Mixed Solvents in Multilayer Ceramic Capacitors (MLCC) Electronic Paste and Their Effects on the Properties of Organic Vehicle
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Characterization
2.2.1. Constant Temperature Volatility of Solvent
2.2.2. Wettability of Copper Powder and Glass Powder to Mixed Solvents
2.2.3. Intrinsic Viscosity of Polyacrylate Resins in the Mixed Solvents
2.2.4. Thermogravimetric Analysis (TGA)
3. Results and Discussion
3.1. Constant Temperature Volatility of Solvent
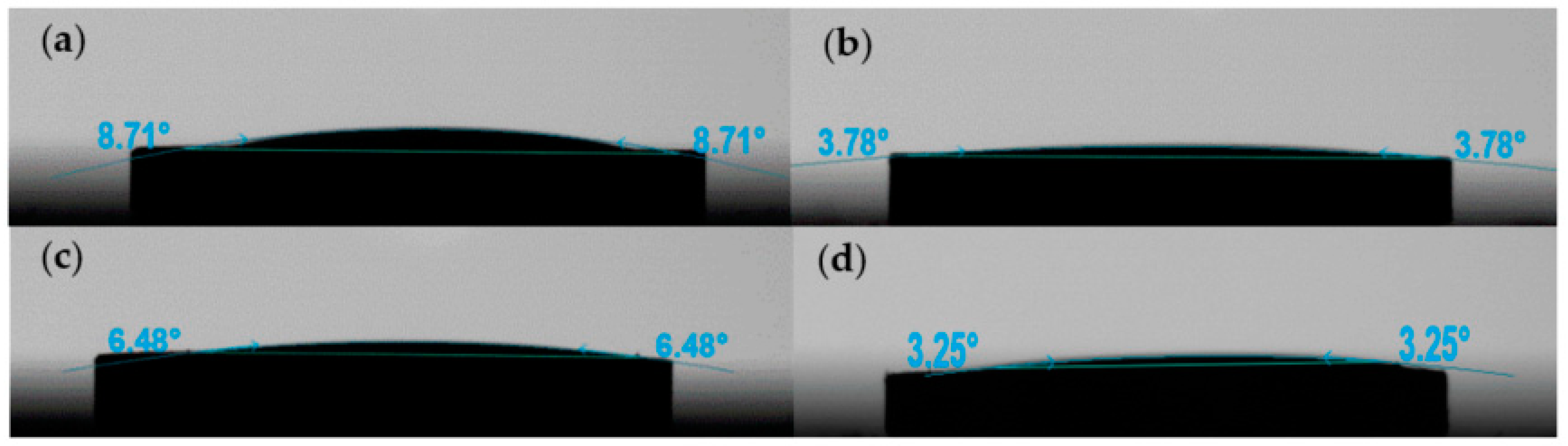
3.2. Wettability of Mixed Solvents to Glass Powder and Glass Powder
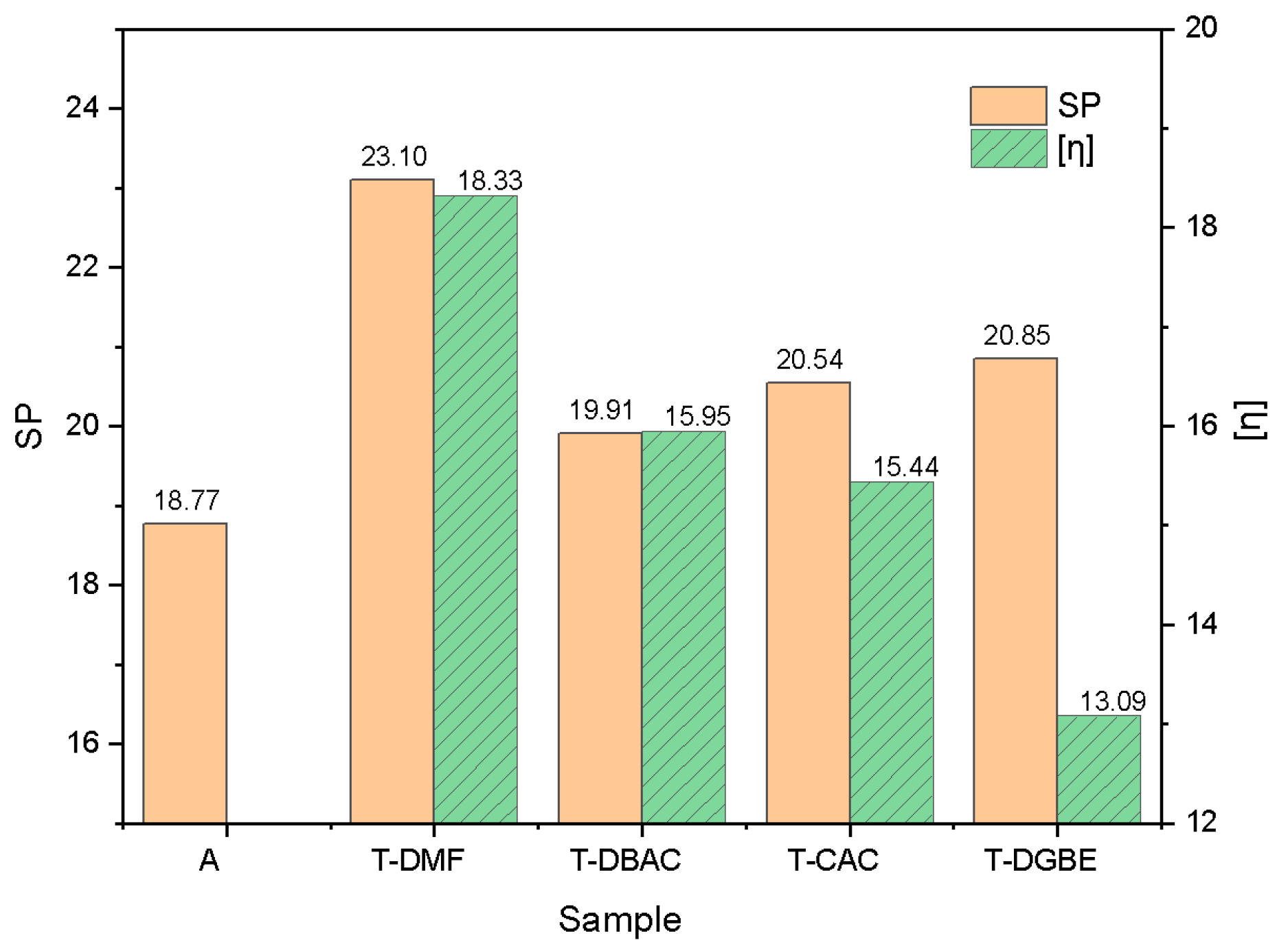
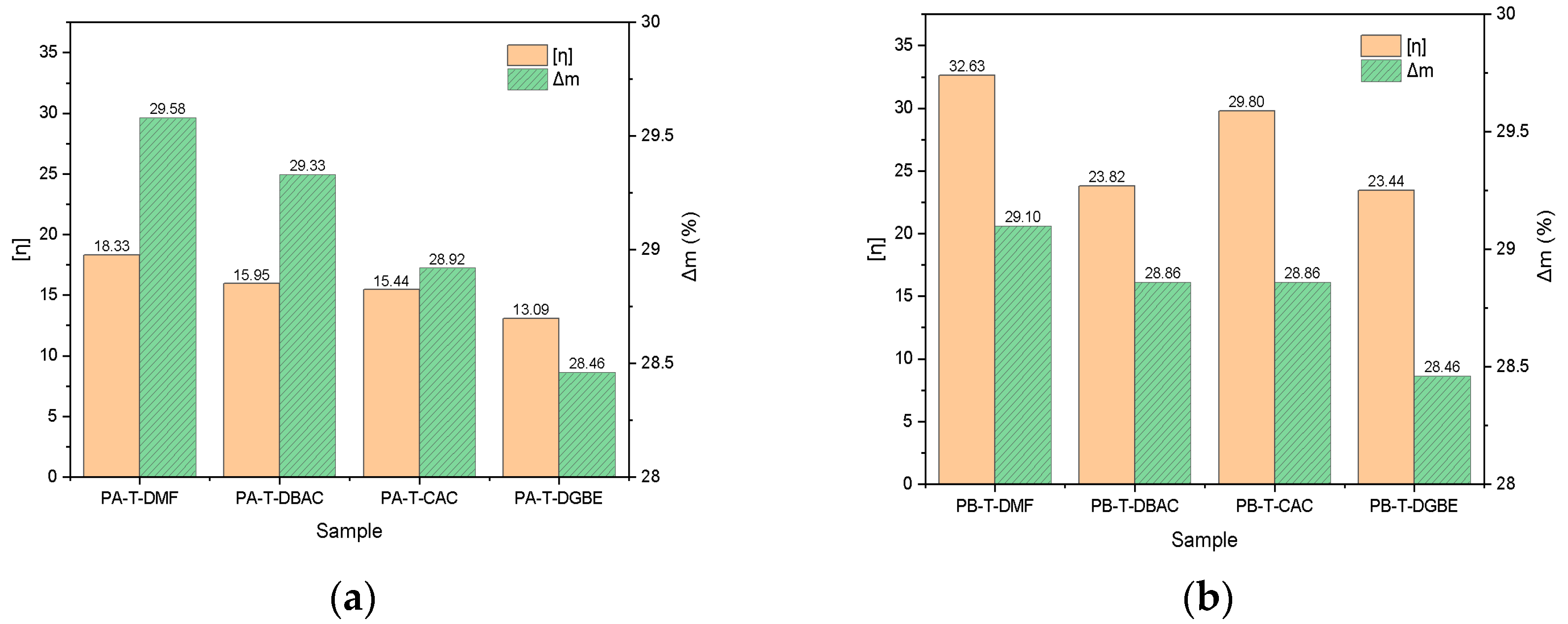
3.3. Solubility of Polyacrylate Resins in Mixed Solvents
3.4. Analysis of Thermogravimetric Results
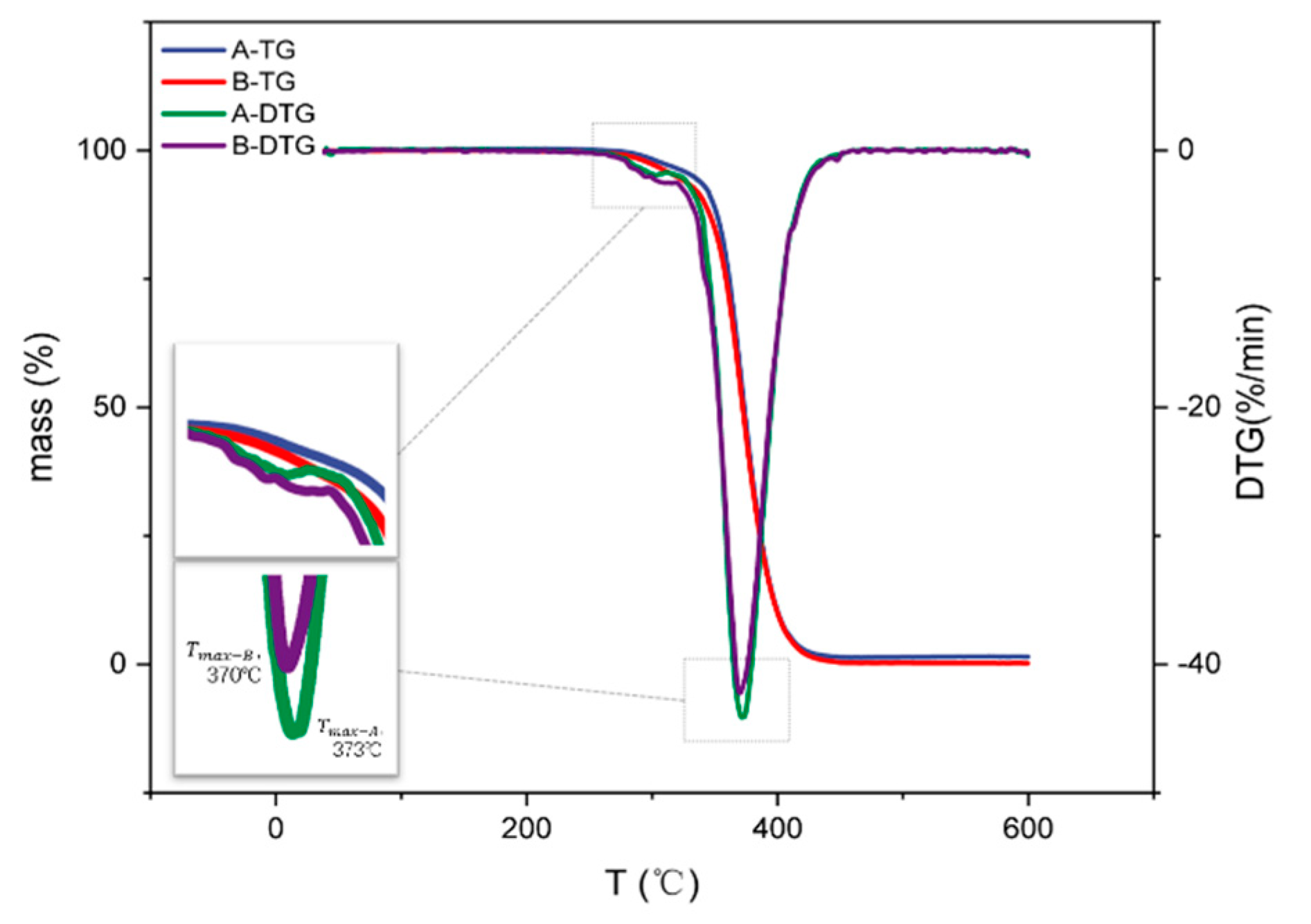
3.4.1. Thermogravimetric Analysis of Polyacrylate Resins
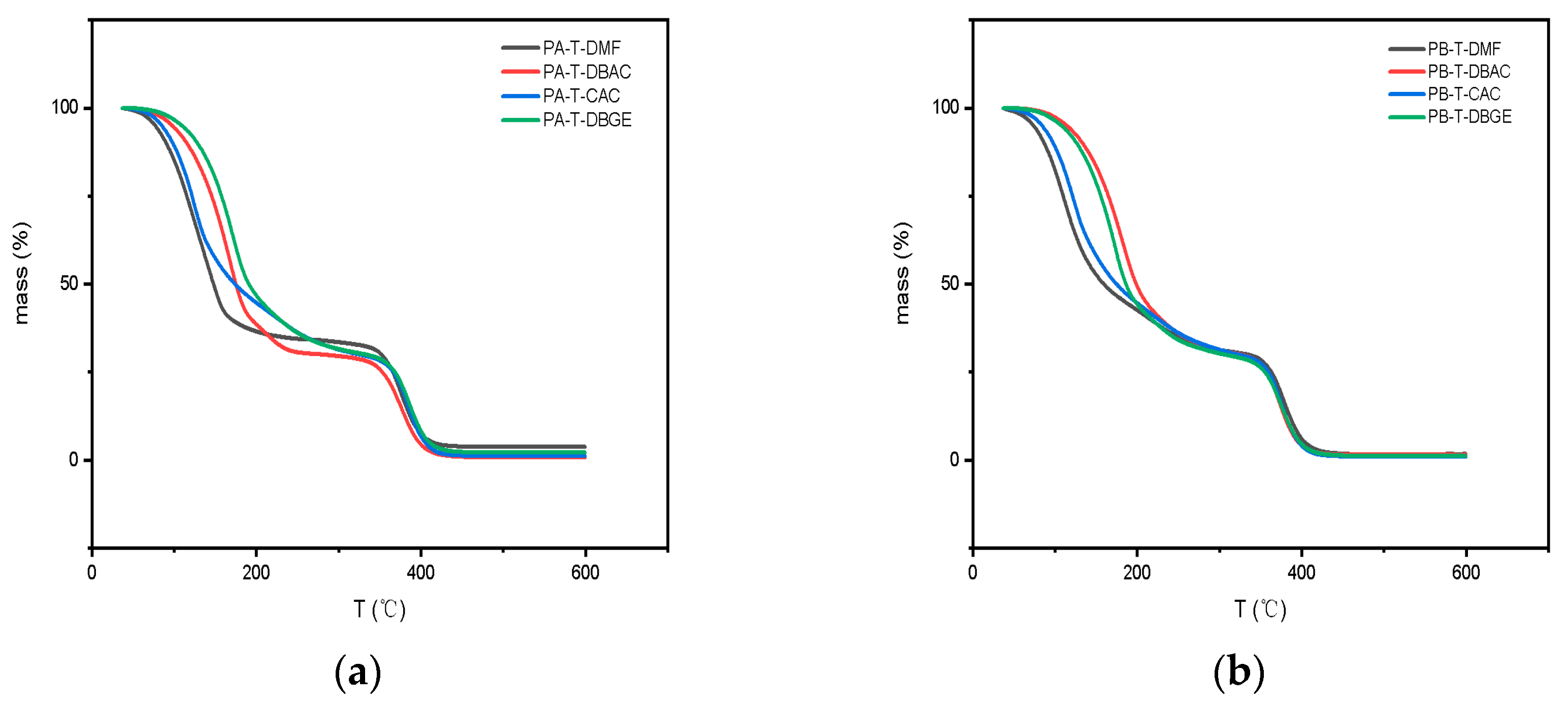
3.4.2. Thermogravimetric Analysis of Organic Vehicles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.R.; Tang, H.; Song, Z.F.; An, K.R. Development tendency and applications of MLCCs in 5G field. Electron. Compon. Mater. 2018, 37, 1–4. [Google Scholar]
- Lu, G.G.; Xuan, T.P. Development tendency and research process of the electronic paste. Met. Funct. Mater. 2008, 15, 48–52. [Google Scholar]
- Akhtar, M.; Anklekar, R.M. Characterization of copper pastes for end termination application of base metal electrode MLCCs. Microelectron. Int. 2004, 21, 36–40. [Google Scholar] [CrossRef]
- Paik, U.; Kang, K.M.; Jung, Y.G.; Kim, J. Binder removal and microstructure with burnout conditions in BaTiO3based Ni–MLCCs. Ceram. Int. 2003, 29, 939–946. [Google Scholar] [CrossRef]
- Wang, T.; Chen, X.; Lu, G.Q.; Lei, G.Y. Low-Temperature Sintering with Nano-Silver Paste in Die-Attached Interconnection. J. Electron. Mater. 2007, 36, 1333–1340. [Google Scholar] [CrossRef]
- Khazaka, R.; Mendizabal, L.; Henry, D. Review on Joint Shear Strength of Nano-Silver Paste and Its Long-Term High Temperature Reliability. J. Electron. Mater. 2014, 43, 2459–2466. [Google Scholar] [CrossRef]
- Bai, J.G.; Lei, T.G.; Calata, J.N.; Lu, G.Q. Control of nanosilver sintering attained through organic binder burnout. J. Mater. Res. 2007, 22, 3494–3500. [Google Scholar] [CrossRef]
- Liu, X.Q.; Su, X.L.; Liu, X.F.; Qu, Y.H. Effects of sintering processing on conductivity of copper paste. J. Mater. Sci. Eng. 2015, 33, 112–116. [Google Scholar]
- Gan, W.P.; Zhou, H.; Zhang, J.L. Investigation of sintering process and electrical conductivity of the lead–free Ag paste. Electron. Compon. Mater. 2010, 29, 65–69. [Google Scholar]
- Ma, X.Q.; Zhu, X.Y.; Long, J.M.; Cao, M. Research status and development tendency of sintering process for electronic paste. Hot. Work. Technol. 2017, 46, 14–19. [Google Scholar]
- Luo, S.Y.; Peng, Y.Y.; Hao, Y.P.; Chen, Q. Volatility of organic vehicle for electronic paste. Electron. Compon. Mater. 2006, 25, 49–51. [Google Scholar]
- Fan, M.N.; Tian, X.L.; Xiong, Z.; Zhang, Y.; Xing, Q.S.; Li, J.P.; Zhao, L. Effect of solvent type on the volatility characteristics and rheological properties of silver paste. Precious. Met. 2015, 36, 104–107. [Google Scholar]
- Fu, X.C.; Shen, W.X.; Yao, T.Y.; Hou, W.H. Physical Chemistry, 5th ed.; Higher Education Press: Beijing, China, 2005. [Google Scholar]
- Zhang, J.Q.; Du, Y.G.; Zhang, W.J.; Yang, J.; Zheng, X.H. Study on Volatility of Organic Vehicle in Thick Film Resistance Paste. Electron. Compon. Mater. 2003, 22, 40–42. [Google Scholar]
- Gan, W.P.; Pan, Q.Z.; Zhou, J. Effect of Organic Vehicle and Sintering Process on Conductive Properties of Silver Paste. Min. Metall. Eng. 2014, 34, 118–121. [Google Scholar]
- Chen, X.L.; Pu, Y.P.; Wu, S.H.; Zhang, N.; Zhao, X. Influence of volatility of organic vehicle on the performances of Al electrode of PTC ceramics. Electron. Compon. Mater. 2010, 29, 22–24. [Google Scholar]
- da Costa, F.A.; da Silva, A.G.P.; Filho, F.A.; Gomes, U.U. Influence of the composition and the tungsten particle size on the sintering of W–Cu composites. In Proceedings of the World Powder Metallurgy Congress and Exhibition, Florence, Italy, 10–14 October 2010; pp. 1884–2022. [Google Scholar]
- Sun, W.T. Conduction Mechanism of Electronic Thick Film Material of Base Metal. Electron. Compon. Mater. 1996, 16, 14–19. [Google Scholar]
- Eom, Y.S.; Son, J.H.; Lee, H.S.; Choi, K.S.; Bae, H.C.; Choi, J.Y.; Moon, J.T. Development of Copper Electro–Plating Technology on a Screen–Printed Conductive Pattern with Copper Paste. J. Microelectron. Packag. Soc. 2015, 22, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Small, P.A. Some factors affecting the solubility of polymers. J. Appl. Chem. 1953, 3, 71–80. [Google Scholar] [CrossRef]
- Fedors, R.F. A Method for Estimating Both the Solubility Parameters and Molar Volumes of Liquids. Polym. Eng. Sci. 1974, 14, 147–154. [Google Scholar] [CrossRef]
- Sun, Z.J.; Zhang, X.Y.; Huang, H.; Fu, H.Q.; Chen, H.Q. Application of Solubility Parameters in the Synthesis of High solids Acrylic Resin. J. Shenyang Jianzhu Univ. Nat. Sci. 2006, 22, 592–595. [Google Scholar]
- Yang, L.; Le, Y.L. Nonlinear mathematical model for calculating solubility parameters of mixed solvents. Paint Coat. Ind. 2001, 31, 20–21. [Google Scholar]
- Han, K.H.; Jeon, G.S.; Hong, I.K.; Lee, S.B. Prediction of solubility parameter from intrinsic viscosity. J. Ind. Eng. Chem. 2013, 19, 1130–1136. [Google Scholar] [CrossRef]
- Eom, Y.; Kim, B.C. Solubility parameter–based analysis of polyacrylonitrile solutions in N, N–dimethyl formamide and dimethyl sulfoxide. Polymer 2014, 55, 2570–2577. [Google Scholar] [CrossRef]
- Flory, P.J.; Fox, T.G. Treatment of intrinsic viscosities. J. Am. Chem. Soc. 1951, 73, 1904–1908. [Google Scholar] [CrossRef]
- Alfrey, T.; Bartovics, A.; Mark, H. The effect of temperature and solvent type on the intrinsic viscosity of high polymer solutions. J. Am. Chem. Soc. 1942, 64, 1557–1560. [Google Scholar] [CrossRef]
- Hansen, C.M. Applications—Coatings and other filled polymer systems. In Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2000; pp. 137–149. [Google Scholar]
- Ye, L.N.; Liu, M.F.; Huang, Y.W.; Zhang, Z.W.; Yang, J.X. Effects of Molecular Weight on Thermal Degradation of Poly(α–methyl styrene) in Nitrogen. J. Macromol. Sci. Part B Phys. 2015, 54, 1479–1494. [Google Scholar] [CrossRef]
- Reich, L.; Stivala, S.S. Elements of Polymer Degradation; McGraw–Hill: New York, NY, USA, 1971; p. 186. [Google Scholar]
- Vollhardt, K.P.C.; Schore, N.E. Organic Chemistry, 2nd ed.; Freeman: New York, NY, USA, 1994; p. 111. [Google Scholar]
- Chen, Z.B.; Yang, S.S.; Meng, T. Study on Thermal Life of Acrylic Resin for Fireproof Coatings. J. Armed Police Acad. 2018, 34, 14–16. [Google Scholar]
- Czech, Z.; Pełech, R. Thermal degradation of butyl acrylate-methyl acrylate-acrylic acid-copolymers. J. Therm. Anal. Calorim. 2009, 96, 583–586. [Google Scholar] [CrossRef]
- Czech, Z.; Pełech, R. The thermal degradation of acrylic pressure-sensitive adhesives based on butyl acrylate and acrylic acid. Prog. Org. Coat. 2009, 65, 84–87. [Google Scholar] [CrossRef]
- Yang, X.F.; Xie, H.H.; He, Q.L.; Zhou, Z.; Xu, X.W.; Zhang, L.; Xie, Z.P. Study of thermal degradation mechanism of binders for ceramic injection molding by TGA–FTIR. Ceram. Int. 2019, 45, 10707–10717. [Google Scholar]
- Czech, Z.; Agnieszka, K.; Ragańska, P.; Antosik, A. Thermal stability and degradation of selected poly (alkyl methacrylates) used in the polymer industry. J. Therm. Anal. Calorim. 2015, 119, 1157–1161. [Google Scholar] [CrossRef] [Green Version]


| Sample | T (wt/%) | DBAC (wt/%) | CAC (wt/%) | Vol (%) | |
|---|---|---|---|---|---|
| 150 °C | 190 °C | ||||
| 1 | 50 | 40 | 10 | 20.96 | 46.24 |
| 2 | 50 | 50 | 0 | 19.20 | 42.51 |
| 3 | 30 | 60 | 10 | 17.44 | 41.28 |
| 4 | 60 | 30 | 10 | 18.56 | 48.48 |
| Sample | ||||
|---|---|---|---|---|
| A and B | 16.59 | 4.20 | 7.71 | 18.77 |
| T-DMF | 17.25 | 10.41 | 11.30 | 23.10 |
| T-DBAC | 16.56 | 4.87 | 9.93 | 19.91 |
| T-CAC | 16.56 | 5.13 | 11.01 | 20.54 |
| T-DGBE | 16.56 | 6.26 | 11.01 | 20.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, R.; Li, J.; Cao, X.; Huang, J.; Qian, L. Mixed Solvents in Multilayer Ceramic Capacitors (MLCC) Electronic Paste and Their Effects on the Properties of Organic Vehicle. Polymers 2022, 14, 685. https://doi.org/10.3390/polym14040685
Gan R, Li J, Cao X, Huang J, Qian L. Mixed Solvents in Multilayer Ceramic Capacitors (MLCC) Electronic Paste and Their Effects on the Properties of Organic Vehicle. Polymers. 2022; 14(4):685. https://doi.org/10.3390/polym14040685
Chicago/Turabian StyleGan, Ruolong, Junrong Li, Xiuhua Cao, Jun Huang, and Liying Qian. 2022. "Mixed Solvents in Multilayer Ceramic Capacitors (MLCC) Electronic Paste and Their Effects on the Properties of Organic Vehicle" Polymers 14, no. 4: 685. https://doi.org/10.3390/polym14040685






