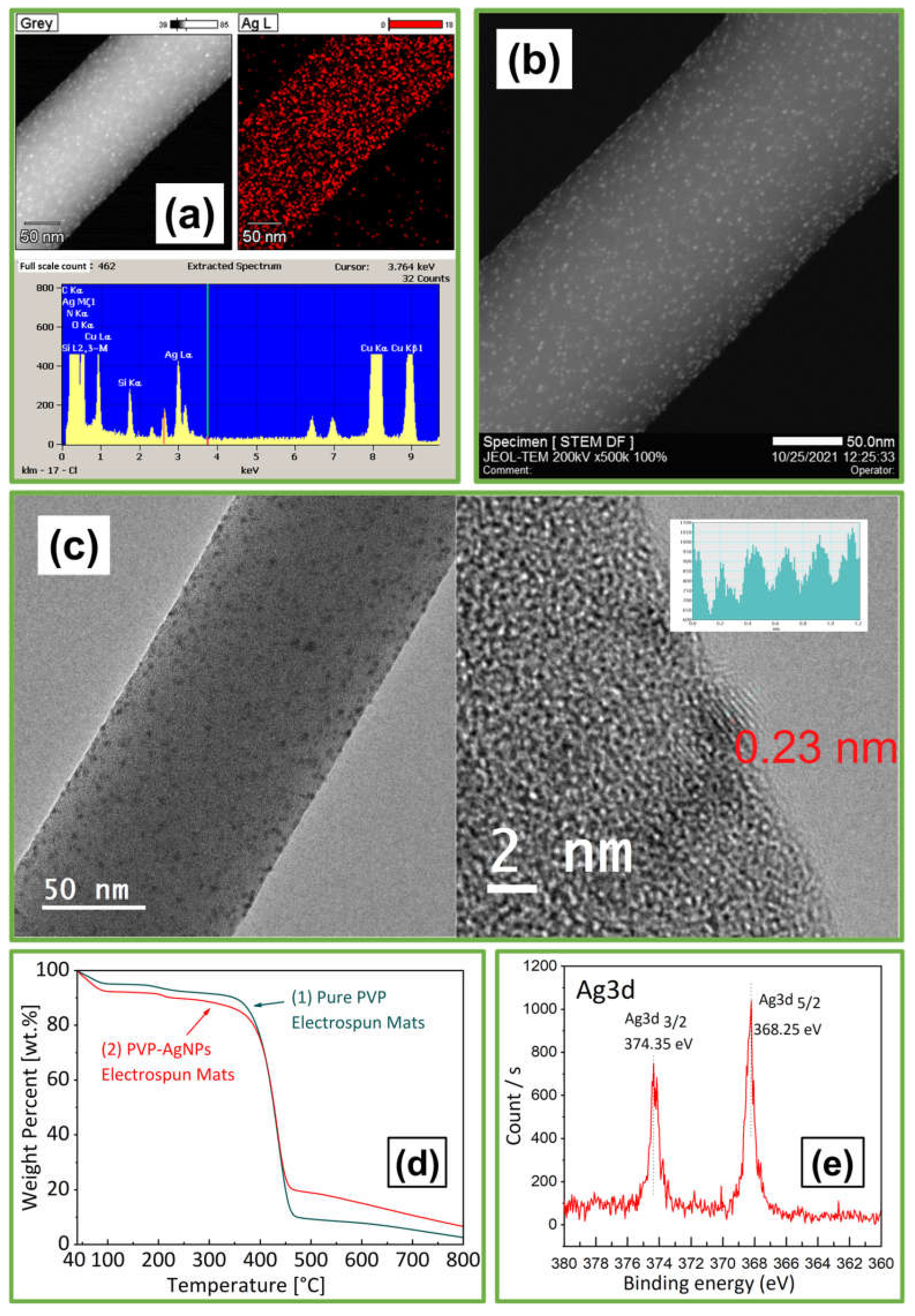
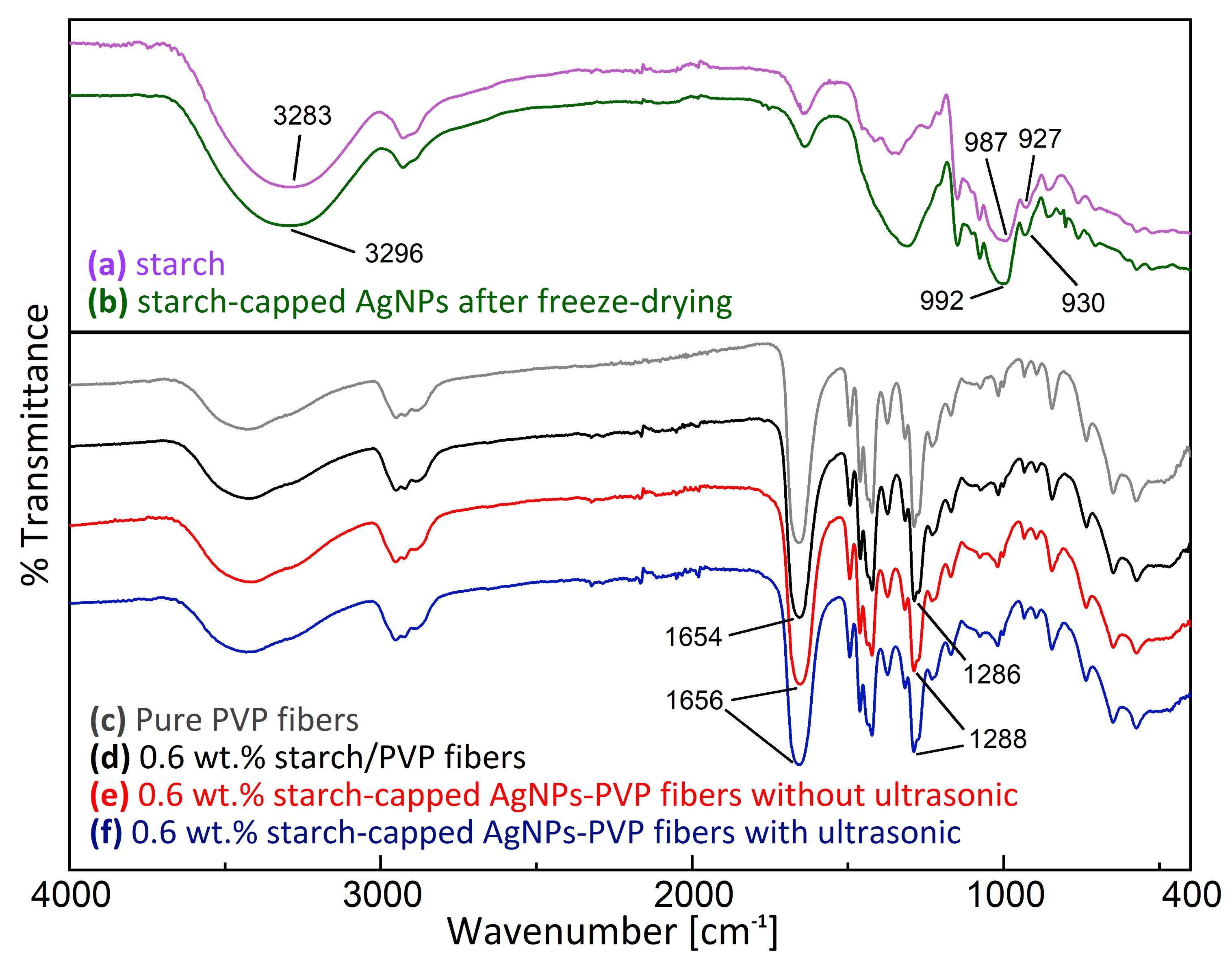
3.1.1. Analysis of AgNPs
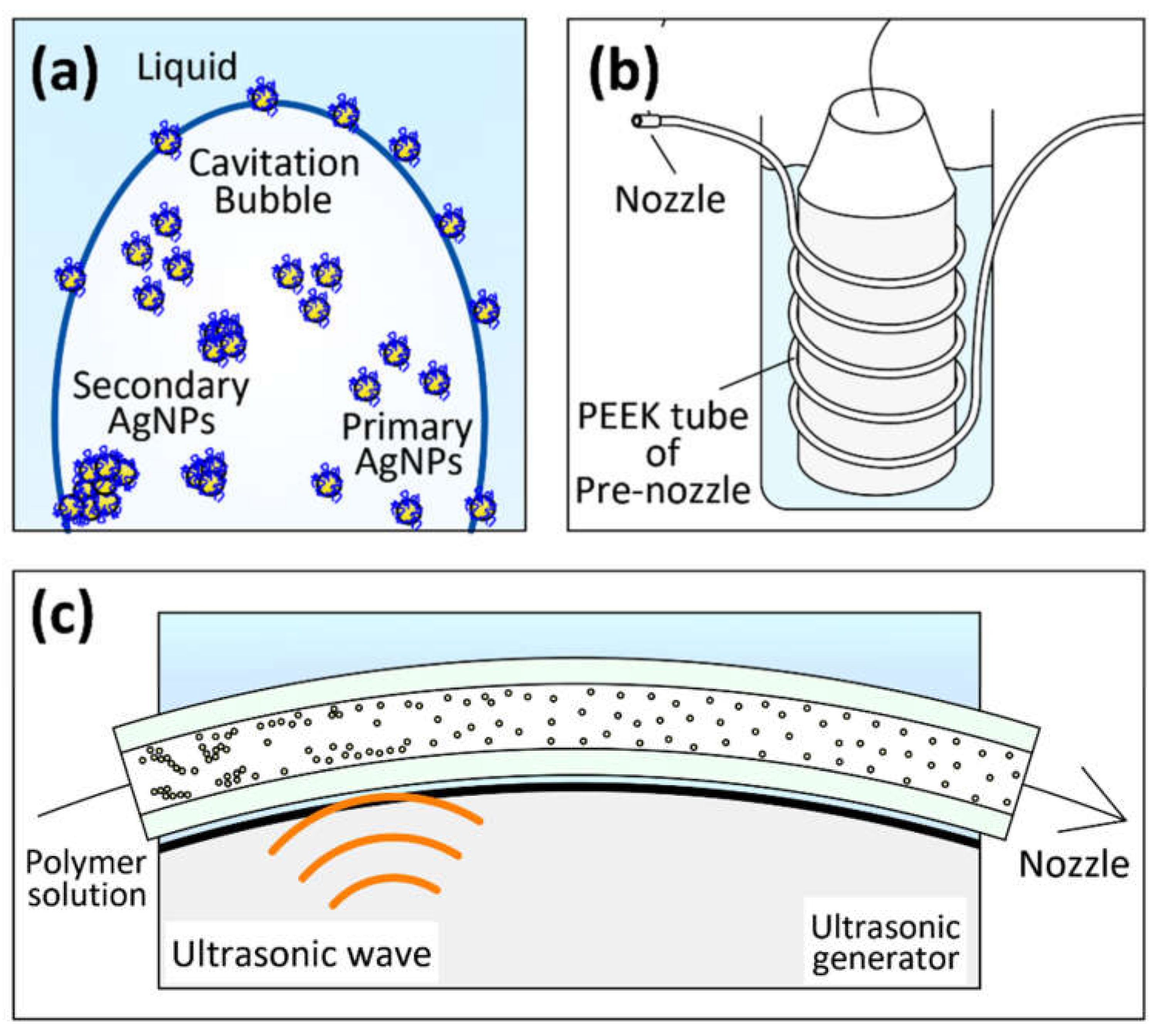
3.1.2. Synthesis Mechanism of AgNPs
3.2. Fabrication of AgNPs-PVP Electrospun Fibers
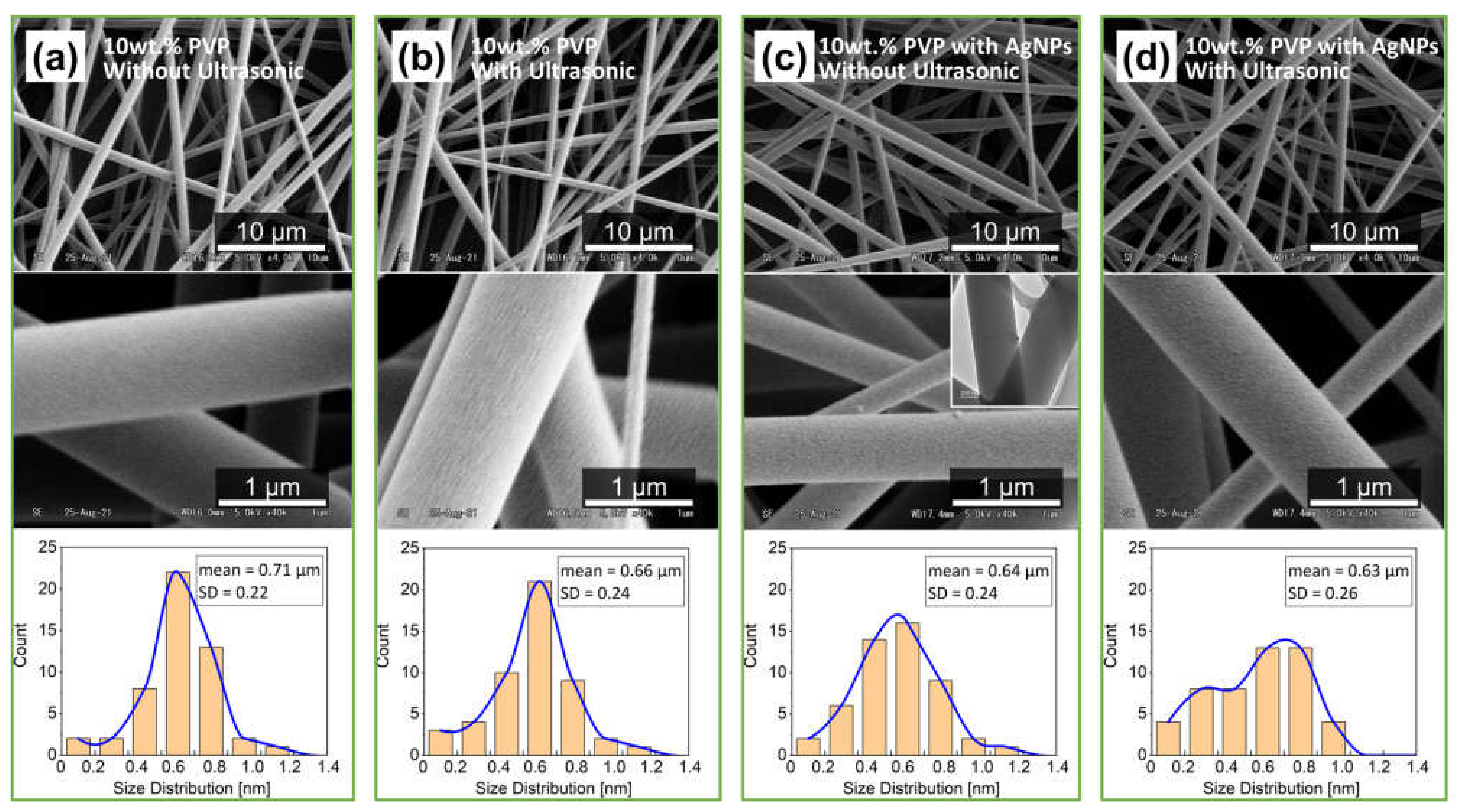
3.2.1. Fibers Morphologies
3.2.2. Dispersion of AgNPs at Different Concentrations on Electrospun Fibers
3.2.3. Analysis of AgNPs-PVP Electrospun Fibers
3.2.4. Mechanism of Nanoparticles Dispersion by US-ES
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Zaarour, M.; El Roz, M.; Dong, B.; Retoux, R.; Aad, R.; Cardin, J.; Dufour, C.; Gourbilleau, F.; Gilson, J.-P.; Mintova, S. Photochemical preparation of silver nanoparticles supported on zeolite crystals. Langmuir 2014, 30, 6250–6256. [Google Scholar] [CrossRef]
- Hu, X.; Takada, N.; Machmudah, S.; Wahyudiono; Kanda, H.; Goto, M. Ultrasonic-Enhanced Fabrication of Metal Nanoparticles by Laser Ablation in Liquid. Ind. Eng. Chem. Res. 2020, 59, 7512–7519. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Preparation of silver nanoparticles by chemical reduction method. Colloids Surf. A Physicochem. Eng. Asp. 2005, 256, 111–115. [Google Scholar] [CrossRef]
- Yamada, M.; Wahyudiono; Machmudah, S.; Kanda, H.; Zhao, Y.; Goto, M. Atmospheric-Pressure Pulsed Discharge Plasma in a Slug Flow Reactor System for the Synthesis of Gold Nanoparticles. ACS Omega 2020, 5, 17679–17685. [Google Scholar] [CrossRef]
- Yamada, M.; Takahashi, S.; Takada, N.; Kanda, H.; Goto, M. Synthesis of silver nanoparticles by atmospheric-pressure pulsed discharge plasma in a slug flow system. Jpn. J. Appl. Phys. 2018, 58, 016001. [Google Scholar] [CrossRef]
- Zhu, W.; Lin, Y.; Zhu, L.; Wahyudiono; Honda, M.; Kanda, H.; Goto, M. Synthesis of Cerium Dioxide Nanoparticles by Gas/Liquid Pulsed Discharge Plasma in a Slug Flow Reactor. ACS Omega 2021, 6, 20966–20974. [Google Scholar] [CrossRef]
- Mohanty, S.; Mishra, S.; Jena, P.; Jacob, B.; Sarkar, B.; Sonawane, A. An investigation on the antibacterial, cytotoxic, and antibiofilm efficacy of starch-stabilized silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 916–924. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V. Design, fabrication and drug release potential of dual stimuli-responsive composite hydrogel nanoparticle interfaces. Colloids Surf. B Biointerfaces 2021, 204, 111819. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V.; Barrera, E.V. Covalently Functionalized Carbon Nano-Onions Integrated Gelatin Methacryloyl Nanocomposite Hydrogel Containing γ-Cyclodextrin as Drug Carrier for High-Performance pH-Triggered Drug Release. Pharmaceuticals 2021, 14, 291. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V.; González-Ortiz, A. Engineering of carbon nano-onion bioconjugates for biomedical applications. Mater. Sci. Eng. C 2021, 120, 111698. [Google Scholar] [CrossRef]
- Cui, H.; Yuan, L.; Li, W.; Lin, L. Antioxidant property of SiO2-eugenol liposome loaded nanofibrous membranes on beef. Food Packag. Shelf Life 2017, 11, 49–57. [Google Scholar] [CrossRef]
- Zou, H.; Lv, P.F.; Wang, X.; Wu, D.; Yu, D.G. Electrospun poly (2-aminothiazole)/cellulose acetate fiber membrane for removing Hg (II) from water. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Rehman, A.U.; Kan, K.; Li, L.; Shi, K. Synthesis, characterization, and ammonia gas sensing properties of Co 3 O 4@ CuO nanochains. J. Mater. Sci. 2017, 52, 3757–3770. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. Nanocomposite electrospun nanofiber membranes for environmental remediation. Materials 2014, 7, 1017–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamidi, N.; Delgadillo, R.M.V.; Castrejón, J.V. Unconventional and facile production of stimuli-responsive multifunctional system for simultaneous drug delivery and environmental remediation. Environ. Sci. Nano 2021, 8, 2081–2097. [Google Scholar] [CrossRef]
- Lim, C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef]
- Mamidi, N.; Zuníga, A.E.; Villela-Castrejón, J. Engineering and evaluation of forcespun functionalized carbon nano-onions reinforced poly (ε-caprolactone) composite nanofibers for pH-responsive drug release. Mater. Sci. Eng. C 2020, 112, 110928. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Chronakis, I.S. Micro-/nano-fibers by electrospinning technology: Processing, properties and applications. Micromanuf. Eng. Technol. 2010, 2010, 264–286. [Google Scholar]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Lim, T.-C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005; p. 396. [Google Scholar]
- GhavamiNejad, A.; Unnithan, A.R.; Sasikala, A.R.K.; Samarikhalaj, M.; Thomas, R.G.; Jeong, Y.Y.; Nasseri, S.; Murugesan, P.; Wu, D.; Park, C.H.; et al. Mussel-Inspired Electrospun Nanofibers Functionalized with Size-Controlled Silver Nanoparticles for Wound Dressing Application. ACS Appl. Mater. Interfaces 2015, 7, 12176–12183. [Google Scholar] [CrossRef] [PubMed]
- Rujitanaroj, P.-o.; Pimpha, N.; Supaphol, P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer 2008, 49, 4723–4732. [Google Scholar] [CrossRef]
- Hassiba, A.J.; El Zowalaty, M.E.; Webster, T.J.; Abdullah, A.M.; Nasrallah, G.K.; Khalil, K.A.; Luyt, A.S.; Elzatahry, A.A. Synthesis, characterization, and antimicrobial properties of novel double layer nanocomposite electrospun fibers for wound dressing applications. Int. J. Nanomed. 2017, 12, 2205–2213. [Google Scholar] [CrossRef] [Green Version]
- Selatile, M.K.; Ojijo, V.; Sadiku, R.; Ray, S.S. Development of bacterial-resistant electrospun polylactide membrane for air filtration application: Effects of reduction methods and their loadings. Polym. Degrad. Stab. 2020, 178, 109205. [Google Scholar] [CrossRef]
- Kowsalya, E.; Mosa, K.; Balashanmugam, P. Biocompatible silver nanoparticles/poly(vinyl alcohol) electrospun nanofibers for potential antimicrobial food packaging applications. Food Packag. Shelf Life 2019, 21, 100379. [Google Scholar]
- Zhang, P.; Shao, C.; Zhang, Z.; Zhang, M.; Mu, J.; Guo, Z.; Liu, Y. In situ assembly of well-dispersed Ag nanoparticles (AgNPs) on electrospun carbon nanofibers (CNFs) for catalytic reduction of 4-nitrophenol. Nanoscale 2011, 3, 3357–3363. [Google Scholar] [CrossRef] [PubMed]
- Ajitha, B.; Ahn, C.W.; Yadav, P.V.K.; Reddy, Y.A.K. Silver nanoparticle embedded polymethacrylic acid/polyvinylpyrrolidone nanofibers for catalytic application. J. Environ. Chem. Eng. 2021, 9, 106291. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Baji, A.; Tien, H.-W.; Yang, Y.-K.; Yang, S.-Y.; Wu, S.-Y.; Ma, C.-C.M.; Liu, H.-Y.; Mai, Y.-W.; Wang, N.-H. Self-assembly of silver–graphene hybrid on electrospun polyurethane nanofibers as flexible transparent conductive thin films. Carbon 2012, 50, 3473–3481. [Google Scholar] [CrossRef]
- Ahmadi, T.; Monshi, A.; Mortazavi, V.; Fathi, M.H.; Sharifi, S.; Kharaziha, M.; Khazdooz, L.; Zarei, A.; Taghian Dehaghani, M. Fabrication and characterization of polycaprolactone fumarate/gelatin-based nanocomposite incorporated with silicon and magnesium co-doped fluorapatite nanoparticles using electrospinning method. Mater. Sci. Eng. C 2020, 106, 110172. [Google Scholar] [CrossRef]
- Srivatsan, T.S.; Ibrahim, I.; Mohamed, F.; Lavernia, E.J. Processing techniques for particulate-reinforced metal aluminium matrix composites. J. Mater. Sci. 1991, 26, 5965–5978. [Google Scholar] [CrossRef]
- Sauter, C.; Emin, M.A.; Schuchmann, H.P.; Tavman, S. Influence of hydrostatic pressure and sound amplitude on the ultrasound induced dispersion and de-agglomeration of nanoparticles. Ultrason. Sonochem. 2008, 15, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Chan, Y.S.; Jeevanandam, J.; Pal, K.; Bechelany, M.; Elkodous, M.A.; El-Sayyad, G.S. Response Surface Methodology Optimization of Mono-dispersed MgO Nanoparticles Fabricated by Ultrasonic-Assisted Sol–Gel Method for Outstanding Antimicrobial and Antibiofilm Activities. J. Clust. Sci. 2020, 31, 367–389. [Google Scholar] [CrossRef]
- Sato, K.; Li, J.-G.; Kamiya, H.; Ishigaki, T. Ultrasonic Dispersion of TiO2 Nanoparticles in Aqueous Suspension. J. Am. Ceram. Soc. 2008, 91, 2481–2487. [Google Scholar] [CrossRef]
- Mandzy, N.; Grulke, E.; Druffel, T. Breakage of TiO2 agglomerates in electrostatically stabilized aqueous dispersions. Powder Technol. 2005, 160, 121–126. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Sonawane, S.H. Ultrasound assisted in situ emulsion polymerization for polymer nanocomposite: A review. Chem. Eng. Process. Process Intensif. 2014, 85, 86–107. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, C.; Wang, Q. Study on ultrasonic induced encapsulating emulsion polymerization in the presence of nanoparticles. J. Appl. Polym. Sci. 2001, 80, 1130–1139. [Google Scholar] [CrossRef]
- Sharma, C.; Desai, M.A.; Patel, S.R. Effect of surfactants and polymers on morphology and particle size of telmisartan in ultrasound-assisted anti-solvent crystallization. Chem. Pap. 2019, 73, 1685–1694. [Google Scholar] [CrossRef]
- Thorat, A.A.; Dalvi, S.V. Liquid antisolvent precipitation and stabilization of nanoparticles of poorly water soluble drugs in aqueous suspensions: Recent developments and future perspective. Chem. Eng. J. 2012, 181–182, 1–34. [Google Scholar] [CrossRef]
- Nguyen, V.S.; Rouxel, D.; Vincent, B. Dispersion of nanoparticles: From organic solvents to polymer solutions. Ultrason. Sonochem. 2014, 21, 149–153. [Google Scholar] [CrossRef]
- Tee, D.I.; Mariatti, M.; Azizan, A.; See, C.H.; Chong, K.F. Effect of silane-based coupling agent on the properties of silver nanoparticles filled epoxy composites. Compos. Sci. Technol. 2007, 67, 2584–2591. [Google Scholar] [CrossRef]
- Goyat, M.S.; Ray, S.; Ghosh, P.K. Innovative application of ultrasonic mixing to produce homogeneously mixed nanoparticulate-epoxy composite of improved physical properties. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1421–1431. [Google Scholar] [CrossRef]
- Bittmann, B.; Haupert, F.; Schlarb, A.K. Ultrasonic dispersion of inorganic nanoparticles in epoxy resin. Ultrason. Sonochem. 2009, 16, 622–628. [Google Scholar] [CrossRef]
- Goyat, M.S.; Ghosh, P.K. Impact of ultrasonic assisted triangular lattice like arranged dispersion of nanoparticles on physical and mechanical properties of epoxy-TiO2 nanocomposites. Ultrason. Sonochem. 2018, 42, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Singh, D.; Kumar, A.; Jain, A. An Analysis of Mechanical and Viscoelastic Behaviour of Nano-SiO2 Dispersed Epoxy Composites. Silicon 2020, 12, 2465–2477. [Google Scholar] [CrossRef]
- Kuo, S.-L.; Chen, Y.-C.; Ger, M.-D.; Hwu, W.-H. Nano-particles dispersion effect on Ni/Al2O3 composite coatings. Mater. Chem. Phys. 2004, 86, 5–10. [Google Scholar] [CrossRef]
- Ding, P.; Pacek, A. De-agglomeration of goethite nano-particles using ultrasonic comminution device. Powder Technol. 2008, 187, 1–10. [Google Scholar] [CrossRef]
- Nair, B. Final Report On the Safety Assessment of Polyvinylpyrrolidone (PVP). Int. J. Toxicol. 1998, 17, 95–130. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [Green Version]
- Tardajos, M.G.; Nash, M.; Rochev, Y.; Reinecke, H.; Elvira, C.; Gallardo, A. Homologous copolymerization route to functional and biocompatible polyvinylpyrrolidone. Macromol. Chem. Phys. 2012, 213, 529–538. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Scheckel, K.G.; Suidan, M.; Tolaymat, T. The impact of stabilization mechanism on the aggregation kinetics of silver nanoparticles. Sci. Total Environ. 2012, 429, 325–331. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, G.; Yao, A.; Xiao, Y.; Du, J.; Guo, Y.; Xiao, D.; Hu, Q.; Choi, M.M.F. A sensitive AgNPs/CuO nanofibers non-enzymatic glucose sensor based on electrospinning technology. Sens. Actuators B Chem. 2014, 195, 431–438. [Google Scholar] [CrossRef]
- Tian, L.; Wang, P.; Zhao, Z.; Ji, J. Antimicrobial Activity of Electrospun Poly(butylenes succinate) Fiber Mats Containing PVP-Capped Silver Nanoparticles. Appl. Biochem. Biotechnol. 2013, 171, 1890–1899. [Google Scholar] [CrossRef]
- Yang, J.; Wang, K.; Yu, D.-G.; Yang, Y.; Bligh, S.W.A.; Williams, G.R. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Mater. Sci. Eng. C 2020, 111, 110805. [Google Scholar] [CrossRef] [PubMed]
- Mendis, P.; de Silva, R.M.; de Silva, K.N.; Wijenayaka, L.A.; Jayawardana, K.; Yan, M. Nanosilver rainbow: A rapid and facile method to tune different colours of nanosilver through the controlled synthesis of stable spherical silver nanoparticles. RSC Adv. 2016, 6, 48792–48799. [Google Scholar] [CrossRef]
- Masui, T.; Hirai, H.; Imanaka, N.; Adachi, G.; Sakata, T.; Mori, H. Synthesis of cerium oxide nanoparticles by hydrothermal crystallization with citric acid. J. Mater. Sci. Lett. 2002, 21, 489–491. [Google Scholar] [CrossRef]
- Vasileva, P.; Donkova, B.; Karadjova, I.; Dushkin, C. Synthesis of starch-stabilized silver nanoparticles and their application as a surface plasmon resonance-based sensor of hydrogen peroxide. Colloids Surf. A Physicochem. Eng. Asp. 2011, 382, 203–210. [Google Scholar] [CrossRef]
- Wahyudiono; Mano, K.; Hayashi, Y.; Yamada, M.; Takahashi, S.; Takada, N.; Kanda, H.; Goto, M. Atmospheric-pressure pulsed discharge plasma in capillary slug flow system for dye decomposition. Chem. Eng. Process. Process Intensif. 2019, 135, 133–140. [Google Scholar] [CrossRef]
- Nakamura, T.; Magara, H.; Herbani, Y.; Sato, S. Fabrication of silver nanoparticles by highly intense laser irradiation of aqueous solution. Appl. Phys. A 2011, 104, 1021–1024. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef]
- Chairam, S.; Somsook, E. Facile, versatile and green synthesis of silver nanoparticles by mung bean starch and their catalytic activity in the reduction of 4-nitrophenol. Chiang Mai J. Sci. 2016, 43, 609–619. [Google Scholar]
- Jia, Y.; Huang, G.; Dong, F.; Liu, Q.; Nie, W. Preparation and characterization of electrospun poly(ε-caprolactone)/poly(vinyl pyrrolidone) nanofiber composites containing silver particles. Polym. Compos. 2016, 37, 2847–2854. [Google Scholar] [CrossRef]
- Subramanian, S.B.; Francis, A.P.; Devasena, T. Chitosan-starch nanocomposite particles as a drug carrier for the delivery of bis-desmethoxy curcumin analog. Carbohydr. Polym. 2014, 114, 170–178. [Google Scholar] [CrossRef]
- Raghavendra, G.M.; Jung, J.; Seo, J. Step-reduced synthesis of starch-silver nanoparticles. Int. J. Biol. Macromol. 2016, 86, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Sibiya, P.N.; Xaba, T.; Moloto, M.J. Green synthetic approach for starch capped silver nanoparticles and their antibacterial activity. Pure Appl. Chem. 2016, 88, 61–69. [Google Scholar] [CrossRef]
- Bryaskova, R.; Pencheva, D.; Nikolov, S.; Kantardjiev, T. Synthesis and comparative study on the antimicrobial activity of hybrid materials based on silver nanoparticles (AgNps) stabilized by polyvinylpyrrolidone (PVP). J. Chem. Biol. 2011, 4, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.L.; Pan, C.T.; Chen, Y.C.; Lin, L.W.; Wu, I.C.; Hung, K.H.; Lin, Y.R.; Huang, H.L.; Liu, C.F.; Mao, S.W.; et al. Synthesis and fabrication of silver nanowires embedded in PVP fibers by near-field electrospinning process. Opt. Mater. 2015, 39, 118–124. [Google Scholar] [CrossRef]
- Lan, J.; Yang, Y.; Li, X. Microstructure and microhardness of SiC nanoparticles reinforced magnesium composites fabricated by ultrasonic method. Mater. Sci. Eng. A 2004, 386, 284–290. [Google Scholar] [CrossRef]
- Suslick, K.S.; Didenko, Y.; Fang, M.M.; Hyeon, T.; Kolbeck, K.J.; McNamara, W.B., III; Mdleleni, M.M.; Wong, M. Acoustic cavitation and its chemical consequences. Philos. Trans. R. Soc. London Ser. A Math. Phys. Eng. Sci. 1999, 357, 335–353. [Google Scholar] [CrossRef]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef]
- Tian, Y.; Li, D.; Zhao, J.; Xu, X.; Jin, Z. Effect of high hydrostatic pressure (HHP) on slowly digestible properties of rice starches. Food Chem. 2014, 152, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Dai, Y.; Hou, H.; Li, X.; Dong, H.; Wang, W.; Zhang, H. Preparation of high quality starch acetate under grinding and its influence mechanism. Int. J. Biol. Macromol. 2018, 120, 2026–2034. [Google Scholar] [CrossRef]
- Grönroos, A.; Pirkonen, P.; Heikkinen, J.; Ihalainen, J.; Mursunen, H.; Sekki, H. Ultrasonic depolymerization of aqueous polyvinyl alcohol. Ultrason. Sonochem. 2001, 8, 259–264. [Google Scholar] [CrossRef]
- Behrend, O. Mechanisches Emulgieren mit Ultraschall; GCA-Verlag: Herdecke, Germany, 2002. [Google Scholar]
- Ma, L.; Chen, F.; Shu, G. Preparation of fine particulate reinforced metal matrix composites by high intensity ultrasonic treatment. J. Mater. Sci. Lett. 1995, 14, 649–650. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, J.; Li, X. Study on bulk aluminum matrix nano-composite fabricated by ultrasonic dispersion of nano-sized SiC particles in molten aluminum alloy. Mater. Sci. Eng. A 2004, 380, 378–383. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Zhu, W.; Hu, X.; Lin, Y.; Machmudah, S.; Wahyudiono; Kanda, H.; Goto, M. PVP/Highly Dispersed AgNPs Nanofibers Using Ultrasonic-Assisted Electrospinning. Polymers 2022, 14, 599. https://doi.org/10.3390/polym14030599
Zhu L, Zhu W, Hu X, Lin Y, Machmudah S, Wahyudiono, Kanda H, Goto M. PVP/Highly Dispersed AgNPs Nanofibers Using Ultrasonic-Assisted Electrospinning. Polymers. 2022; 14(3):599. https://doi.org/10.3390/polym14030599
Chicago/Turabian StyleZhu, Li, Wanying Zhu, Xin Hu, Yingying Lin, Siti Machmudah, Wahyudiono, Hideki Kanda, and Motonobu Goto. 2022. "PVP/Highly Dispersed AgNPs Nanofibers Using Ultrasonic-Assisted Electrospinning" Polymers 14, no. 3: 599. https://doi.org/10.3390/polym14030599
APA StyleZhu, L., Zhu, W., Hu, X., Lin, Y., Machmudah, S., Wahyudiono, Kanda, H., & Goto, M. (2022). PVP/Highly Dispersed AgNPs Nanofibers Using Ultrasonic-Assisted Electrospinning. Polymers, 14(3), 599. https://doi.org/10.3390/polym14030599







