Preparation of a Novel Organic Phosphonic Acid Intercalated Phosphate Tailings Based Hydrotalcite and Its Application in Enhancing Fire Safety for Epoxy Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TM-DTPMP LDHs/EP Composite Materials
2.3. Characterization
3. Results and Discussion
3.1. Characterization of TM-DTPMP LDHs
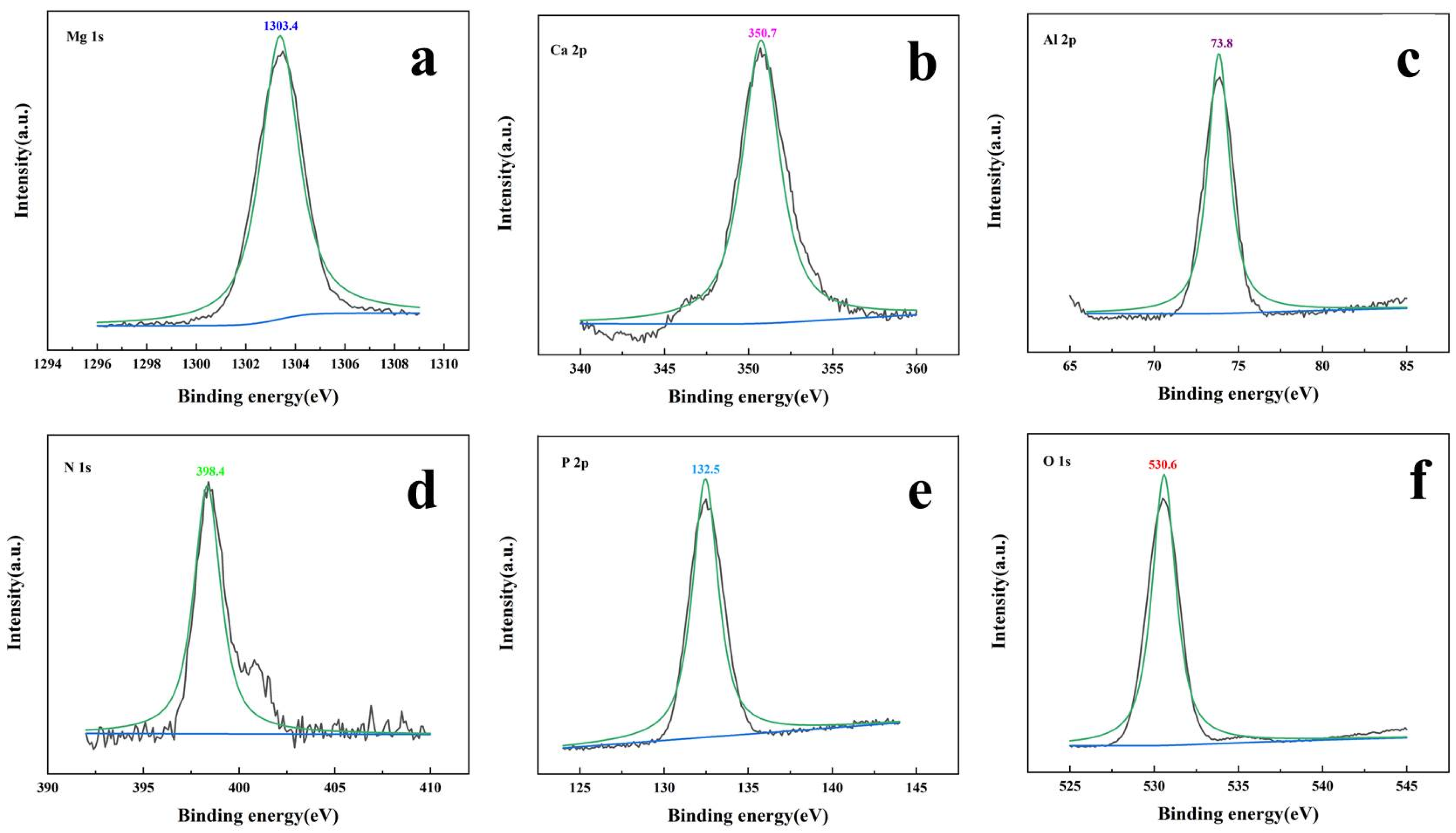
3.1.1. Chemical Properties of TM-DTPMP LDHs
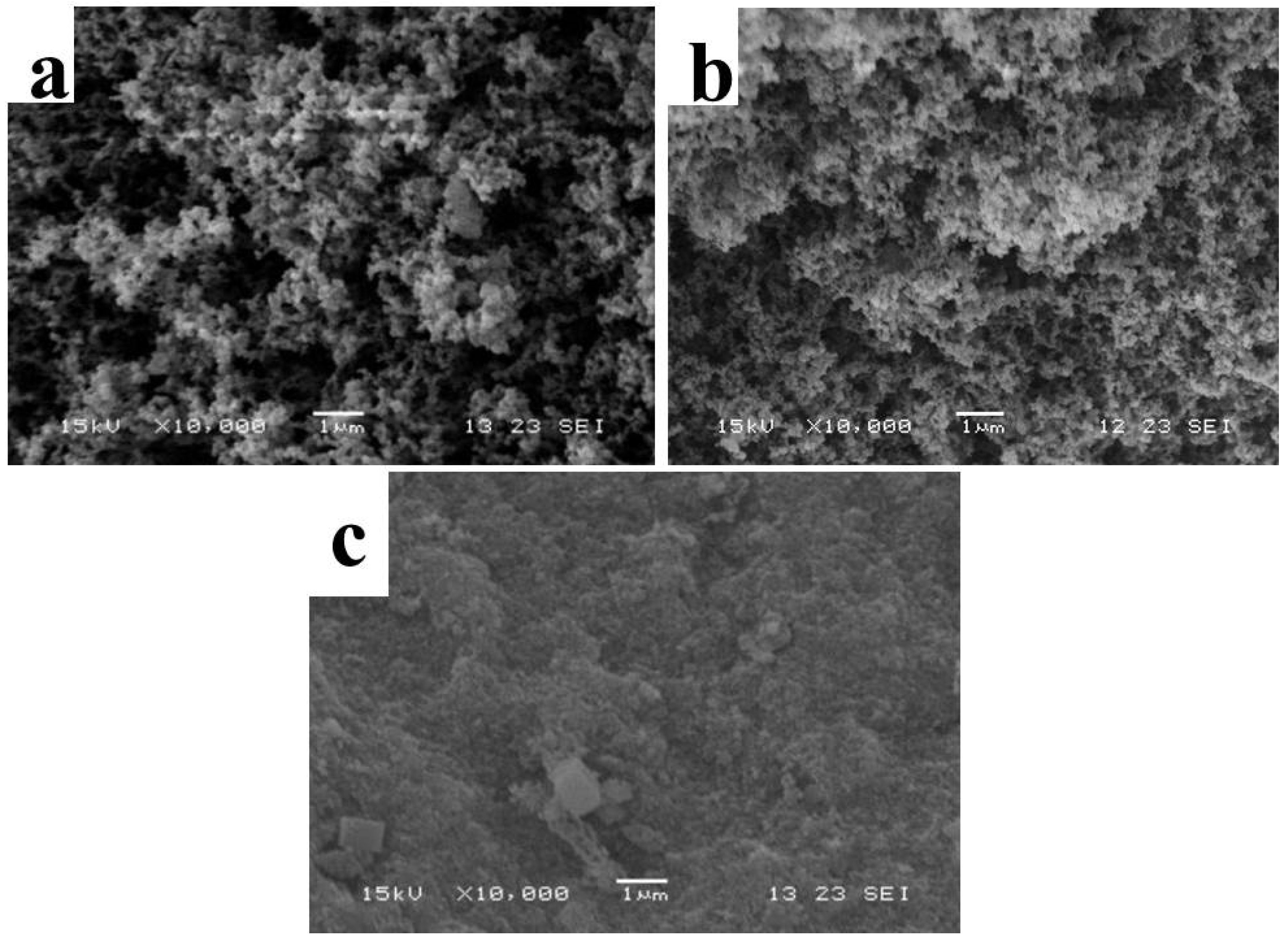
3.1.2. Microstructure of TM LDHs and TM-DTPMP LDHs
3.1.3. Thermal Stability of TM-DTPMP LDHs
3.2. Flame Retardant Performance Analysis of TM-DTPMP LDHs/EP Composites
3.2.1. TG Analysis
3.2.2. Analysis of Oxygen Index
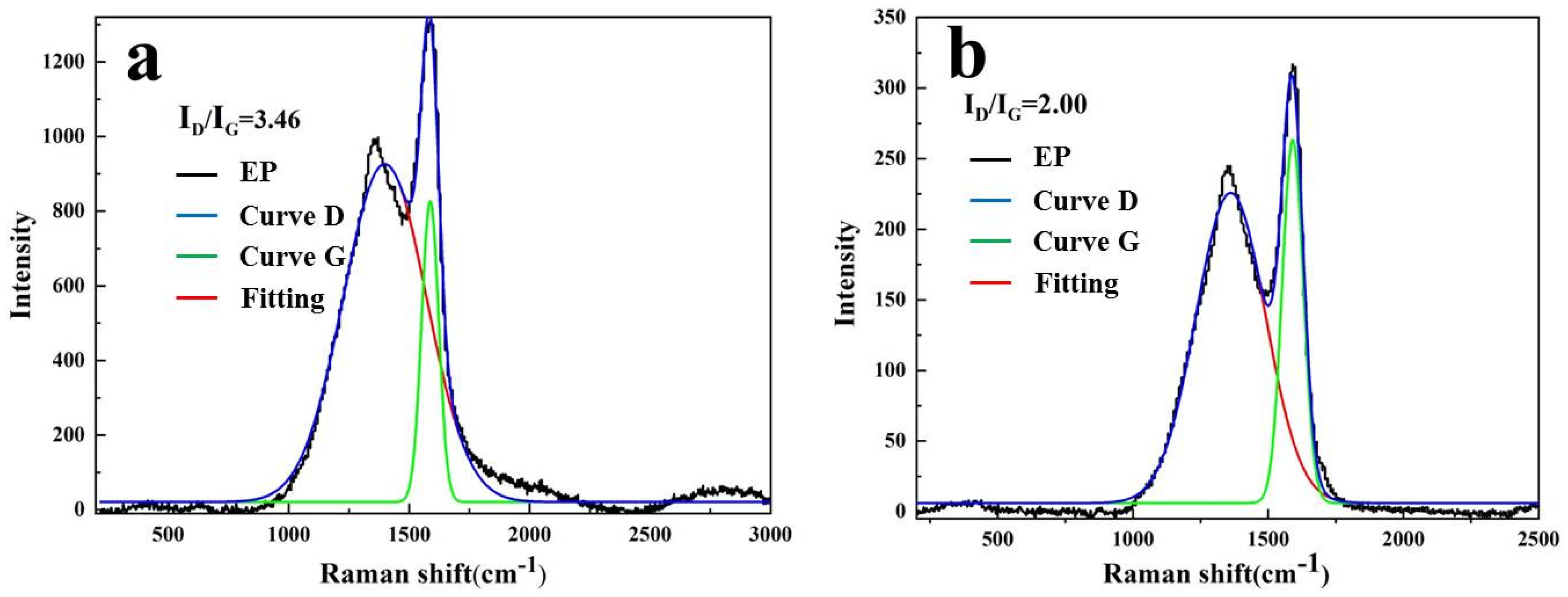
3.2.3. Solid Phase Products of Composites after Combustion Analysis
3.2.4. Combustion Behavior Analysis of TM-DTPMP LDHs/EP Composites
3.2.5. Flame Retardant Mechanism Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Li, J.; Rao, F. Mechanical property and structural evolution of alkali-activated slag-phosphate mine tailings mortars. Chemosphere 2020, 251, 126367. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Dai, J.; Hou, Y. An efficient and environmentally friendly process for the reduction of SO2 by using waste phosphate mine tailings as adsorbent. J. Hazard. Mater. 2020, 388, 121748. [Google Scholar] [CrossRef] [PubMed]
- Perumal, P.; Piekkari, K.; Sreenivasan, H. One-part geopolymers from mining residues-Effect of thermal treatment on three different tailings. Miner. Eng. 2019, 144, 106026. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, Z.; Irfan, M. Laboratory investigation of the relationship between electrical resistivity and geotechnical properties of phosphate tailings. Measurement 2018, 126, 289–298. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiang, C.; Lei, H. Effect of change of Ca, P and Mg on the surface of catalyst prepared from phosphate tailing on urea alcoholysis. Catal. Commun. 2019, 128, 105712. [Google Scholar] [CrossRef]
- Lomakin, S.M.; Zaikov, G.E. New type of ecologically safe flame retardant based on polymer char former. Polym. Degrad. Stabil. 1996, 51, 343–350. [Google Scholar] [CrossRef]
- Chen, L.; Ruan, C.; Yang, R. Phosphorus-containing thermotropic liquid crystalline polymers: A class of efficient polymeric flame retardants. Polym. Chem. 2014, 5, 3737–3749. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.Y.; Lu, H.D.; Lv, P.; Jie, G.X. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer 2010, 51, 2435–2445. [Google Scholar] [CrossRef]
- Hobbs, C.E. Recent Advances in Bio-Based Flame Retardant Additives for Synthetic Polymeric Materials. Polymers 2019, 11, 224. [Google Scholar] [CrossRef] [Green Version]
- Sag, J.; Goedderz, D.; Kukla, P.; Greiner, L.; Schonberger, F.; Doring, M. Phosphorus-Containing Flame Retardants from Biobased Chemicals and Their Application in Polyesters and Epoxy Resins. Molecules 2019, 24, 3746. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Jin, F.L.; Park, S.J. Synthesis of a novel phosphorus-nitrogen-containing intumescent flame retardant and its application to fabrics. J. Ind. Eng. Chem. 2015, 27, 40–43. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Chen, M.J.; Wang, X.; Tao, M.C. Full substitution of petroleum-based polyols by phosphorus-containing soy-based polyols for fabricating highly flame-retardant polyisocyanurate foams. Polym. Degrad. Stabil. 2018, 154, 312–322. [Google Scholar] [CrossRef]
- Zammarano, M.; Bellayer, S.; Gilman, J.W.; Franceschi, M.; Beyer, F.L.; Harris, R.H.; Meriani, S. Delamination of organo-modified layered double hydroxides in polyamide 6 by melt processing. Polymer 2006, 47, 652–662. [Google Scholar] [CrossRef]
- Ding, J.M.; Zhang, Y.; Zhang, X.; Kong, Q.H.; Zhang, J.H.; Liu, H.; Zhang, F. Improving the flame-retardant efficiency of layered double hydroxide with disodium phenylphosphate for epoxy resin. J. Therm. Anal. Calorim. 2020, 140, 149–156. [Google Scholar] [CrossRef]
- Naderi Kalali, E.; Wang, X.; Wang, D.Y. Functionalized layered double hydroxide-based epoxy nanocomposites with improved flame retardancy and mechanical properties. J. Mater. Chem. A 2015, 3, 6819–6826. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Naderi Kalali, E.; Wang, D.Y. Renewable Cardanol-Based Surfactant Modified Layered Double Hydroxide as a Flame Retardant for Epoxy Resin. ACS Sustain. Chem. Eng. 2015, 3, 3281–3290. [Google Scholar] [CrossRef]
- Jiang, S.D.; Bai, Z.M.; Tang, G.; Song, L.; Stec, A.A.; Hull, T.R.; Hu, Y. Synthesis of Mesoporous Silica@Co−Al Layered Double Hydroxide Spheres: Layer-by-Layer Method and Their Effects on the Flame Retardancy of Epoxy Resins. ACS Appl. Mater. Interfaces 2014, 6, 14076–14086. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Qin, J.Y.; Yang, R.J. Synthesis of a novel dual layered double hydroxide hybrid nanomaterial and its application in epoxy nanocomposites. Chem. Eng. J. 2020, 381, 122777. [Google Scholar] [CrossRef]
- Kong, Q.H.; Wu, T.; Tang, Y.Q.; Xiong, L.M.; Liu, H.; Zhang, J.H.; Guo, R.H.; Zhang, F. Improving Thermal and Flame Retardant Properties of Epoxy Resin with Organic NiFe-Layered Double Hydroxide-Carbon Nanotubes Hybrids. Chin. J. Chem. 2017, 35, 1875–1880. [Google Scholar] [CrossRef]
- Kiaei, Z.; Haghtalab, A. Experimental study of using Ca-DTPMP nanoparticles in inhibition of CaCO3 scaling in a bulk water process. Desalination 2014, 338, 84–92. [Google Scholar] [CrossRef]
- Zhang, H.L.; Zhang, J.X.; Wu, H.J.; Pan, Y.; Xia, Y.; Pan, Z.Q.; Wang, D.S. Synthesis and characterization of heterogeneous catalyst EDTMPA-Cu-LDH and study of the mechanism of visible-light photocatalytic degradation of Rhodamine B. Desalin. Water. Treat. 2020, 196, 177–188. [Google Scholar] [CrossRef]
- Mantilla, A.; Jácome-Acatitla, G.; Morales-Mendoza, G. Photoassisted Degradation of 4-Chlorophenol andp-Cresol Using MgAl Hydrotalcites. Ind. Eng. Chem. Res. 2011, 50, 2762–2767. [Google Scholar] [CrossRef]
- Lestari, F.; Green, A.R.; Chattopadhyay, G. An alternative method for fire smoke toxicity assessment using human lung cells. Fire Saf. J. 2006, 41, 605–615. [Google Scholar] [CrossRef]
- Fang, L.; Li, W.; Chen, H. Synergistic effect of humic and fulvic acids on Ni removal by the calcined Mg/Al layered double hydroxide. RSC Adv. 2015, 5, 18866–18874. [Google Scholar] [CrossRef]
- Stojilovic, N.; Isaacs, D.E. Inquiry-Based Experiment with Powder XRD and FeS2 Crystal: “Discovering” the (400) Peak. J. Chem. Educ. 2019, 96, 1449–1452. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Fan, F. Effect of Divalent Metals on the UV-Shielding Properties of M(II)/MgAl Layered Double Hydroxides. ACS Omega 2019, 4, 10151–10159. [Google Scholar] [CrossRef]
- Li, J.; Cui, H.; Song, X. Adsorption and intercalation of organic pollutants and heavy metal ions into MgAl-LDHs nanosheets with high capacity. RSC Adv. 2016, 6, 92402–92410. [Google Scholar] [CrossRef]
- Song, J.X.; Yu, Z.X.; Gordin, M.L.; Li, X.L.; Peng, H.S.; Wang, D.H. Advanced Sodium Ion Battery Anode Constructed via Chemical Bonding between Phosphorus, Carbon Nanotube, and Cross-Linked Polymer Binder. ACS Nano 2015, 12, 11933–11941. [Google Scholar] [CrossRef]
- Fonder, G.; Minet, I.; Volcke, C. Anchoring of alkylphosphonic derivatives molecules on copper oxide surfaces. Appl. Surf. Sci. 2011, 257, 6300–6307. [Google Scholar] [CrossRef]
- Gao, T.; Hou, S.; Huynh, K. Existence of Solid Electrolyte Interphase in Mg Batteries: Mg/S Chemistry as an Example. ACS Appl. Mater. Interfaces 2018, 10, 14767–14776. [Google Scholar] [CrossRef] [PubMed]
- Connell, J.G.; Genorio, B.; Lopes, P.P. Tuning the Reversibility of Mg Anodes via Controlled Surface Passivation by H2O/Cl− in Organic Electrolytes. Chem. Mater. 2016, 28, 8268–8277. [Google Scholar] [CrossRef]
- Gao, D.Q.; Zhang, J.; Yang, G.J.; Zhang, J.L.; Shi, Z.H.; Qi, J.; Zhang, Z.H.; Xue, D.S. Ferromagnetism in ZnO Nanoparticles Induced by Doping of a Nonmagnetic Element: Al. J. Chem. Phys. 2010, 114, 13477–13481. [Google Scholar] [CrossRef]
- Mao, N.; Zhou, C.H.; Keeling, J. Tracked changes of dolomite into Ca-Mg-Al layered double hydroxide. Appl. Clay Sci. 2018, 159, 25–36. [Google Scholar] [CrossRef]
- Zhang, W.; He, X.; Ye, G. Americium(III) capture using phosphonic acid-functionalized silicas with different mesoporous morphologies: Adsorption behavior study and mechanism investigation by EXAFS/XPS. Environ. Sci. Technol. 2014, 48, 6874–6881. [Google Scholar] [CrossRef]
- Kim, Y.J.P.; Chong, R. Analysis of Problematic Complexing Behavior of Ferric Chloride with N,N-Dimethylformamide Using Combined Techniques of FT-IR, XPS, and TGA/DTG. Inorg. Chem. 2002, 41, 6211–6216. [Google Scholar] [CrossRef]
- Nayak, S.; Swain, G.; Parida, K. Enhanced Photocatalytic Activities of RhB Degradation and H2 Evolution from in Situ Formation of the Electrostatic Heterostructure MoS2/NiFe LDH Nanocomposite through the Z-Scheme Mechanism via p-n Heterojunctions. ACS Appl. Mater. Interfaces 2019, 11, 20923–20942. [Google Scholar] [CrossRef]
- Wu, H.J.; Zhang, H.L.; Zhang, W.J.; Yang, X.F.; Zhou, H.; Pan, Z.Q.; Wang, D.S. Preparation of magnetic polyimide@ Mg-Fe layered double hydroxides core-shell composite for effective removal of various organic contaminants from aqueous solution. Chemosphere 2019, 219, 66–75. [Google Scholar] [CrossRef]
- Wu, H.J.; Zhang, W.J.; Zhang, H.L.; Pan, Y.; Yang, X.F.; Pan, Z.Q.; Yu, X.J.; Wang, D.S. Preparation of the novel g-C3N4 and porous polyimide supported hydrotalcite-like compounds materials for water organic contaminants removal. Colloid Surf. A 2020, 607, 125517. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.S.; Zhang, Z.; Wang, Q. Preparation of ammonium polyphosphate and dye co-intercalated LDH/polypropylene composites with enhanced flame retardant and UV resistance properties. Chemosphere 2021, 277, 130370. [Google Scholar] [CrossRef]
- Guo, X.; Wang, H.S.; Ma, D.L.; He, J.N.; Lei, Z.Q. Synthesis of a novel, multifunctional inorganic curing agent and its effect on the flame-retardant and mechanical properties of intrinsically flame retardant epoxy resin. Appl. Polym. Sci. 2018, 29, 46410. [Google Scholar] [CrossRef]
- Qian, X.D.; Song, L.; Yu, B.; Wang, B.B.; Yuan, B.H.; Shi, Y.Q.; Hu, Y.; Yuen, R.K.K. Novel organic-inorganic flame retardants containing exfoliated graphene: Preparation and their performance on the flame retardancy of epoxy resins. J. Mater. Chem. A 2013, 1, 6822–6830. [Google Scholar] [CrossRef]
- Shi, C.L.; Qian, X.D.; Jing, J.Y. Phosphorylated cellulose/Fe(3+)complex: A novel flame retardant for epoxy resins. Polym. Adv. Technol. 2021, 32, 183–189. [Google Scholar] [CrossRef]
- Gupta, S.S.; Sreeprasad, T.S.; Maliyekkal, S.M. Graphene from sugar and its application in water purification. ACS Appl. Mater. Interfaces 2012, 4, 4156–4163. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Wang, B.; Jiang, S. Facile Preparation of Nickel Phosphide (Ni12P5) and Synergistic Effect with Intumescent Flame Retardants in Ethylene-Vinyl Acetate Copolymer. Ind. Eng. Chem. Res. 2013, 52, 6303–6310. [Google Scholar] [CrossRef]
- Wang, L.; Yang, W.; Wang, B. The Impact of Metal Oxides on the Combustion Behavior of Ethylene-Vinyl Acetate Coploymers Containing an Intumenscent Flame Retardant. Ind. Eng. Chem. Res. 2012, 51, 7884–7890. [Google Scholar] [CrossRef]
- Lu, H.D.; Wilkie, C.A. Study on intumescent flame retarded polystyrene composites with improved flame retardancy. Polym. Degrad. Stabil. 2010, 95, 2388–2395. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.M.; Lin, P.L.; Wang, H.; Wang, L.X.; Yu, B.; Yang, F.H. A facile one-step synthesis of highly efficient melamine salt reactive flame retardant for epoxy resin. J. Mater. Sci. 2020, 55, 12836–12847. [Google Scholar] [CrossRef]
- Zhu, Z.M.; Wang, L.X.; Lin, X.B.; Dong, L.P. Synthesis of a novel phosphorus-nitrogen flame retardant and its application in epoxy resin. Polym. Degrad. Stabil. 2019, 169, 108981. [Google Scholar] [CrossRef]
- Mei, Y.J.; Xu, J.X.; Jiang, L.H.; Chen, P.; Tan, Q.P. Protecting of steel from chloride-induced corrosion by cement slurry coatings with calcined Mg-Al layered double hydroxides. Mater. Rev. 2018, 32, 3941–3947. [Google Scholar]
- Zhang, X.; Li, Y.; Yan, S. Improved flame-retardant properties of HIPS/ATH system by organo Fe-montmorillonite. Nanomater. Energy 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Branca, C.; D’Angelo, G.; Crupi, C. Role of the OH and NH vibrational groups in polysaccharide-nanocomposite interactions: A FTIR-ATR study on chitosan and chitosan/clay films. Polymer 2016, 99, 614–622. [Google Scholar] [CrossRef]
- Liang, S.H.; Zhang, L.F.; Chen, Z.L.; Fu, F. Flame Retardant Efficiency of Melamine Pyrophosphate with Added Mg-Al-Layered Double Hydroxide in Medium Density Fiberboards. Bioresources 2017, 12, 533–545. [Google Scholar] [CrossRef] [Green Version]


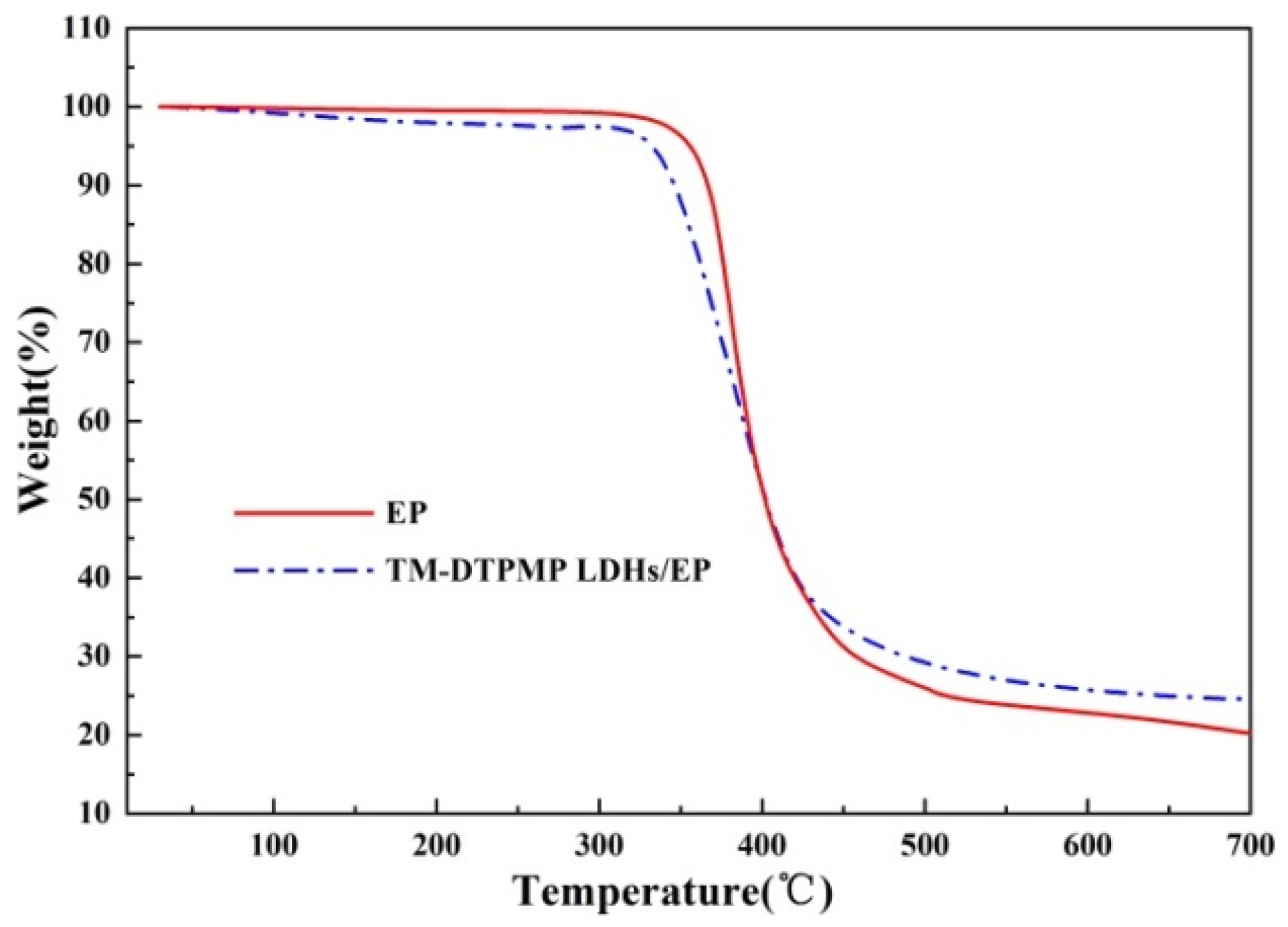


| Sample | T5% (°C) | Tmax (°C) | Residue at 750 °C (%) |
|---|---|---|---|
| TM LDHs | 102.3 | 328.4 | 60.7 |
| TM-DTPMP LDHs | 103.5 | 325.1 | 67.3 |
| EP | 367.5 | 391.4 | 22 |
| TM-DTPMP LDHs/EP | 340.3 | 385.6 | 27.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Jin, L.; Wu, H.; Zhang, Z.; Yu, J.; Zhang, W.; Pan, Y.; Pan, Z. Preparation of a Novel Organic Phosphonic Acid Intercalated Phosphate Tailings Based Hydrotalcite and Its Application in Enhancing Fire Safety for Epoxy Resin. Polymers 2022, 14, 725. https://doi.org/10.3390/polym14040725
Zhang H, Jin L, Wu H, Zhang Z, Yu J, Zhang W, Pan Y, Pan Z. Preparation of a Novel Organic Phosphonic Acid Intercalated Phosphate Tailings Based Hydrotalcite and Its Application in Enhancing Fire Safety for Epoxy Resin. Polymers. 2022; 14(4):725. https://doi.org/10.3390/polym14040725
Chicago/Turabian StyleZhang, Huali, Lingzi Jin, Hanjun Wu, Zhenyue Zhang, Junxia Yu, Wenjun Zhang, Yi Pan, and Zhiquan Pan. 2022. "Preparation of a Novel Organic Phosphonic Acid Intercalated Phosphate Tailings Based Hydrotalcite and Its Application in Enhancing Fire Safety for Epoxy Resin" Polymers 14, no. 4: 725. https://doi.org/10.3390/polym14040725
APA StyleZhang, H., Jin, L., Wu, H., Zhang, Z., Yu, J., Zhang, W., Pan, Y., & Pan, Z. (2022). Preparation of a Novel Organic Phosphonic Acid Intercalated Phosphate Tailings Based Hydrotalcite and Its Application in Enhancing Fire Safety for Epoxy Resin. Polymers, 14(4), 725. https://doi.org/10.3390/polym14040725







