Influence of Biomimetically Mineralized Collagen Scaffolds on Bone Cell Proliferation and Immune Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Mineralization of the Scaffolds
2.1.1. Preparation of Collagen Scaffolds
2.1.2. Biomimetic Mineralization of Collagen Scaffolds with SBF
2.2. Characterization of the Scaffolds
2.2.1. Morphology of the Scaffolds
2.2.2. Evaluation of Texture Stability under a Mechanical Load
2.2.3. Elemental Composition of the Scaffolds
2.2.4. Structural Changes in the Scaffolds after Mineralization
2.3. Cell Model and Culture Conditions
2.4. Cell Adhesion, Growth and Viability
2.4.1. Visualization of Cells within the Scaffolds
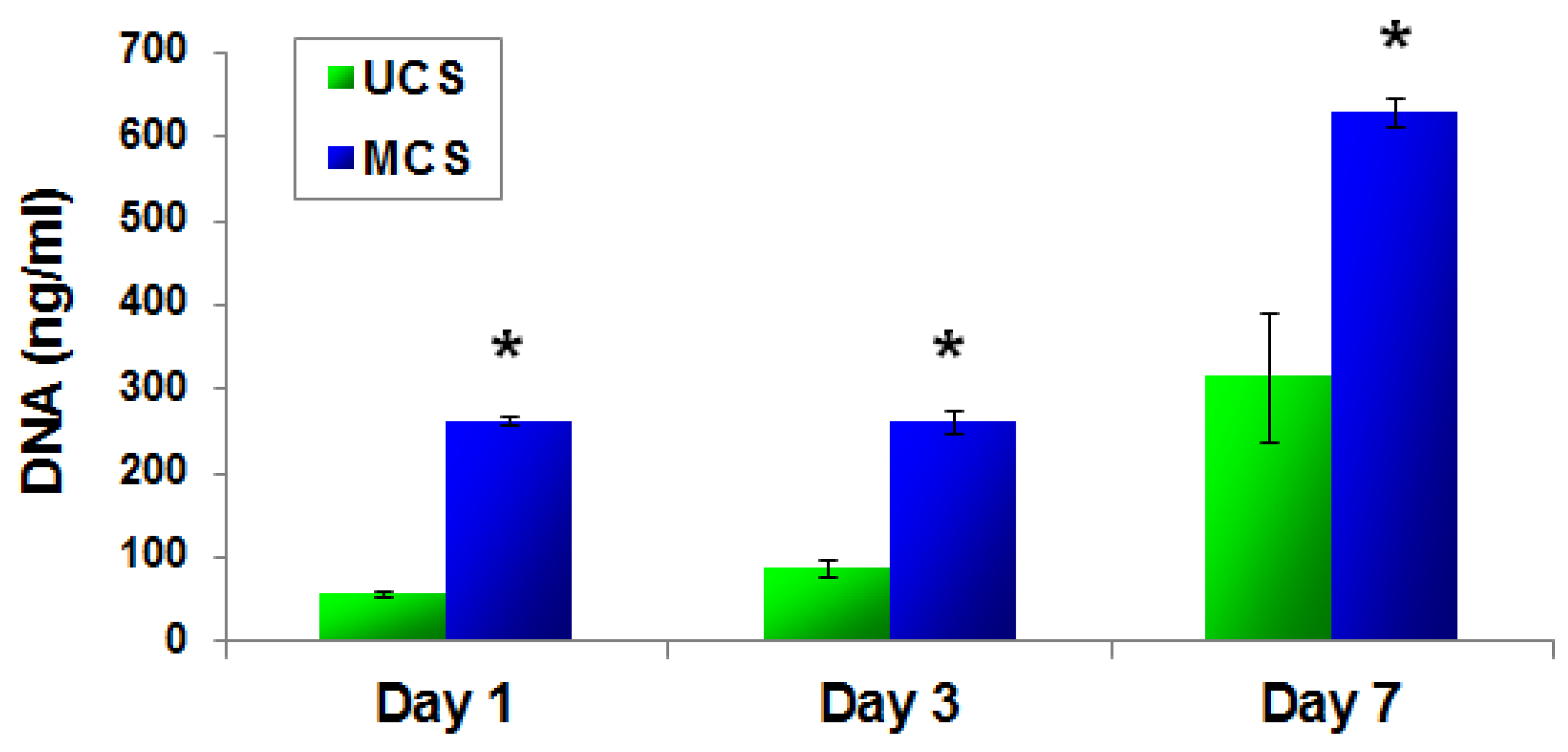
2.4.2. PicoGreen dsDNA Assay
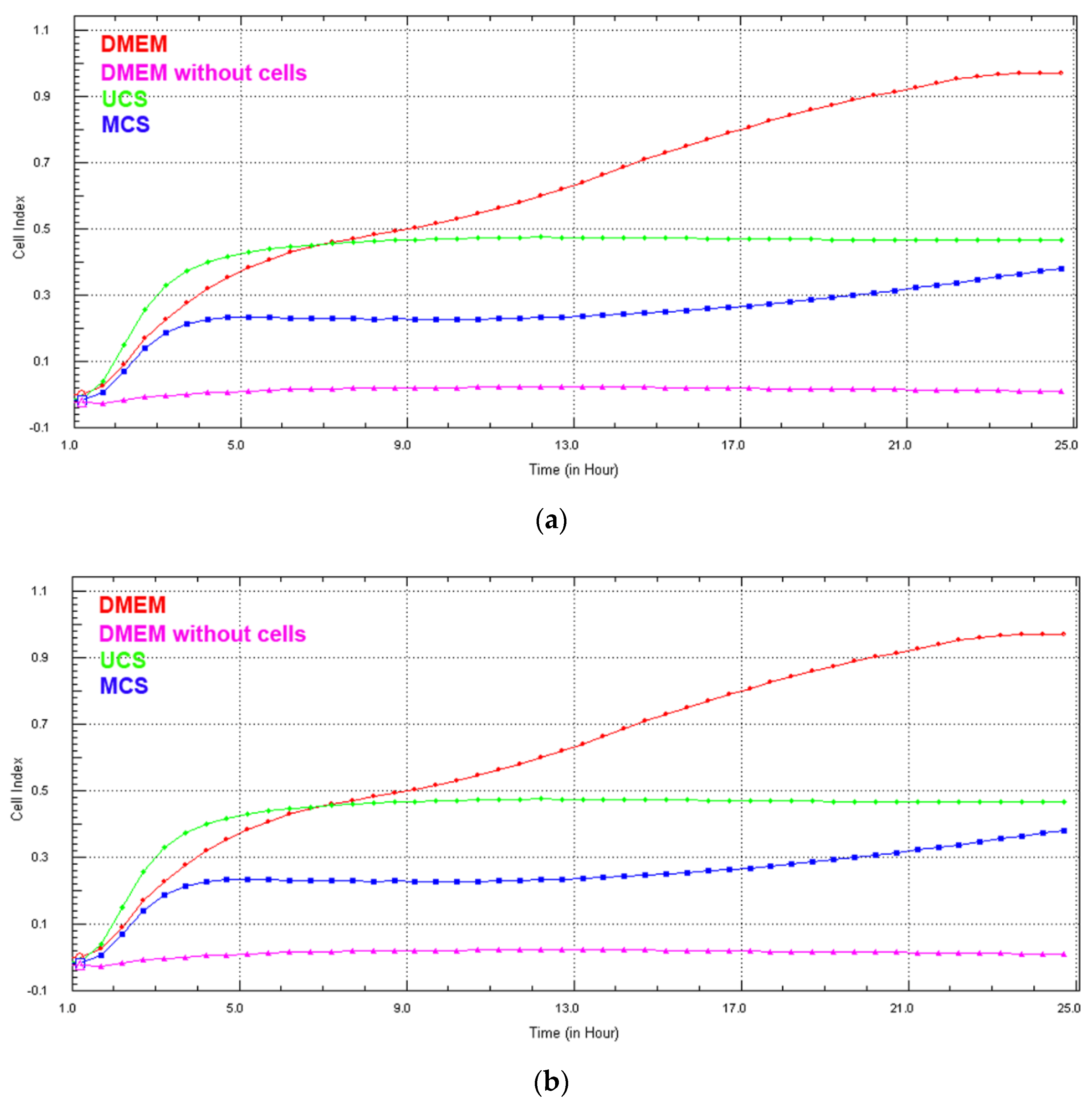
2.4.3. Real-Time Monitoring of Cell Growth in Extracts from the Scaffolds
2.5. Molecular Markers of Cell Adhesion, Spreading and Osteogenic Differentiation
2.6. Potential Immune Activation of Cells on the Scaffolds
2.7. Statistics
3. Results
3.1. Morphology of the Scaffolds
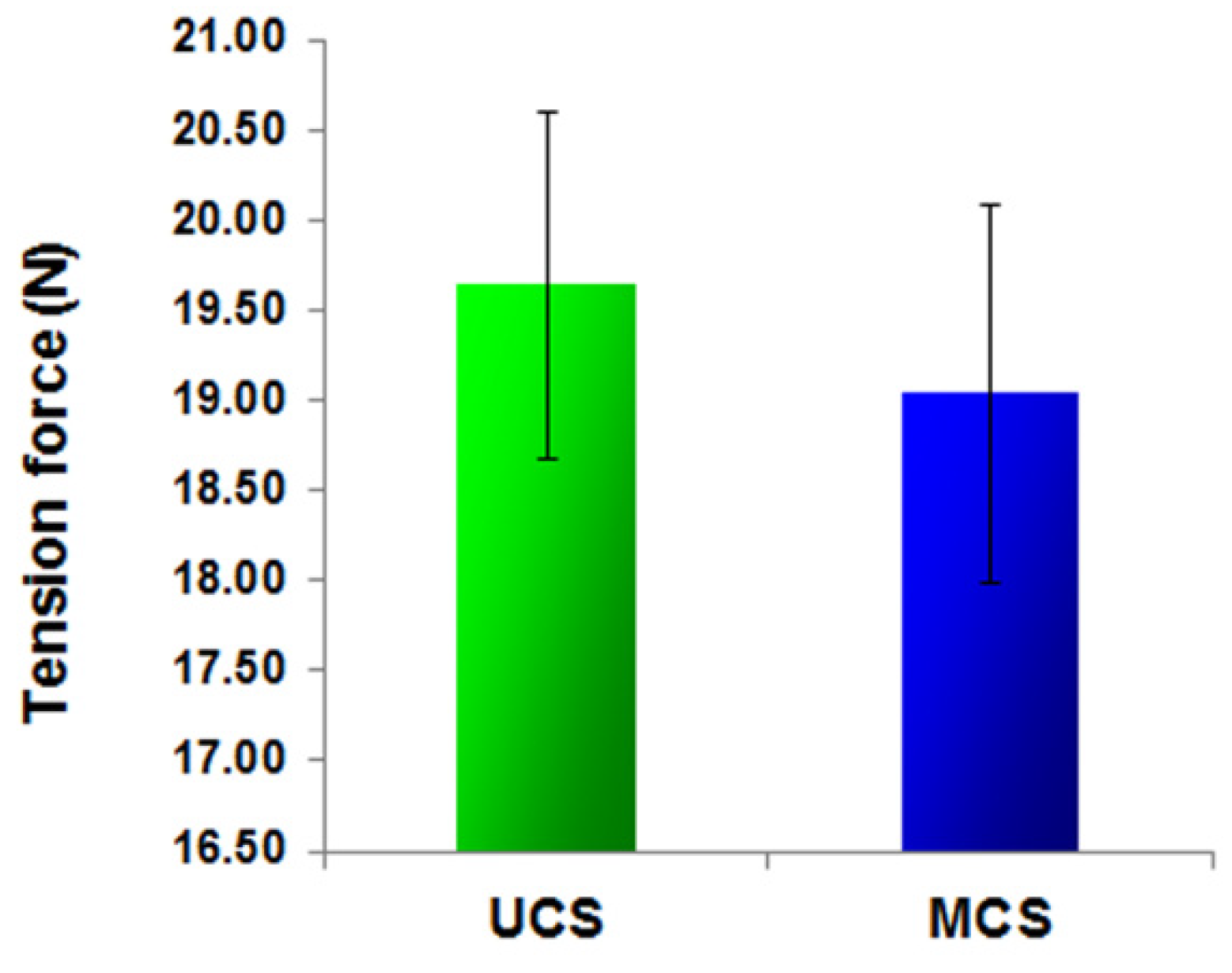
3.2. Mechanical Stability of the Scaffolds
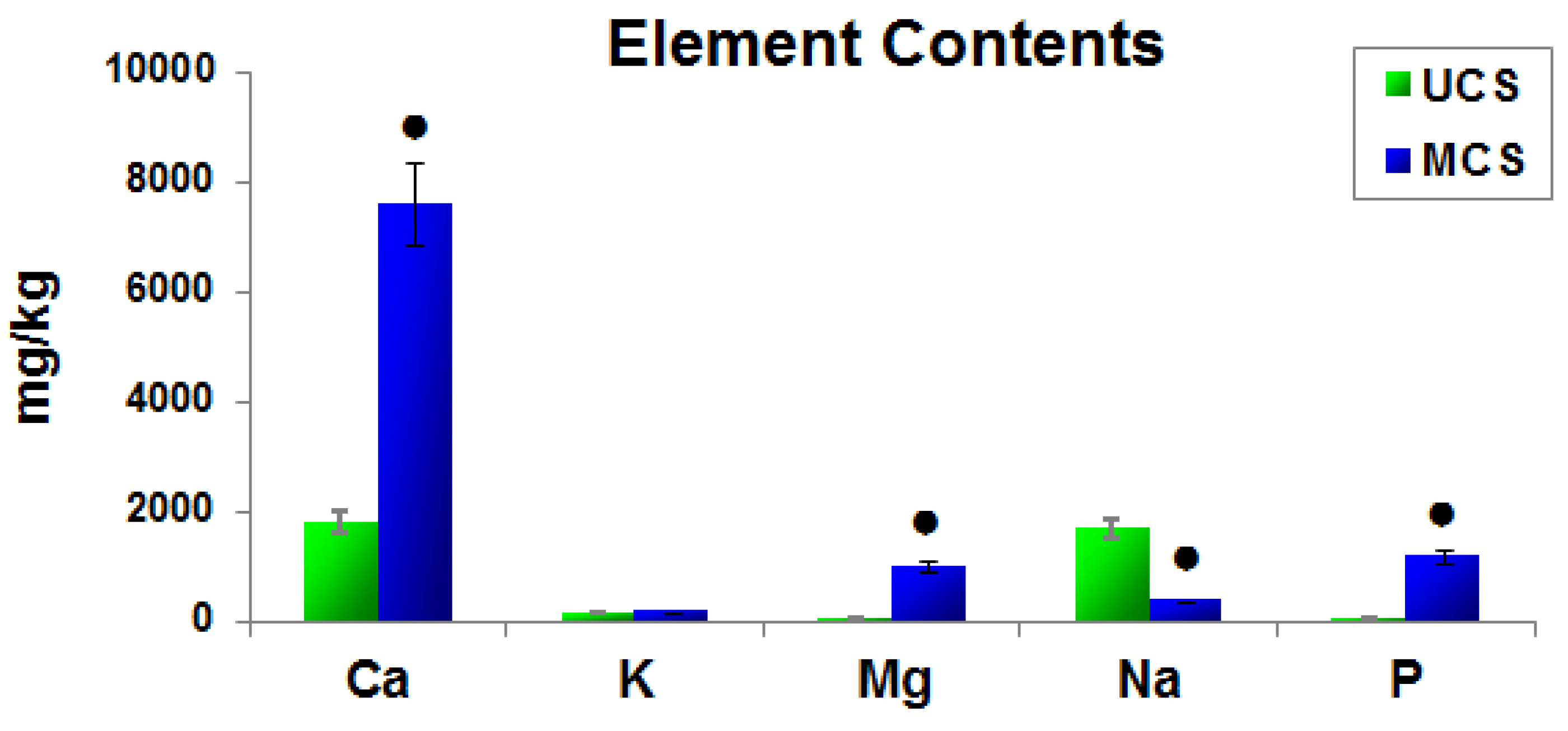
3.3. Elemental Composition of the Scaffolds
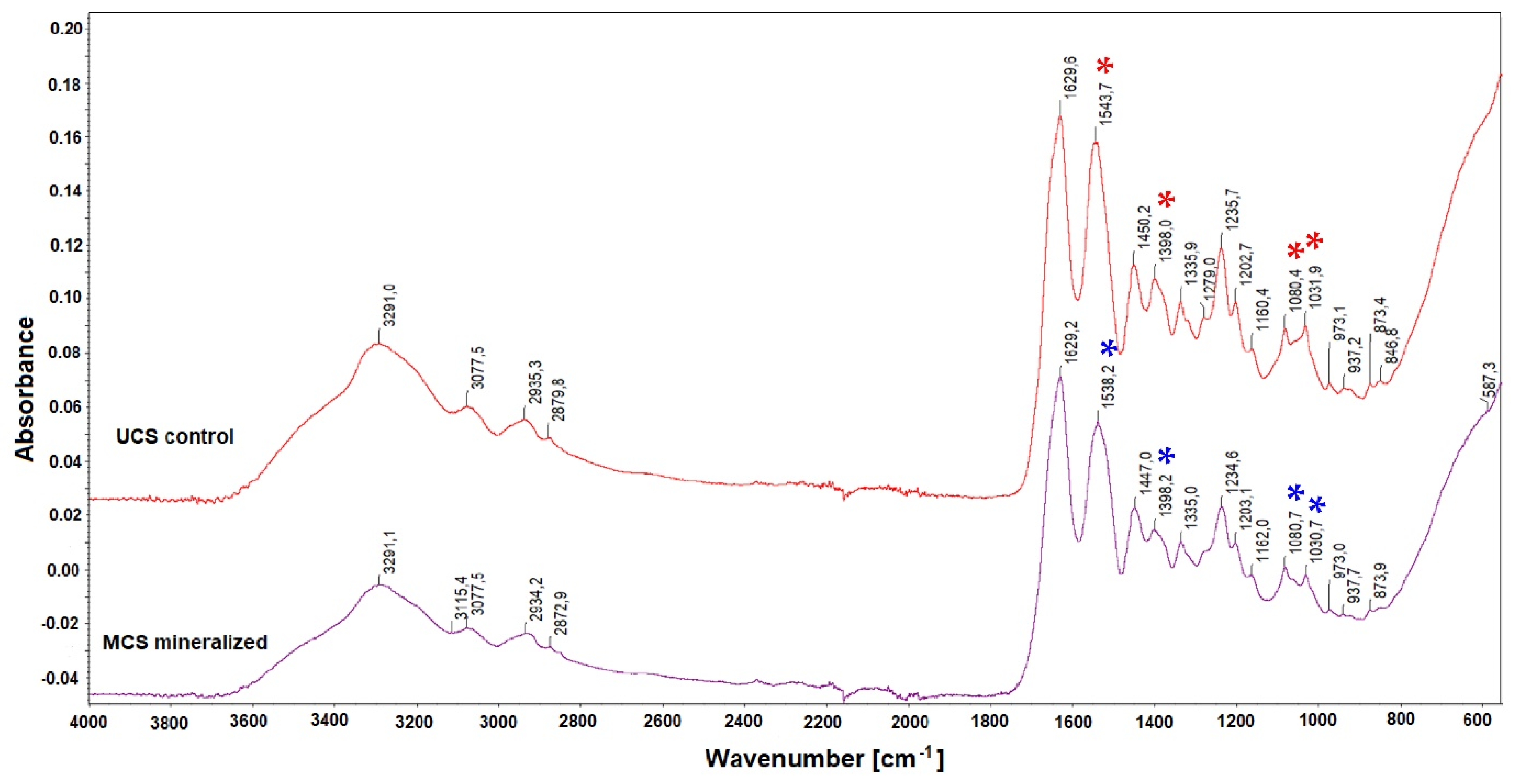
3.4. Structural Changes in the Scaffolds after Mineralization
3.5. Adhesion, Growth and Maturation of Cells on the Scaffolds
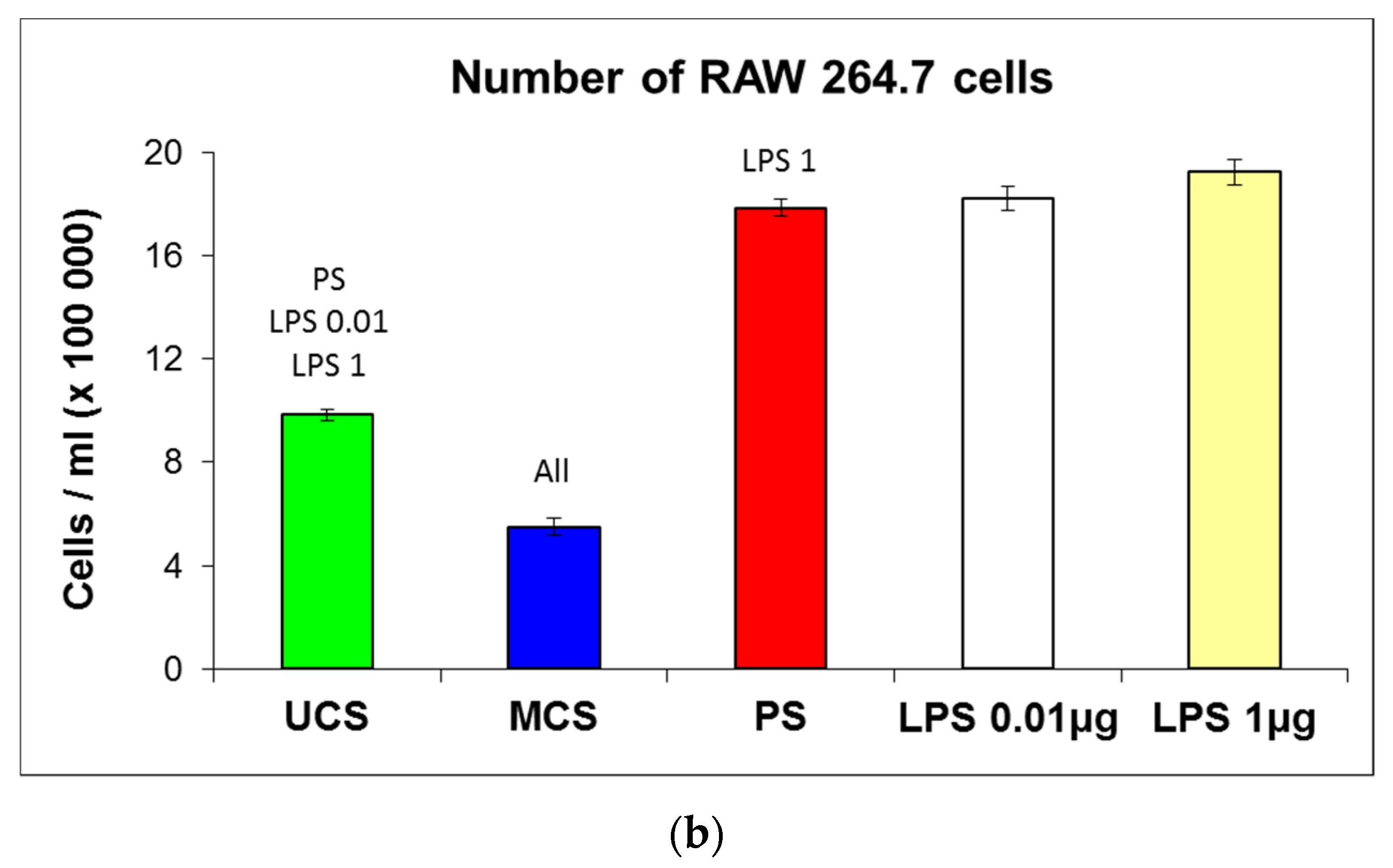
3.6. Cell Growth in Extracts from the Scaffolds
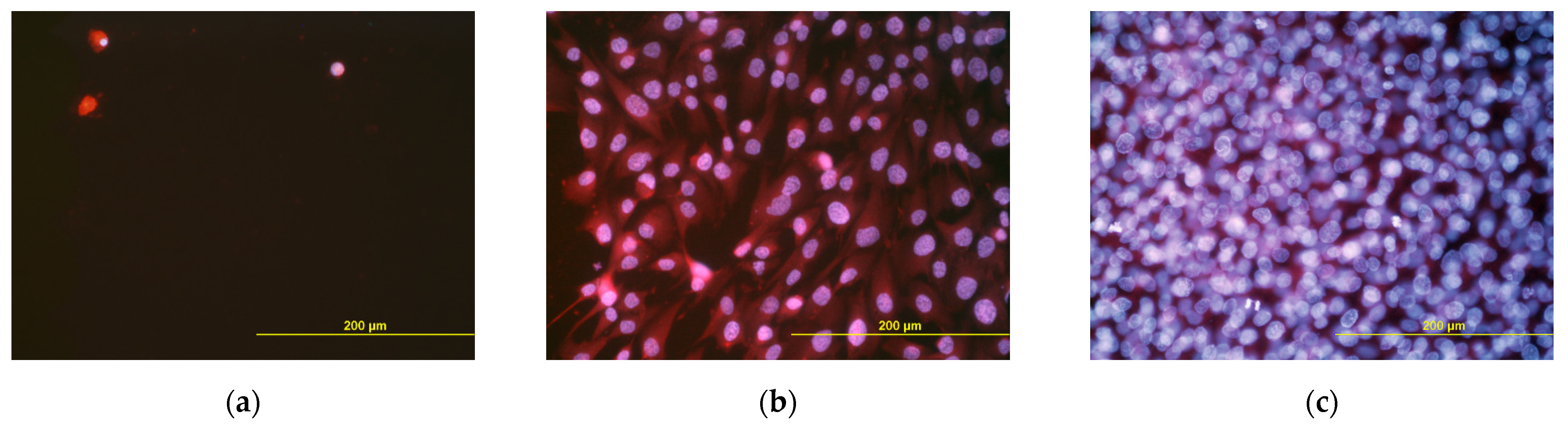
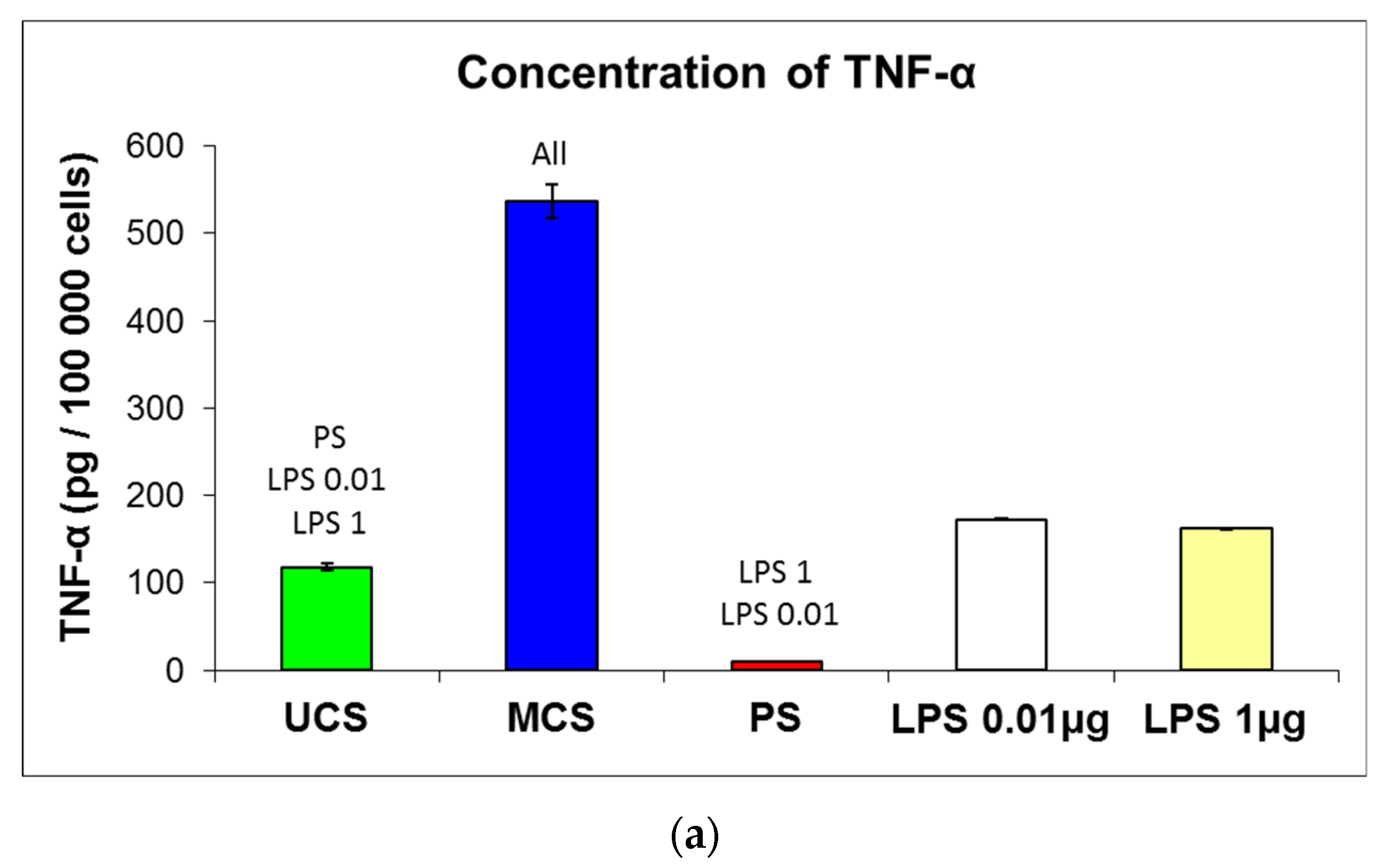
3.7. Potential Immune Activation of Cells on the Scaffolds
4. Discussion
4.1. Properties of the Scaffolds and Their Colonization with Cells
4.2. Positive Effect of Scaffold Mineralization on Cell Colonization
4.3. Extrafibrillar versus Intrafibrillar Mineralization of Collagen
4.4. Immunological Acceptance of the Scaffolds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mallis, P.; Kostakis, A.; Stavropoulos-Giokas; Michalopoulos, E. Future perspectives in small-diameter vascular graft engineering. Bioengineering 2020, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Naung, N.; Shehata, E.; Van Sickels, J.E. Resorbable versus nonresorbable membranes: When and why? Dent. Clin. N. Am. 2019, 63, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Imoto, K.; Yamauchi, K.; Odashima, K.; Nogami, S.; Shimizu, Y.; Kessler, P.; Lethaus, B.; Unuma, H.; Takahashi, T. Periosteal expansion osteogenesis using an innovative, shape-memory polyethylene terephthalate membrane: An experimental study in rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1327–1333. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Bačáková, L.; Pajorová, J.; Zikmundová, M.; Filová, E.; Mikeš, P.; Jenčová, V.; Kuželová Košťáková, E.; Sinica, A. Nanofibrous scaffolds for skin tissue engineering and wound healing based on nature-derived polymers. In Current and Future Aspects of Nanomedicine; Khalil, I., Ed.; IntechOpen: London, UK, 2020; pp. 1–30. ISBN 9781789858709. [Google Scholar] [CrossRef] [Green Version]
- Frosch, K.H.; Barvencik, F.; Lohmann, C.H.; Viereck, V.; Siggelkow, H.; Breme, J.; Dresing, K.; Stürmer, K.M. Migration, matrix production and lamellar bone formation of human osteoblast-like cells in porous titanium implants. Cells Tissues Organs 2002, 170, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Yu, X.; Jiang, X.; Brody, H.D.; Rowe, D.W.; Wei, M. Fabrication and characterization of biomimetic collagen-apatite scaffolds with tunable structures for bone tissue engineering. Acta Biomater. 2013, 9, 7308–7319. [Google Scholar] [CrossRef] [Green Version]
- Maher, M.; Castilho, M.; Yue, Z.; Glattauer, V.; Hughes, T.C.; Ramshaw, J.A.M.; Wallace, G.G. Shaping collagen for engineering hard tissues: Towards a printomics approach. Acta Biomater. 2021, 131, 41–61. [Google Scholar] [CrossRef]
- Yu, L.; Wei, M. Biomineralization of collagen-based materials for hard tissue repair. Int. J. Mol. Sci. 2021, 22, 944. [Google Scholar] [CrossRef]
- Knight, C.G.; Morton, L.F.; Peachey, A.R.; Tuckwell, D.S.; Farndale, R.W.; Barnes, M.J. The collagen-binding A-domains of integrins α1β1 and α2β1 recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J. Biol. Chem. 2000, 275, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, M.; Yamato, M.; Aoyagi, M.; Yamamoto, K. Identification of integrins involved in cell adhesion to native and denatured type I collagens and the phenotypic transition of rabbit arterial smooth muscle cells. Exp. Cell Res. 1995, 219, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Isaksson, P.; Ferguson, S.J.; Persson, C. Young’s modulus of trabecular bone at the tissue level: A review. Acta Biomater. 2018, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; van der Werf, K.O.; Fitié, C.F.; Bennink, M.L.; Dijkstra, P.J.; Feijen, J. Mechanical properties of native and cross-linked type I collagen fibrils. Biophys. J. 2008, 94, 2204–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenger, M.P.; Bozec, L.; Horton, M.A.; Mesquida, P. Mechanical properties of collagen fibrils. Biophys. J. 2007, 93, 1255–1263. [Google Scholar] [CrossRef] [Green Version]
- Varley, M.C.; Neelakantan, S.; Clyne, T.W.; Dean, J.; Brooks, R.A.; Markaki, A.E. Cell structure, stiffness and permeability of freeze-dried collagen scaffolds in dry and hydrated states. Acta Biomater. 2016, 33, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; He, Z.; Han, F.; Zhong, Z.; Chen, L.; Li, B. Preparation of collagen/hydroxyapatite/alendronate hybrid hydrogels as potential scaffolds for bone regeneration. Colloids Surf. B Biointerfaces 2016, 143, 81–87. [Google Scholar] [CrossRef]
- Schuster, L.; Ardjomandi, N.; Munz, M.; Umrath, F.; Klein, C.; Rupp, F.; Reinert, S.; Alexander, D. Establishment of collagen: Hydroxyapatite/BMP-2 mimetic peptide composites. Materials 2020, 13, 1203. [Google Scholar] [CrossRef] [Green Version]
- Piard, C.; Luthcke, R.; Kamalitdinov, T.; Fisher, J. Sustained delivery of vascular endothelial growth factor from mesoporous calcium-deficient hydroxyapatite microparticles promotes in vitro angiogenesis and osteogenesis. J. Biomed. Mater. Res. A 2021, 109, 1080–1087. [Google Scholar] [CrossRef]
- Sen, K.S.; Duarte Campos, D.F.; Köpf, M.; Blaeser, A.; Fischer, H. The Effect of addition of calcium phosphate particles to hydrogel-based composite materials on stiffness and differentiation of mesenchymal stromal cells toward osteogenesis. Adv. Health Mater. 2018, 7, e1800343. [Google Scholar] [CrossRef]
- Ferreira, S.A.; Young, G.; Jones, J.R.; Rankin, S. Bioglass/carbonate apatite/collagen composite scaffold dissolution products promote human osteoblast differentiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111393. [Google Scholar] [CrossRef] [PubMed]
- Al-Munajjed, A.A.; Plunkett, N.A.; Gleeson, J.P.; Weber, T.; Jungreuthmayer, C.; Levingstone, T.; Hammer, J.; O’Brien, F.J. Development of a biomimetic collagen-hydroxyapatite scaffold for bone tissue engineering using a SBF immersion technique. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 584–591. [Google Scholar] [CrossRef]
- Quan, B.D.; Wojtas, M.; Sone, E.D. Polyaminoacids in biomimetic collagen mineralization: Roles of isomerization and disorder in polyaspartic and polyglutamic acids. Biomacromolecules 2021, 22, 2996–3004. [Google Scholar] [CrossRef] [PubMed]
- Gkioni, K.; Leeuwenburgh, S.C.G.; Douglas, T.E.L.; Mikos, A.G.; Jansen, J.A. Mineralization of hydrogels for bone regeneration. Tissue Eng. Part B Rev. 2010, 16, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Coyac, B.R.; Chicatun, F.; Hoac, B.; Nelea, V.; Chaussain, C.; Nazhat, S.N.; McKee, M.D. Mineralization of dense collagen hydrogel scaffolds by human pulp cells. J. Dent. Res. 2013, 92, 648–654. [Google Scholar] [CrossRef]
- Liu, G.; Pastakia, M.; Fenn, M.B.; Kishore, V. Saos-2 cell-mediated mineralization on collagen gels: Effect of densification and bioglass incorporation. J. Biomed. Mater. Res. A 2016, 104, 1121–1134. [Google Scholar] [CrossRef]
- Liskova, J.; Douglas, T.E.L.; Wijnants, R.; Samal, S.K.; Mendes, A.C.; Chronakis, I.; Bacakova, L.; Skirtach, A.G. Phytase-mediated enzymatic mineralization of chitosan-enriched hydrogels. Mater. Lett. 2018, 214, 186–189. [Google Scholar] [CrossRef] [Green Version]
- Antebi, B.; Cheng, X.; Harris, J.N.; Gower, L.B.; Chen, X.D.; Ling, J. Biomimetic collagen-hydroxyapatite composite fabricated via a novel perfusion-flow mineralization technique. Tissue Eng. Part C Methods 2013, 19, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Van Manh, N.; Wang, H.; Zhong, X.; Zhang, X.; Li, C. Synergistic intrafibrillar/extrafibrillar mineralization of collagen scaffolds based on a biomimetic strategy to promote the regeneration of bone defects. Int. J. Nanomed. 2016, 11, 2053–2067. [Google Scholar] [CrossRef] [Green Version]
- Diogo, G.S.; Marques, C.F.; Sotelo, C.G.; Pérez-Martín, R.I.; Pirraco, R.P.; Reis, R.L.; Silva, T.H. Cell-laden biomimetically mineralized shark-skin-collagen-based 3D printed hydrogels for the engineering of hard tissues. ACS Biomater. Sci. Eng. 2020, 6, 3664–3672. [Google Scholar] [CrossRef]
- Acri, T.M.; Laird, N.Z.; Jaidev, L.R.; Meyerholz, D.K.; Salem, A.K.; Shin, K. Nonviral gene delivery embedded in biomimetically mineralized matrices for bone tissue engineering. Tissue Eng. Part A 2021, 27, 1074–1083. [Google Scholar] [CrossRef]
- Chen, L.; Wu, C.; Wei, D.; Chen, S.; Xiao, Z.; Zhu, H.; Luo, H.; Sun, J.; Fan, H. Biomimetic mineralized microenvironment stiffness regulated BMSCs osteogenic differentiation through cytoskeleton mediated mechanical signaling transduction. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111613. [Google Scholar] [CrossRef] [PubMed]
- Alijotas-Reig, J.; Fernández-Figueras, M.T.; Puig, L. Inflammatory, immune-mediated adverse reactions related to soft tissue dermal fillers. Semin. Arthritis Rheum. 2013, 43, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Rücker, M.; Laschke, M.W.; Junker, D.; Carvalho, C.; Schramm, A.; Mülhaupt, R.; Gellrich, N.C.; Menger, M.D. Angiogenic and inflammatory response to biodegradable scaffolds in dorsal skinfold chambers of mice. Biomaterials 2006, 27, 5027–5038. [Google Scholar] [CrossRef] [PubMed]
- Panda, N.N.; Jonnalagadda, S.; Pramanik, K. Development and evaluation of cross-linked collagen-hydroxyapatite scaffolds for tissue engineering. J. Biomater. Sci. Polym. Ed. 2013, 24, 2031–2044. [Google Scholar] [CrossRef]
- Nishimoto, S.; Goto, Y.; Morishige, H.; Shiraishi, R.; Doi, M.; Akiyama, K.; Yamauchi, S.; Sugahara, T. Mode of action of the immunostimulatory effect of collagen from jellyfish. Biosci. Biotechnol. Biochem. 2008, 72, 2806–2814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, L.; Novakova, M.; Lukac, P.; Cechova, D.; Sukenikova, L.; Hrdy, J.; Mlcek, M.; Chlup, H.; Suchy, T.; Grus, T. Evaluation of the immunogenicity of a vascular graft covered with collagen derived from the European carp (Cyprinus carpio) and bovine collagen. Biomed. Res. Int. 2019, 2019, 5301405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Liu, S.; Fu, Y.; Kou, X.X.; He, D.Q.; Wang, G.N.; Fu, C.C.; Liu, Y.; Zhou, Y.H. Mineralized collagen regulates macrophage polarization during bone regeneration. J. Biomed. Nanotechnol. 2016, 12, 2029–2040. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.D.; Chen, L.W.; Li, S.W.; Sun, X.D.; Cui, F.Z.; Ma, H.M. The observed difference of RAW264.7 macrophage phenotype on mineralized collagen and hydroxyapatite. Biomed. Mater. 2018, 13, 041001. [Google Scholar] [CrossRef]
- Oyane, A.; Kim, H.M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. A 2003, 65, 188–195. [Google Scholar] [CrossRef]
- Clover, J.; Gowen, M. Are MG-63 and HOS TE85 human osteosarcoma cell lines representative models of the osteoblastic phenotype? Bone 1994, 15, 585–591. [Google Scholar] [CrossRef]
- Hoberg, M.; Gratz, H.H.; Noll, M.; Jones, D.B. Mechanosensitivity of human osteosarcoma cells and phospholipase C β2 expression. Biochem. Biophys. Res. Commun. 2005, 333, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Parizek, M.; Douglas, T.E.L.; Novotna, K.; Kromka, A.; Brady, M.A.; Renzing, A.; Voss, E.; Jarosova, M.; Palatinus, L.; Tesarek, P.; et al. Nanofibrous poly(lactide-co-glycolide) membranes loaded with diamond nanoparticles as promising substrates for bone tissue engineering. Int. J. Nanomed. 2012, 7, 1931–1951. [Google Scholar] [CrossRef] [Green Version]
- Novotna, K.; Zajdlova, M.; Suchy, T.; Hadraba, D.; Lopot, F.; Zaloudkova, M.; Douglas, T.E.; Munzarova, M.; Juklickova, M.; Stranska, D.; et al. Polylactide nanofibers with hydroxyapatite as growth substrates for osteoblast-like cells. J. Biomed. Mater. Res. A 2014, 102, 3918–3930. [Google Scholar] [CrossRef]
- Suchý, T.; Bartoš, M.; Sedláček, R.; Šupová, M.; Žaloudková, M.; Martynková, G.S.; Foltán, R. Various simulated body fluids lead to significant differences in collagen tissue engineering scaffolds. Materials 2021, 14, 4388. [Google Scholar] [CrossRef]
- Jiang, W.; Griffanti, G.; Tamimi, F.; McKee, M.D.; Nazhat, S.N. Multiscale structural evolution of citrate-triggered intrafibrillar and interfibrillar mineralization in dense collagen gels. J. Struct. Biol. 2020, 212, 107592. [Google Scholar] [CrossRef]
- Song, Q.; Jiao, K.; Tonggu, L.; Wang, L.G.; Zhang, S.L.; Yang, Y.D.; Zhang, L.; Bian, J.H.; Hao, D.X.; Wang, C.Y.; et al. Contribution of biomimetic collagen-ligand interaction to intrafibrillar mineralization. Sci. Adv. 2019, 5, eaav9075. [Google Scholar] [CrossRef] [Green Version]
- Nesseri, E.; Boyatzis, S.C.; Boukos, N.; Panagiaris, G. Optimizing the biomimetic synthesis of hydroxyapatite for the consolidation of bone using diammonium phosphate, simulated body fluid, and gelatin. SN Appl. Sci. 2020, 2, 1892. [Google Scholar] [CrossRef]
- Vallecillo, C.; Toledano-Osorio, M.; Vallecillo-Rivas, M.; Toledano, M.; Osorio, R. In vitro biodegradation pattern of collagen matrices for soft tissue augmentation. Polymers 2021, 13, 2633. [Google Scholar] [CrossRef]
- Doktor, T.; Valach, J.; Kytyr, D.; Jirousek, O. Pore size distribution of human trabecular bone—Comparison of intrusion measurements with image analysis. In Proceedings of the 17th International Conference Engineering Mechanics, Svratka, Czech Republic, 9–12 May 2011. [Google Scholar]
- Pamula, E.; Filova, E.; Bacakova, L.; Lisa, V.; Adamczyk, D. Resorbable polymeric scaffolds for bone tissue engineering: The influence of their microstructure on the growth of human osteoblast-like MG 63 cells. J. Biomed. Mater. Res. A 2009, 89, 432–443. [Google Scholar] [CrossRef]
- Stankova, L.; Fraczek-Szczypta, A.; Blazewicz, M.; Filova, E.; Blazewicz, S.; Lisa, V.; Bacakova, L. Human osteoblast-like MG 63 cells on polysulfone modified with carbon nanotubes or carbon nanohorns. Carbon 2014, 67, 578–591. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopal, S.; Multhaupt, H.A.B.; Couchman, J.R. Calcium in Cell-Extracellular Matrix Interactions. Adv. Exp. Med. Biol. 2020, 1131, 1079–1102. [Google Scholar] [CrossRef]
- Lee, M.N.; Hwang, H.S.; Oh, S.H.; Roshanzadeh, A.; Kim, J.W.; Song, J.H.; Kim, E.S.; Koh, J.T. Elevated extracellular calcium ions promote proliferation and migration of mesenchymal stem cells via increasing osteopontin expression. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef]
- Resende, R.R.; Andrade, L.M.; Oliveira, A.G.; Guimarães, E.S.; Guatimosim, S.; Leite, M.F. Nucleoplasmic calcium signaling and cell proliferation: Calcium signaling in the nucleus. Cell Commun. Signal. 2013, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various aspects of calcium signaling in the regulation of apoptosis, autophagy, cell proliferation, and cancer. Int. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef] [PubMed]
- Obata, A.; Ogasawara, T.; Kasuga, T. Combinatorial effects of inorganic ions on adhesion and proliferation of osteoblast-like cells. J. Biomed. Mater. Res. A 2019, 107, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Camalier, C.E.; Yi, M.; Yu, L.R.; Hood, B.L.; Conrads, K.A.; Lee, Y.J.; Lin, Y.; Garneys, L.M.; Bouloux, G.F.; Young, M.R.; et al. An integrated understanding of the physiological response to elevated extracellular phosphate. J. Cell Physiol. 2013, 228, 1536–1550. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Xu, C.; Yu, S.; Wu, X.; Jie, Z.; Dai, H. Citric acid enhances the physical properties, cytocompatibility and osteogenesis of magnesium calcium phosphate cement. J. Mech. Behav. Biomed. Mater. 2019, 94, 42–50. [Google Scholar] [CrossRef]
- Song, W.; Wang, Q.; Wan, C.; Shi, T.; Markel, D.; Blaiser, R.; Ren, W. A novel alkali metals/strontium co-substituted calcium polyphosphate scaffolds in bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Sang Cho, J.; Um, S.H.; Su Yoo, D.; Chung, Y.C.; Hye Chung, S.; Lee, J.C.; Rhee, S.H. Enhanced osteoconductivity of sodium-substituted hydroxyapatite by system instability. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1046–1062. [Google Scholar] [CrossRef]
- Klimek, K.; Belcarz, A.; Pazik, R.; Sobierajska, P.; Han, T.; Wiglusz, R.J.; Ginalska, G. “False” cytotoxicity of ions-adsorbing hydroxyapatite—Corrected method of cytotoxicity evaluation for ceramics of high specific surface area. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 65, 70–79. [Google Scholar] [CrossRef]
- Mestres, G.; Le Van, C.; Ginebra, M.P. Silicon-stabilized α-tricalcium phosphate and its use in a calcium phosphate cement: Characterization and cell response. Acta Biomater. 2012, 8, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Isom, L.L. The role of sodium channels in cell adhesion. Front. Biosci. 2002, 7, 12–23. [Google Scholar] [CrossRef]
- Brackenbury, W.J.; Djamgoz, M.B.; Isom, L.L. An emerging role for voltage-gated Na+ channels in cellular migration: Regulation of central nervous system development and potentiation of invasive cancers. Neuroscientist 2008, 14, 571–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffanti, G.; Jiang, W.; Nazhat, S.N. Bioinspired mineralization of a functionalized injectable dense collagen hydrogel through silk sericin incorporation. Biomater. Sci. 2019, 7, 1064–1077. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology, 5th ed.; The Immune System in Health and Disease; Garland Science: New York, NY, USA, 2001; ISBN 081533642X. [Google Scholar]
- Velard, F.; Braux, J.; Amedee, J.; Laquerriere, P. Inflammatory cell response to calcium phosphate biomaterial particles: An overview. Acta Biomater. 2013, 9, 4956–4963. [Google Scholar] [CrossRef]
- Wang, M.; Chen, F.; Wang, J.; Chen, X.; Liang, J.; Yang, X.; Zhu, X.; Fan, Y.; Zhang, X. Calcium phosphate altered the cytokine secretion of macrophages and influenced the homing of mesenchymal stem cells. J. Mater. Chem. B 2018, 6, 4765–4774. [Google Scholar] [CrossRef]
- Matesanz, M.C.; Feito, M.J.; Oñaderra, M.; Ramírez-Santillán, C.; da Casa, C.; Arcos, D.; Vallet-Regí, M.; Rojo, J.M.; Portolés, M.T. Early in vitro response of macrophages and T lymphocytes to nanocrystalline hydroxyapatites. J. Colloid Interface Sci. 2014, 416, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Curran, J.M.; Gallagher, J.A.; Hunt, J.A. The inflammatory potential of biphasic calcium phosphate granules in osteoblast/macrophage co-culture. Biomaterials 2005, 26, 5313–5320. [Google Scholar] [CrossRef] [PubMed]
- Branch, D.R.; Guilbert, L.J. Autocrine regulation of macrophage proliferation by tumor necrosis factor-alpha. Exp. Hematol. 1996, 24, 675–681. [Google Scholar] [PubMed]
- Witzel, A.L.; Schook, L.B. Tumor necrosis factor alpha is an autocrine growth regulator during macrophage differentiation. Proc. Natl. Acad. Sci. USA 1992, 89, 4754–4758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, K.N.; Mak, N.K.; Fung, M.C.; Hapel, A.J. Synergistic effect of IL-4 and TNF-alpha in the induction of monocytic differentiation of a mouse myeloid leukaemic cell line (WEHI-3B JCS). Immunology 1994, 81, 65–72. [Google Scholar] [PubMed]
- Xie, B.; Laouar, A.; Huberman, E. Autocrine regulation of macrophage differentiation and 92-kDa gelatinase production by tumor necrosis factor-α via α5β1 integrin in HL-60 cells. J. Biol. Chem. 1998, 273, 11583–11588. [Google Scholar] [CrossRef] [Green Version]
- Raschke, W.C.; Baird, S.; Ralph, P.; Nakoinz, I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell 1978, 15, 261–267. [Google Scholar] [CrossRef]
- Introna, M.; Hamilton, T.A.; Kaufman, R.E.; Adams, D.O.; Bast, R.C., Jr. Treatment of murine peritoneal macrophages with bacterial lipopolysaccharide alters expression of c-fos and c-myc oncogenes. J. Immunol. 1986, 137, 2711–2715. [Google Scholar]
- Cooper, P.H.; Mayer, P.; Baggiolini, M. Stimulation of phagocytosis in bone marrow-derived mouse macrophages by bacterial lipopolysaccharide: Correlation with biochemical and functional parameters. J. Immunol. 1984, 133, 913–922. [Google Scholar]
- Sánchez-Tilló, E.; Comalada, M.; Farrera, C.; Valledor, A.F.; Lloberas, J.; Celada, A. Macrophage-colony-stimulating factor-induced proliferation and lipo-polysaccharide-dependent activation of macrophages requires Raf-1 phosphorylation to induce mitogen kinase phosphatase-1 expression. J. Immunol. 2006, 176, 6594–6602. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.; Hassan, F.; Tumurkhuu, G.; Dagvadorj, J.; Koide, N.; Naiki, Y.; Mori, I.; Yoshida, T.; Yokochi, T. Bacterial lipopolysaccharide induces osteoclast formation in RAW 264.7 macrophage cells. Biochem. Biophys. Res. Commun. 2007, 360, 346–351. [Google Scholar] [CrossRef]

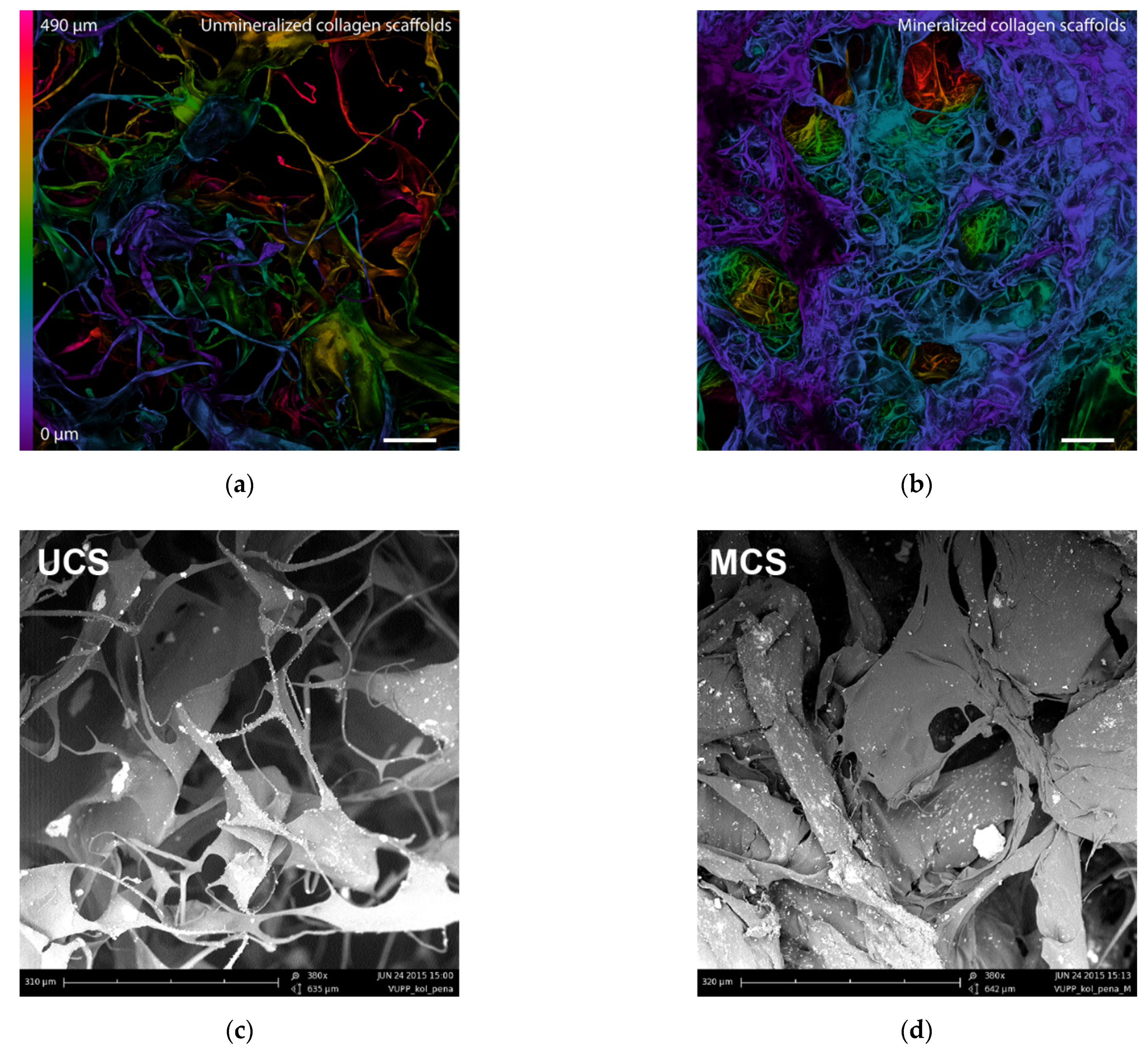


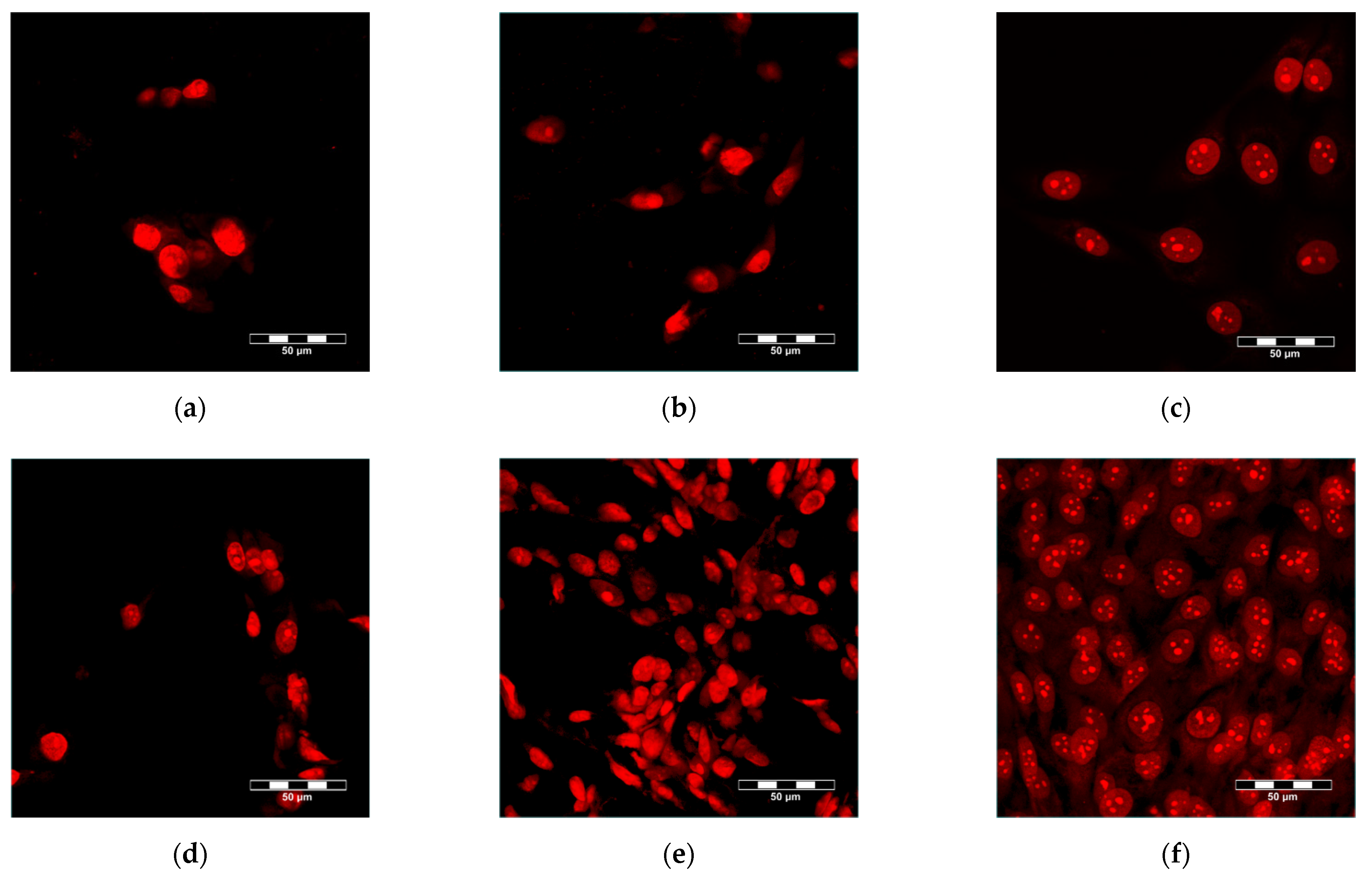


| Area [µm2] | UCS | MCS | ||
|---|---|---|---|---|
| Counts | Percent | Counts | Percent | |
| 102–103 | 0 | 0 | 11 | 10 |
| 103–104 | 24 | 20 | 64 | 53.3 |
| 104–105 | 83 | 69.2 | 39 | 32.5 |
| 105–106 | 13 | 10.8 | 5 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacakova, L.; Novotna, K.; Hadraba, D.; Musilkova, J.; Slepicka, P.; Beran, M. Influence of Biomimetically Mineralized Collagen Scaffolds on Bone Cell Proliferation and Immune Activation. Polymers 2022, 14, 602. https://doi.org/10.3390/polym14030602
Bacakova L, Novotna K, Hadraba D, Musilkova J, Slepicka P, Beran M. Influence of Biomimetically Mineralized Collagen Scaffolds on Bone Cell Proliferation and Immune Activation. Polymers. 2022; 14(3):602. https://doi.org/10.3390/polym14030602
Chicago/Turabian StyleBacakova, Lucie, Katarina Novotna, Daniel Hadraba, Jana Musilkova, Petr Slepicka, and Milos Beran. 2022. "Influence of Biomimetically Mineralized Collagen Scaffolds on Bone Cell Proliferation and Immune Activation" Polymers 14, no. 3: 602. https://doi.org/10.3390/polym14030602
APA StyleBacakova, L., Novotna, K., Hadraba, D., Musilkova, J., Slepicka, P., & Beran, M. (2022). Influence of Biomimetically Mineralized Collagen Scaffolds on Bone Cell Proliferation and Immune Activation. Polymers, 14(3), 602. https://doi.org/10.3390/polym14030602







