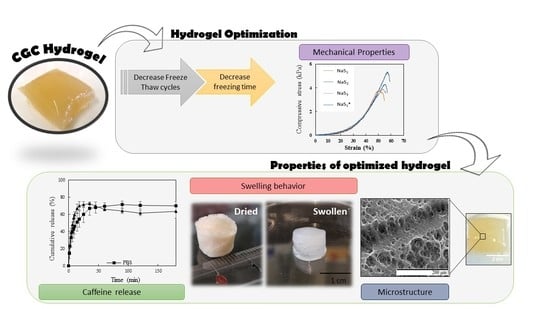
Chitin-Glucan Complex Hydrogels: Optimization of Gel Formation and Demonstration of Drug Loading and Release Ability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CGC Hydrogels
2.3. CGC Hydrogels Characterization
2.3.1. Chemical Characterization
2.3.2. Morphology, Density, and Porosity
2.4. Compressive Mechanical Analysis
2.5. Rheological Properties
2.6. Swelling and Water Retention Behavior
2.7. Drug Loading
2.8. Characterization of the Loaded Hydrogels
2.9. In Vitro Drug Release Studies
2.10. Statistical Analysis
3. Results
3.1. Hydrogels Formation
3.2. Chemical Characterization of the Hydrogels
3.3. Morphological Characterization
3.4. Hydrogels’ Porosity and Density
3.5. Mechanical Properties
3.6. Rheological Properties
3.7. Swelling Behavior and Water Retention Kinetics
3.8. Hydrogels Loading and Release Ability
3.8.1. Loading Na51* Hydrogels with Caffeine
3.8.2. Characterization of the Na51* Loaded Hydrogels
3.8.3. Release of Caffeine from the Na51* Hydrogels
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahsan, A.; Tian, W.-X.; Farooq, M.A.; Khan, D.H. An overview of hydrogels and their role in transdermal drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 574–584. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem. 2016, 18, 53–75. [Google Scholar] [CrossRef] [Green Version]
- Klein, M.; Poverenov, E. Natural biopolymer-based hydrogels for use in food and agriculture. J. Sci. Food Agric. 2020, 100, 2337–2347. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A.K. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 1–14. [Google Scholar] [CrossRef]
- Chen, G.; Tang, W.; Wang, X.; Zhao, X.; Chen, C.; Zhu, Z. Applications of hydrogels with special physical properties in bio-medicine. Polymers 2019, 11, 1420. [Google Scholar] [CrossRef] [Green Version]
- Xiang, J.; Shen, L.; Hong, Y. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Wu, J. Physically crosslinked hydrogels from polysaccharides prepared by freeze–thaw technique. React. Funct. Polym. 2013, 73, 923–928. [Google Scholar] [CrossRef]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef]
- Tang, S.; Zhao, L.; Yuan, J.; Chen, Y.; Leng, Y. Physical hydrogels based on natural polymers. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 51–89. [Google Scholar] [CrossRef]
- Farinha, I.; Duarte, P.; Pimentel, A.; Plotnikova, E.; Chagas, B.; Mafra, L.; Grandfils, C.; Freitas, F.; Fortunato, E.; Reis, M.A. Chitin–glucan complex production by Komagataella pastoris: Downstream optimization and product characterization. Carbohydr. Polym. 2015, 130, 455–464. [Google Scholar] [CrossRef]
- Roca, C.; Chagas, B.; Farinha, I.; Freitas, F.; Mafra, L.; Aguiar, F.; Oliveira, R.; Reis, M.A. Production of yeast chitin–glucan complex from biodiesel industry byproduct. Process Biochem. 2012, 47, 1670–1675. [Google Scholar] [CrossRef]
- Meichik, N.R.; Vorob’Ev, D.V. Chitin-glucan complex in cell walls of the Peltigera aphthosa lichen. Appl. Biochem. Microbiol. 2012, 48, 307–311. [Google Scholar] [CrossRef]
- Araújo, D.; Ferreira, I.C.; Torres, C.A.; Neves, L.; Freitas, F. Chitinous polymers: Extraction from fungal sources, characteri-zation and processing towards value-added applications. J. Chem. Technol. Biotechnol. 2020, 95, 1277–1289. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Dutta, P.K.; Kumar, H.; Kureel, A.K.; Rai, A.K. Improved antibacterial and antioxidant activities of gallic acid grafted chitin-glucan complex. J. Polym. Res. 2019, 26, 234. [Google Scholar] [CrossRef]
- Kulev, D.; Negrutsa, I. Chitin-glucan complex-food additive with sorbent properties. J. Hyg. Eng. Des. 2015, 11, 53–56. [Google Scholar]
- Marzorati, M.; Maquet, V.; Possemiers, S. Fate of chitin-glucan in the human gastrointestinal tract as studied in a dynamic gut simulator (SHIME®). J. Funct. Foods 2017, 30, 313–320. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.M.; Jancar, J.; Massoud, D.; Fohlerova, Z.; Elhadidy, H.; Spotz, Z.; Hebeish, A. Novel chitin/chitosan-glucan wound dressing: Isolation, characterization, antibacterial activity and wound healing properties. Int. J. Pharm. 2016, 510, 86–99. [Google Scholar] [CrossRef]
- Araújo, D.; Alves, V.D.; Marques, A.C.; Fortunato, E.; Reis, M.A.M.; Freitas, F. Low Temperature Dissolution of Yeast Chitin-Glucan Complex and Characterization of the Regenerated Polymer. Bioengineering 2020, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, A.; Chen, G.; Guan, J. Solubility of Chitin: Solvents, Solution Behaviors and Their Related Mechanisms. In Solubility of Polysaccharides; Xu, Z., Ed.; InTech: Rjeka, Croatia, 2017. [Google Scholar] [CrossRef] [Green Version]
- Araújo, D.; Alves, V.D.; Lima, S.A.; Reis, S.; Freitas, F.; Reis, M.A. Novel hydrogels based on yeast chitin-glucan complex: Characterization and safety assessment. Int. J. Biol. Macromol. 2020, 156, 1104–1111. [Google Scholar] [CrossRef]
- Araújo, D.; Freitas, F.; Sevrin, C.; Grandfils, C.; Reis, M.A. Co-production of chitin-glucan complex and xylitol by Koma-gataella pastoris using glucose and xylose mixtures as carbon source. Carbohydr. Polym. 2017, 166, 24–30. [Google Scholar] [CrossRef]
- Pawar, V.; Dhanka, M.; Srivastava, R. Cefuroxime conjugated chitosan hydrogel for treatment of wound infections. Colloids Surf. B Biointerfaces 2019, 173, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.H.C.S.; Mota, J.P.; De Almeida, T.S.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Rosado, C.; Freire, C.S.R. Topical Drug Delivery Systems Based on Bacterial Nanocellulose: Accelerated Stability Testing. Int. J. Mol. Sci. 2020, 21, 1262. [Google Scholar] [CrossRef] [Green Version]
- Zarzycki, R.; Modrzejewska, Z.; Nawrotek, K. Drug release from hydrogel matrices. Ecol. Chem. Eng. S 2010, 17, 117–136. [Google Scholar]
- Ferreira, L.; Figueiredo, M.M.; Gil, M.H.; Ramos, M.A. Structural analysis of dextran-based hydrogels obtained chemoen-zymatically. J. Biomed. Mater. Res. B: Appl. Biomater. 2006, 77, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Chen, S.; Zhang, L. Novel hydrogels prepared via direct dissolution of chitin at low temperature: Structure and biocompatibility. J. Mater. Chem. 2011, 21, 3865–3871. [Google Scholar] [CrossRef]
- Figueroa-Pizano, M.D.; Vélaz, I.; Peñas, F.J.; Zavala-Rivera, P.; Rosas-Durazo, A.J.; Maldonado-Arce, A.D.; Martínez-Barbosa, M.E. Effect of freeze-thawing conditions for preparation of chitosan-poly (vinyl alcohol) hydrogels and drug release studies. Carbohydr. Polym. 2018, 195, 476–485. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Zhu, C.; Fan, D.; Mi, Y.; Li, X.; Fu, R.Z.; Duan, Z.; Wang, Y.; Feng, R.R. A Novel Human-Like Collagen Hydrogel Scaffold with Porous Structure and Sponge-Like Properties. Polymers 2017, 9, 638. [Google Scholar] [CrossRef] [Green Version]
- Hurler, J.; Engesland, A.; Poorahmary, B.K.; Škalko-Basnet, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- Wong, R.S.H.; Dodou, K. Effect of Drug Loading Method and Drug Physicochemical Properties on the Material and Drug Release Properties of Poly (Ethylene Oxide) Hydrogels for Transdermal Delivery. Polymers 2017, 9, 286. [Google Scholar] [CrossRef] [PubMed]
- Baby, D.K. Rheology of hydrogels. In Rheology of Polymer Blends and Nanocomposites: Theory, Modelling and Applications, 1st ed.; Thomas, S., Chandrasekharakurup, S., Chandran, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 193–204. [Google Scholar]
- Mushi, N.E.; Kochumalayil, J.; Cervin, N.T.; Zhou, Q.; Berglund, L.A. Nanostructurally Controlled Hydrogel Based on Small-Diameter Native Chitin Nanofibers: Preparation, Structure, and Properties. ChemSusChem 2016, 9, 989–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, I.C.; Araújo, D.; Voisin, P.; Alves, V.D.; Rosatella, A.A.; Afonso, C.A.; Freitas, F.; Neves, L.A. Chitin-glucan complex—Based biopolymeric structures using biocompatible ionic liquids. Carbohydr. Polym. 2020, 247, 116679. [Google Scholar] [CrossRef]
- Kim, S.W.; Bae, Y.H.; Okano, T. Hydrogels: Swelling, drug loading, and release. Pharm. Res. 1992, 9, 283–290. [Google Scholar] [CrossRef]
- Kong, B.J.; Kim, A.; Park, S.N. Properties and in vitro drug release of hyaluronic acid-hydroxyethyl cellulose hydrogels for transdermal delivery of isoliquiritigenin. Carbohydr. Polym. 2016, 147, 473–481. [Google Scholar] [CrossRef]
- Wu, S.; Duan, B.; Lu, A.; Wang, Y.; Ye, Q.; Zhang, L. Biocompatible chitin/carbon nanotubes composite hydrogels as neuronal growth substrates. Carbohydr. Polym. 2017, 174, 830–840. [Google Scholar] [CrossRef]
- Udeni Gunathilake, T.M.S.; Ching, Y.C.; Chuah, C.H. Enhancement of curcumin bioavailability using nanocellulose rein-forced chitosan hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Raza, H.; Ranjha, N.M.; Razzaq, R.; Ansari, M.; Mahmood, A.; Rashid, Z. Fabrication and in vitro evaluation of 5-florouracil loaded chondroitin sulfate-sodium alginate microspheres for colon specific delivery. Acta Pol. Pharm. 2016, 73, 495–507. [Google Scholar]
- Zhang, S.; Guan, Y.; Fu, G.-Q.; Chen, B.-Y.; Peng, F.; Yao, C.-L.; Sun, R.-C. Organic/Inorganic Superabsorbent Hydrogels Based on Xylan and Montmorillonite. J. Nanomater. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Noor, N.; Shah, A.; Gani, A.; Gani, A.; Masoodi, F.A. Microencapsulation of caffeine loaded in polysaccharide based delivery systems. Food Hydrocoll. 2018, 82, 312–321. [Google Scholar] [CrossRef]
- Abosabaa, S.A.; ElMeshad, A.N.; Arafa, M.G. Chitosan Nanocarrier Entrapping Hydrophilic Drugs as Advanced Polymeric System for Dual Pharmaceutical and Cosmeceutical Application: A Comprehensive Analysis Using Box-Behnken Design. Polymers 2021, 13, 677. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Komes, D.; Karlović, S.; Djaković, S.; Špoljarić, I.; Mršić, G.; Ježek, D. Improving the controlled delivery formulations of caffeine in alginate hydrogel beads combined with pectin, carrageenan, chitosan and psyllium. Food Chem. 2015, 167, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Seyedabadi, M.M.; Rostami, H.; Jafari, S.M.; Fathi, M. Development and characterization of chitosan-coated nanoliposomes for encapsulation of caffeine. Food Biosci. 2020, 40, 100857. [Google Scholar] [CrossRef]
- Alves, V.D.; Costa, N.; Coelhoso, I.M. Barrier properties of biodegradable composite films based on kappa-carrageenan/pectin blends and mica flakes. Carbohydr. Polym. 2010, 79, 269–276. [Google Scholar] [CrossRef]
- Tavagnacco, L.; Engström, O.; Schnupf, U.; Saboungi, M.-L.; Himmel, M.; Widmalm, G.; Cesàro, A.; Brady, J.W. Caffeine and Sugars Interact in Aqueous Solutions: A Simulation and NMR Study. J. Phys. Chem. B 2012, 116, 11701–11711. [Google Scholar] [CrossRef] [Green Version]
- Prince, D.A.; Villamagna, I.J.; Hopkins, C.C.; de Bruyn, J.R.; Gillies, E.R. Effect of drug loading on the properties of tem-perature-responsive polyester–poly(ethylene glycol)–polyester hydrogels. Polym. Int. 2019, 68, 1074–1083. [Google Scholar] [CrossRef]
- Moradi, S.; Barati, A.; Salehi, E.; Tonelli, A.E.; Hamedi, H. Preparation and characterization of chitosan based hydrogels containing cyclodextrin inclusion compounds or nanoemulsions of thyme oil. Polym. Int. 2019, 68, 1891–1902. [Google Scholar] [CrossRef]
- Damiri, F.; Bachra, Y.; Bounacir, C.; Laaraibi, A.; Berrada, M. Synthesis and Characterization of Lyophilized Chitosan-Based Hydrogels Cross-Linked with Benzaldehyde for Controlled Drug Release. J. Chem. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Lim, L.Y.; Go, M.-L. Caffeine and nicotinamide enhances the aqueous solubility of the antimalarial agent halofantrine. Eur. J. Pharm. Sci. 2000, 10, 17–28. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, Z.; Du, Y.; Kennedy, J.F. Controlled release of ciprofloxacin hydrochloride from chitosan/polyethylene glycol blend films. Carbohydr. Polym. 2007, 69, 336–343. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, X.Y.; Xu, J.R.; Guo, J. Study on the preparation and drug release property of soybean selenoprotein/carboxymethyl chitosan composite hydrogel. J. Polym. Eng. 2018, 38, 963–970. [Google Scholar] [CrossRef]
- De Stéfano, J.C.Q.; Abundis-Correa, V.; Herrera-Flores, S.D.; Alvarez, A.J. pH-sensitive starch-based hydrogels: Synthesis and effect of molecular components on drug release behavior. Polymers 2020, 12, 1974. [Google Scholar] [CrossRef] [PubMed]

 ) and density (
) and density (  ) of the CGC hydrogels.
) of the CGC hydrogels.







| Samples | Na51 | Na52 | Na53 | Na51* | Na5 [22] |
|---|---|---|---|---|---|
| No. of cycles | 1 | 2 | 3 | 1 | 4 |
| Freezing time (h) | 48 | 48 | 48 | 18 | 48 |
| Polymer content (wt%) | 1.68 ± 0.17 | 1.58 ± 0.04 | 1.42 ± 0.02 | 1.66 ± 0.11 | 2.28 |
| Water content (wt%) | 98.22 ± 0.20 | 98.45 ± 0.06 | 98.58 ± 0.02 | 97.63 ± 0.12 | 97.72 |
| Chitin content (%) | 25.63 ± 0.78 | 24.71 ± 2.98 | 23.85 ± 0.14 | 21.51 ± 1.49 | n.a. |
| Sample | Na51* Hydrogel | Na51* Rehydrated Hydrogel | Na51* Loaded Hydrogel | |
|---|---|---|---|---|
| Mechanical properties | Compressive modulus (kPa) | 23.0 ± 0.89 | 38.06 ± 4.46 | 120.0 ± 61.64 |
| Toughness (kJ/m3) | 0.78 ± 0.01 | 1.67 ± 0.09 | 1.8 ± 0.33 | |
| Hardness (kPa) | 5.04 ± 0.14 | 11.50 ± 0.58 | 15.6 ± 2.53 | |
| Rheological properties | Storage modulus1 Hz (G′, kPa) | 149.9 ± 9.8 | 186.8 ± 22.0 | 315.0 ± 76.7 |
| Loss modulus1 Hz (G″, kPa) | 11.9 ± 0.5 | 16.8 ± 2.5 | 29.3 ± 8.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, D.; Rodrigues, T.; Alves, V.D.; Freitas, F. Chitin-Glucan Complex Hydrogels: Optimization of Gel Formation and Demonstration of Drug Loading and Release Ability. Polymers 2022, 14, 785. https://doi.org/10.3390/polym14040785
Araújo D, Rodrigues T, Alves VD, Freitas F. Chitin-Glucan Complex Hydrogels: Optimization of Gel Formation and Demonstration of Drug Loading and Release Ability. Polymers. 2022; 14(4):785. https://doi.org/10.3390/polym14040785
Chicago/Turabian StyleAraújo, Diana, Thomas Rodrigues, Vítor D. Alves, and Filomena Freitas. 2022. "Chitin-Glucan Complex Hydrogels: Optimization of Gel Formation and Demonstration of Drug Loading and Release Ability" Polymers 14, no. 4: 785. https://doi.org/10.3390/polym14040785
APA StyleAraújo, D., Rodrigues, T., Alves, V. D., & Freitas, F. (2022). Chitin-Glucan Complex Hydrogels: Optimization of Gel Formation and Demonstration of Drug Loading and Release Ability. Polymers, 14(4), 785. https://doi.org/10.3390/polym14040785