Polymeric Nanostructures Containing Proteins and Peptides for Pharmaceutical Applications
Abstract
:1. Introduction
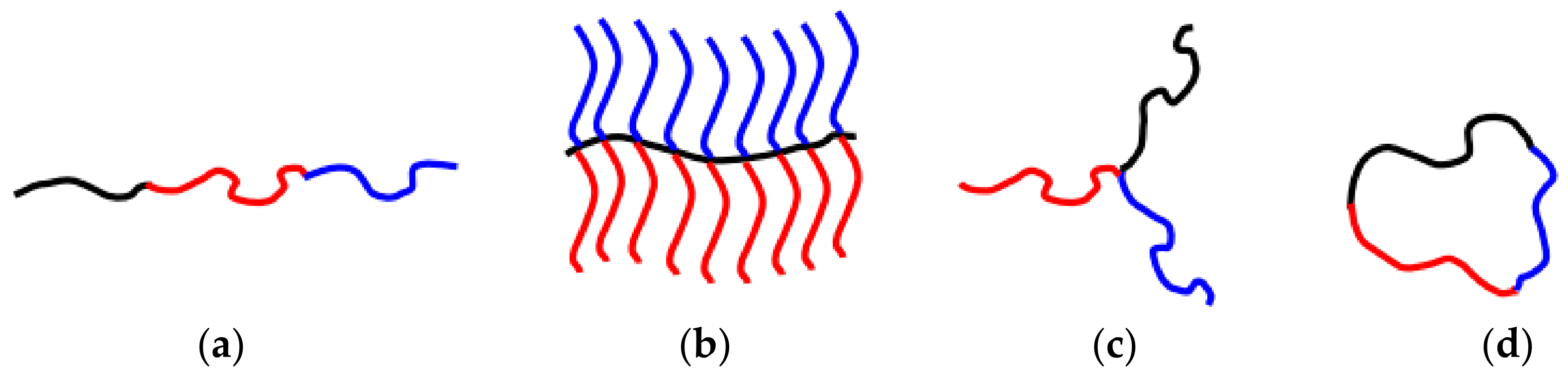
2. Polymeric Nanostructures
3. Linking Polymers, Proteins, and Peptides towards the Formation of Polymer–Protein–Peptide Nanostructures
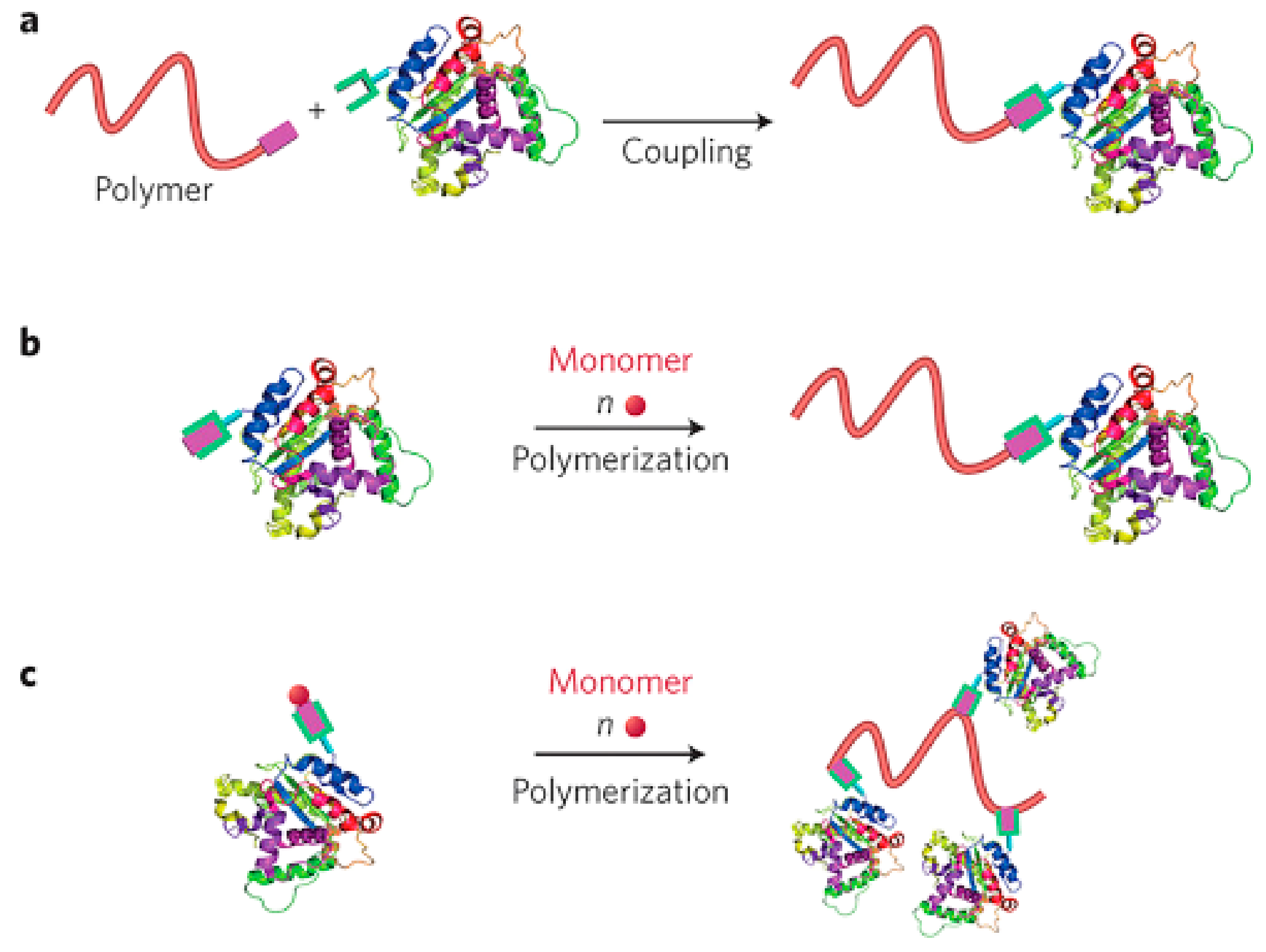
3.1. Polymer–Protein Conjugation
3.2. Chemical Bonding of Polymer Chains with Protein
3.3. Protein Encapsulation
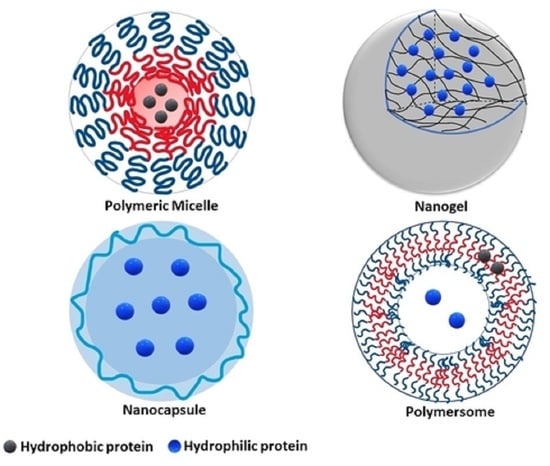
4. Classes of Polymeric Protein and Peptide Nanocarriers
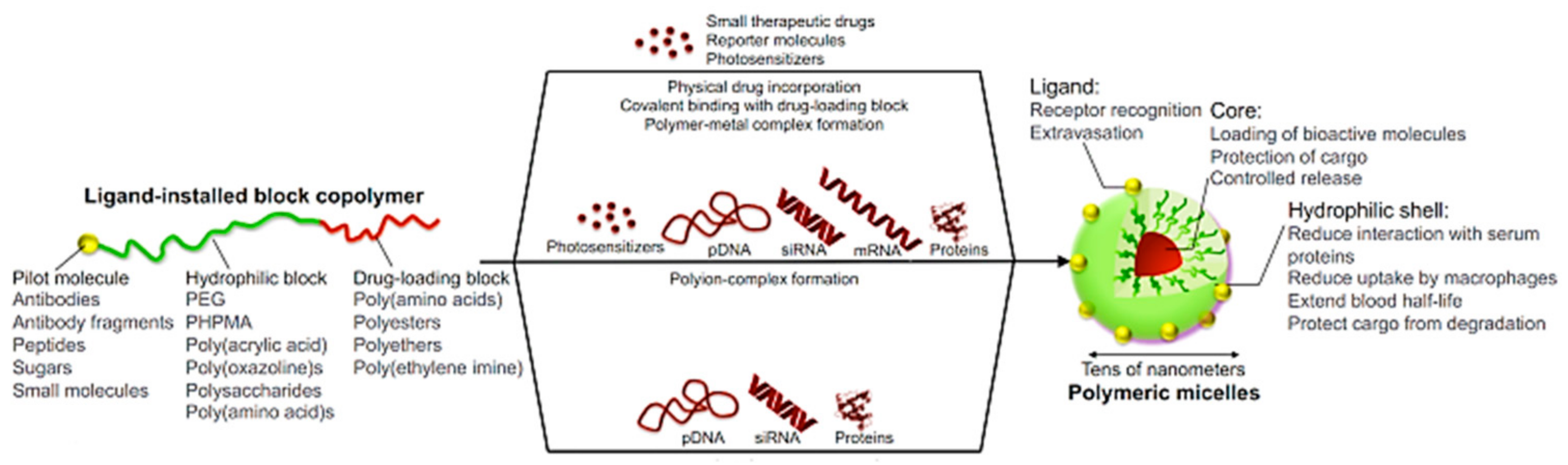
4.1. Micelles
4.1.1. Conventional Micelles
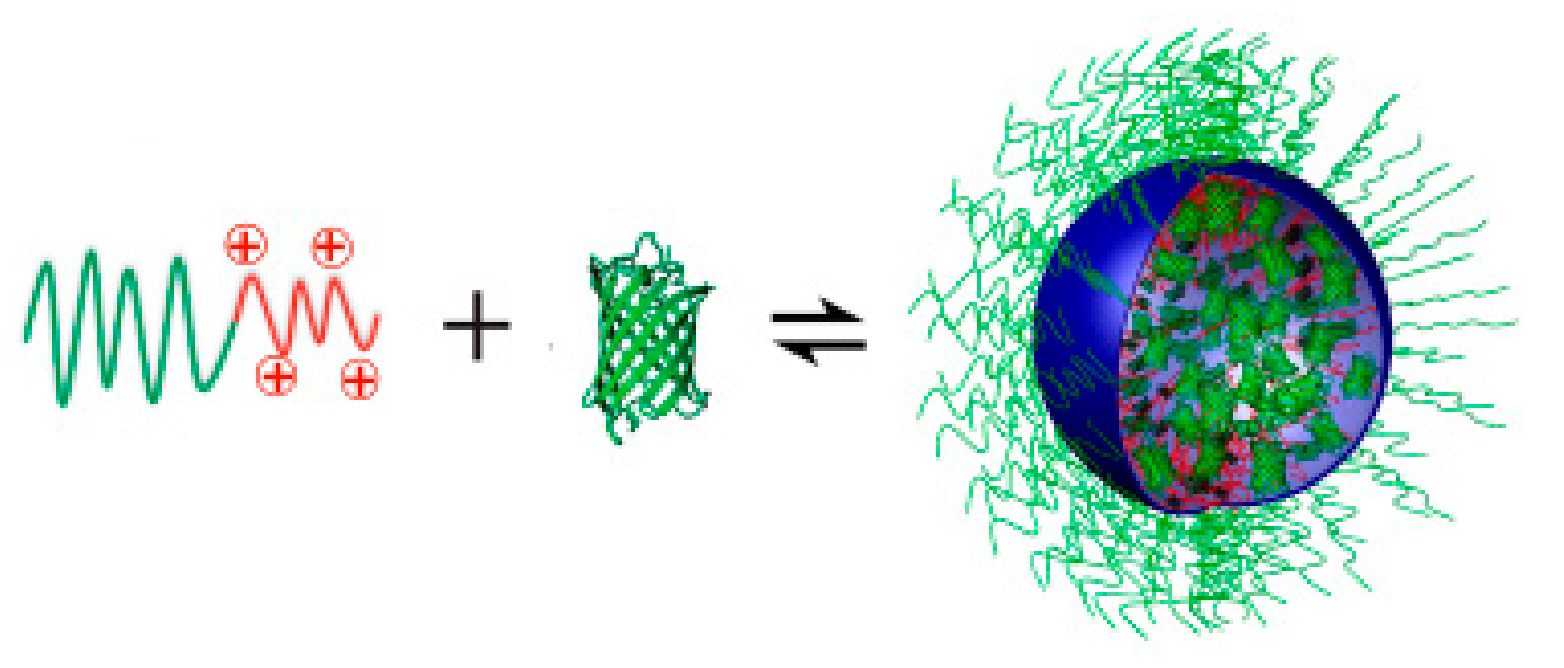
4.1.2. Polyion Complex Micelles (PICs)
4.2. Hydrogels
4.3. Polymeric Vesicles
4.3.1. Polymersomes
4.3.2. Nanocapsules
5. Potential Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, C.; Venkatraman, S. The long-term delivery of proteins and peptides using micro/nanoparticles: Overview and perspectives. Ther. Deliv. 2019, 10, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zou, Y.; Deng, C.; Meng, F.; Zhang, J.; Zhong, Z. Multifunctional Click Hyaluronic Acid Nanogels for Targeted Protein Delivery and Effective Cancer Treatment in Vivo. Chem. Mater. 2016, 28, 8792–8799. [Google Scholar] [CrossRef]
- Al-Azzam, S.; Ding, Y.; Liu, J.; Pandya, P.; Ting, J.P.; Afshar, S. Peptides to combat viral infectious diseases. Peptides 2020, 134, 170402. [Google Scholar] [CrossRef] [PubMed]
- Washburn, R.L.; Mueller, K.; Kaur, G.; Moreno, T.; Moustaid-Moussa, N.; Ramalingam, L.; Dufour, J.M. C-Peptide as a Therapy for Type 1 Diabetes Mellitus. Biomedicines 2021, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Shah, M.R.; Shafiullah. Chapter 10—Amphiphilic block copolymers–based micelles for drug delivery. In Design and Development of New Nanocarriers, 1st ed.; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 365–400. [Google Scholar]
- Walsh, G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 2018, 36, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.K.; Al-Attraqchi, O.; Chandrasekaran, B.; Paradkar, A.; Tekade, R.K. Chapter 16—Protein/Peptide Drug Delivery Systems: Practical Considerations in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery, 1st ed.; Tekade, R.K., Ed.; Elsevier, Academic Press: Cambridge, MA, USA, 2019; pp. 651–684. [Google Scholar]
- Stratton, M.R.; Campbell, P.J.; Futreal, P.A. The cancer genome. Nature 2009, 458, 719–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. 2015, 20, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Dastider, D.; Jyoti Sen, D.; Kumar Mandal, S.; Bose, S.; Ray, S.; Mahanti, B. Hand santizers bid farewell to germs on surface area of hands. Eur. J. Pharm. Sci. 2020, 7, 648–656. [Google Scholar]
- Lagassé, H.A.D.; Alexaki, A.; Simhadri, V.L.; Katagiri, N.H.; Jankowski, W.; Sauna, Z.E.; Kimchi-Sarfaty, C. Recent advances in (therapeutic protein) drug development. F1000Research 2017, 6, 113. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Lin, Z.Y.; Yildirimer, L.; Dhinakar, A.; Zhao, X.; Wu, J. Polymer-based nanoparticles for protein delivery: Design, strategies and applications. J. Mater. Chem. B 2016, 4, 4060–4071. [Google Scholar] [CrossRef]
- Hou, Y.; Lu, H. Protein PEPylation: A New Paradigm of Protein–Polymer Conjugation. Bioconjug. Chem. 2019, 30, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Sharma, V.; Bhushan, B.; Malviya, R.; Awasthi, R.; Kulkarni, G.T. Nanocarriers for protein and peptide delivery: Recent advances and progress. J. Res. Pharm. 2021, 25, 99–116. [Google Scholar] [CrossRef]
- Khodabakhsh, F.; Salimian, M.; Hedayati, M.H.; Ahangari Cohan, R.; Norouzian, D. Challenges and advancements in the pharmacokinetic enhancement of therapeutic proteins. Prep. Biochem. Biotechnol. 2021, 51, 519–529. [Google Scholar] [CrossRef]
- Dellas, N.; Liu, J.; Botham, R.C.; Huisman, G.W. Adapting protein sequences for optimized therapeutic efficacy. Curr. Opin. Chem. Biol. 2021, 64, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Zhang, N.; Lyu, Z.; Zhu, W.; Chang, Y.-C.; Hu, X.; Du, D.; Lin, Y. Protein-based nanomaterials and nanosystems for biomedical applications: A review. Mater. Today 2021, 43, 166–184. [Google Scholar] [CrossRef]
- Le Saux, S.; Aubert-Pouëssel, A.; Ouchait, L.; Mohamed, K.E.; Martineau, P.; Guglielmi, L.; Devoisselle, J.-M.; Legrand, P.; Chopineau, J.; Morille, M. Nanotechnologies for Intracellular Protein Delivery: Recent Progress in Inorganic and Organic Nanocarriers. Adv. Ther. 2021, 4, 2100009. [Google Scholar] [CrossRef]
- Zeb, A.; Rana, I.; Choi, H.-I.; Lee, C.-H.; Baek, S.-W.; Lim, C.-W.; Khan, N.; Arif, S.T.; Sahar, N.U.; Alvi, A.M.; et al. Potential and Applications of Nanocarriers for Efficient Delivery of Biopharmaceuticals. Pharmaceutics 2020, 12, 1184. [Google Scholar] [CrossRef] [PubMed]
- Hirai, Y.; Hirose, H.; Imanishi, M.; Asai, T.; Futaki, S. Cytosolic protein delivery using pH-responsive, charge-reversible lipid nanoparticles. Science 2021, 11, 19896. [Google Scholar] [CrossRef]
- Jash, A.; Ubeyitogullari, A.; Rizvi, S.S.H. Liposomes for oral delivery of protein and peptide-based therapeutics: Challenges, formulation strategies, and advances. J. Mater. Chem. B 2021, 9, 4773–4792. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Polymer-Based Nanoparticle Strategies for Insulin Delivery. Polymers 2019, 11, 1380. [Google Scholar] [CrossRef] [Green Version]
- Rebekah, A.; Sivaselvam, S.; Viswanathan, C.; Prabhu, D.; Gautam, R.; Ponpandian, N. Magnetic nanoparticle-decorated graphene oxide-chitosan composite as an efficient nanocarrier for protein delivery. Colloids Surf. A Physicochem Eng. Asp. 2021, 610, 125913. [Google Scholar] [CrossRef]
- Abasian, P.; Ghanavati, S.; Rahebi, S.; Nouri Khorasani, S.; Khalili, S. Polymeric nanocarriers in targeted drug delivery systems: A review. Polym. Adv. Technol. 2020, 31, 2939–2954. [Google Scholar] [CrossRef]
- Agrahari, V. Advances and applications of block-copolymer-based nanoformulations. Drug Discov. 2018, 23, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Karayianni, M.; Pispas, S. Self-Assembly of Amphiphilic Block Copolymers in Selective Solvents. In Fluorescence Studies of Polymer Containing Systems, 1st ed.; Procházka, K., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 27–63. [Google Scholar]
- Perin, F.; Motta, A.; Maniglio, D. Amphiphilic copolymers in biomedical applications: Synthesis routes and property control. Mater. Sci. Eng. C 2021, 123, 111952. [Google Scholar] [CrossRef]
- Gao, S.; Holkar, A.; Srivastava, S. Protein-Polyelectrolyte Complexes and Micellar Assemblies. Polymers 2019, 11, 1097. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Venditti, I. Morphologies and functionalities of polymeric nanocarriers as chemical tools for drug delivery: A review. J. King Saud. Univ. Sci. 2019, 31, 398–411. [Google Scholar] [CrossRef]
- Kopeček, J.; Yang, J. Polymer nanomedicines. Adv. Drug Deliv. Rev. 2020, 156, 40–64. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Zhang, H.; Mi, P. 12—Polymeric Micelles for Tumor Theranostics. In Theranostic Bionanomaterials, 1st ed.; Cui, W., Zhao, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 289–302. [Google Scholar]
- Javan Nikkhah, S.; Thompson, D. Molecular Modelling Guided Modulation of Molecular Shape and Charge for Design of Smart Self-Assembled Polymeric Drug Transporters. Pharmaceutics 2021, 13, 141. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Magazù, S.; Calandra, P. Amphiphiles Self-Assembly: Basic Concepts and Future Perspectives of Supramolecular Approaches. Adv. Condens. Matter Phys. 2015, 2015, 151683. [Google Scholar] [CrossRef] [Green Version]
- Díez-García, I.; Santamaria-Echart, A.; Eceiza, A.; Tercjak, A. Triblock copolymers containing hydrophilic PEO blocks as effective polyols for organic solvent-free waterborne poly(urethane-urea)s. React. Funct Polym. 2018, 131, 1–11. [Google Scholar] [CrossRef]
- El Jundi, A.; Buwalda, S.J.; Bakkour, Y.; Garric, X.; Nottelet, B. Double hydrophilic block copolymers self-assemblies in biomedical applications. Adv. Colloid Interface Sci. 2020, 283, 102213. [Google Scholar] [CrossRef]
- Feng, H.; Lu, X.; Wang, W.; Kang, N.G.; Mays, J.W. Block Copolymers: Synthesis, Self-Assembly, and Applications. Polymers 2017, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Gunatillake, P.A.; Meagher, L. Biomedical applications of polymers derived by reversible addition—Fragmentation chain-transfer (RAFT). Adv. Drug Deliv. Rev. 2015, 91, 141–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, N.P.; Jones, G.R.; Bradford, K.G.E.; Konkolewicz, D.; Anastasaki, A. A comparison of RAFT and ATRP methods for controlled radical polymerization. Nat. Rev. Chem. 2021, 5, 859–869. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, J.; Dhas, N.; Longhi, M.; García, M.C. Overcoming Biological Barriers with Block Copolymers-Based Self-Assembled Nanocarriers. Recent Advances in Delivery of Anticancer Therapeutics. Front. Pharmacol. 2020, 11, 1840. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Amphiphilic block copolymers in drug delivery: Advances in formulation structure and performance. Expert. Opin. Drug Deliv. 2018, 15, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, H.; He, H.; Ribbe, A.E.; Thayumanavan, S. Blended Assemblies of Amphiphilic Random and Block Copolymers for Tunable Encapsulation and Release of Hydrophobic Guest Molecules. Macromolecules 2020, 53, 2713–2723. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Xia, G.; Feng, Z.-J.; Hao, Q.-H.; Tan, H.-G. Self-assembly of polyelectrolyte diblock copolymers at monovalent and multivalent counterions. Soft Matter 2019, 15, 3689–3699. [Google Scholar] [CrossRef] [PubMed]
- Demetzos, C. Application of Nanotechnology in Drug Delivery and Targeting. In Pharmaceutical Nanotechnology: Fundamentals and Practical Applications; Springer: Singapore, 2016; pp. 77–145. [Google Scholar]
- Jayasuriya, A.C. 8—Production of micro- and nanoscale chitosan particles for biomedical applications. In Chitosan Based Biomaterials Volume 1; Jennings, J.A., Bumgardner, J.D., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2017; pp. 185–209. [Google Scholar]
- Theodorou, A.; Liarou, E.; Haddleton, D.M.; Stavrakaki, I.G.; Skordalidis, P.; Whitfield, R.; Anastasaki, A.; Velonia, K. Protein-polymer bioconjugates via a versatile oxygen tolerant photoinduced controlled radical polymerization approach. Nat. Commun. 2020, 11, 1486. [Google Scholar] [CrossRef] [Green Version]
- Matyjaszewski, K. Advanced Materials by Atom Transfer Radical Polymerization. Adv. Mater. 2018, 30, e1706441. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Wei, L.; Liu, L.; Zhao, H. Surface Coassembly of Polymer Brushes and Polymer–Protein Bioconjugates: An Efficient Approach to the Purification of Bioconjugates under Mild Conditions. Biomacromolecules 2018, 19, 4463–4471. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, C. Site-Specific Conjugation of Polymers to Proteins. Biomacromolecules 2018, 19, 1804–1825. [Google Scholar] [CrossRef]
- Abuchowski, A.; van Es, T.; Palczuk, N.C.; Davis, F.F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J. Biol. Chem. 1977, 252, 3578–3581. [Google Scholar] [CrossRef]
- Kariduraganavar, M.Y.; Heggannavar, G.B.; Amado, S.; Mitchell, G.R. Chapter 6—Protein Nanocarriers for Targeted Drug Delivery for Cancer Therapy. In Nanocarriers for Drug Delivery, 1st ed.; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 173–204. [Google Scholar]
- Rondon, A.; Mahri, S.; Morales-Yanez, F.; Dumoulin, M.; Vanbever, R. Protein Engineering Strategies for Improved Pharmacokinetics. Adv. Funct. Mater. 2021, 31, 2101633. [Google Scholar] [CrossRef]
- Dozier, J.K.; Distefano, M.D. Site-Specific PEGylation of Therapeutic Proteins. Int. J. Mol. Sci. 2015, 16, 25831–25864. [Google Scholar] [CrossRef] [Green Version]
- Harijan, M.; Singh, M. Zwitterionic polymers in drug delivery: A review. J. Mol. Recognit. 2022, 35, e2944. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, Y.; Zhang, Y.; Zhao, H. Fabrication of Polymer–Protein Hybrids. Macromol. Rapid Commun. 2018, 39, 1700737. [Google Scholar] [CrossRef]
- Kurinomaru, T.; Kuwada, K.; Tomita, S.; Kameda, T.; Shiraki, K. Noncovalent PEGylation through Protein–Polyelectrolyte Interaction: Kinetic Experiment and Molecular Dynamics Simulation. J. Phys. Chem. B. 2017, 121, 6785–6791. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.; Borchard, G. Noncovalent PEGylation, An Innovative Subchapter in the Field of Protein Modification. J. Pharm. Sci. 2016, 105, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Chi, H. A review of recent progress in drug and protein encapsulation: Approaches, applications and challenges. Mater. Sci. Eng. C 2018, 83, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, G.; Ma, R.; Liu, Y.; Liu, Y.; Lv, J.; An, Y.; Shi, L. Nitrilotriacetic Acid (NTA) and Phenylboronic Acid (PBA) Functionalized Nanogels for Efficient Encapsulation and Controlled Release of Insulin. ACS Biomater. Sci. Eng. 2018, 4, 2007–2017. [Google Scholar] [CrossRef]
- Chen, F.; Stenzel, M.H. Polyion Complex Micelles for Protein Delivery. Aust. J. Chem. 2018, 71, 768–780. [Google Scholar] [CrossRef]
- Yu, M.; Wu, J.; Shi, J.; Farokhzad, O.C. Nanotechnology for protein delivery: Overview and perspectives. J. Control. Release 2016, 240, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Fan, Q.; Wang, H.; Cheng, Y. Polymers for cytosolic protein delivery. Biomaterials 2019, 218, 119358. [Google Scholar] [CrossRef]
- Shelma, R. Chapter 15: Polymeric Nanoparticles for Drug Delivery. In A Holistic and Integrated Approach to Lifestyle Diseases, 1st ed.; George, J.S., George, A., Mathew, S., Kalarikkal, N., Thomas, S., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2022; pp. 319–332. [Google Scholar]
- Pham, D.T.; Chokamonsirikun, A.; Phattaravorakarn, V.; Tiyaboonchai, W. Polymeric micelles for pulmonary drug delivery: A comprehensive review. J. Mater. Sci. 2021, 56, 2016–2036. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric micelles: Basic research to clinical practice. Int. J. Pharm. 2017, 532, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Joint MHLW/EMA Reflection Paper on the Development of Block Copolymer Micelle Medicinal Products. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/01/WC500159411.pdf (accessed on 21 January 2022).
- Simoes, S.; Figueiras, A.; Veiga, F.; Concheiro, A.; Alvarez-Lorenzo, C. Polymeric micelles for oral drug administration enabling locoregional and systemic treatments. Expert. Opin. Drug Deliv. 2014, 12, 297–318. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, B.P.; Yadav, H.K.; Nagesha, D.K.; Raizaday, A.; Karim, A. Polymeric Micelles as Novel Carriers for Poorly Soluble Drugs--A Review. J. Nanosci. Nanotechnol. 2015, 15, 4009–4018. [Google Scholar] [CrossRef]
- Xu, F.J.; Yang, W.-T. Polymer vectors via controlled/living radical polymerization for gene delivery. Prog. Polym. Sci. 2011, 36, 1099–1131. [Google Scholar] [CrossRef]
- Pires-Oliveira, R.; Tang, J.; Percebom, A.M.; Petzhold, C.L.; Tam, K.C.; Loh, W. Effect of Molecular Architecture and Composition on the Aggregation Pathways of POEGMA Random Copolymers in Water. Langmuir 2020, 36, 15018–15029. [Google Scholar] [CrossRef]
- Andrade, F.; Neves, J.d.; Gener, P.; Schwartz, S.; Ferreira, D.; Oliva, M.; Sarmento, B. Biological assessment of self-assembled polymeric micelles for pulmonary administration of insulin. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1621–1631. [Google Scholar] [CrossRef]
- Kamenova, K.; Haladjova, E.; Grancharov, G.; Kyulavska, M.; Tzankova, V.; Aluani, D.; Yoncheva, K.; Pispas, S.; Petrov, P. Co-assembly of block copolymers as a tool for developing novel micellar carriers of insulin for controlled drug delivery. Eur. Polym. J. 2018, 104, 1–9. [Google Scholar] [CrossRef]
- Li, X.; Shang, H.; Wu, W.; Li, S.; Lin, Z.; Duan, J.; Xu, L.; Li, J. Glucose-Responsive Micelles for Controlled Insulin Release Based on Transformation from Amphiphilic to Double Hydrophilic. J. Nanosci. Nanotechnol. 2016, 16, 5457–5463. [Google Scholar] [CrossRef]
- Sun, J.; Li, Z. Polyion Complexes via Electrostatic Interaction of Oppositely Charged Block Copolymers. Macromolecules 2020, 53, 8737–8740. [Google Scholar] [CrossRef]
- Uchida, S.; Kataoka, K. Design concepts of polyplex micelles for in vivo therapeutic delivery of plasmid DNA and messenger RNA. J. Biomed. Mater. Res. A 2019, 107, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Marras, A.E.; Ting, J.M.; Stevens, K.C.; Tirrell, M.V. Advances in the Structural Design of Polyelectrolyte Complex Micelles. J. Phys. Chem. B 2021, 125, 7076–7089. [Google Scholar] [CrossRef]
- Pippa, N.; Karayianni, M.; Pispas, S.; Demetzos, C. Complexation of cationic-neutral block polyelectrolyte with insulin and in vitro release studies. Int. J. Pharm. 2015, 491, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Kataoka, K. Formation of Polyion Complex Micelles in an Aqueous Milieu from a Pair of Oppositely-Charged Block Copolymers with Poly(ethylene glycol) Segments. Macromolecules 1995, 28, 5294–5299. [Google Scholar] [CrossRef]
- Nolles, A.; Westphal, A.H.; de Hoop, J.A.; Fokkink, R.G.; Kleijn, J.M.; van Berkel, W.J.H.; Borst, J.W. Encapsulation of GFP in Complex Coacervate Core Micelles. Biomacromolecules 2015, 16, 1542–1549. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, H.; Chen, F.; Callari, M.; Pourgholami, M.; Morris, D.L.; Stenzel, M.H. PEGylated Albumin-Based Polyion Complex Micelles for Protein Delivery. Biomacromolecules 2016, 17, 808–817. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Chen, T.; Xian, W.; Zhang, H.; Wu, L.; Zhu, W.; Zeng, Q. Nanoscale cationic micelles of amphiphilic copolymers based on star-shaped PLGA and PEI cross-linked PEG for protein delivery application. J. Mater. Sci. Mater. Med. 2019, 30, 93. [Google Scholar] [CrossRef]
- Chen, T.; Xu, Y.; Yang, W.; Li, A.; Wang, Y.; Sun, J.; Liu, J. Design of Enzyme Micelles with Controllable Concavo-Convex Micromorphologies for Highly Enhanced Stability and Catalytical Activity. Macromol. Biosci. 2018, 18, 1700312. [Google Scholar] [CrossRef]
- Skandalis, A.; Murmiliuk, A.; Štěpánek, M.; Pispas, S. Physicochemical Evaluation of Insulin Complexes with QPDMAEMA-b-PLMA-b-POEGMA Cationic Amphiphlic Triblock Terpolymer Micelles. Polymers 2020, 12, 309. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.-H.; Sheu, S.-C.; Chen, C.-W.; Huang, S.-C.; Li, B.-R. Wearable hydrogel patch with noninvasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Van Blarcom, D.S. Hydrogel-based biosensors and sensing devices for drug delivery. J. Control. Release 2016, 240, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Zhang, T.; Lin, X. 3D printed hydrogel scaffolds with macro pores and interconnected microchannel networks for tissue engineering vascularization. Chem. Eng. J. 2022, 430, 132926. [Google Scholar] [CrossRef]
- Xin, H.; Naficy, S. Drug Delivery Based on Stimuli-Responsive Injectable Hydrogels for Breast Cancer Therapy: A Review. Gels 2022, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, H.; Buksh, K.; Noreen, S.; Pervaiz, F.; Maqbool, I. Hydrogels as potential drug-delivery systems: Network design and applications. Ther. Deliv. 2021, 12, 375–396. [Google Scholar] [CrossRef]
- Bajracharya, R.; Song, J.G.; Back, S.Y.; Han, H.-K. Recent Advancements in Non-Invasive Formulations for Protein Drug Delivery. Comput. Struct. Biotechnol. J. 2019, 17, 1290–1308. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, X.; Li, X.; Ma, C.; Chu, X.; Wang, L.; Xu, W. A review on recent advances of Protein-Polymer hydrogels. Eur. Polym. J. 2022, 162, 110881. [Google Scholar] [CrossRef]
- Baghban Taraghdari, Z.; Imani, R.; Mohabatpour, F. A Review on Bioengineering Approaches to Insulin Delivery: A Pharmaceutical and Engineering Perspective. Macromol. Biosci. 2019, 19, 1800458. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Le, T.M.D.; Janarthanan, G.; Ngo, P.-K.T.; Lee, D.S.; Thambi, T. Development of bioresorbable smart injectable hydrogels based on thermo-responsive copolymer integrated bovine serum albumin bioconjugates for accelerated healing of excisional wounds. J. Ind. Eng. Chem. 2021, 96, 345–355. [Google Scholar] [CrossRef]
- Xu, R.; Ma, S.; Lin, P.; Yu, B.; Zhou, F.; Liu, W. High Strength Astringent Hydrogels Using Protein as the Building Block for Physically Cross-linked Multi-Network. ACS Appl. Mater. Interfaces. 2018, 10, 7593–7601. [Google Scholar] [CrossRef]
- Lima, D.S.; Tenório-Neto, E.T.; Lima-Tenório, M.K.; Guilherme, M.R.; Scariot, D.B.; Nakamura, C.V.; Muniz, E.C.; Rubira, A.F. pH-responsive alginate-based hydrogels for protein delivery. J. Mol. Liq. 2018, 262, 29–36. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Hanna, D.H.; Abu Elella, M.H.; Mohamed, R.R. Encapsulation of bovine serum albumin within novel xanthan gum based hydrogel for protein delivery. Mater. Sci. Eng. C. 2019, 94, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qin, T.; Chen, T.; Wang, J.; Zeng, Q. Poly(vinyl alcohol)/poly(hydroxypropyl methacrylate-co-methacrylic acid) as pH-sensitive semi-IPN hydrogels for oral insulin delivery: Preparation and characterization. Iran Polym. J. 2021, 30, 343–353. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, D.; Liu, L.; Li, X. Development of poly(hydroxyethyl methacrylate) nanogel for effective oral insulin delivery. Pharm. Dev. Technol. 2018, 23, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.J.; Thambi, T.; Lee, D.S. Heparin-based temperature-sensitive injectable hydrogels for protein delivery. J. Mater. Chem B 2015, 3, 8892–8901. [Google Scholar] [CrossRef]
- Turabee, M.H.; Thambi, T.; Duong, H.T.T.; Jeong, J.H.; Lee, D.S. A pH- and temperature-responsive bioresorbable injectable hydrogel based on polypeptide block copolymers for the sustained delivery of proteins in vivo. Biomater. Sci. 2018, 6, 661–671. [Google Scholar] [CrossRef]
- Nomani, A.; Nosrati, H.; Manjili, H.K.; Khesalpour, L.; Danafar, H. Preparation and Characterization of Copolymeric Polymersomes for Protein Delivery. Drug Res. 2017, 67, 458–465. [Google Scholar] [CrossRef]
- Araste, F.; Aliabadi, A.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Self-assembled polymeric vesicles: Focus on polymersomes in cancer treatment. J. Control. Release 2021, 330, 502–528. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Li, B.; Cheng, Y.; Zhou, D.; Chen, X.; Jing, X.; Huang, Y. Compact Vesicles Self-Assembled from Binary Graft Copolymers with High Hydrophilic Fraction for Potential Drug/Protein Delivery. ACS Macro Lett. 2017, 6, 1186–1190. [Google Scholar] [CrossRef]
- Iqbal, S.; Blenner, M.; Alexander-Bryant, A.; Larsen, J. Polymersomes for Therapeutic Delivery of Protein and Nucleic Acid Macromolecules: From Design to Therapeutic Applications. Biomacromolecules 2020, 21, 1327–1350. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.; Qian, C.; Lu, Y.; Kahkoska, A.R.; Xie, Z.; Jing, X.; Buse, J.B.; Gu, Z. H2O2-Responsive Vesicles Integrated with Transcutaneous Patches for Glucose-Mediated Insulin Delivery. ACS Nano 2017, 11, 613–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Erdman Iii, W.; Yuan, Y.; Mohamed, M.A.; Xie, R.; Wang, Y.; Gong, S.; Cheng, C. Crosslinked polymer nanocapsules for therapeutic, diagnostic, and theranostic applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1653. [Google Scholar] [CrossRef] [PubMed]
- Villegas, M.R.; Baeza, A.; Vallet-Regí, M. Nanotechnological Strategies for Protein Delivery. Molecules 2018, 23, 1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Forero Ramirez, L.M.; Boudier, A.; Gaucher, C.; Babin, J.; Durand, A.; Six, J.-L.; Nouvel, C. Dextran-covered pH-sensitive oily core nanocapsules produced by interfacial Reversible Addition-Fragmentation chain transfer miniemulsion polymerization. J. Colloid Interface Sci. 2020, 569, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Forero Ramirez, L.M.; Babin, J.; Schmutz, M.; Durand, A.; Six, J.-L.; Nouvel, C. Multi-reactive surfactant and miniemulsion Atom Transfer Radical Polymerization: An elegant controlled one-step way to obtain dextran-covered nanocapsules. Eur. Polym. J. 2018, 109, 317–325. [Google Scholar] [CrossRef]
- Liang, S.; Liu, Y.; Jin, X.; Liu, G.; Wen, J.; Zhang, L.; Li, J.; Yuan, X.; Chen, I.S.Y.; Chen, W.; et al. Phosphorylcholine polymer nanocapsules prolong the circulation time and reduce the immunogenicity of therapeutic proteins. Nano Res. 2016, 9, 1022–1031. [Google Scholar] [CrossRef]
- Zhao, M.; Biswas, A.; Hu, B.; Joo, K.I.; Wang, P.; Gu, Z.; Tang, Y. Redox-responsive nanocapsules for intracellular protein delivery. Biomaterials 2011, 32, 5223–5230. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, Z.; Yu, J.; Kahkoska, A.R.; Buse, J.B.; Gu, Z. Glucose-Responsive Insulin and Delivery Systems: Innovation and Translation. Adv. Mater. 2020, 32, 1902004. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Guan, M.; Zhou, T.; Duan, X.; Xiang, Z. Growth Factor and Its Polymer Scaffold-Based Delivery System for Cartilage Tissue Engineering. Int. J. Nanomed. 2020, 15, 6097–6111. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, M.A.; Shin, J.Y.; Jeon, J.H.; Lee, S.J.; Yoon, M.Y.; Kim, H.-J.; Choi, E.-J.; Do, S.H.; Yang, V.C.; et al. Intra-articular delivery of synovium-resident mesenchymal stem cells via BMP-7-loaded fibrous PLGA scaffolds for cartilage repair. J. Control. Release 2019, 302, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, C.; Qi, H.; Zhou, J.; Wen, J.; Wu, D.; Xu, D.; Qin, M.; Ren, J.; Wang, Q.; et al. Systemic Delivery of Monoclonal Antibodies to the Central Nervous System for Brain Tumor Therapy. Adv. Mater. 2019, 31, 1805697. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vardaxi, A.; Kafetzi, M.; Pispas, S. Polymeric Nanostructures Containing Proteins and Peptides for Pharmaceutical Applications. Polymers 2022, 14, 777. https://doi.org/10.3390/polym14040777
Vardaxi A, Kafetzi M, Pispas S. Polymeric Nanostructures Containing Proteins and Peptides for Pharmaceutical Applications. Polymers. 2022; 14(4):777. https://doi.org/10.3390/polym14040777
Chicago/Turabian StyleVardaxi, Antiopi, Martha Kafetzi, and Stergios Pispas. 2022. "Polymeric Nanostructures Containing Proteins and Peptides for Pharmaceutical Applications" Polymers 14, no. 4: 777. https://doi.org/10.3390/polym14040777
APA StyleVardaxi, A., Kafetzi, M., & Pispas, S. (2022). Polymeric Nanostructures Containing Proteins and Peptides for Pharmaceutical Applications. Polymers, 14(4), 777. https://doi.org/10.3390/polym14040777