Current Advances of Polysaccharide-Based Nanogels and Microgels in Food and Biomedical Sciences
Abstract
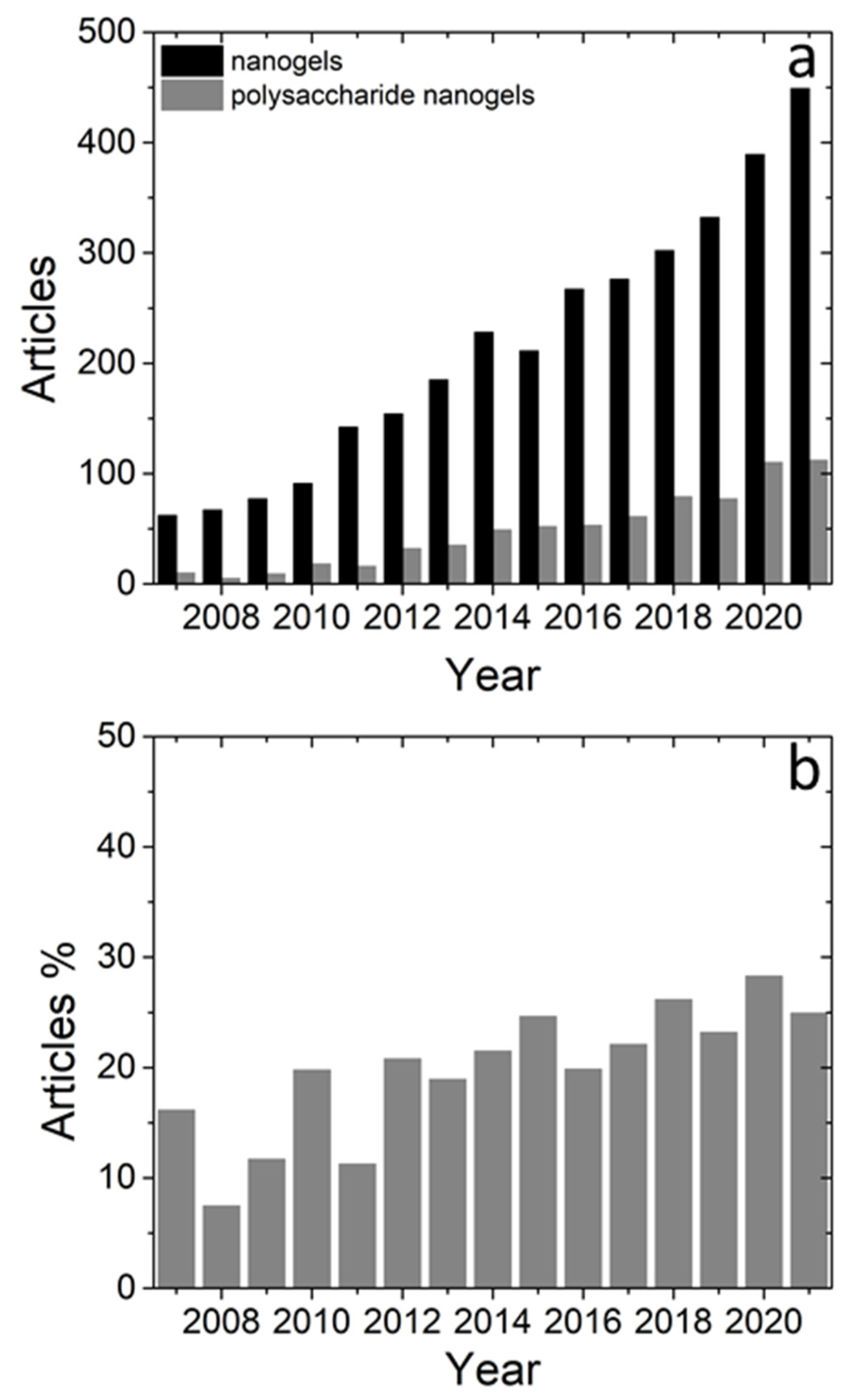
:1. Introduction
2. Motivation for Investigations on Polysaccharide NGs and MGs
3. Polysaccharide NGs and MGs Applications in the Food Industry
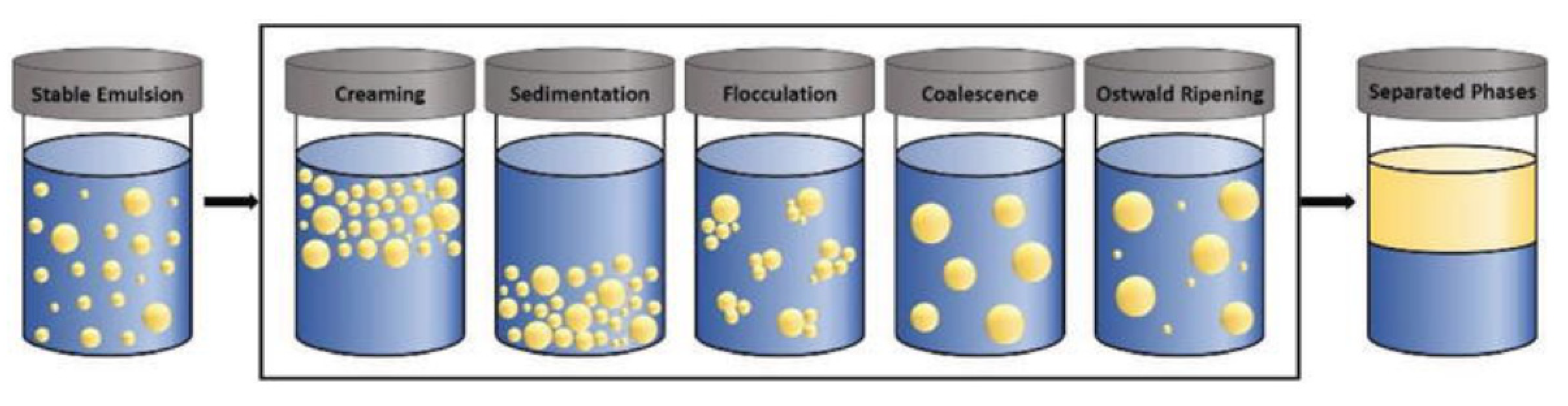
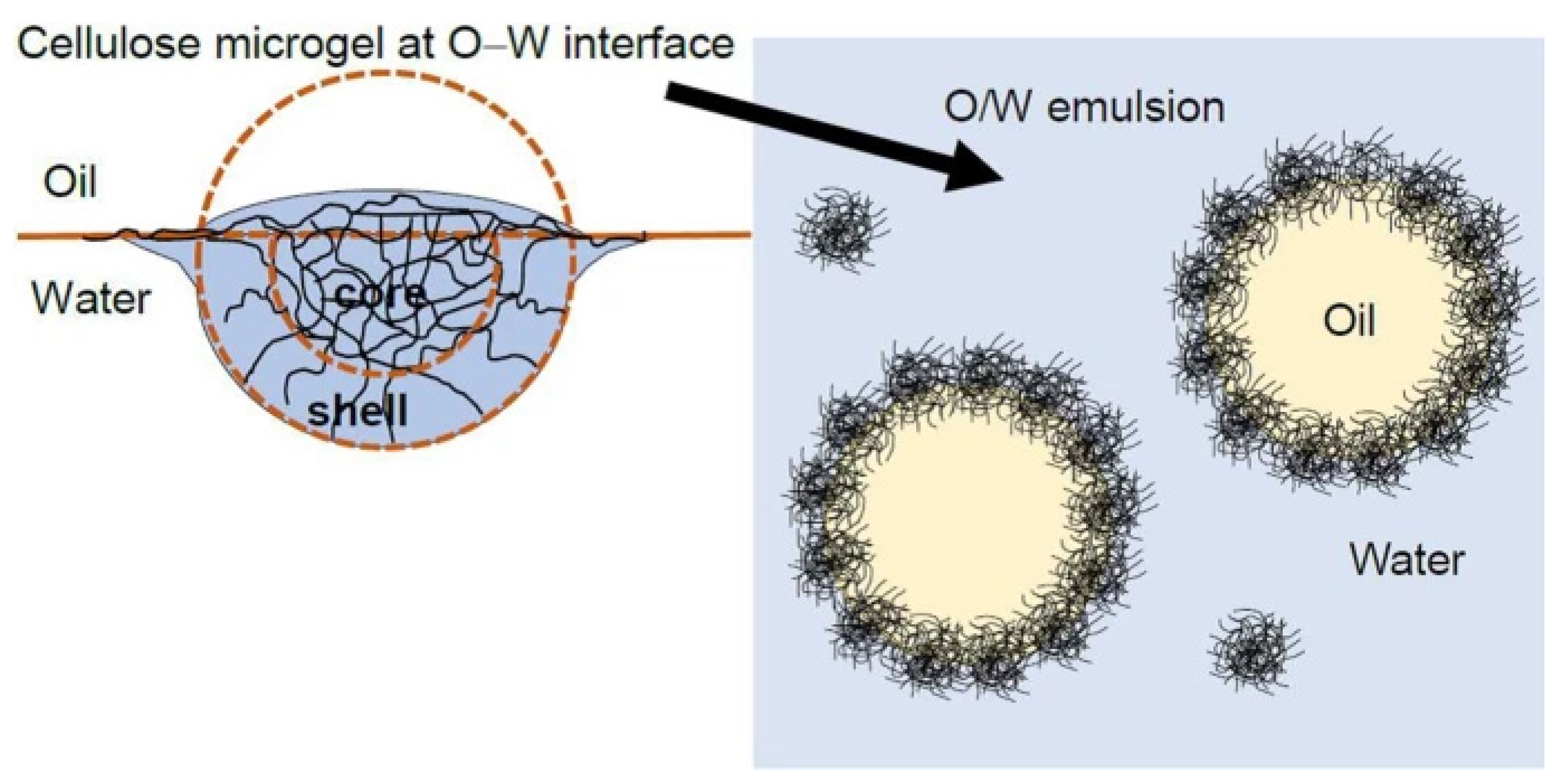
3.1. NGs and MGs as Stabilizers in Edible Emulsions
3.2. Polysaccharide NGs and MGs as Delivery Systems of Nutrients
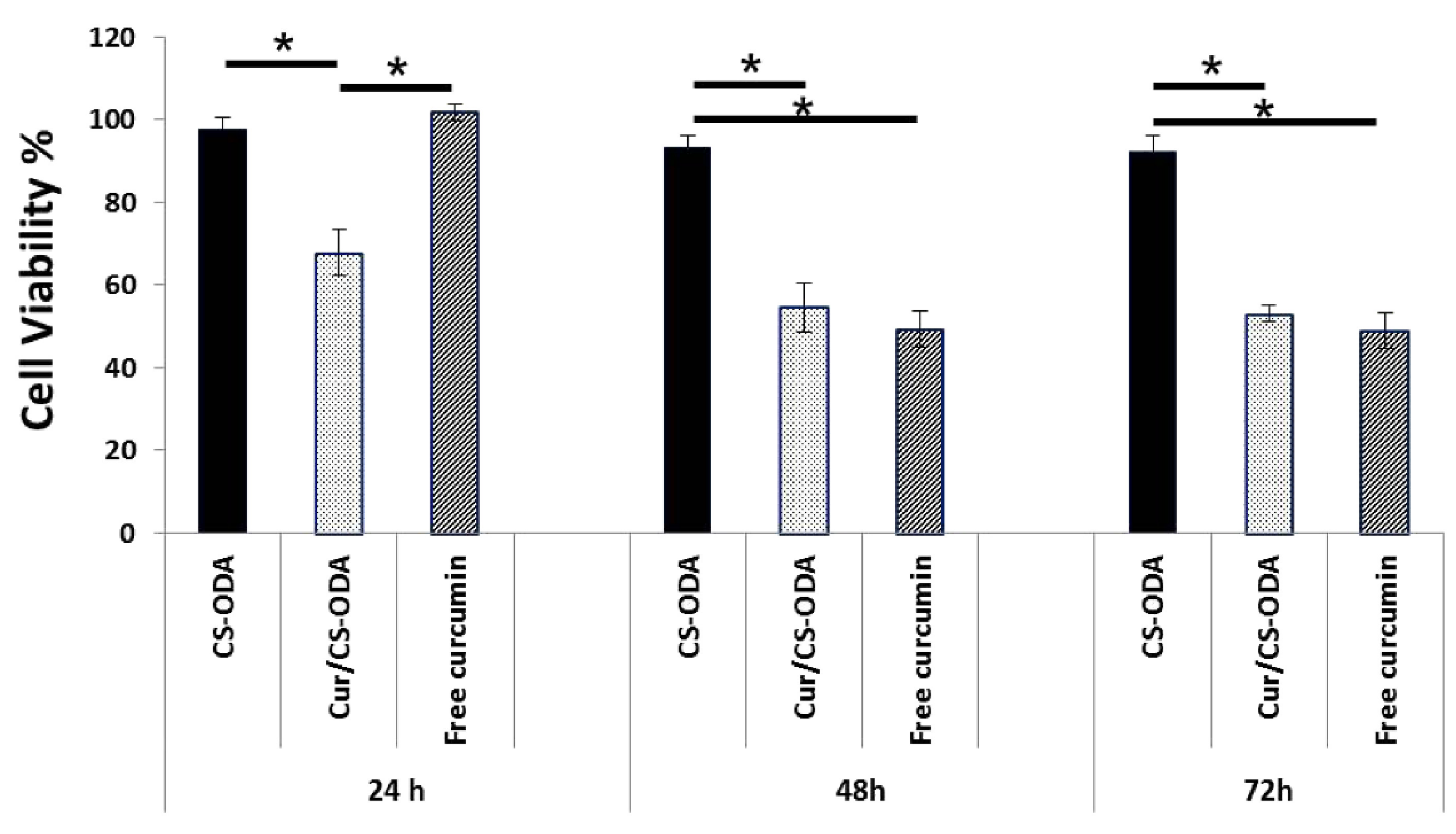
4. Medical Applications of Polysaccharide NGs and MGs
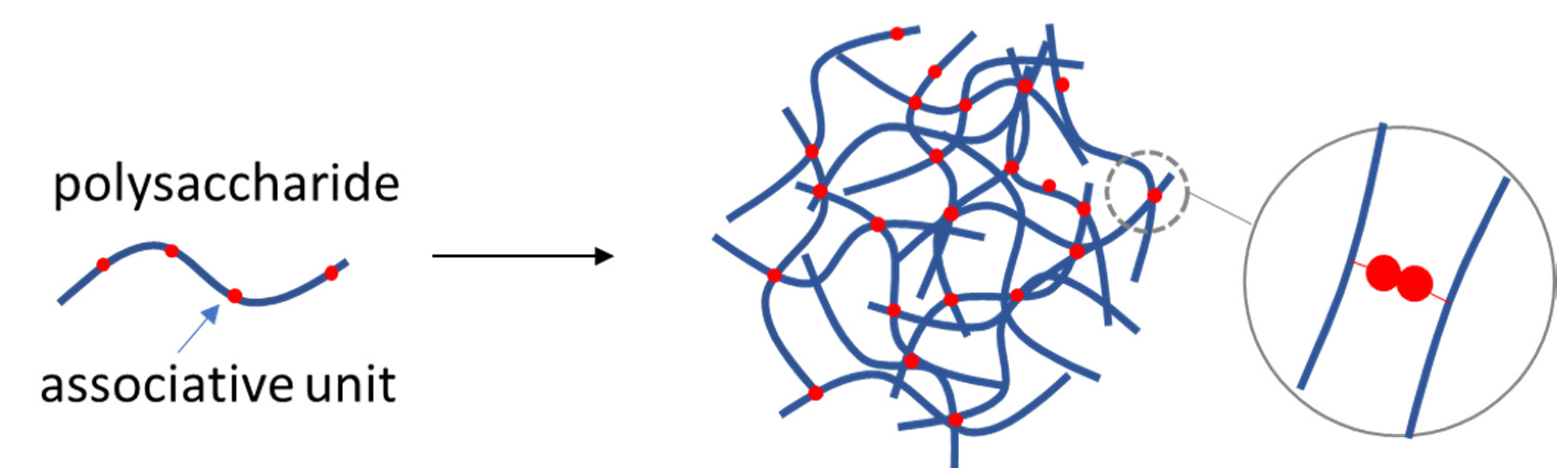
4.1. Self-Assembled Polysaccharide NGs
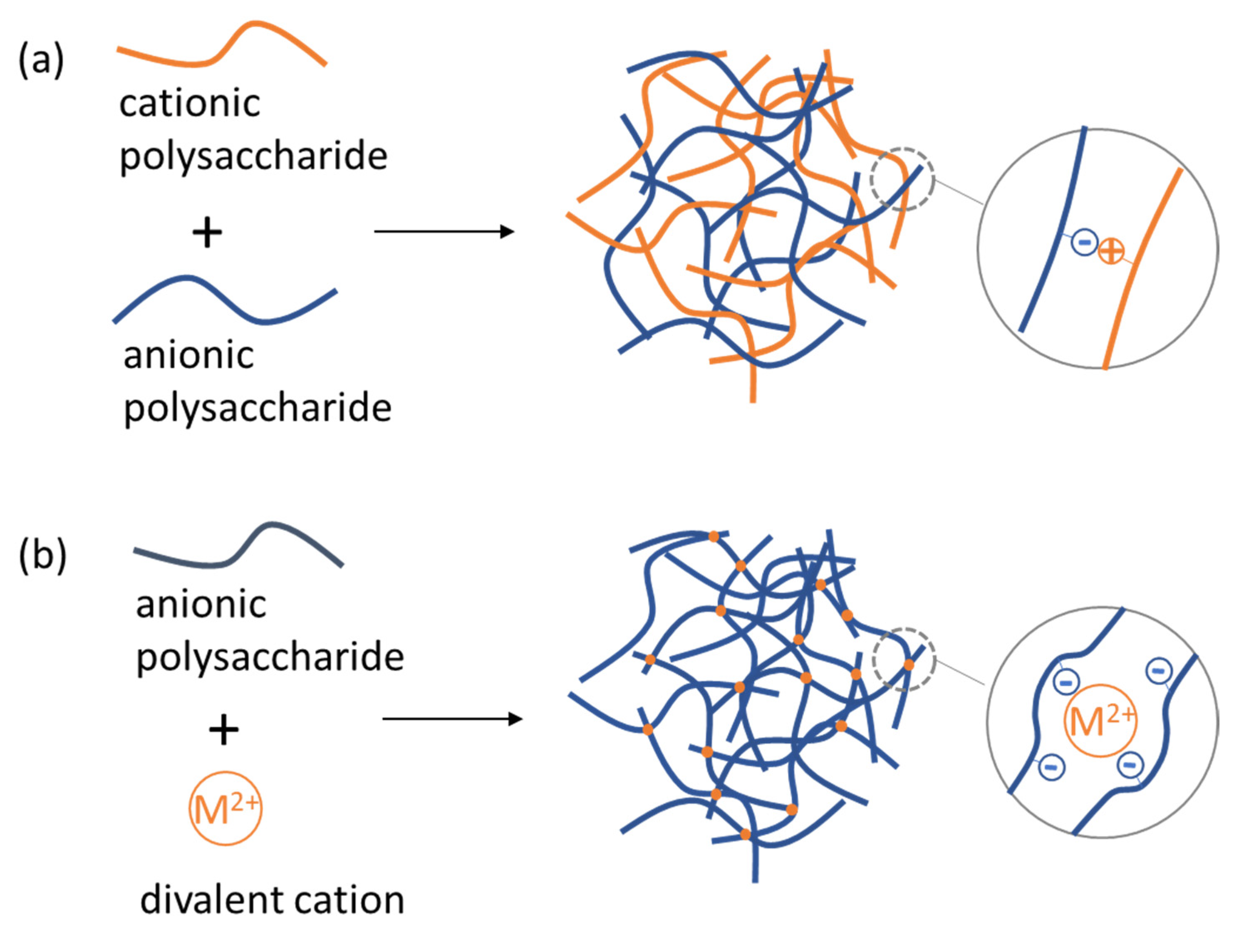
4.2. Polysaccharide NGs and MGs Formed by Electrostatic Complexation with Other Biopolymers and by Ionic Crosslinking
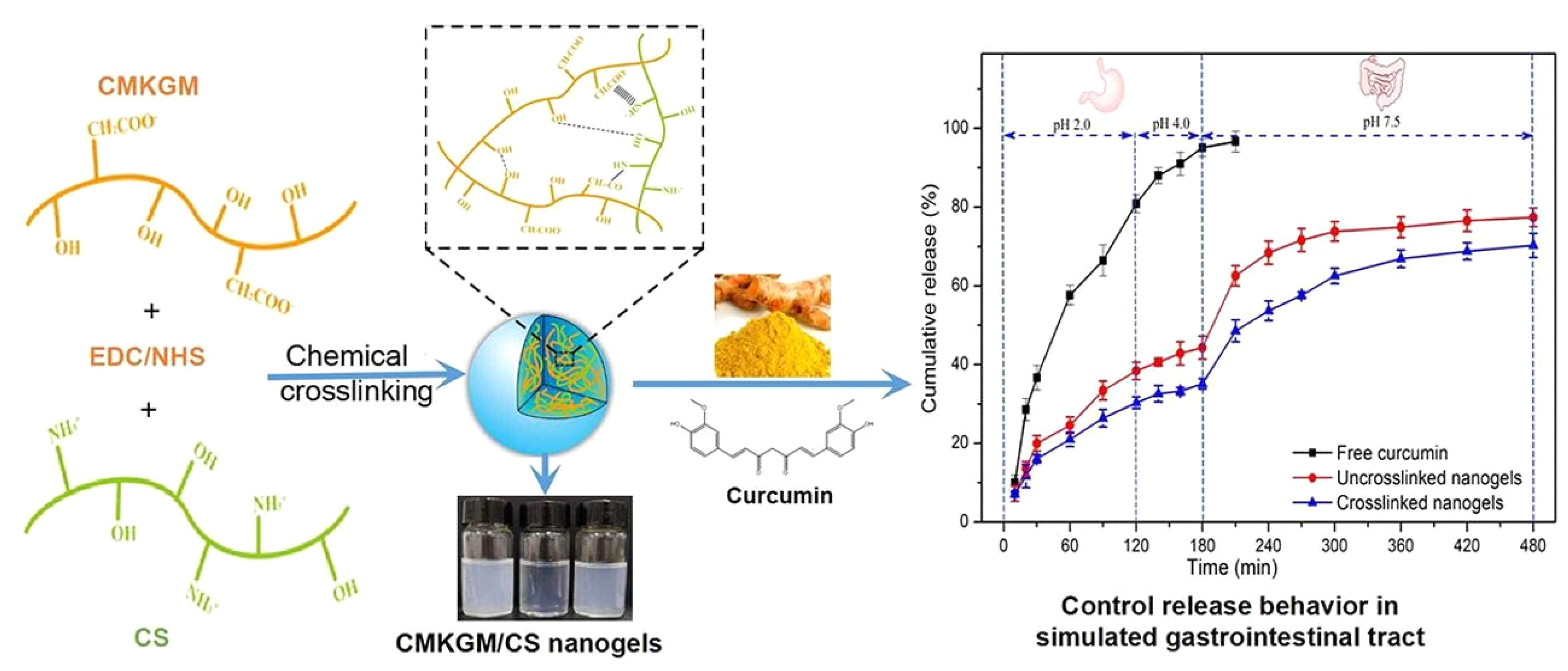
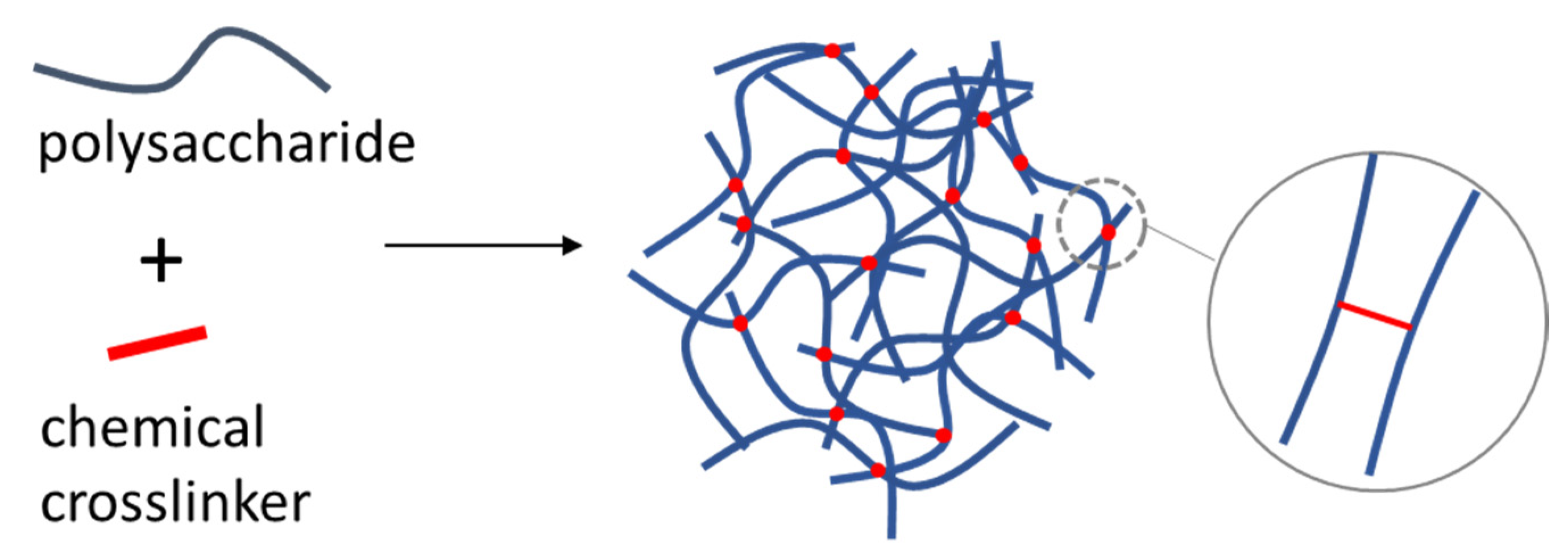
4.3. Chemically Crosslinked Polysaccharide NGs and MGs
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abedini, F.; Ebrahimi, M.; Roozbehani, A.H.; Domb, A.J.; Hosseinkhani, H. Overview on natural hydrophilic polysaccharide polymers in drug delivery. Polym. Adv. Technol. 2018, 29, 2564–2573. [Google Scholar] [CrossRef]
- Jindal, N.; Khattar, J.S. Microbial Polysaccharides in Food Industry. In Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 95–123. [Google Scholar]
- Sood, A.; Gupta, A.; Agrawal, G. Recent advances in polysaccharides based biomaterials for drug delivery and tissue engineering applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100067. [Google Scholar] [CrossRef]
- Campos, E.V.R.; de Oliveira, J.L.; Fraceto, L.F.; Singh, B. Polysaccharides as safer release systems for agrochemicals. Agron. Sustain. Dev. 2015, 35, 47–66. [Google Scholar] [CrossRef]
- Plucinski, A.; Lyu, Z.; Schmidt, B.V.K.J. Polysaccharide nanoparticles: From fabrication to applications. J. Mater. Chem. B 2021, 9, 7030–7062. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, J.; Xiao, H.; Xu, X.; Qian, Z. Polysaccharide hydrogels: Functionalization, construction and served as scaffold for tissue engineering. Carbohydr. Polym. 2021, 278, 118952. [Google Scholar] [CrossRef]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for Protein Delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohajer, F.; Khanzadi, S.; Keykhosravy, K.; Noori, S.M.A.; Azizzadeh, M.; Hashemi, M. Impact of gelatin nanogel coating containing thymol and nisin on the microbial quality of rainbow trout fillets and the inoculated Listeria monocytogenes. Aquac. Res. 2021, 52, 3958–3965. [Google Scholar] [CrossRef]
- Thorne, J.B.; Vine, G.J.; Snowden, M.J. Microgel applications and commercial considerations. Colloid Polym. Sci. 2011, 289, 625–646. [Google Scholar] [CrossRef]
- Zembyla, M.; Murray, B.S.; Sarkar, A. Water-in-oil emulsions stabilized by surfactants, biopolymers and/or particles: A review. Trends Food Sci. Technol. 2020, 104, 49–59. [Google Scholar] [CrossRef]
- Ngai, T.; Behrens, S.H.; Auweter, H. Novel emulsions stabilized by pH and temperature sensitive microgels. Chem. Commun. 2004, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dai, Y.; Gao, J.; Deng, Q.; Wan, C.; Li, B.; Zhou, B. Desalted duck egg white nanogels combined with κ-carrageenan as stabilisers for food-grade Pickering emulsion. Int. J. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Atarian, M.; Rajaei, A.; Tabatabaei, M.; Mohsenifar, A.; Bodaghi, H. Formulation of Pickering sunflower oil-in-water emulsion stabilized by chitosan-stearic acid nanogel and studying its oxidative stability. Carbohydr. Polym. 2019, 210, 47–55. [Google Scholar] [CrossRef]
- Huang, X.-M.; Luo, Z.-J.; Guo, J.; Ruan, Q.-J.; Wang, J.-M.; Yang, X.-Q. Enzyme-Adsorbed Chitosan Nanogel Particles as Edible Pickering Interfacial Biocatalysts and Lipase-Responsive Phase Inversion of Emulsions. J. Agric. Food Chem. 2020, 68, 8890–8899. [Google Scholar] [CrossRef]
- Li, W.; Nian, Y.; Huang, Y.; Zeng, X.; Chen, Q.; Hu, B. High loading contents, distribution and stability of β-carotene encapsulated in high internal phase emulsions. Food Hydrocoll. 2019, 96, 300–309. [Google Scholar] [CrossRef]
- Li, X.-M.; Xie, Q.-T.; Zhu, J.; Pan, Y.; Meng, R.; Zhang, B.; Chen, H.-Q.; Jin, Z.-Y. Chitosan hydrochloride/carboxymethyl starch complex nanogels as novel Pickering stabilizers: Physical stability and rheological properties. Food Hydrocoll. 2019, 93, 215–225. [Google Scholar] [CrossRef]
- Hosseini, E.; Rajaei, A.; Tabatabaei, M.; Mohsenifar, A.; Jahanbin, K. Preparation of Pickering Flaxseed Oil-in-Water Emulsion Stabilized by Chitosan-Myristic Acid Nanogels and Investigation of Its Oxidative Stability in Presence of Clove Essential Oil as Antioxidant. Food Biophys. 2019, 15, 216–228. [Google Scholar] [CrossRef]
- Calahorra, A.A.; Glover, Z.; Akhtar, M.; Sarkar, A. Conjugate microgel-stabilized Pickering emulsions: Role in delaying gastric digestion. Food Hydrocoll. 2020, 105, 105794. [Google Scholar] [CrossRef]
- Isusi, G.S.; Lohner, N.; Karbstein, H.; van der Schaaf, U. Emulsions stabilised with pectin-based microgels: Investigations into the break-up of droplets in the presence of microgels. J. Food Eng. 2020, 294, 110421. [Google Scholar] [CrossRef]
- Isusi, G.S.; Madlindl, L.; Karbstein, H.; van der Schaaf, U. Microstructures and conformational arrangement in emulsions caused by concentration ratios of pectin-based microgels and oil. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 602, 125166. [Google Scholar] [CrossRef]
- Kawano, S.; Kida, T.; Akashi, M.; Sato, H.; Shizuma, M.; Ono, D. Preparation of Pickering emulsions through interfacial adsorption by soft cyclodextrin nanogels. Beilstein J. Org. Chem. 2015, 11, 2355–2364. [Google Scholar] [CrossRef] [Green Version]
- Ravera, F.; Dziza, K.; Santini, E.; Cristofolini, L.; Liggieri, L. Emulsification and emulsion stability: The role of the interfacial properties. Adv. Colloid Interface Sci. 2020, 288, 102344. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Fernandes, I.P.; Silva, S.C.; Rezende, S.C.; Colucci, G.; Dias, M.M.; Barreiro, M.F. New Trends in Natural Emulsifiers and Emulsion Technology for the Food Industry; IntechOpen: London, UK, 2011. [Google Scholar]
- Chen, L.; Ao, F.; Ge, X.; Shen, W. Food-Grade Pickering Emulsions: Preparation, Stabilization and Applications. Molecules 2020, 25, 3202. [Google Scholar] [CrossRef]
- Kaptay, G. On the equation of the maximum capillary pressure induced by solid particles to stabilize emulsions and foams and on the emulsion stability diagrams. Colloids Surfaces A: Physicochem. Eng. Asp. 2006, 282–283, 387–401. [Google Scholar] [CrossRef]
- Dickinson, E. Microgels—An alternative colloidal ingredient for stabilization of food emulsions. Trends Food Sci. Technol. 2015, 43, 178–188. [Google Scholar] [CrossRef]
- Lefroy, K.S.; Murray, B.S.; Ries, M.E. Advances in the use of microgels as emulsion stabilisers and as a strategy for cellulose functionalisation. Cellulose 2020, 28, 647–670. [Google Scholar] [CrossRef]
- Hosseini, R.S.; Rajaei, A. Potential Pickering emulsion stabilized with chitosan-stearic acid nanogels incorporating clove essential oil to produce fish-oil-enriched mayonnaise. Carbohydr. Polym. 2020, 241, 116340. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Matsumiya, K.; Aoshima, M.; Matsumura, Y. Microgelation imparts emulsifying ability to surface-inactive polysaccharides—bottom-up vs top-down approaches. NPJ Sci. Food 2018, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, M.; Sha, L.; Lu, P.; Wu, M. “Bottom-Up” Assembly of Nanocellulose Microgels as Stabilizer for Pickering Foam Forming. Biomacromolecules 2021, 22, 3960–3970. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Murray, B.S.; Sarkar, A. Combination of egg white protein and microgels to stabilize foams: Impact of processing treatments. J. Food Eng. 2019, 275, 109860. [Google Scholar] [CrossRef]
- Isusi, G.I.S.; Weilandt, M.; Majollari, I.; Karbstein, H.P.; van der, S.U.S. Emulsions stabilised with pectin-based microgels: Investigations into the effect of pH and ionic strength on emulsion stability. Food Funct. 2021, 12, 7227–7238. [Google Scholar] [CrossRef] [PubMed]
- Andablo-Reyes, E.; Yerani, D.; Fu, M.; Liamas, E.; Connell, S.; Torres, O.; Sarkar, A. Microgels as viscosity modifiers influence lubrication performance of continuum. Soft Matter 2019, 15, 9614–9624. [Google Scholar] [CrossRef] [Green Version]
- Scheffold, F. Pathways and challenges towards a complete characterization of microgels. Nat. Commun. 2020, 11, 4315. [Google Scholar] [CrossRef]
- Bao, C.; Jiang, P.; Chai, J.; Jiang, Y.; Li, D.; Bao, W.; Liu, B.; Liu, B.; Norde, W.; Li, Y. The delivery of sensitive food bioactive ingredients: Absorption mechanisms, influencing factors, encapsulation techniques and evaluation models. Food Res. Int. 2019, 120, 130–140. [Google Scholar] [CrossRef]
- Berry, O.P. Stability of vitamins during food processing and storage. In Chemical Deterioration and Physical Instability of Food and Beverages; Skibsted, L.H., Risbo, J., Andersen, M.L., Eds.; Woodhead Publishing: Sawston, UK, 2010; pp. 539–560. [Google Scholar]
- Parisi, O.I.; Puoci, F.; Restuccia, D.; Farina, G.; Iemma, F.; Picci, N. Polyphenols and their formulations: Different strategies to overcome the drawbacks associated with their poor stability and bioavailability. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 29–45. [Google Scholar]
- Gonçalves, R.F.; Martins, J.T.; Duarte, C.M.; Vicente, A.A.; Pinheiro, A.C. Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation. Trends Food Sci. Technol. 2018, 78, 270–291. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Celli, G.B.; Lee, M.; Licker, J.; Abbaspourrad, A. Polyelectrolyte Complex Inclusive Biohybrid Microgels for Tailoring Delivery of Copigmented Anthocyanins. Biomacromolecules 2018, 19, 1517–1527. [Google Scholar] [CrossRef]
- Pravinata, L.C.; Murray, B.S. Encapsulation of water-insoluble polyphenols and β-carotene in Ca-alginate microgel particles produced by the Leeds Jet Homogenizer. Colloids Surf. A Physicochem. Eng. Asp. 2018, 561, 147–154. [Google Scholar] [CrossRef]
- Buosi, F.S.; Alaimo, A.; Di Santo, M.C.; Elías, F.; Liñares, G.G.; Acebedo, S.L.; Cataña, M.A.C.; Spagnuolo, C.C.; Lizarraga, L.; Martínez, K.D.; et al. Resveratrol encapsulation in high molecular weight chitosan-based nanogels for applications in ocular treatments: Impact on human ARPE-19 culture cells. Int. J. Biol. Macromol. 2020, 165, 804–821. [Google Scholar] [CrossRef]
- Piran, F.; Khoshkhoo, Z.; Hosseini, S.E.; Azizi, M.H. Controlling the Antioxidant Activity of Green Tea Extract through Encapsulation in Chitosan-Citrate Nanogel. J. Food Qual. 2020, 2020, 7935420. [Google Scholar] [CrossRef]
- Tarifa, M.C.; Piqueras, C.M.; Genovese, D.B.; Brugnoni, L.I. Microencapsulation of Lactobacillus casei and Lactobacillus rhamnosus in pectin and pectin-inulin microgel particles: Effect on bacterial survival under storage conditions. Int. J. Biol. Macromol. 2021, 179, 457–465. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Jiang, H.; Li, Y.; Pang, J. Construction of carboxymethyl konjac glucomannan/chitosan complex nanogels as potential delivery vehicles for curcumin. Food Chem. 2021, 362, 130242. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yao, P. Soy Protein/Soy Polysaccharide Complex Nanogels: Folic Acid Loading, Protection, and Controlled Delivery. Langmuir 2013, 29, 8636–8644. [Google Scholar] [CrossRef] [PubMed]
- Jooybar, E.; Abdekhodaie, M.J.; Mousavi, A.; Zoetebier, B.; Dijkstra, P.J. Enzymatically crosslinked hyaluronic acid microgels as a vehicle for sustained delivery of cationic proteins. Eur. Polym. J. 2019, 115, 234–243. [Google Scholar] [CrossRef]
- Ravi, H.; Baskaran, V. Biodegradable chitosan-glycolipid hybrid nanogels: A novel approach to encapsulate fucoxanthin for improved stability and bioavailability. Food Hydrocoll. 2015, 43, 717–725. [Google Scholar] [CrossRef]
- Feng, R.; Wang, L.; Zhou, P.; Luo, Z.; Li, X.; Gao, L. Development of the pH responsive chitosan-alginate based microgel for encapsulation of Jughans regia L. polyphenols under simulated gastrointestinal digestion in vitro. Carbohydr. Polym. 2020, 250, 116917. [Google Scholar] [CrossRef]
- Mazza, G.J. Anthocyanins and heart health. Ann. Ist. Super Sanita 2007, 43, 369–374. [Google Scholar]
- He, J.; Giusti, M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Chen, L.; Xin, X.; Yuan, Q. A Study of Controlled Uptake and Release of Anthocyanins by Oxidized Starch Microgels. J. Agric. Food Chem. 2013, 61, 5880–5887. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Dadmohammadi, Y.; Lee, M.C.; Abbaspourrad, A. Combination of copigmentation and encapsulation strategies for the synergistic stabilization of anthocyanins. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3164–3191. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y. Synthesis of porous starch microgels for the encapsulation, delivery and stabilization of anthocyanins. J. Food Eng. 2021, 302, 110552. [Google Scholar] [CrossRef]
- Wang, M.; Doi, T.; McClements, D.J. Encapsulation and controlled release of hydrophobic flavors using biopolymer-based microgel delivery systems: Sustained release of garlic flavor during simulated cooking. Food Res. Int. 2019, 119, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Xie, J.; Du, H.; McClements, D.J.; Xiao, H.; Li, L. Progress in microencapsulation of probiotics: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mwangi, W.W.; Lim, H.P.; Low, L.E.; Tey, B.T.; Chan, E.S. Food-grade Pickering emulsions for encapsulation and delivery of bioactives. Trends Food Sci. Technol. 2020, 100, 320–332. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, L.-M. Casein nanogels as effective stabilizers for Pickering high internal phase emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123662. [Google Scholar] [CrossRef]
- El-Say, K.; El-Sawy, H. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Ahmed, S.A.; Alkahtani, S.; Milivojevic, M.; Kandar, C.C.; Dhara, A.K.; Nayak, A.K. Biopolymers for drug delivery. In Advanced Biopolymeric Systems for Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–29. [Google Scholar]
- Suner, S.S.; Ari, B.; Onder, F.C.; Ozpolat, B.; Ay, M.; Sahiner, N. Hyaluronic acid and hyaluronic acid: Sucrose nanogels for hydrophobic cancer drug delivery. Int. J. Biol. Macromol. 2019, 126, 1150–1157. [Google Scholar] [CrossRef]
- Zhang, H.; Pei, M.; Liu, P. pH-Activated surface charge-reversal double-crosslinked hyaluronic acid nanogels with feather keratin as multifunctional crosslinker for tumor-targeting DOX delivery. Int. J. Biol. Macromol. 2019, 150, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, T.F.; Couturaud, B.; Szarpak-Jankowska, A.; Fournier, D.; Louage, B.; Garcia, F.P.; Nakamura, C.V.; De Geest, B.G.; Woisel, P.; van der Sanden, B.; et al. Coumarin-containing thermoresponsive hyaluronic acid-based nanogels as delivery systems for anticancer chemotherapy. Nanoscale 2017, 9, 12150–12162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Asghar, S.; Tian, C.; Hu, Z.; Ping, Q.; Chen, Z.; Shao, F.; Xiao, Y. Lactoferrin/phenylboronic acid-functionalized hyaluronic acid nanogels loading doxorubicin hydrochloride for targeting glioma. Carbohydr. Polym. 2020, 253, 117194. [Google Scholar] [CrossRef]
- Simonson, A.W.; Lawanprasert, A.; Goralski, T.D.; Keiler, K.; Medina, S.H. Bioresponsive peptide-polysaccharide nanogels—A versatile delivery system to augment the utility of bioactive cargo. Nanomed. Nanotechnol. Biol. Med. 2018, 17, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, C.; Liu, A.; Zhen, X.; Gao, J.; Wu, W.; Cai, W.; Jiang, X. Responsive hyaluronic acid-gold cluster hybrid nanogel theranostic systems. Biomater. Sci. 2020, 9, 1363–1373. [Google Scholar] [CrossRef]
- Vlassi, E.; Papagiannopoulos, A. Nanoformulation of fibrinogen by thermal stabilization of its electrostatic complexes with hyaluronic acid. Int. J. Biol. Macromol. 2020, 158, 251–257. [Google Scholar] [CrossRef]
- Setayesh, A.; Bagheri, F.; Boddohi, S. Self-assembled formation of chondroitin sulfate-based micellar nanogel for curcumin delivery to breast cancer cells. Int. J. Biol. Macromol. 2020, 161, 771–778. [Google Scholar] [CrossRef]
- Onishi, H.; Ikeuchi-Takahashi, Y.; Kawano, K.; Hattori, Y. Preparation of Chondroitin Sulfate-Glycyl-Prednisolone Conjugate Nanogel and Its Efficacy in Rats with Ulcerative Colitis. Biol. Pharm. Bull. 2019, 42, 1155–1163. [Google Scholar] [CrossRef] [Green Version]
- Onishi, H.; Matsuyama, M. Conjugate between Chondroitin Sulfate and Prednisolone with a Glycine Linker: Preparation and in Vitro Conversion Analysis. Chem. Pharm. Bull. 2013, 61, 902–912. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhao, W.; Chen, H.; Qin, A.; Zhu, P. Anti-tumor Study of Chondroitin Sulfate-Methotrexate Nanogels. Nanoscale Res. Lett. 2017, 12, 572. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Vlassi, E. Stimuli-responsive nanoparticles by thermal treatment of bovine serum albumin inside its complexes with chondroitin sulfate. Food Hydrocoll. 2019, 87, 602–610. [Google Scholar] [CrossRef]
- Ding, Y.-F.; Wei, J.; Li, S.; Pan, Y.-T.; Wang, L.-H.; Wang, R. Host–Guest Interactions Initiated Supramolecular Chitosan Nanogels for Selective Intracellular Drug Delivery. ACS Appl. Mater. Interfaces 2019, 11, 28665–28670. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, M.; Kokabi, M. Chitosan/laponite nanocomposite nanogels as a potential drug delivery system. Polym. Bull. 2020, 78, 4593–4607. [Google Scholar] [CrossRef]
- Tao, Q.; Zhong, J.; Wang, R.; Huang, Y. Ionic and Enzymatic Multiple-Crosslinked Nanogels for Drug Delivery. Polymers 2021, 13, 3565. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, G.; Zhang, J.; Song, H.; Niu, J.; Shi, S.; Huang, P.; Wang, Y.; Wang, W.; Li, C.; et al. Targeted antigen delivery to dendritic cell via functionalized alginate nanoparticles for cancer immunotherapy. J. Control. Release 2017, 256, 170–181. [Google Scholar] [CrossRef]
- Mizuno, K.; Ikeuchi-Takahashi, Y.; Hattori, Y.; Onishi, H. Preparation and evaluation of conjugate nanogels of glycyl-prednisolone with natural anionic polysaccharides as anti-arthritic delivery systems. Drug Deliv. 2020, 28, 136–144. [Google Scholar] [CrossRef]
- Onishi, H.; Mizuno, K.; Ikeuchi-Takahashi, Y.; Hattori, Y. Targeting potential of alginate-glycyl-prednisolone conjugate nanogel to inflamed joints in rats with adjuvant-induced arthritis. J. Drug Target. 2021, 29, 892–899. [Google Scholar] [CrossRef]
- Podgórna, K.; Szczepanowicz, K.; Piotrowski, M.; Gajdošová, M.; Štěpánek, F.; Warszynski, P. Gadolinium alginate nanogels for theranostic applications. Colloids Surf. B Biointerfaces 2017, 153, 183–189. [Google Scholar] [CrossRef]
- Peng, N.; Ding, X.; Wang, Z.; Cheng, Y.; Gong, Z.; Xu, X.; Gao, X.; Cai, Q.; Huang, S.; Liu, Y. Novel dual responsive alginate-based magnetic nanogels for onco-theranostics. Carbohydr. Polym. 2018, 204, 32–41. [Google Scholar] [CrossRef]
- Wang, Q.-S.; Gao, L.-N.; Zhu, X.-N.; Zhang, Y.; Zhang, C.-N.; Xu, D.; Cui, Y.-L. Co-delivery of glycyrrhizin and doxorubicin by alginate nanogel particles attenuates the activation of macrophage and enhances the therapeutic efficacy for hepatocellular carcinoma. Theranostics 2019, 9, 6239–6255. [Google Scholar] [CrossRef]
- Kinoshita, N.; Sasaki, Y.; Marukawa, E.; Hirose, R.; Sawada, S.-I.; Harada, H.; Akiyoshi, K. Crosslinked nanogel-based porous hydrogel as a functional scaffold for tongue muscle regeneration. J. Biomater. Sci. Polym. Ed. 2020, 31, 1254–1271. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Yamamoto, K.; Kishida, T.; Kotani, S.-I.; Sato, Y.; Horiguchi, S.; Yamanobe, H.; Adachi, T.; Boschetto, F.; Marin, E.; et al. Osteogenic Response to Polysaccharide Nanogel Sheets of Human Fibroblasts After Conversion into Functional Osteoblasts by Direct Phenotypic Cell Reprogramming. Front. Bioeng. Biotechnol. 2021, 9, 713932. [Google Scholar] [CrossRef] [PubMed]
- Miura, R.; Sawada, S.-I.; Mukai, S.-A.; Sasaki, Y.; Akiyoshi, K. Antigen Delivery to Antigen-Presenting Cells for Adaptive Immune Response by Self-Assembled Anionic Polysaccharide Nanogel Vaccines. Biomacromolecules 2019, 21, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Moraes, F.C.; Ramirez, L.M.F.; Aid, R.; Benadda, S.; Maire, M.; Chauvierre, C.; Antunes, J.C.; Chaubet, F.; Letourneur, D. P-selectin targeting polysaccharide-based nanogels for miRNA delivery. Int. J. Pharm. 2021, 597, 120302. [Google Scholar] [CrossRef]
- Rahmani, Z.; Ghaemy, M.; Olad, A. Preparation of nanogels based on kappa-carrageenan/chitosan and N-doped carbon dots: Study of drug delivery behavior. Polym. Bull. 2020, 78, 2709–2726. [Google Scholar] [CrossRef]
- Liu, K.; Zheng, D.; Zhao, J.; Tao, Y.; Wang, Y.; He, J.; Lei, J.; Xi, X. pH-Sensitive nanogels based on the electrostatic self-assembly of radionuclide131I labeled albumin and carboxymethyl cellulose for synergistic combined chemo-radioisotope therapy of cancer. J. Mater. Chem. B 2018, 6, 4738–4746. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Sklapani, A. Xanthan-based polysaccharide/protein nanoparticles: Preparation, characterization, encapsulation and stabilization of curcumin. Carbohydr. Polym. Technol. Appl. 2021, 2, 100075. [Google Scholar] [CrossRef]
- Cai, H.; Yao, P. In situ preparation of gold nanoparticle-loaded lysozyme–dextran nanogels and applications for cell imaging and drug delivery. Nanoscale 2013, 5, 2892–2900. [Google Scholar] [CrossRef]
- Sahiner, N.; Umut, E.; Suner, S.S.; Sahiner, M.; Culha, M.; Ayyala, R.S. Hyaluronic acid (HA)-Gd(III) and HA-Fe(III) microgels as MRI contrast enhancing agents. Carbohydr. Polym. 2021, 277, 118873. [Google Scholar] [CrossRef]
- Sahiner, N.; Suner, S.S.; Ayyala, R.S. Mesoporous, degradable hyaluronic acid microparticles for sustainable drug delivery application. Colloids Surf. B Biointerfaces 2019, 177, 284–293. [Google Scholar] [CrossRef]
- De Carvalho, B.G.; Taketa, T.B.; Garcia, B.B.M.; Han, S.W.; de la Torre, L.G. Hybrid microgels produced via droplet microfluidics for sustainable delivery of hydrophobic and hydrophilic model nanocarriers. Mater. Sci. Eng. C 2020, 118, 111467. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Chen, X.; Chen, X.; Li, S.; Yuan, G.; Zhou, T.; Li, J.; Jia, Y.; Xiong, D.; Tan, H. Covalent Chitosan-Cellulose Hydrogels via Schiff-Base Reaction Containing Macromolecular Microgels for pH-Sensitive Drug Delivery and Wound Dressing. Macromol. Chem. Phys. 2019, 220, 1900399. [Google Scholar] [CrossRef]
- Yan, Y.; Wu, Q.; Ren, P.; Liu, Q.; Zhang, N.; Ji, Y.; Liu, J. Zinc ions coordinated carboxymethyl chitosan-hyaluronic acid microgel for pulmonary drug delivery. Int. J. Biol. Macromol. 2021, 193, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Pellá, M.C.G.; Simão, A.R.; Lima-Tenório, M.K.; Tenório-Neto, E.; Scariot, D.B.; Nakamura, C.V.; Rubira, A.F. Chitosan hybrid microgels for oral drug delivery. Carbohydr. Polym. 2020, 239, 116236. [Google Scholar] [CrossRef]
- Marín-Payá, J.C.; Díaz-Benito, B.; Martins, L.A.; Trujillo, S.C.; Cordón, L.; Lanceros-Méndez, S.; Ferrer, G.G.; Sempere, A.; Ribelles, J.L.G. Biomimetic 3D Environment Based on Microgels as a Model for the Generation of Drug Resistance in Multiple Myeloma. Materials 2021, 14, 7121. [Google Scholar] [CrossRef]
- Zhang, Y.; An, C.; Zhang, Y.; Zhang, H.; Mohammad, A.F.; Li, Q.; Liu, W.; Shao, F.; Sui, J.; Ren, C.; et al. Microfluidic-templating alginate microgels crosslinked by different metal ions as engineered microenvironment to regulate stem cell behavior for osteogenesis. Mater. Sci. Eng. C 2021, 131, 112497. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; He, D.; Ma, Z.; Liu, K.; Xue, K.; Li, H. An effective strategy for preparing macroporous and self-healing bioactive hydrogels for cell delivery and wound healing. Chem. Eng. J. 2021, 425, 130677. [Google Scholar] [CrossRef]
- Chai, N.; Zhang, J.; Zhang, Q.; Du, H.; He, X.; Yang, J.; Zhou, X.; He, J.; He, C. Construction of 3D printed constructs based on microfluidic microgel for bone regeneration. Compos. Part B Eng. 2021, 223, 109100. [Google Scholar] [CrossRef]
- He, L.; Zheng, R.; Min, J.; Lu, F.; Wu, C.; Zhi, Y.; Shan, S.; Su, H. Preparation of magnetic microgels based on dextran for stimuli-responsive release of doxorubicin. J. Magn. Magn. Mater. 2020, 517, 167394. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Chiu, C.-C.; Chen, H.-Y.; Chen, S.-H.; Wang, L.F. Preparation of Chondroitin Sulfate-g-poly(ε-caprolactone) Copolymers as a CD44-Targeted Vehicle for Enhanced Intracellular Uptake. Mol. Pharm. 2014, 11, 1164–1175. [Google Scholar] [CrossRef]
- Wang, K.; Liu, M.; Mo, R. Polysaccharide-Based Biomaterials for Protein Delivery. Med. Drug Discov. 2020, 7, 100031. [Google Scholar] [CrossRef]
- Sun, Z.; Yi, Z.; Cui, X.; Chen, X.; Su, W.; Ren, X.; Li, X. Tumor-targeted and nitric oxide-generated nanogels of keratin and hyaluronan for enhanced cancer therapy. Nanoscale 2018, 10, 12109–12122. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhou, J.; Huang, L.; Jing, J.; Wang, N.; Wang, L. Curcumin encapsulation and protection based on lysozyme nanoparticles. Food Sci. Nutr. 2019, 7, 2702–2707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.N.; Ha, P.T.; Nguyen, A.S.; Nguyen, D.T.; Do, H.D.; Thi, Q.N.; Thi, M.N.H. Curcumin as fluorescent probe for directly monitoring in vitro uptake of curcumin combined paclitaxel loaded PLA-TPGS nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 25001. [Google Scholar] [CrossRef]
- Cho, A.R.; Chun, Y.G.; Kim, B.K.; Park, D.J. Preparation of alginate–CaCl2 microspheres as resveratrol carriers. J. Mater. Sci. 2014, 49, 4612–4619. [Google Scholar] [CrossRef]
- Abdi, F.; Michel, R.; Poirot, R.; Dakir, M.; Sancey, L.; Ravaine, V.; Auzély-Velty, R. Dynamic Covalent Chemistry Enables Reconfigurable All-Polysaccharide Nanogels. Macromol. Rapid Commun. 2020, 41, e2000213. [Google Scholar] [CrossRef]





| Polysaccharide | Size | Formation Mechanism | Ref. |
|---|---|---|---|
| Chitosan | NG | Amide bonding with stearic acid (EDC-mediated reaction) | [18] |
| NG | Crosslinking with genipin | [19] | |
| MG | Crosslinking with genipin | [20] | |
| Chitosan/carboxymethyl starch | NG | Amide bonding with stearic acid (EDC-mediated reaction) | [21] |
| Chitosan/Myristic acid | NG | Amide bonding(EDC-mediated reaction) | [22] |
| Whey protein/dextran | MG | Maillard conjugation, top-down method | [23] |
| Pectin | MG | Crosslinking with CaCl2 | [24] |
| Pectin | MG | Crosslinking with CaCl2 | [25] |
| Cyclodextrin | NG | Crosslinking with 1,4-phenylene diisocyanate (PDI) | [26] |
| Polysaccharide | Size | Crosslinking Method | Loaded Substance | Potential for Applications | Ref. |
|---|---|---|---|---|---|
| Alginate | MG | Emulsification/internal gelation, Polyelectrolyte complexes (Chitosan-Chondroitin sulfate) | Anthocyanins | Controlled delivery | [44] |
| MG | CaCl2 crosslinking | Rutin, tiliroside, β-carotene, curcumin | Therapeutic | [45] | |
| Pectin | NG | Ionic gelation with sodium tripolyphosphate | Resveratrol | Ocular treatments | [46] |
| NG | Covalent bonding with citrate | Green tea | Antioxidant activity | [47] | |
| MG | Ionotropic gelation, CaCl2 crosslinking | Lactobacillus casei/rhamnosus | Storage stability enhancement, protected delivery | [48] | |
| Carboxymethyl konjac glucomannan/chitosan | NG | Covalent bonding, electrostatic interactions, EDC/NHS crosslinking | Curcumin | Controlled release | [49] |
| Soy polysaccharide | NG | Self-assembly with Soy protein | Folic acid | Controlled release, protected delivery | [50] |
| Hyaluronic acid | MG | Enzymatic crosslinking of tyramine conjugated HA in the presence of HRP and H2O2 | Lysozyme, TGF-β1 | Sustained release | [51] |
| Chitosan | NG | Ionic gelation with sodium tripolyphosphate | Fucoxanthin | Controlled release, Storage stability enhancement | [52] |
| Chit/Alginate | MG | Electrostatic interactions | Juglans regia L. polyphenols | Sustained release | [53] |
| Polysaccharide | Formation Mechanism | Loaded Substance | Potential for Applications | Ref. |
|---|---|---|---|---|
| Hyaluronic acid | crosslinking by glycerol diglycidyl ether in emulsion | 3 ((E) 3 (4 hydroxyphenyl)acryloyl) 2H chromen 2 one | Blood compatibility, loading and release in biological fluids | [65] |
| electrostatic complexation with keratin and crosslinking by peroxide | DOX | Antitumor activity | [66] | |
| Self-assembled by DEGMA side chains and coumarin | paclitaxel | Activity against ovarian cancer cells | [67] | |
| Disulfide bonds by methacrylating with cystamine | Cationic DOX | Glioma therapy | [68] | |
| electrostatic complexation with poly-l-lysine | GFP, DOX and VAN | chemotherapy and antibiotic activity | [69] | |
| radical polymerization of methacrylated hyaluronic acid with cystamine bisacrylamide | DOX and Au nanoclusters | tumor cell inhibition | [70] | |
| electrostatic complexation with Fbg and thermal treatment | Curcumin | therapeutic and diagnostic | [71] | |
| Chondroitin sulfate | Self-assembling grafted by octadecylamine | Curcumin | Activity against human breast cancer cells | [72] |
| Self-assembly by conjugated prednisolone | Prednisolone | treatment of ulcerative colitis | [73] | |
| Self-assembly by conjugated prednisolone | Prednisolone | Treatment of rheumatoid arthritis | [74] | |
| self-assembly by conjugated methotrexate | Methotrexate | activity against A549T and Hela tumor cells | [75] | |
| electrostatic complexation with BSA and thermal treatment | β-Carotene | Therapeutic | [76] | |
| Chitosan | grafted phenylamine for host-guest interaction in the presence cucurbit[8]uril | DOX | Hindering the growth of human lung cancer cells | [77] |
| Ionic gelation with tripolyphosphate | Honey | Effect of laponite on drug loading/release | [78] | |
| Ionic gelation with tripolyphosphate and enzymatic by peroxide | 5-Fluorouracil | Effect of crosslinking and pH conditions on drug release | [79] | |
| Alginate | Crosslinking by CaCl2 | Ovalbumin | Dendritic cell targeting | [80] |
| Self-assembly by conjugated prednisolone | Prednisolone | Arthritis therapy | [81,82] | |
| ionic crosslinking with gadolinium in reverse microemulsion | Hydrophilic drugs and rhodamine b | Treatment of neurodegenerative diseases and MRI | [83] | |
| disulfide modification and CaCl2 crosslinking | DOX and superparamagnetic iron oxide NPs | chemotherapy and MRI | [84] | |
| CaCl2 crosslinking | DOX and GL | treatment of hepatocellular carcinoma | [85] | |
| Pullulan | Physical crosslinking by conjugated cholesterol units | Insulin | Tongue muscle regeneration | [86] |
| crosslinked with terminal thiol group polyethylene glycol | Human dermal fibroblasts/osteoblasts | Osteogenic NG transplants | [87] | |
| Physical crosslinking by conjugated cholesterol units | Ovalbumin | Anticancer immunotherapy | [88] | |
| electrostatic complexation with fucoidan and genipin crosslinking | miRNA | Atherothrombosis treatment | [89] | |
| κ-Carrageenan/Chitosan | copolymerization of acrylamide and sodium acrylate | Rivastigmine | Biocompatibility, drug release, incorporation of carbon dots | [90] |
| carboxymethyl cellulose | electrostatic complexation with BSA and thermal treatment | Camptothecin, 132I | Therapeutic and diagnostic action | [91] |
| Xanthan gum | electrostatic complexation with BSA and thermal treatment | Curcumin | Therapeutic | [92] |
| Dextran | thermal treatment with lysozyme | DOX and Au NPs | Optical cell imaging and cancer treatment | [93] |
| Polysaccharide | Formation Mechanism | Loaded Substance | Potential for Applications | Ref. |
|---|---|---|---|---|
| Hyaluronic acid | Fe3+ and Gd3+ crosslinking in reverse micelle emulsion medium | Fe and Gd | Blood contacting applications and MRI signal enhancers | [94] |
| Crosslinikng by divinyl sulfone | VAN | Sustainable drug delivery | [95] | |
| Alginate/Chondroitin sulfate/Silk fibroin | Droplet microfluidics and Ca2+/Zn2+ crosslinking | PS NPs and BSA-coated PS NPs | Controlled release of drug-loaded NPs | [96] |
| Chitosan | Schiff-base crosslinking reaction | BSA and AgSD | Wound dressings | [97] |
| Schiff-base crosslinking reaction | BSA | Pulmonary drug delivery | [98] | |
| Emulsion polymerization | vitamin-B12 | Oral drug delivery | [99] | |
| Alginate | microspheres crosslinking by CaCl2 | RPMI 8226 cells | Model for drug resistance in multiple myeloma | [100] |
| metal ions crosslinked hydrogel and microfluidic preparation | mesenchymal stem cells | Microenvironment for osteogenesis | [101] | |
| chemically bonded interpenetrating hydrogels with HA and squeezing | Human umbilical vein/mouse aortic endothelial cells | Tissue regeneration | [102] | |
| crosslinking by CaCl2 in a microfluidic device | Bone marrow mesenchymal stem cells | Scaffolds for bone regeneration | [103] | |
| Dextran | Schiff base reaction with diamine in w/o inverse emulsion | magnetic NPs and DOX | DOX release | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papagiannopoulos, A.; Sotiropoulos, K. Current Advances of Polysaccharide-Based Nanogels and Microgels in Food and Biomedical Sciences. Polymers 2022, 14, 813. https://doi.org/10.3390/polym14040813
Papagiannopoulos A, Sotiropoulos K. Current Advances of Polysaccharide-Based Nanogels and Microgels in Food and Biomedical Sciences. Polymers. 2022; 14(4):813. https://doi.org/10.3390/polym14040813
Chicago/Turabian StylePapagiannopoulos, Aristeidis, and Konstantinos Sotiropoulos. 2022. "Current Advances of Polysaccharide-Based Nanogels and Microgels in Food and Biomedical Sciences" Polymers 14, no. 4: 813. https://doi.org/10.3390/polym14040813
APA StylePapagiannopoulos, A., & Sotiropoulos, K. (2022). Current Advances of Polysaccharide-Based Nanogels and Microgels in Food and Biomedical Sciences. Polymers, 14(4), 813. https://doi.org/10.3390/polym14040813







