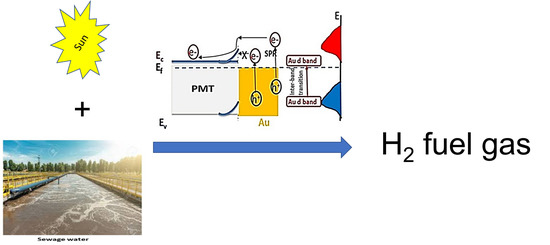
Effect of Au Plasmonic Material on Poly M-Toluidine for Photoelectrochemical Hydrogen Generation from Sewage Water
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Electropolymerization of MT and Preparation of the Electrode
2.3. Characterization Process
3. Results and Discussion
3.1. Electropolymerization of M-Toluidine
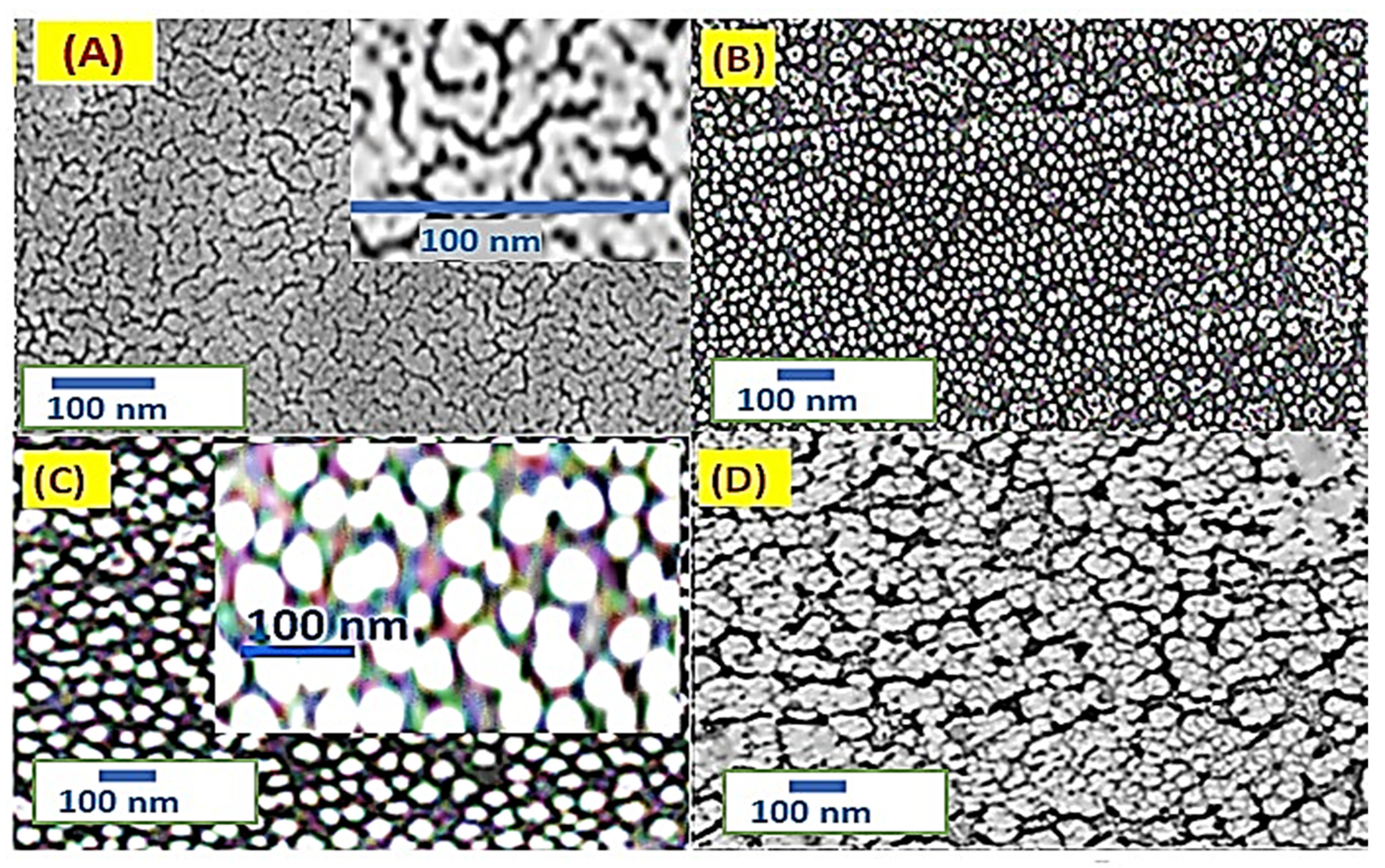
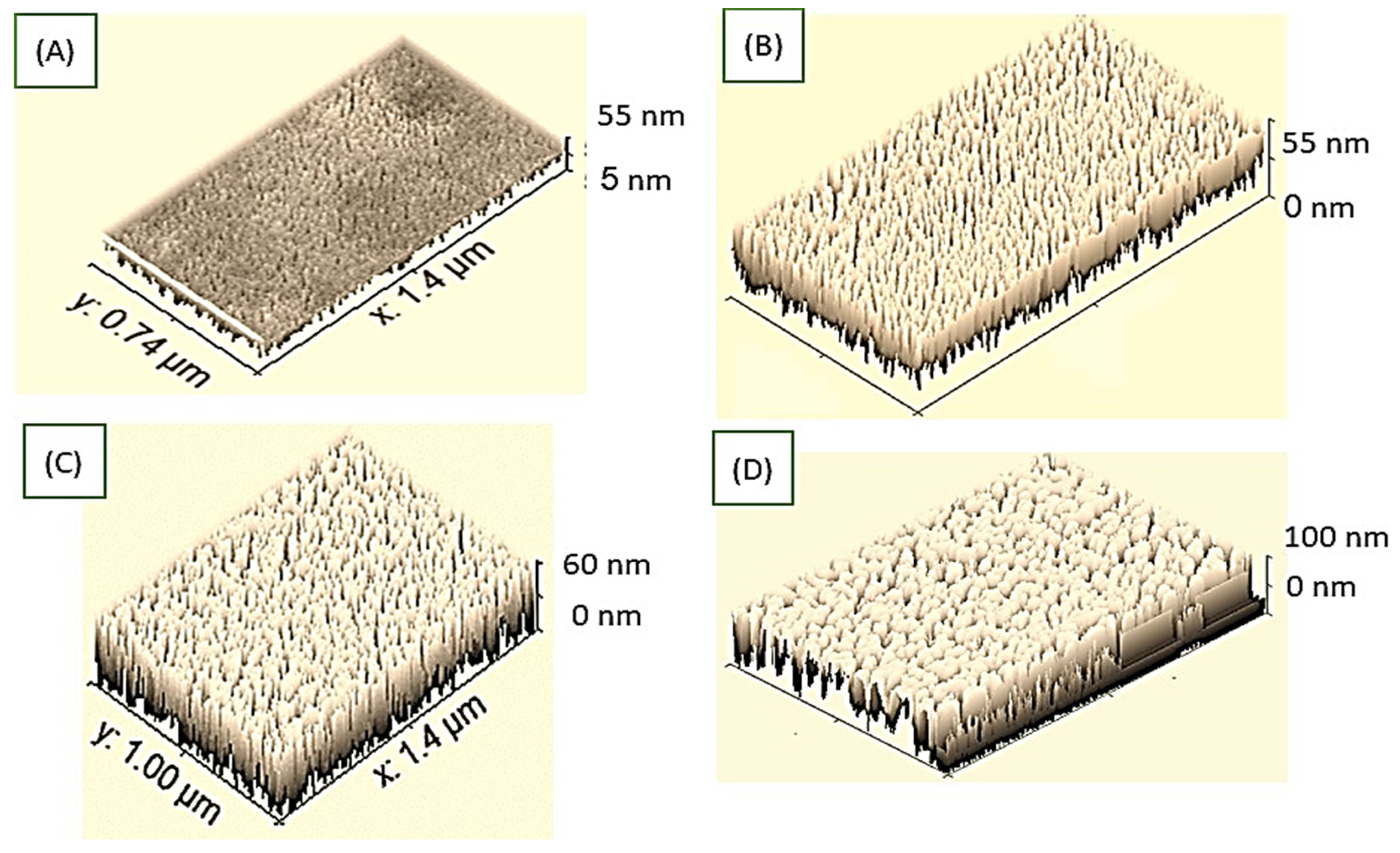
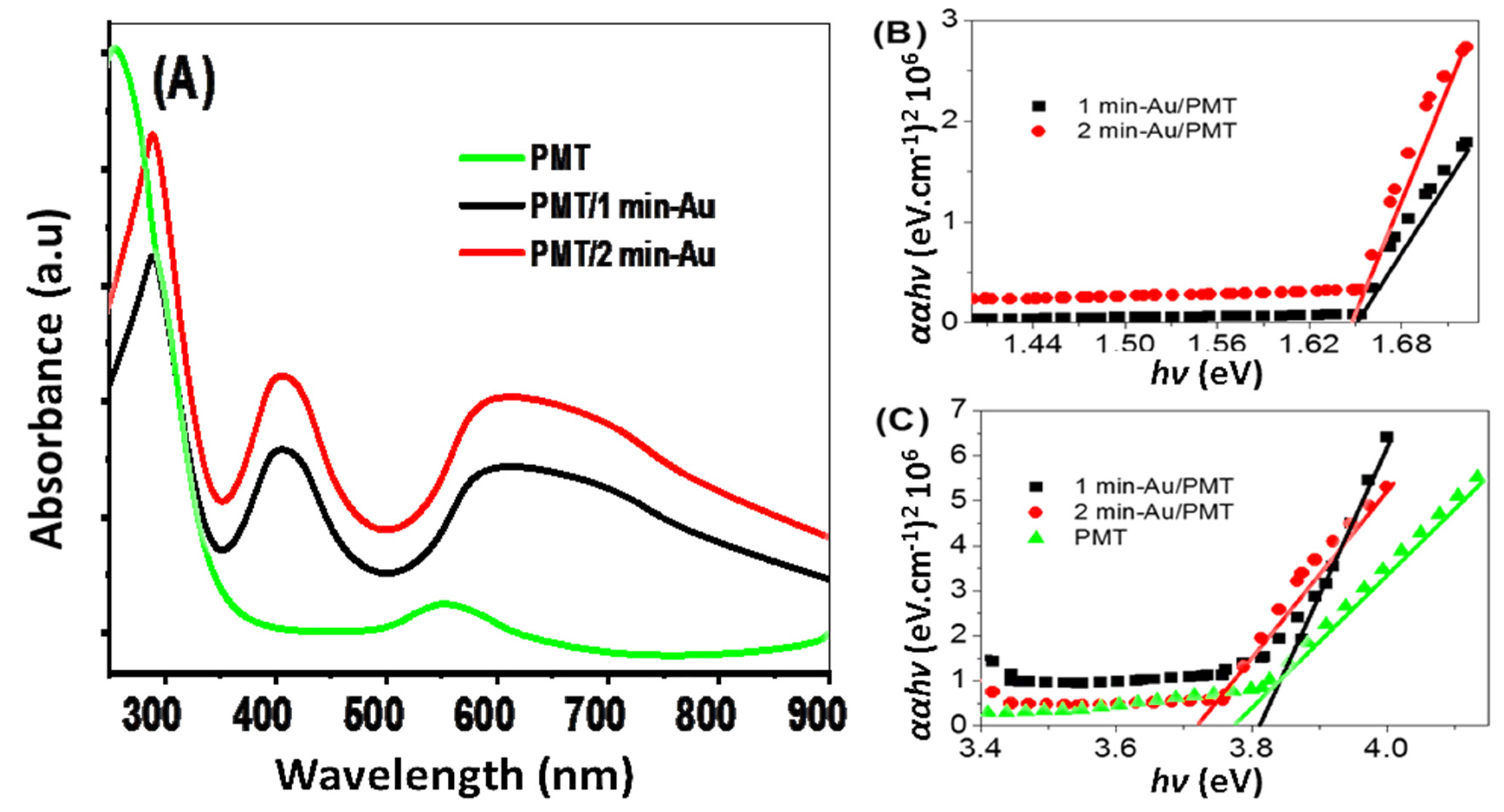
3.2. PMT and PMT/Au Analyses
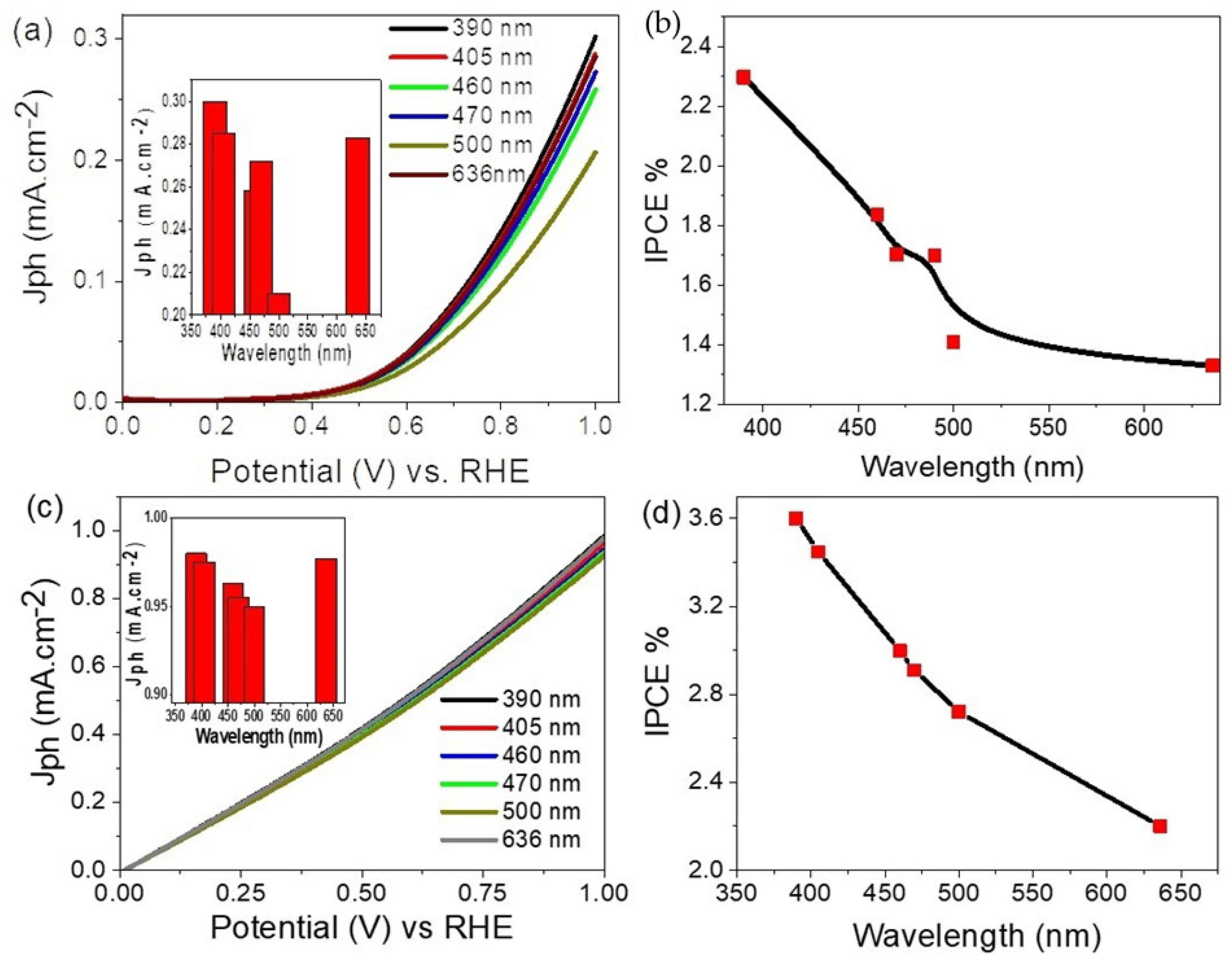
3.3. Electrochemical Hydrogen Generation
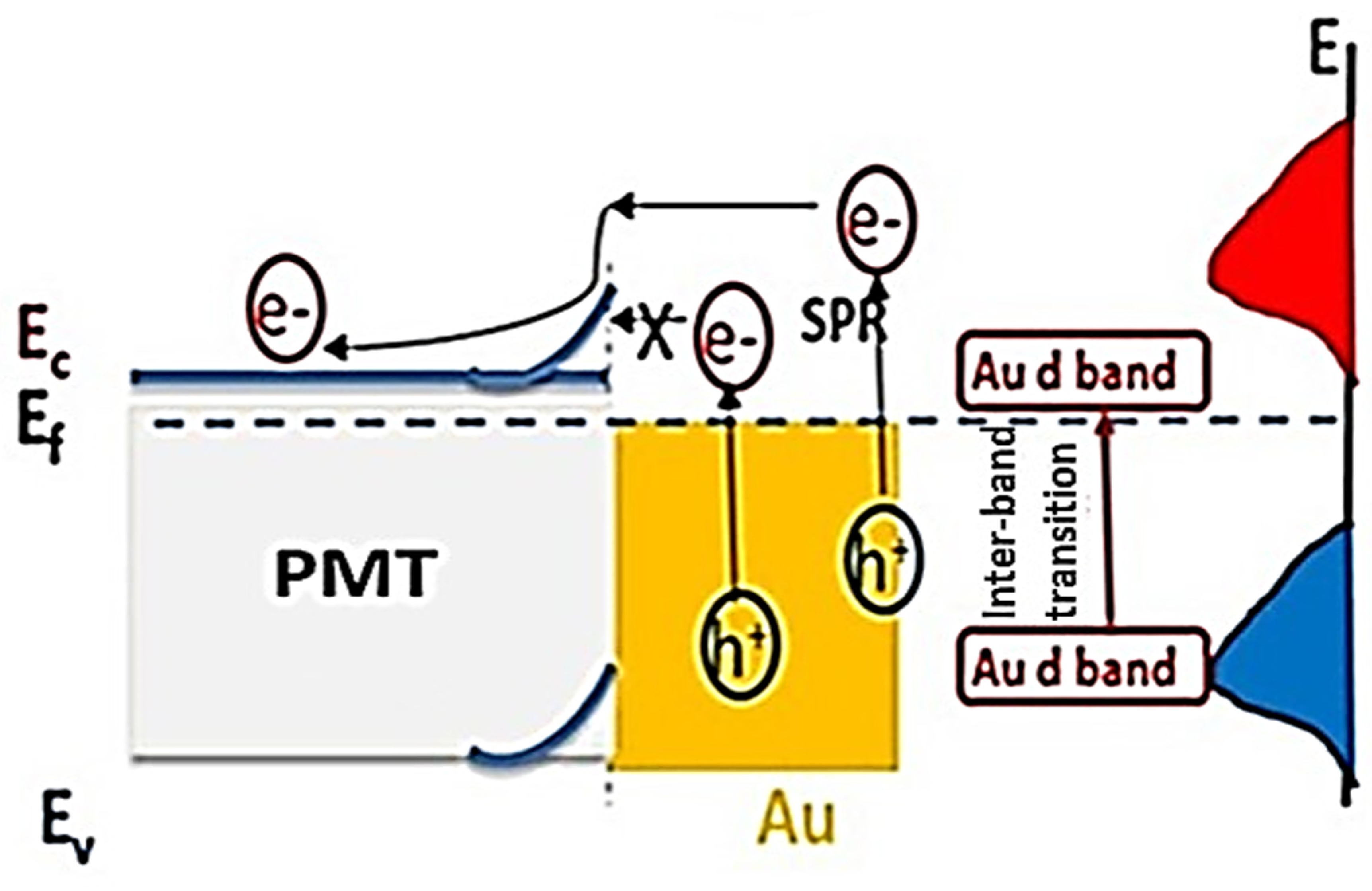
3.4. Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brandt, J.; Salerno, F.; Fuchter, M.J. The added value of small-molecule chirality in technological applications. Nat. Rev. Chem. 2017, 1, 0045. [Google Scholar] [CrossRef]
- Wade, J.; Hilfiker, J.N.; Brandt, J.R.; Liirò-Peluso, L.; Wan, L.; Shi, X.; Salerno, F.; Ryan, S.T.J.; Schöche, S.; Arteaga, O.; et al. Natural optical activity as the origin of the large chiroptical properties in π-conjugated polymer thin films. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Worch, J. Stereochemical enhancement of polymer properties. Nat. Rev. Chem. 2019, 3, 514–535. [Google Scholar] [CrossRef]
- Vøllestad, E.; Strandbakke, R.; Tarach, M.; Catalán-Martínez, D.; Fontaine, M.L.; Beeaff, D.; Clark, D.R.; Serra, J.M.; Norby, T. Mixed proton and electron conducting double perovskite anodes for stable and efficient tubular proton ceramic electrolysers. Nat. Mater. 2019, 18, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Kee, R.; Zhu, H.; Sullivan, N.; Zhu, L.; Bian, L.; Jennings, D.; O’Hayre, R. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 2019, 4, 230–240. [Google Scholar] [CrossRef]
- Service, R.F. New electrolyzer splits water on the cheap. Science 2020, 367, 80. [Google Scholar] [CrossRef]
- Spitaleri, L.; Nicotra, G.; Zimbone, M.; Contino, A.; Maccarrone, G.; Alberti, A.; Gulino, A. Fast and Efficient Sun Light Photocatalytic Activity of Au_ZnO Core-Shell Nanoparticles Prepared by a One-Pot Synthesis. ACS Omega 2019, 4, 15061–15066. [Google Scholar] [CrossRef] [Green Version]
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; Eldakrory, M.G.; Maree, R.M.; Ahmed, A.M. Efficient photoselectrochemical hydrogen production utilizing of APbI 3 (A = Na, Cs, and Li) perovskites nanorods. Int. J. Energy Res. 2020, 45, 7436. [Google Scholar] [CrossRef]
- Mohamed, F.; Rabia, M.; Shaban, M. Synthesis and characterization of biogenic iron oxides of different nanomorphologies from pomegranate peels for efficient solar hydrogen production. J. Mater. Res. Technol. 2020, 9, 4255–4271. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Zhao, H.; Romanus, H.; El-Hossary, F.M.; EL-Kassem, M.A.; Awad, M.A.; Rabia, M.; Lei, Y. Optical, water splitting and wettability of titanium nitride/titanium oxynitride bilayer films for hydrogen generation and solar cells applications. Mater. Sci. Semicond. Process. 2020, 105, 104704. [Google Scholar] [CrossRef]
- Shaban, M.; Ali, S.; Rabia, M. Design and application of nanoporous graphene oxide film for CO2, H2, and C2H2 gases sensing. J. Mater. Res. Technol. 2019, 8, 4510–4520. [Google Scholar] [CrossRef]
- Almohammedi, A.; Shaban, M.; Mostafa, H.; Rabia, M. Nanoporous TiN/TiO2/Alumina Membrane for Photoelectrochemical Hydrogen Production from Sewage Water. Nanomaterials 2021, 11, 2617. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.M.; Rabia, M.; Shaban, M.; Aly, A.H.; Ahmed, A.M. Preparation of hexagonal nanoporous Al2O3/TiO2/TiN as a novel photodetector with high efficiency. Sci. Rep. 2021, 11, 17572. [Google Scholar] [CrossRef]
- Rabia, M.; Shaban, M.; Adel, A.; Abdel-Khaliek, A.A. Effect of plasmonic au nanoparticles on the photoactivity of polyaniline/indium tin oxide electrodes for water splitting. Environ. Prog. Sustain. Energy 2019, 38, 13171. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, D.; Khare, N. Plasmonic Ag nanoparticles decorated Bi 2 S 3 nanorods and nanoflowers: Their comparative assessment for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2019, 44, 3538–3552. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.; Zhang, H.; Li, B.; Lu, H.; Ma, T.; Hao, C. Effects of 4-tert-butylpyridine on perovskite formation and performance of solution-processed perovskite solar cells. J. Mater. Chem. A 2015, 3, 22191–22198. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, M.; Liu, J.; Deng, Z.; Li, Y.; Su, B.L. Synergistic promotion of solar-driven H2 generation bythree-dimensionally ordered macroporous structured TiO2-Au-CdS ternary photocatalyst. Appl. Catal. B Environ. 2016, 184, 182–190. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; El-Sayed, A.M.A.; Ahmed, A.; Sayed, S. Photocatalytic properties of PbS/graphene oxide/polyaniline electrode for hydrogen generation. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Atta, A.; Abdelhamied, M.M.; Essam, D.; Shaban, M.; Alshammari, A.H.; Rabia, M. Structural and physical properties of polyaniline/silver oxide/silver nanocomposite electrode for supercapacitor applications. Int. J. Energy Res. 2021; accepted. [Google Scholar] [CrossRef]
- Sengodan, S.; Choi, S.; Jun, A.; Shin, T.H.; Ju, Y.W.; Jeong, H.Y.; Shin, J.; Irvine, J.T.S.; Kim, G. Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat. Mater. 2015, 14, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.H.; Neagu, D.; Miller, D.N.; Irvine, J.T.S. Switching on electrocatalytic activity in solid oxide cells. Nature 2016, 537, 528–531. [Google Scholar] [CrossRef] [Green Version]
- Sayyah, E.-S.M.; Shaban, M.; Rabia, M. A sensor of m -cresol nanopolymer/Pt-electrode film for detection of lead ions by potentiometric methods. Adv. Polym. Technol. 2018, 37, 1296–1304. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. A sensor of m-toluidine/m-cresol polymer film for detection of lead ions by potentiometric methods. Sens. Lett. 2016, 14, 522–529. [Google Scholar] [CrossRef]
- Jung, J.W.; Lee, C.L.; Yu, S.; Kim, I.D. Electrospun nanofibers as a platform for advanced secondary batteries: A comprehensive review. J. Mater. Chem. A 2016, 4, 703–750. [Google Scholar] [CrossRef]
- Ognibene, G.; Gangemi, C.M.A.; Spitaleri, L.; Gulino, A.; Purrello, R.; Cicala, G.; Fragalà, M.E. Role of the surface composition of the polyethersulfone–TiiP–H2T4 fibers on lead removal: From electrostatic to coordinative binding. J. Mater. Sci. 2019, 54, 8023–8033. [Google Scholar] [CrossRef]
- Ghosh, S.; Kouamé, N.A.; Ramos, L.; Remita, S.; Dazzi, A.; Deniset-Besseau, A.; Beaunier, P.; Goubard, F.; Aubert, P.H.; Remita, H. Conducting polymer nanostructures for photocatalysis under visible light. Nat. Mater. 2015, 14, 505–511. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, Q. Controlled synthesis and energy applications of one-dimensional conducting polymer nanostructures: An overview. Adv. Energy Mater. 2012, 2, 179–218. [Google Scholar] [CrossRef]
- Modibane, K.D.; Waleng, N.J.; Ramohlola, K.E.; Maponya, T.C.; Monama, G.R.; Makgopa, K.; Hato, M.J. Poly(3-aminobenzoic acid) decorated with cobalt zeolitic benzimidazolate framework for electrochemical production of clean hydrogen. Polymers 2020, 12, 1581. [Google Scholar] [CrossRef]
- Xiao, K.; Tu, B.; Chen, L.; Heil, T.; Wen, L.; Jiang, L.; Antonietti, M. Photo-Driven Ion Transport for a Photodetector Based on an Asymmetric Carbon Nitride Nanotube Membrane. Angew. Chemie Int. Ed. 2019, 58, 12574–12579. [Google Scholar] [CrossRef] [Green Version]
- Belabed, C.; Abdi, A.; Benabdelghani, Z.; Rekhila, G.; Etxeberria, A.; Trari, M. Photoelectrochemical properties of doped polyaniline: Application to hydrogen photoproduction. Int. J. Hydrogen Energy 2013, 38, 6593–6599. [Google Scholar] [CrossRef]
- Mejia, E.; Cherupurakal, N.; Mourad, A.H.I.; Al Hassanieh, S.; Rabia, M. Effect of Processing Techniques on the Microstructure and Mechanical Performance of High-Density Polyethylene. Polymers 2021, 13, 3346. [Google Scholar] [CrossRef] [PubMed]
- Sayyah, S.M.; Shaban, M.; Rabia, M. M-toluidine polymer film coated platinum electrode as a pH sensor by potentiometric methods. Sens. Lett. 2015, 13, 961–966. [Google Scholar] [CrossRef]
- Sayyah, S.M.; El-Deeb, M.M.; Mohamed, A.S. Electrodeposition of poly(3-hydroxyaniline) from acidic aqueous/acetonitrile solution. Int. J. Polym. Mater. Polym. Biomater. 2007, 56, 1079–1097. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. Electropolymerization of m -Toluidin on Platinum Electrode from Aqueous Acidic Solution and Character of the Obtained Polymer. Adv. Polym. Technol. 2018, 37, 126–136. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. A High-Sensitivity Potentiometric Mercuric Ion Sensor Based on m-Toluidine Films. IEEE Sens. J. 2016, 16, 1541–1548. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; Fathallah, W.; El-Mawgoud, N.A.; Mahmoud, A.; Hussien, H.; Said, O. Preparation and Characterization of Polyaniline and Ag/Polyaniline Composite Nanoporous Particles and Their Antimicrobial Activities. J. Polym. Environ. 2018, 26, 434–442. [Google Scholar] [CrossRef]
- Schindler, A.; Neumann, G.; Rager, A.; Füglein, E.; Blumm, J.; Denner, T. A novel direct coupling of simultaneous thermal analysis (STA) and Fourier transform-infrared (FT-IR) spectroscopy. J. Therm. Anal. Calorim. 2013, 113, 1091–1102. [Google Scholar] [CrossRef] [Green Version]
- Buda, S.; Nawój, M.; Mlynarski, J. Recent Advances in NMR Studies of Carbohydrates. Annu. Rep. NMR Spectrosc. 2016, 89, 185–223. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Rabia, M.; Shaban, M.; Verpoort, F. Heulandite/polyaniline hybrid composite for efficient removal of acidic dye from water; kinetic, equilibrium studies and statistical optimization. Adv. Powder Technol. 2018, 29, 2501–2511. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Rabia, M.; Elkader, Y.A.; Abd El-Halim, M.R. Investigation the adsorption properties of graphene oxide and polyaniline nano/micro structures for efficient removal of toxic Cr(VI) contaminants from aqueous solutions; kinetic and equilibrium studies. Rend. Lincei. Sci. Fis. Nat. 2018, 29, 141. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, N.; Zhang, N.; Song, Y.; Stanislaus, M.S.; Zhao, C.; Yang, Y. Efficient photocatalytic removal of RhB, MO and MB dyes by optimized Ni/NiO/TiO2 composite thin films under solar light irradiation. J. Environ. Chem. Eng. 2018, 6, 2724–2732. [Google Scholar] [CrossRef]
- Contino, A.; Maccarrone, G.; Fragalà, M.E.; Spitaleri, L.; Gulino, A. Conjugated Gold–Porphyrin Monolayers Assembled on Inorganic Surfaces. Chem.–A Eur. J. 2017, 23, 14937–14943. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Thimsen, E.; Le Formal, F.; Grätzel, M.; Warren, S.C. Influence of plasmonic Au nanoparticles on the photoactivity of Fe 2O3 electrodes for water splitting. Nano Lett. 2011, 11, 35–43. [Google Scholar] [CrossRef]
- Peleg, M.; Normand, M.D.; Corradini, M.G. The Arrhenius equation revisited. Crit. Rev. Food Sci. Nutr. 2012, 52, 830–851. [Google Scholar] [CrossRef] [PubMed]
- Rabia, M.; Mohamed, H.S.H.; Shaban, M.; Taha, S. Preparation of polyaniline/PbS core-shell nano/microcomposite and its application for photocatalytic H2 electrogeneration from H2O. Sci. Rep. 2018, 8, 1107. [Google Scholar] [CrossRef] [Green Version]
- Evingür, G.A.; Pekcan, Ö. Optical energy band gap of PAAm-GO composites. Compos. Struct. 2018, 183, 212–215. [Google Scholar] [CrossRef]
- Pan, L.; Liu, Y.; Yao, L.; Ren, D.; Sivula, K.; Grätzel, M.; Hagfeldt, A. Cu2O photocathodes with band-tail states assisted hole transport for standalone solar water splitting. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmy, A.; Rabia, M.; Shaban, M.; Ashraf, A.M.; Ahmed, S.; Ahmed, A.M. Graphite/rolled graphene oxide/carbon nanotube photoelectrode for water splitting of exhaust car solution. Int. J. Energy Res. 2020, 44, 7687–7697. [Google Scholar] [CrossRef]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 63–71. [Google Scholar] [CrossRef]
- Rabia, M.; Mohamed, S.H.; Zhao, H.; Shaban, M.; Lei, Y.; Ahmed, A.M. TiO2/TiOxNY hollow mushrooms-like nanocomposite photoanode for hydrogen electrogeneration. J. Porous Mater. 2020, 27, 133–139. [Google Scholar] [CrossRef]
- Kolivand, A.; Sharifnia, S. Enhanced photocatalytic hydrogen evolution from water splitting by Z-scheme CdS/BiFeO3 heterojunction without using sacrificial agent. Int. J. Energy Res. 2021, 45, 2739–2752. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, D.; Wen, L.; Xu, R.; Obergfell, M.; Mi, Y.; Zhan, Z.; Nasori, N.; Demsar, J.; Lei, Y. Manipulation of charge transfer and transport in plasmonic-ferroelectric hybrids for photoelectrochemical applications. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, F.X.; Miao, J.; Wang, H.Y.; Yang, H.; Chen, J.; Liu, B. Electrochemical construction of hierarchically ordered CdSe-sensitized TiO2 nanotube arrays: Towards versatile photoelectrochemical water splitting and photoredox applications. Nanoscale 2014, 6, 6727–6737. [Google Scholar] [CrossRef] [PubMed]
- Taffa, D.H.; Hamm, I.; Dunkel, C.; Sinev, I.; Bahnemann, D.; Wark, M. Electrochemical deposition of Fe2O3 in the presence of organic additives: A route to enhanced photoactivity. RSC Adv. 2015, 5, 103512–103522. [Google Scholar] [CrossRef] [Green Version]
- Sherman, B.D.; Ashford, D.L.; Lapides, A.M.; Sheridan, M.V.; Wee, K.R.; Meyer, T.J. Light-Driven Water Splitting with a Molecular Electroassembly-Based Core/Shell Photoanode. J. Phys. Chem. Lett. 2015, 6, 3213–3217. [Google Scholar] [CrossRef]
- Wei, Y.; Ke, L.; Kong, J.; Liu, H.; Jiao, Z.; Lu, X.; Du, H.; Sun, X.W. Enhanced photoelectrochemical water-splitting effect with a bent ZnO nanorod photoanode decorated with Ag nanoparticles. Nanotechnology 2012, 23, 235401. [Google Scholar] [CrossRef]
- Freeman, E.; Kumar, S.; Thomas, S.R.; Pickering, H.; Fermin, D.J.; Eslava, S. PrFeO3 Photocathodes Prepared Through Spray Pyrolysis. ChemElectroChem 2020, 7, 1365–1372. [Google Scholar] [CrossRef]
- Feng, S.; Ji, W. Advanced Nanoporous Anodic Alumina-Based Optical Sensors for Biomedical Applications. Front. Nanotechnol. 2021, 3, 36. [Google Scholar] [CrossRef]
- Langhammer, C.; Schwind, M.; Kasemo, B.; Zorić, I. Localized Surface Plasmon Resonances in Aluminum Nanodisks. Nano Lett. 2008, 8, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gu, Q.; Hou, L.; Zhang, C.; Lu, Y.; Wang, X.; Long, J. Molecular p–n heterojunction-enhanced visible-light hydrogen evolution over a N-doped TiO2 photocatalyst. Catal. Sci. Technol. 2017, 7, 2039–2049. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Shaban, M.; Aly, A.H.; Ahmed, A.M.; Rabia, M. Preparation and characterization of a high-efficiency photoelectric detector composed of hexagonal Al2O3/TiO2/TiN/Au nanoporous array. Mater. Sci. Semicond. Process. 2022, 139, 106348. [Google Scholar] [CrossRef]

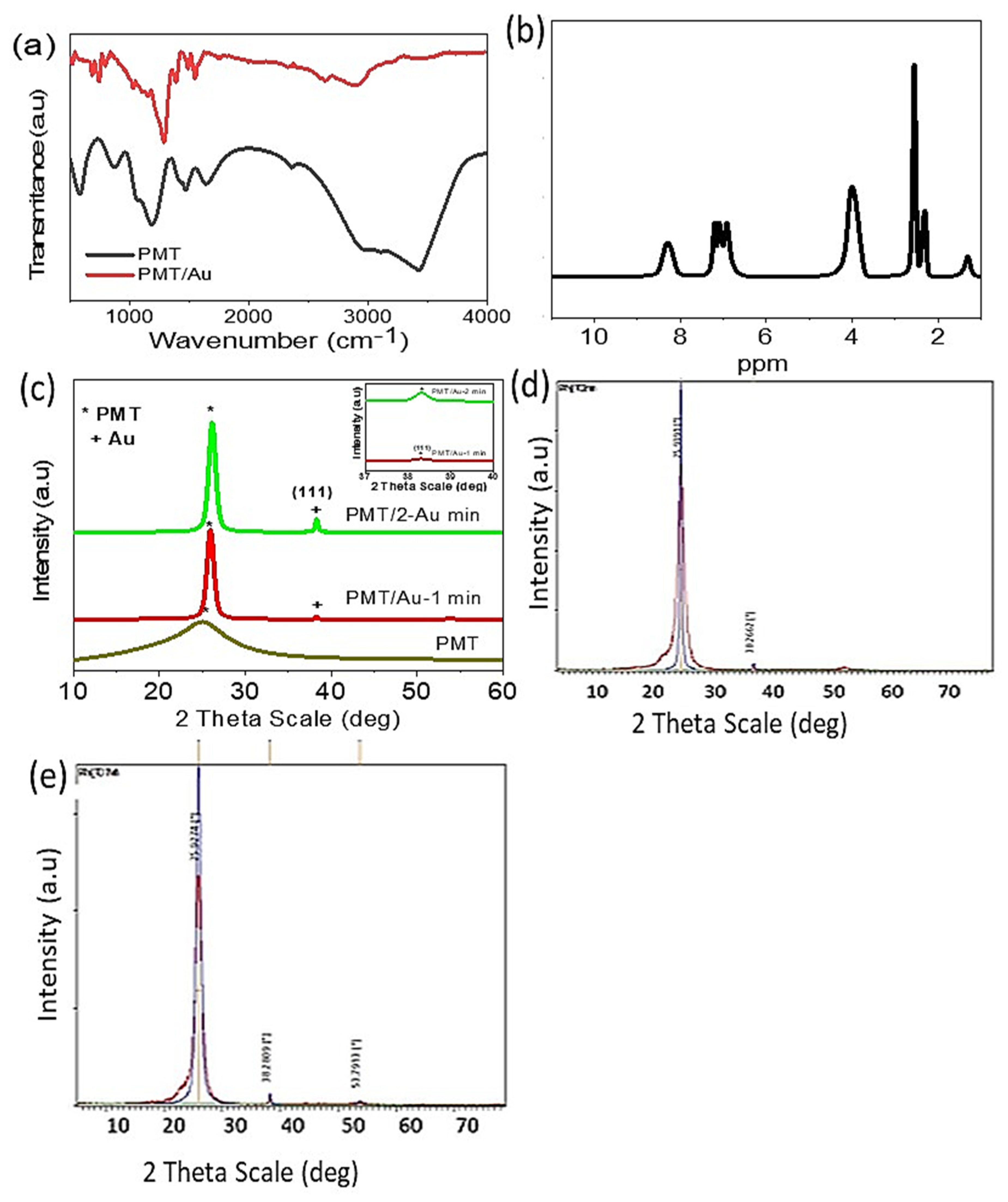


| Band Position (cm−1) | Assignment |
|---|---|
| 3429 | N–H group [35,36,37] |
| 3106 | Aromatic C–H group |
| 2950 | Methyl group stretching [32] |
| 2357 | Adsorbed H2O or CO2 from the atmosphere [38] |
| 1465 and 1407 | C=C benzenoid [32] |
| 1631 | C=C quinoid |
| 1339 | C–N vibrations |
| 1051 | C–H in-plane |
| 595 | C–H out of plane |
| 879 | Aromatic rings para-disubstituted |
| Chemical Shift (ppm) | Assignment and Structure |
|---|---|
| 1.23 | The proton of the methyl group [39] |
| 2.56 | DMSO proton (solvent) [35] |
| 2.3 | Adsorbed H2O |
| 4.07 | Singlet signal for NH proton [35] |
| 6.93 to 7.27 | The protons of the benzene ring [39] |
| Material or Element | Concentration (mg/L) |
|---|---|
| Phenols | 0.015 |
| F− | 1.0 |
| Al3+ | 3.0 |
| NH3 | 5.0 |
| Hg2+ | 0.005 |
| Pb2+ | 0.5 |
| Cd3+ | 0.05 |
| As3+ | 0.05 |
| Cr3+ | 1.0 |
| Cu2+ | 1.5 |
| Ni3+ | 0.1 |
| Fe3+ | 1.5 |
| Mn2+ | 1.0 |
| Zn2+ | 5.0 |
| Ag+ | 0.1 |
| Ba3+ | 2.0 |
| Co2+ | 2.0 |
| Other cations | 0.1 |
| Pesticides | 0.2 |
| CN−1 | 0.1 |
| Industrial washing | 0.5 |
| Coli groups | 4000/100 cm3 |
| Electrode Materials | Electrolyte | IPCE% (390 nm) | Jph (mA cm−2) |
|---|---|---|---|
| BiFeO3 [53] | Na2SO4 | -- | 0.16 |
| Au/Pb(Zr,Ti)O3 [54] | Na2SO4 | -- | 0.06 |
| CdSe/TiO2 nanotube electrode [55] | NaOH | 0.45 | 0.13 |
| Fe2O3/sodium dodecyl sulfonate electrodes [56] | NaOH | 2 | 0.05 |
| SnO2/TiO2 [57] | Na2S2O3 | -- | 0.4 |
| ZnO/Ag [58] | Na2SO4 | 0.5 | 1 |
| PrFeO3 [59] | Na2SO4 | 1.2 | 0.13 |
| ITO/PMT/2 min-Au (present work) | Na2S2O3 | 2.3 | 0.3 |
| ITO/PMT/2 min-Au (present work) | Sewage water | 3.6 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelazeez, A.A.A.; Hadia, N.M.A.; Mourad, A.-H.I.; El-Fatah, G.A.; Shaban, M.; Ahmed, A.M.; Alzaid, M.; Cherupurakal, N.; Rabia, M. Effect of Au Plasmonic Material on Poly M-Toluidine for Photoelectrochemical Hydrogen Generation from Sewage Water. Polymers 2022, 14, 768. https://doi.org/10.3390/polym14040768
Abdelazeez AAA, Hadia NMA, Mourad A-HI, El-Fatah GA, Shaban M, Ahmed AM, Alzaid M, Cherupurakal N, Rabia M. Effect of Au Plasmonic Material on Poly M-Toluidine for Photoelectrochemical Hydrogen Generation from Sewage Water. Polymers. 2022; 14(4):768. https://doi.org/10.3390/polym14040768
Chicago/Turabian StyleAbdelazeez, Ahmed Adel A., N.M.A. Hadia, Abdel-Hamid I. Mourad, Gehad Abd El-Fatah, Mohamed Shaban, Ashour M. Ahmed, Meshal Alzaid, Nizamudeen Cherupurakal, and Mohamed Rabia. 2022. "Effect of Au Plasmonic Material on Poly M-Toluidine for Photoelectrochemical Hydrogen Generation from Sewage Water" Polymers 14, no. 4: 768. https://doi.org/10.3390/polym14040768