Effect of Solution Properties in the Development of Cellulose Derivative Nanostructures Processed via Electrospinning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanofabrication of Cellulose Derivatives
2.2.1. Solution Preparation
2.2.2. Electrospinning
2.3. Characterization
2.3.1. Characterization of the Solutions
Rheological Properties
Physical Properties
2.3.2. Characterization of the Nanostructures
Chemical Properties
Thermal Properties
Microstructural Properties
2.4. Statistical Analysis
3. Results
3.1. Characterization of the Solutions
3.2. Characterization of the Nanostructures
3.2.1. Chemical Properties
3.2.2. Thermal Properties
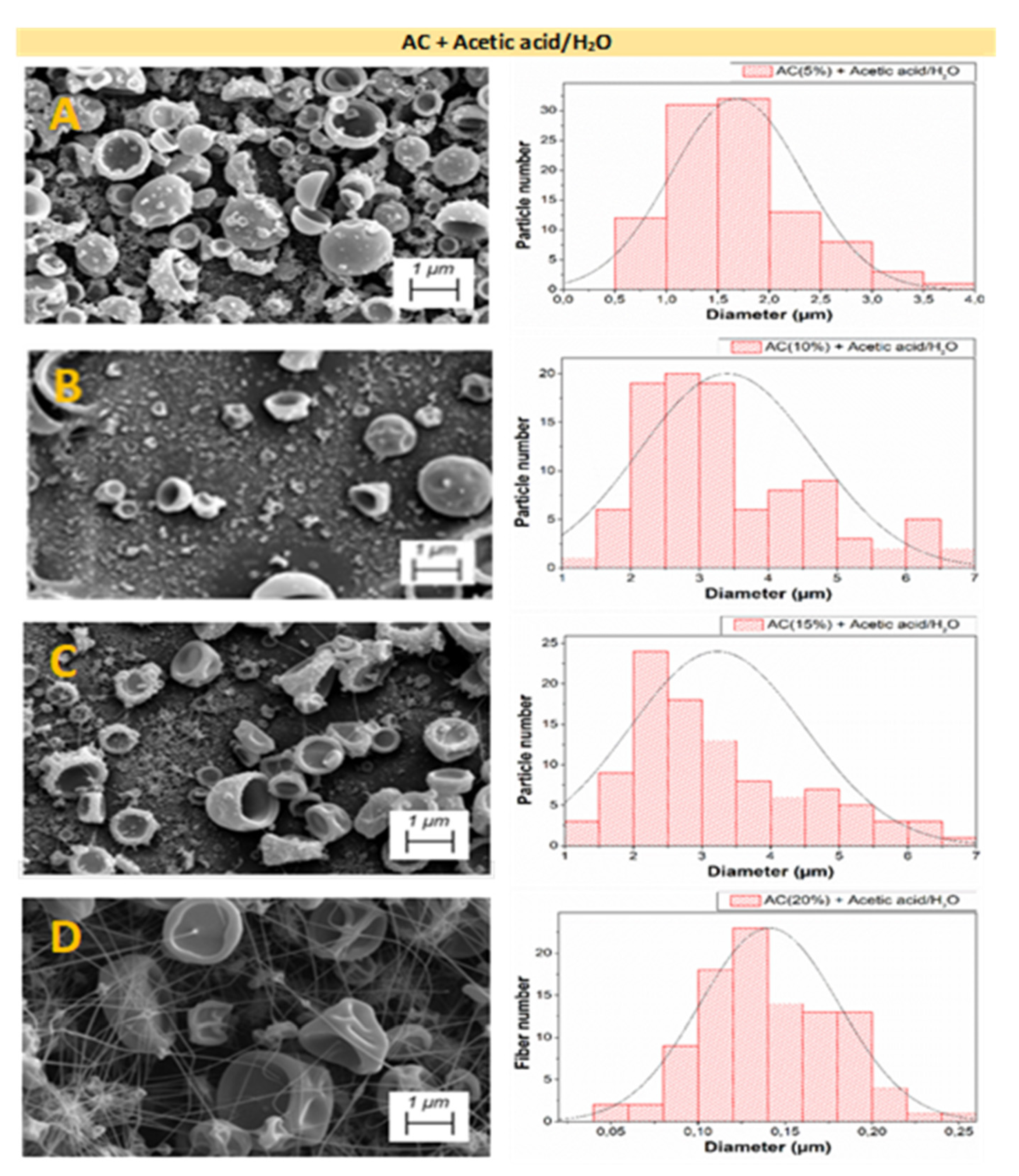
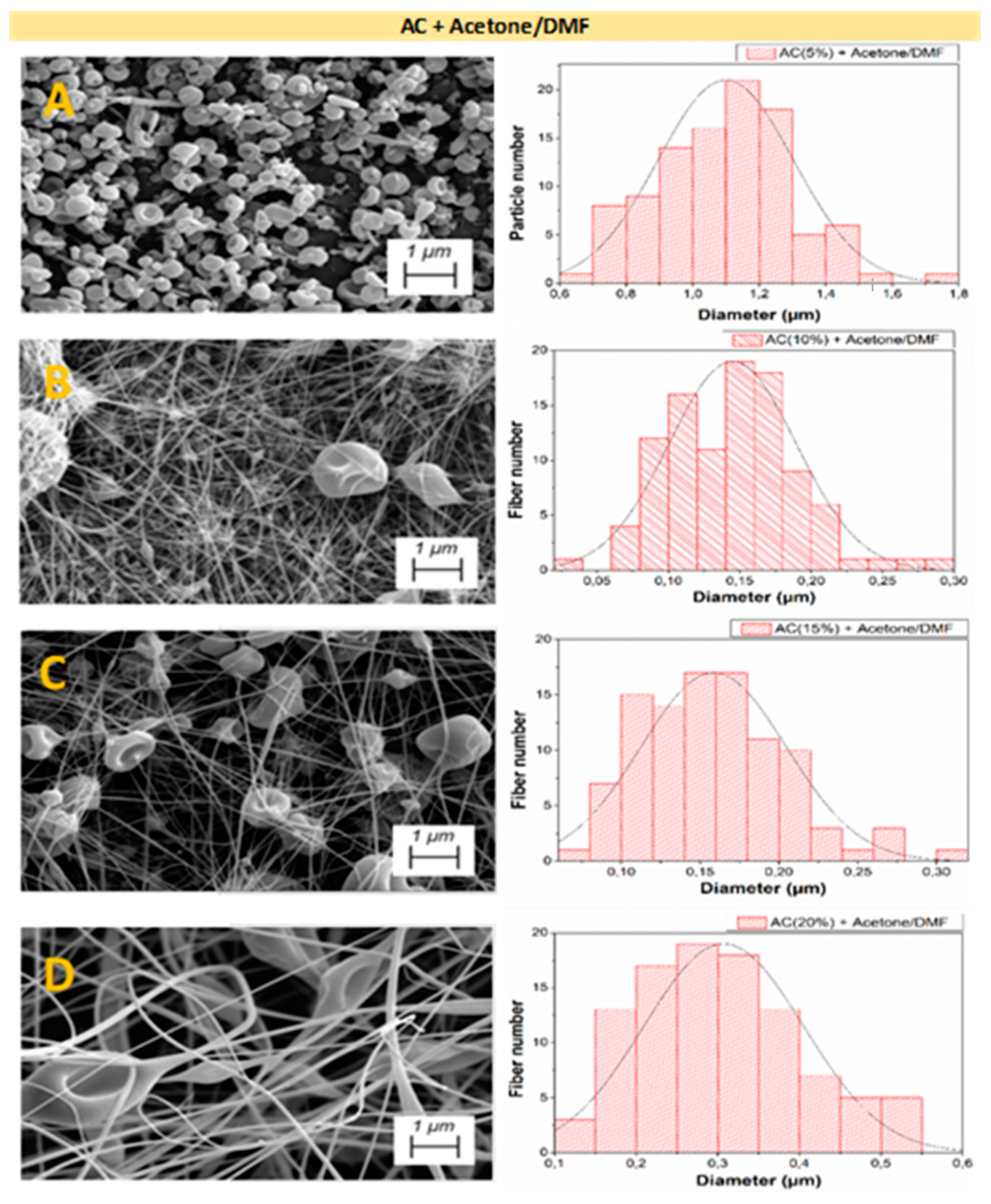
3.2.3. Microstructural Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duque Sánchez, L.M.; Rodriguez, L.; López, M. Electrospinning: The Nanofibers Age. Rev. Iberoam. Polímeros Vol. Iber. Polímeros 2014, 14, 10–27. [Google Scholar]
- Pérez-Puyana, V.; Guerrero, A.; Romero, A. Biomateriales; Gandulfo Impresores S.L.: Sevilla, Spain, 2020; ISBN 978-84-617-5006-1. [Google Scholar]
- Gritsch, L.; Liverani, L.; Lovell, C.; Boccaccini, A.R. Polycaprolactone Electrospun Fiber Mats Prepared Using Benign Solvents: Blending with Copper(II)-Chitosan Increases the Secretion of Vascular Endothelial Growth Factor in a Bone Marrow Stromal Cell Line. Macromol. Biosci. 2020, 20, 1900355. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Rubio-Valle, J.F.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Comparison between pea and soy protein-based bioplastics obtained by injection molding. J. Appl. Polym. Sci. 2021, 138, 50412. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Bouroudian, E.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Evaluation of different strengthening methods in the mechanical and functional properties of soy protein-based bioplastics. J. Clean. Prod. 2020, 262, 121517. [Google Scholar] [CrossRef]
- El-Aswar, E.I.; Ramadan, H.; Elkik, H.; Taha, A.G. A comprehensive review on preparation, functionalization and recent applications of nanofiber membranes in wastewater treatment. J. Environ. Manage. 2022, 301, 113908. [Google Scholar] [CrossRef]
- Elnaggar, M.; Shalaby, E.; Abd-Al-Aleem, A.-A.-A.; Youssef, A. Nanomaterials and nanofibers as wound dressing mats: An overview of the fundamentals, properties and applications. Egypt. J. Chem. 2021, 64, 7447–7473. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Wang, Y. Recent progress in cellulose-based electrospun nanofibers as multifunctional materials. Nanoscale Adv. 2021, 3, 6040–6047. [Google Scholar] [CrossRef]
- Brown, R.M. Cellulose Structure and Biosynthesis: What is in Store for the 21st Century? J. Polym. Sci. Part A Polym. Chem. 2004, 42, 487–495. [Google Scholar] [CrossRef]
- Rafael, V. Bacterial degradation of lignin. Enzyme Microb. Technol. 1988, 10, 646–655. [Google Scholar]
- Kobayasi, S.; Kashiwa, K.; Shimada, J.; Kawasaki, T.; Shoda, S.-I. Enzymatic polymerization: The first in vitro of cellulose via non biosynthetic path catalyzed by cellulase. Makromol. Chemie, Macromol. Symp. 1992, 54–55, 509–518. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Mascal, M.; Nikitin, E.B. Comment on Processes for the Direct Conversion of Cellulose or Cellulosic Biomass into Levulinate Esters. ChemSusChem 2010, 3, 1349–1351. [Google Scholar] [CrossRef]
- Muqeet, M.; Mahar, R.B.; Gadhi, T.A.; Ben Halima, N. Insight into cellulose-based-nanomaterials—A pursuit of environmental remedies. Int. J. Biol. Macromol. 2020, 163, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.; Paiva, M.C.; Amorim, M.T.P.; Felgueiras, H.P. Electrospun nanocomposites containing cellulose and its derivatives modified with specialized biomolecules for an enhanced wound healing. Nanomaterials 2020, 10, 557. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.K.; Zhang, K.; Gong, Q.; Yu, D.G.; Wang, J.; Tan, X.; Quan, H. Ethylcellulose-based drug nano depots fabricated using a modified triaxial electrospinning. Int. J. Biol. Macromol. 2020, 152, 68–76. [Google Scholar] [CrossRef]
- Ahmadi, P.; Jahanban-Esfahlan, A.; Ahmadi, A.; Tabibiazar, M.; Mohammadifar, M. Development of Ethyl Cellulose-based Formulations: A Perspective on the Novel Technical Methods. Food Rev. Int. 2020, 10, 1–48. [Google Scholar] [CrossRef]
- Konwarh, R.; Karak, N.; Misra, M. Electrospun cellulose acetate nanofibers: The present status and gamut of biotechnological applications. Biotechnol. Adv. 2013, 31, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Wsoo, M.A.; Shahir, S.; Mohd Bohari, S.P.; Nayan, N.H.M.; Razak, S.I.A. A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: A new perspective. Carbohydr. Res. 2020, 491, 107978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, B.; Qin, X.; Ye, S.; Shi, Y.; Feng, Y.; Han, W.; Liu, C.; Shen, C. Cellulose acetate monolith with hierarchical micro/nano-porous structure showing superior hydrophobicity for oil/water separation. Carbohydr. Polym. 2020, 241, 116361. [Google Scholar] [CrossRef] [PubMed]
- Crabbe-Mann, M.; Tsaoulidis, D.; Parhizkar, M.; Edirisinghe, M. Ethyl cellulose, cellulose acetate and carboxymethyl cellulose microstructures prepared using electrohydrodynamics and green solvents. Cellulose 2018, 25, 1687–1703. [Google Scholar] [CrossRef]
- Um-i-Zahra, S.; Shen, X.X.; Li, H.; Zhu, L. Study of sustained release drug-loaded nanofibers of cellulose acetate and ethyl cellulose polymer blends prepared by electrospinning and their in-vitro drug release profiles. J. Polym. Res. 2014, 21, 602. [Google Scholar] [CrossRef]
- Souza, P.R.; de Oliveira, A.C.; Vilsinski, B.H.; Kipper, M.J.; Martins, A.F. Polysaccharide-based materials created by physical processes: From preparation to biomedical applications. Pharmaceutics 2021, 13, 621. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent advances in applications of cellulose derivatives-based composite membranes with hydroxyapatite. Materials 2020, 13, 2481. [Google Scholar] [CrossRef]
- Gopiraman, M.; Bang, H.; Yuan, G.; Yin, C.; Song, K.-H.; Lee, J.S.; Chung, I.M.; Karvembu, R.; Kim, I.S. Noble metal/functionalized cellulose nanofiber composites for catalytic applications. Carbohydr. Polym. 2015, 132, 554–564. [Google Scholar] [CrossRef]
- Morsi, R.E.; Elsawy, M.; Manet, I.; Ventura, B. Cellulose acetate fabrics loaded with rhodamine B hydrazide for optical detection of Cu(II). Molecules 2020, 25, 3751. [Google Scholar] [CrossRef]
- Fatima, A.; Yasir, S.; Khan, M.S.; Manan, S.; Ullah, M.W.; Ul-Islam, M. Plant extract-loaded bacterial cellulose composite membrane for potential biomedical applications. J. Bioresour. Bioprod. 2021, 6, 26–32. [Google Scholar] [CrossRef]
- Deeksha, B.; Sadanand, V.; Hariram, N.; Rajulu, A.V. Preparation and properties of cellulose nanocomposite fabrics with in situ generated silver nanoparticles by bioreduction method. J. Bioresour. Bioprod. 2021, 6, 75–81. [Google Scholar] [CrossRef]
- Bekturov, E.A.; Bakauova, Z.K. Synthetic Water-Soluble Polymers in Solution, 6th ed.; Hüthig and Wepf Verlag: Mainz, Germany, 1986. [Google Scholar]
- Painter, P.C.; Coleman, M.M. Fundamentals of Polymer Science: An Introductory Text, 3rd ed.; Technocomic Publishing Company Inc.: Lancaster, PA, USA, 1994. [Google Scholar]
- Rubio-Valle, J.F.; Sánchez, M.C.; Valencia, C.; Martín-Alfonso, J.E.; Franco, J.M. Electrohydrodynamic Processing of PVP-Doped Kraft Lignin Micro- and Nano-Structures and Application of Electrospun Nanofiber Templates to Produce Oleogels. Polymers 2021, 13, 2206. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Kirk-Othmer Encyclopedia of Chemical Technology; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Gupta, P.; Elkins, C.; Long, T.E.; Wilkes, G.L. Electrospinning of linear homopolymers of poly(methyl methacrylate): Exploring relationships between fiber formation, viscosity, molecular weight and concentration in a good solvent. Polymer 2005, 46, 4799–4810. [Google Scholar] [CrossRef]
- Aslanzadeh, S.; Ahvazi, B.; Boluk, Y.; Ayranci, C. Carbon Fiber Production from Electrospun Sulfur Free Softwood Lignin Precursors. J. Eng. Fiber. Fabr. 2017, 12, 155892501701200. [Google Scholar] [CrossRef] [Green Version]
- Khatri, M.; Ahmed, F.; Jatoi, A.W.; Mahar, R.B.; Khatri, Z.; Kim, I.S. Ultrasonic dyeing of cellulose nanofibers. Ultrason. Sonochem. 2016, 31, 350–354. [Google Scholar] [CrossRef]
- Wang, X.; He, G.; Liu, H.; Zheng, G.; Sun, D. Fabrication and morphological control of electrospun ethyl cellulose nanofibers. In Proceedings of the 8th Annual IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Suzhou, China, 7–10 April 2013; pp. 324–327. [Google Scholar]
- Arribas-Jimeno, S.; Burriel-Martí, F.; Hernández-Méndez, J.; Lucena-Conde, F. Química Analítica Cualitativa, 1st ed.; Paraninfo: Madrid, Spain, 2006. [Google Scholar]
- Jiménez-Álvarez, M.D.; Gómez-del Río, M.I. Guía Didáctica Química Analítica II, 14th ed.; UNED: Madrid, Spain, 1999. [Google Scholar]
- Bermejo-Barrera, M.P. Química Analítica General, Cuantitativa e Instrumental, 7th ed.; Paraninfo: Madrid, Spain, 1990. [Google Scholar]
- Blanco, M.; Cerdá, V.; Sanz-Medel, A. Espectroscopía Atómica Analítica, 1st ed.; Universidad Autónoma de Barcelona: Barcelona, Spain, 1990. [Google Scholar]
- Burriel-Martí, F. Química Analítica Cualitativa, 6th ed.; Paraninfo: Madrid, Spain, 2003. [Google Scholar]
- Burriel-Martí, F.; Lucena-Conde, C.F.; Arribas-Jimeno, S. Química Analítica Cuantitativa, 18th ed.; Revolucionaria: Madrid, Spain, 1978. [Google Scholar]
- Pal, S.; Srivastava, R.K.; Nandan, B. Effect of spinning solvent on crystallization behavior of confined polymers in electrospun nanofibers. Polym. Cryst. 2021, 4, e10209. [Google Scholar] [CrossRef]
- Nuamcharoen, P.; Kobayashi, T.; Potiyaraj, P. Influence of volatile solvents and mixing ratios of binary solvent systems on morphology and performance of electrospun poly(vinylidene fluoride) nanofibers. Polym. Int. 2021, 70, 1465–1477. [Google Scholar] [CrossRef]
- Nayak, R.; Padhye, R.; Kyratzis, I.L.; Truong, Y.B.; Arnold, L. Effect of viscosity and electrical conductivity on the morphology and fiber diameter in melt electrospinning of polypropylene. Text. Res. J. 2013, 83, 606–617. [Google Scholar] [CrossRef]
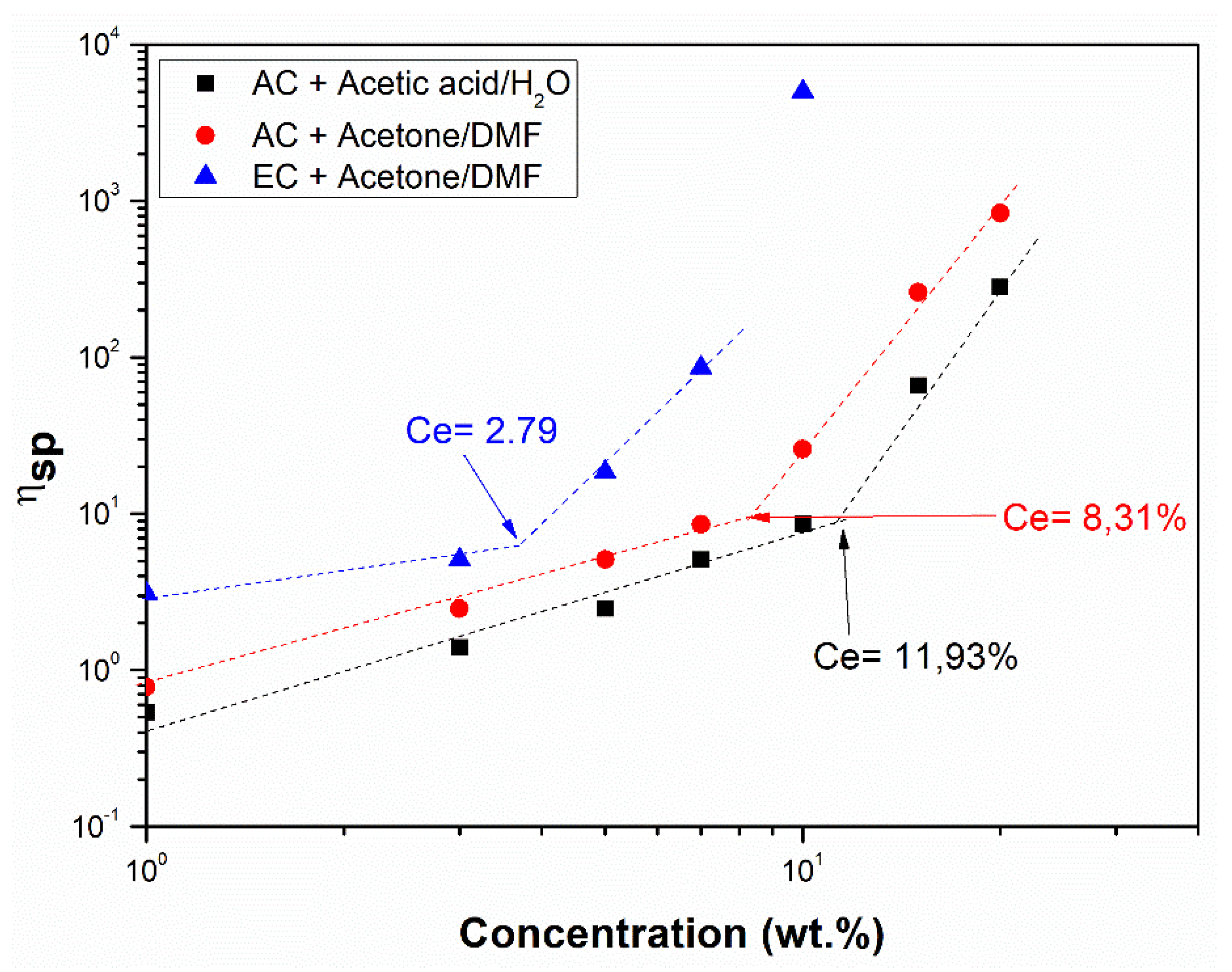



| Systems | η (Pa·s) | ηext (Pa·s) | ρ (g·cm−3) | γ (mN·m−1) | σ (μS·cm−1) | |
|---|---|---|---|---|---|---|
| AC + Acetic acid/H2O (2:1) | 5 wt.% | 0.004 a | 0.011 A | 1.067 α | 29.75 I | 184.0 a |
| 10 wt.% | 0.011 a | 0.035 B | 1.074 α | 30.15 II | 197.7 b | |
| 15 wt.% | 0.185 b | 0.575 C | 1.087 β | 31.25 III | 174.3 c | |
| 20 wt.% | 0.311 c | 0.983 D | 1.095 β | 30.75 II | 157.7 d | |
| AC + Acetone/DMF (2:1) | 5 wt.% | 0.006 a | 0.015 A | 0.862 γ | 30.35 II | 128.1 e |
| 10 wt.% | 0.105 d | 0.335 E | 0.881 δ | 31.54 III | 113.1 f | |
| 15 wt.% | 0.385 e | 1.105 F | 0.892 δ | 32.15 IV | 96.71 g | |
| 20 wt.% | 0.523 f | 1.529 G | 0.902 ε | 31.74 III | 73.13 h | |
| EC + Acetone/DMF (2:1) | 2.5 wt.% | 0.009 a | 0.029 B | 0.859 γ | 30.58 II | 144.5 i |
| 5 wt.% | 0.132 g | 0.356 H | 0.869 γ | 32.51 IV | 97.57 g | |
| 10 wt.% | 0.678 h | 2.104 I | 0.877 γ | 33.58 V | 69.27 h | |
| 15 wt.% | 1.115 i | 3.395 J | 0.886 δ | 33.47 V | 25.20 j | |
| Systems | Tonset (°C) | Tmax (°C) | Weight Loss (%) | Residue (%) | Tg (°C) | |
|---|---|---|---|---|---|---|
| AC | 272.0/345.4 | 285.6/367.9 | 81.9 | 18.1 | 171.1 | |
| AC + Acetic acid/H2O (2:1) | 5 wt.% | 258.7/330.8 | 305.8/356.7 | 88.7 | 11.3 | -- |
| 10 wt.% | 259.9/335.3 | 307.2/358.8 | 88.1 | 11.9 | 190.1 | |
| 15 wt.% | 260.8/337.4 | 309.9/358.8 | 87.5 | 12.5 | -- | |
| 20 wt.% | 261.6/337.7 | 311.9/359.8 | 87.3 | 12.7 | -- | |
| AC + Acetone/DMF (2:1) | 10 wt.% | 257.2/322.8 | 314.4/356.8 | 87.9 | 12.1 | -- |
| 15 wt.% | 259.0/329.0 | 314.8/357.2 | 87.8 | 12.2 | 188.2 | |
| 20 wt.% | 260.0/334.7 | 315.4/359.3 | 87.5 | 12.5 | -- | |
| EC | 261.2/322.9 | 283.1/354.2 | 95.2 | 4.8 | 181.5 | |
| EC + Acetone/DMF (2:1) | 5 wt.% | 253.1/337.2 | 308.3/359.8 | 95.5 | 4.5 | 183.0 |
| Systems | Particle Diameter (μm) | Fiber Diameter (μm) | Porosity (%) | |
|---|---|---|---|---|
| AC + Acetic acid/H2O (2:1) | 5 wt.% | 1.70 ± 0.12 a | -- | 24.29 ± 0.18 α |
| 10 wt.% | 3.40 ± 0.09 b | -- | 22.72 ± 0.16 β | |
| 15 wt.% | 3.05 ± 0.14 b | 0.06 ± 0.01 | 28.19 ± 0.27 γ | |
| 20 wt.% | 5.00 c ± 0.27 | 0.14 ± 0.01 A | 25.30 ± 0.25 α,γ | |
| AC + Acetone/DMF (2:1) | 5 wt.% | 1.10 ± 0.04 d | -- | 30.42 ± 0.28 δ |
| 10 wt.% | 3.10 ± 0.11 e | 0.15 ± 0.01 A | 26.65 ± 0.15 γ | |
| 15 wt.% | 3.90 ± 0.19 f | 0.16 ± 0.01 A | 33.32 ± 0.38 ε | |
| 20 wt.% | -- | 0.31 ± 0.03 B | 25.67 ± 0.41 γ | |
| EC + Acetone/DMF (2:1) | 2.5 wt.% | 1.25 ± 0.03 d | -- | 35.45 ± 0.21 ζ |
| 5 wt.% | 2.75 ± 0.08 g | 0.19 ± 0.03 C | 26.21 ± 0.26 γ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Cid, P.; Rubio-Valle, J.F.; Jiménez-Rosado, M.; Pérez-Puyana, V.; Romero, A. Effect of Solution Properties in the Development of Cellulose Derivative Nanostructures Processed via Electrospinning. Polymers 2022, 14, 665. https://doi.org/10.3390/polym14040665
Sánchez-Cid P, Rubio-Valle JF, Jiménez-Rosado M, Pérez-Puyana V, Romero A. Effect of Solution Properties in the Development of Cellulose Derivative Nanostructures Processed via Electrospinning. Polymers. 2022; 14(4):665. https://doi.org/10.3390/polym14040665
Chicago/Turabian StyleSánchez-Cid, Pablo, José Fernando Rubio-Valle, Mercedes Jiménez-Rosado, Víctor Pérez-Puyana, and Alberto Romero. 2022. "Effect of Solution Properties in the Development of Cellulose Derivative Nanostructures Processed via Electrospinning" Polymers 14, no. 4: 665. https://doi.org/10.3390/polym14040665
APA StyleSánchez-Cid, P., Rubio-Valle, J. F., Jiménez-Rosado, M., Pérez-Puyana, V., & Romero, A. (2022). Effect of Solution Properties in the Development of Cellulose Derivative Nanostructures Processed via Electrospinning. Polymers, 14(4), 665. https://doi.org/10.3390/polym14040665








