Smart 3D Printed Hydrogel Skin Wound Bandages: A Review
Abstract
1. Introduction
2. Skin Structure
2.1. Epidermis
2.2. Dermis
2.3. Hypodermis
3. Skin Wounds
3.1. Common Skin Wounds and Clinical Treatment
3.2. Acute and Chronic Skin Wounds
3.2.1. Acute Wounds
3.2.2. Chronic Wounds
| Gauge used | Measurement | Indication | Ref. |
|---|---|---|---|
| iDr or mobile app. | 3D imaging of the wound (Figure 2a) | By applying the optical imaging principle and surface feet per minutes (SFM), using a smartphone video, iDr can accurately and non-invasively reconstruct a 3D wound model and measure the wound’s area and volume in 3D digital space. Using recorded history data on volume and area, iDr can help clinicians analyze wound healing effectiveness during treatment. | [69] |
| Matrix metalloproteinase (MMP) | Collecting wound fluids (22 samples) and chronic wounds (25 samples) of various etiologies, including mixed vessel disease ulcers, decubitus and diabetic foot ulcers (Figure 2b). | Chronic wounds (median 22.8 μg MMP Eq/mL) compared to acute wounds (median 0.76 μg MMP Eq/mL) (p < 0.001). | [70] |
3.3. Skin Wound Healing Process
3.3.1. Hemostasis (Blood Clotting)
3.3.2. Inflammation
3.3.3. Tissue Growth (Proliferation)
3.3.4. Tissue Remodeling (Maturation)
4. Smart Hydrogel Wound Dressings
Effect of the Hydrogel Crosslinking Process on Mechanical Strength and Water Absorption
| Type of Crosslinking | Monomers | Common Crosslinkers | Ref. |
|---|---|---|---|
| Homopolymer (single network) | Poly(2-hydroxyethyl methacrylate) (Figure 3) | Polyethylene glycol dimethacrylate | [90,93] |
| Triethylene glycol dimethacrylate (TEGDMA) | |||
| Co-polymer (double or more) | Polyethylene glycol (PEG)/ | ||
| methacrylic acid (MAA) (Figure 3) | Tetra(ethylene glycol) dimethacrylate | [90,92,122] | |
| Carboxymethyl acid cellulose (CMC)/ | |||
| Poly(vinyl pyrrolid) (PVP) | |||
| Semi-interpenetrating network (semi-IPN) | Acrylamide/acrylic acid copolymer/ Linear cationic polyallylammonium chloride | N,N’-methylene bisacrylamide | [93] |
| Interpenetrating network (IPN) | Poly(N-isopropyl acrylamide)/ Chitosan | N,N’-methylene bisacrylamide | [123] |

5. Additive Manufacturing/3D Printing
5.1. Recent Bio-Printing Technologies Outcomes and Limitations
5.2. D Printed Hydrogel Patches by DLP/SLA
6. Recent Developments in the 3D Printing of Hydrogel Wound Dressings
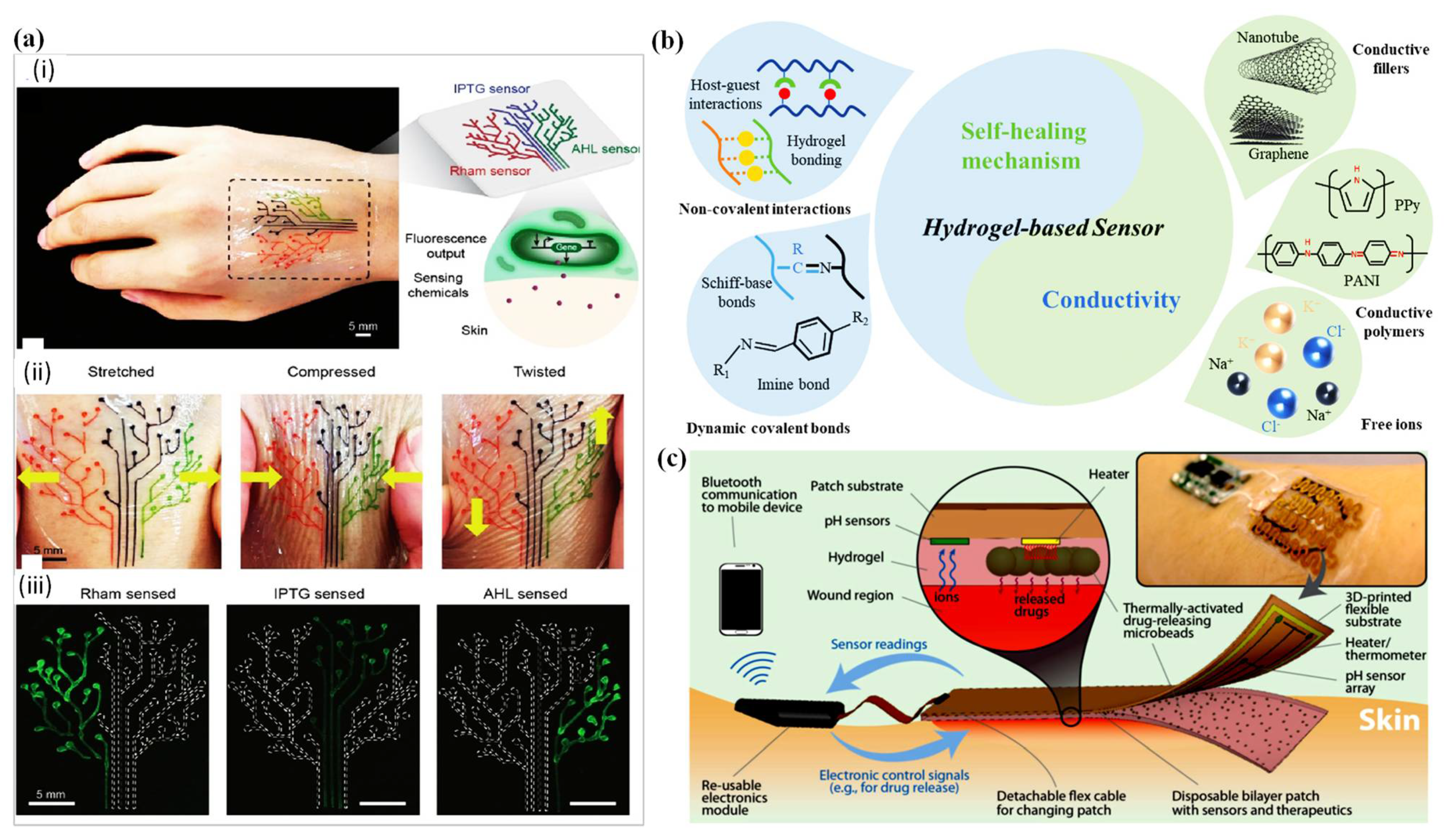
7. D Printed Wound Dressing Integrated with Sensors
7.1. Temperature Sensor-Integrated Wound Dressings
7.2. pH Sensor-Integrated Wound Dressings
8. Fundamental Challenges and Future Outcomes
9. Recommendations and Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kresina, T.; Kaplowitz, L.; Johnson, K. Human Immunodeficiency Virus Infection in Young Adults: Treatment of Substance Use Disorders as a Priority Component of HIV Prevention, Care and Treatment in Low and Middle Income Countries. Int. J. AIDS Res. 2016, 3, 97–104. [Google Scholar]
- Reportlinker. The Global Advanced Wound Care Market Is Projected to Reach USD 12.8 Billion by 2026 from USD 9.4 Billion in 2021, at a CAGR of 6.2%. Available online: https://uk.finance.yahoo.com/news/global-advanced-wound-care-market-103200438.html?guccounter=1 (accessed on 8 December 2021).
- Sen, C.K. Human wounds and its burden: An updated compendium of estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.F.; Ayoub, N.; McIlwraith, T.; Uchegbu, I.; Gerrish, A.; Weidlich, D.; Vowden, K.; Vowden, P. Health economic burden that different wound types impose on the UK’s National Health Service. Int. Wound J. 2017, 14, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Jones, V.; Grey, J.E.; Harding, K.G. Wound dressings. BMJ 2006, 332, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Shen, Y.; Deng, S.; Liu, T.; Qi, F.; Lu, Z.; Liu, L.; Shao, N.; Xie, J.; Ding, F. Dual functional β-peptide polymer-modified resin beads for bacterial killing and endotoxin adsorption. BMC Mater. 2019, 1, 1–8. [Google Scholar] [CrossRef]
- Natarajan, S.; Harini, K.; Gajula, G.P.; Sarmento, B.; Neves-Petersen, M.T.; Thiagarajan, V. Multifunctional magnetic iron oxide nanoparticles: Diverse synthetic approaches, surface modifications, cytotoxicity towards biomedical and industrial applications. BMC Mater. 2019, 1, 1–22. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Wang, F.; Fan, Z.; Wang, H.; Tao, C.; Wang, Z. Preparation of polyurethane/polyvinyl alcohol hydrogel and its performance enhancement via compositing with silver particles. RSC Adv. 2017, 7, 46480–46485. [Google Scholar] [CrossRef]
- Heyer, K.; Augustin, M.; Protz, K.; Herberger, K.; Spehr, C.; Rustenbach, S. Effectiveness of advanced versus conventional wound dressings on healing of chronic wounds: Systematic review and meta-analysis. Dermatology 2013, 226, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Herndon, D.N.; Barrow, R.E.; Rutan, R.L.; Rutan, T.C.; Desai, M.H.; Abston, S. A comparison of conservative versus early excision. Therapies in severely burned patients. Ann. Surg. 1989, 209, 547. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, C.; Stiefel, D.; Meuli, M. Giant naevus, giant excision, eleg (i) ant closure? Reconstructive surgery with Integra Artificial Skin® to treat giant congenital melanocytic naevi in children. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, C.; Neuhaus, K.; Biedermann, T.; Böttcher-Haberzeth, S.; Reichmann, E.; Meuli, M. Novel treatment for massive lower extremity avulsion injuries in children: Slow, but effective with good cosmesis. Eur. J. Pediatr. Surg. 2011, 21, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Böttcher-Haberzeth, S.; Kapoor, S.; Meuli, M.; Neuhaus, K.; Biedermann, T.; Reichmann, E.; Schiestl, C. Osmotic expanders in children: No filling–no control–no problem? Eur. J. Pediatr. Surg. 2011, 21, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Barbour, J.R.; Schweppe, M.; Seung-Jun, O. Lower-extremity burn reconstruction in the child. Eur. J. Pediatr. Surg. 2008, 19, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.; Viera, M.H.; Amini, S.; Huo, R.; Jones, I.S. Prevention and management of hypertrophic scars and keloids after burns in children. Eur. J. Pediatr. Surg. 2008, 19, 989–1006. [Google Scholar] [CrossRef]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Advancement of wound care from grafts to bioengineered smart skin substitutes. Prog. Biomater. 2014, 3, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Pita-López, M.L.; Fletes-Vargas, G.; Espinosa-Andrews, H.; Rodríguez-Rodríguez, R. Physically cross-linked chitosan-based hydrogels for tissue engineering applications: A state-of-the-art review. Eur. Polym. J. 2021, 145, 110176. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Agarwal, R.; Malhotra, S.; Gupta, V.; Jain, V. The application of Three-dimensional printing on foot fractures and deformities: A mini-review. Ann. 3d Print. Med. 2022, 100046. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef] [PubMed]
- Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Razzaghi, M.; Tavakoli, M.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Chen, X.; Berto, F. 3-Dimensional Printing of Hydrogel-Based Nanocomposites: A Comprehensive Review on the Technology Description, Properties, and Applications. Adv. Eng. Mater. 2021, 23, 2100477. [Google Scholar] [CrossRef]
- Zamboulis, A.; Michailidou, G.; Koumentakou, I.; Bikiaris, D.N. Polysaccharide 3D Printing for Drug Delivery Applications. Pharmaceutics 2022, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, S. Electrically conducting hydrogels for health care: Concept, fabrication methods, and applications. Int. J. Bioprinting 2020, 6, 273. [Google Scholar] [CrossRef]
- Athukorala, S.S.; Tran, T.S.; Balu, R.; Truong, V.K.; Chapman, J.; Dutta, N.K.; Roy Choudhury, N. 3D printable electrically conductive hydrogel scaffolds for biomedical applications: A review. Polymers 2021, 13, 474. [Google Scholar] [CrossRef] [PubMed]
- Advincula, R.C.; Dizon, J.R.C.; Caldona, E.B.; Viers, R.A.; Siacor, F.D.C.; Maalihan, R.D.; Espera, A.H. On the progress of 3D-printed hydrogels for tissue engineering. MRS Commun. 2021, 11, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Siree, G.K.; M Amulya, T.; M Pramod Kumar, T.; Sowmya, S.; Divith, K.; G Ramu, B.; P Gowrav, M. Transfiguring Healthcare: Three-Dimensional Printing in Pharmaceutical Sciences; Trends during COVID-19: A Review. J. Pharm. Res. Int. 2021, 33, 207–218. [Google Scholar] [CrossRef]
- Topuz, M.; Dikici, B.; Gavgali, M.; Yilmazer, H. A review on the hydrogels used in 3D Bio-printing. Int. J. 3D Print. Technol. Digital Ind. 2018, 2, 68–75. [Google Scholar]
- Paul, G.M.; Rezaienia, A.; Wen, P.; Condoor, S.; Parkar, N.; King, W.; Korakianitis, T. Medical applications for 3D printing: Recent developments. Mo. Med. 2018, 115, 75. [Google Scholar] [PubMed]
- Fayyazbakhsh, F.; Leu, M.C. A brief review on 3D bioprinted skin substitutes. Procedia Manuf. 2020, 48, 790–796. [Google Scholar] [CrossRef]
- de Oliveira, R.S.; Fantaus, S.S.; Guillot, A.J.; Melero, A.; Beck, R.C.R. 3D-Printed Products for Topical Skin Applications: From Personalized Dressings to Drug Delivery. Pharmaceutics 2021, 13, 1946. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Schaffer, S.; Dai, K.; Yao, L.; Feinberg, A.; Webster-Wood, V. 3D Printing Hydrogel-Based Soft and Biohybrid Actuators: A Mini-Review on Fabrication Techniques, Applications, and Challenges. Front. Robot. AI 2021, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qiao, D.; Zhao, S.; Lin, Q.; Zhang, B.; Xie, F. 3D printing to innovate biopolymer materials for demanding applications: A review. Mater. Today Chem. 2021, 20, 100459. [Google Scholar] [CrossRef]
- Beheshtizadeh, N.; Lotfibakhshaiesh, N.; Pazhouhnia, Z.; Hoseinpour, M.; Nafari, M. A review of 3D bio-printing for bone and skin tissue engineering: A commercial approach. J. Mater. Sci. 2020, 55, 3729–3749. [Google Scholar] [CrossRef]
- Waghmare, V.S.; Wadke, P.R.; Dyawanapelly, S.; Deshpande, A.; Jain, R.; Dandekar, P. Starch based nanofibrous scaffolds for wound healing applications. Bioact. Mater. 2018, 3, 255–266. [Google Scholar] [CrossRef]
- Calonge, W.M.; AlAli, A.B.; Griffin, M.; Butler, P.E. Three-dimensional printing of models of cleft lip and palate. Plast. Reconstr. Surg. Glob. Open 2016, 4, e689. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Naniz, M.A.; Kouhi, M.; Saberi, A.; Zolfagharian, A.; Bodaghi, M. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: A comprehensive review with focus on advanced fabrication techniques. Biomater. Sci. 2021, 9, 535–573. [Google Scholar] [CrossRef]
- Ghosh, S.; Kaushik, G.; Roy, P.; Lahiri, D. Application of 3D Bioprinting in Wound Healing: A Review. Trends Biomater. Artif. Organs 2021, 35. [Google Scholar]
- Gupta, S.; Bissoyi, A.; Bit, A. A review on 3D printable techniques for tissue engineering. BioNanoScience 2018, 8, 868–883. [Google Scholar] [CrossRef]
- He, P.; Zhao, J.; Zhang, J.; Li, B.; Gou, Z.; Gou, M.; Li, X. Bioprinting of skin constructs for wound healing. Burn. Trauma 2018, 6. [Google Scholar] [CrossRef]
- Li, H.; Tan, C.; Li, L. Review of 3D printable hydrogels and constructs. Mater. Des. 2018, 159, 20–38. [Google Scholar] [CrossRef]
- Li, X.; Cui, R.; Sun, L.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.; Watari, F. 3D-printed biopolymers for tissue engineering application. Int. J. Polym. Sci. 2014, 2014, 829145. [Google Scholar] [CrossRef]
- Sahranavard, M.; Zamanian, A.; Ghorbani, F.; Shahrezaee, M.H. A critical review on three dimensional-printed chitosan hydrogels for development of tissue engineering. Bioprinting 2020, 17, 00063. [Google Scholar] [CrossRef]
- Malekmohammadi, S.; Sedghi Aminabad, N.; Sabzi, A.; Zarebkohan, A.; Razavi, M.; Vosough, M.; Bodaghi, M.; Maleki, H. Smart and Biomimetic 3D and 4D Printed Composite Hydrogels: Opportunities for Different Biomedical Applications. Biomedicines 2021, 9, 1537. [Google Scholar] [CrossRef]
- Smandri, A.; Nordin, A.; Hwei, N.M.; Chin, K.-Y.; Abd Aziz, I.; Fauzi, M.B. Natural 3D-printed bioinks for skin regeneration and wound healing: A systematic review. Polymers 2020, 12, 1782. [Google Scholar] [CrossRef] [PubMed]
- Nadhif, M.H.; Assyarify, H.; Irsyad, M.; Pramesti, A.R.; Suhaeri, M. Recent advances in 3D printed wound dressings. In AIP Conference Proceedings; AIP Publishing LLC.: Melville, NY, USA, 2021; p. 020021. [Google Scholar]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, A.D.; Ferguson, M.W. Tissue engineering of replacement skin: The crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc Interface 2007, 4, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Sousa, A.; Barrias, C.C.; Bayat, A.; Granja, P.L.; Bártolo, P.J. Advances in bioprinted cell-laden hydrogels for skin tissue engineering. Biomanufacturing Rev. 2017, 2, 1–26. [Google Scholar] [CrossRef]
- Uccioli, L.; Izzo, V.; Meloni, M.; Vainieri, E.; Ruotolo, V.; Giurato, L. Non-healing foot ulcers in diabetic patients: General and local interfering conditions and management options with advanced wound dressings. J. Wound Care 2015, 24, 35–42. [Google Scholar] [CrossRef]
- Dorishetty, P.; Dutta, N.K.; Choudhury, N.R. Bioprintable tough hydrogels for tissue engineering applications. Adv. Colloid Interface Sci. 2020, 281, 102163. [Google Scholar] [CrossRef] [PubMed]
- Streitz, M.J. How To Cleanse, Irrigate, Debride, and Dress Wounds. Available online: https://www.msdmanuals.com/professional/injuries-poisoning/how-to-care-for-wounds-and-lacerations/how-to-cleanse,-irrigate,-debride,-and-dress-wounds (accessed on 9 December 2021).
- Gottrup, F. Wound closure techniques. J. Wound Care 1999, 8, 397–400. [Google Scholar] [CrossRef]
- Andreassi, A.; Bilenchi, R.; Biagioli, M.; D’Aniello, C. Classification and pathophysiology of skin grafts. Clin. Dermatol. 2005, 23, 332–337. [Google Scholar] [CrossRef]
- Bielefeld, K.A.; Amini-Nik, S.; Alman, B.A. Cutaneous wound healing: Recruiting developmental pathways for regeneration. Cell. Mol. Life Sci. 2013, 70, 2059–2081. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-H.; Pappas, A.; Zhang, L.; Ruvolo, E.; Cavender, D. IL-11, IL-1α, IL-6, and TNF-α are induced by solar radiation in vitro and may be involved in facial subcutaneous fat loss in vivo. J. Dermatol. Sci. 2013, 71, 58–66. [Google Scholar] [CrossRef]
- Greaves, N.S.; Ashcroft, K.J.; Baguneid, M.; Bayat, A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J. Dermatol. Sci. 2013, 72, 206–217. [Google Scholar] [CrossRef] [PubMed]
- FrykbergRobert, G. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.; Chakraborty, B.; Nandi, S.K.; Mandal, B.B. Role of non-mulberry silk fibroin in deposition and regulation of extracellular matrix towards accelerated wound healing. Acta Biomater. 2017, 48, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Percival, N.J. Classification of wounds and their management. Surgery (Oxford) 2002, 20, 114–117. [Google Scholar] [CrossRef]
- Hadian, Y.; Bagood, M.D.; Dahle, S.E.; Sood, A.; Isseroff, R.R. Interleukin-17: Potential target for chronic wounds. Mediat. Inflamm. 2019, 2019, 1297675. [Google Scholar] [CrossRef] [PubMed]
- Golinko, M.S.; Clark, S.; Rennert, R.; Flattau, A.; Boulton, A.J.; Brem, H. Wound emergencies: The importance of assessment, documentation, and early treatment using a wound electronic medical record. Ostomy/Wound Manag. 2009, 55, 54. [Google Scholar]
- Moore, K.; McCallion, R.; Searle, R.J.; Stacey, M.C.; Harding, K.G. Prediction and monitoring the therapeutic response of chronic dermal wounds. Int. Wound J. 2006, 3, 89–98. [Google Scholar] [CrossRef]
- Chandika, P.; Ko, S.-C.; Jung, W.-K. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration. Int. J. Biol. Macromol. 2015, 77, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. Innovations in gene and growth factor delivery systems for diabetic wound healing. J. Tissue Eng. Regen. Med. 2018, 12, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.M.; Phillips, T.J. Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Dermatol. 2016, 74, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Yee, A.; Patel, M.; Wu, E.; Yi, S.; Marti, G.; Harmon, J. IDr: An Intelligent Digital Ruler App for Remote Wound Assessment. In Proceedings of the 2016 IEEE First International Conference on Connected Health: Applications, Systems and Engineering Technologies (CHASE), Washington, DC, USA, 27–29 June 2016; pp. 380–381. [Google Scholar]
- Trengove, N.J.; Stacey, M.C.; Macauley, S.; Bennett, N.; Gibson, J.; Burslem, F.; Murphy, G.; Schultz, G. Analysis of the acute and chronic wound environments: The role of proteases and their inhibitors. Wound Repair Regen. 1999, 7, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, J.; Margraf, A.; Zarbock, A. Role of platelets in leukocyte recruitment and resolution of inflammation. Front. Immunol. 2018, 2712. [Google Scholar] [CrossRef]
- Versteeg, H.; Heemskerk, J.; Levi, M.; Reitsma, P. New fundamentals in coagulation. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. 23 Principles of Wound Healing. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge R, T.M., Ed.; University of Adelaide Press: Adelaide, UK, 2011. [Google Scholar]
- Midwood, K.S.; Williams, L.V.; Schwarzbauer, J.E. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 2004, 36, 1031–1037. [Google Scholar] [CrossRef]
- Desjardins-Park, H.E.; Foster, D.S.; Longaker, M.T. Fibroblasts and wound healing: An update. Regerative Med. 2018, 13, 491–495. [Google Scholar] [CrossRef]
- Bowden, L.; Byrne, H.; Maini, P.; Moulton, D. A morphoelastic model for dermal wound closure. Biomech. Model. Mechanobiol. 2016, 15, 663–681. [Google Scholar] [CrossRef]
- Yee, A.; Harmon, J.; Yi, S. Quantitative monitoring wound healing status through three-dimensional imaging on mobile platforms. J. Am. Coll. Clin. Wound Spéc. 2016, 8, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Mohseni, N.; Moghtader, M. An introduction to hydrogels and some recent applications. In Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: London, UK, 2016. [Google Scholar]
- Darge, H.F.; Andrgie, A.T.; Tsai, H.-C.; Lai, J.-Y. Polysaccharide and polypeptide based injectable thermo-sensitive hydrogels for local biomedical applications. Int. J. Biol. Macromol. 2019, 133, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Dissemond, J.; Augustin, M.; Eming, S.A.; Goerge, T.; Horn, T.; Karrer, S.; Schumann, H.; Stücker, M.; for the working group for wound healing (AGW) of the German Society of Dermatology (DDG). Modern wound care–practical aspects of non-interventional topical treatment of patients with chronic wounds. J. Der Dtsch. Dermatol. Ges. 2014, 12, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.G.; Morton, L.M.; Phillips, T.J. Dressings for chronic wounds. Dermatol. Ther. 2013, 26, 197–206. [Google Scholar] [CrossRef]
- Skórkowska-Telichowska, K.; Czemplik, M.; Kulma, A.; Szopa, J. The local treatment and available dressings designed for chronic wounds. J. Am. Acad. Dermatol. 2013, 68, e117–e126. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, L.; Huang, T.; Wang, Q. Bubble template fabrication of chitosan/poly (vinyl alcohol) sponges for wound dressing applications. Int. J. Biol. Macromol. 2013, 62, 188–193. [Google Scholar] [CrossRef]
- Fan, L.; Yang, J.; Wu, H.; Hu, Z.; Yi, J.; Tong, J.; Zhu, X. Preparation and characterization of quaternary ammonium chitosan hydrogel with significant antibacterial activity. Int. J. Biol. Macromol. 2015, 79, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Khodja, A.N.; Mahlous, M.; Tahtat, D.; Benamer, S.; Youcef, S.L.; Chader, H.; Mouhoub, L.; Sedgelmaci, M.; Ammi, N.; Mansouri, M.B. Evaluation of healing activity of PVA/chitosan hydrogels on deep second degree burn: Pharmacological and toxicological tests. Burns 2013, 39, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Arora, A.; Alam, M.; Gupta, B. Development of antimicrobial and scar preventive chitosan hydrogel wound dressings. Int. J. Pharm. 2016, 508, 92–101. [Google Scholar] [CrossRef]
- Chen, S.-H.; Tsao, C.-T.; Chang, C.-H.; Lai, Y.-T.; Wu, M.-F.; Chuang, C.-N.; Chou, H.-C.; Wang, C.-K.; Hsieh, K.-H. Assessment of reinforced poly (ethylene glycol) chitosan hydrogels as dressings in a mouse skin wound defect model. Mater. Sci. Eng. C 2013, 33, 2584–2594. [Google Scholar] [CrossRef] [PubMed]
- Aïssa, B.; Therriault, D.; Haddad, E.; Jamroz, W. Self-healing materials systems: Overview of major approaches and recent developed technologies. Adv. Mater. Sci. Eng. 2012, 2012, 854203. [Google Scholar] [CrossRef]
- Arnold, R.M.; Huddleston, N.E.; Locklin, J. Utilizing click chemistry to design functional interfaces through post-polymerization modification. J. Mater. Chem. 2012, 22, 19357–19365. [Google Scholar] [CrossRef]
- Billiet, S.; Hillewaere, X.K.; Teixeira, R.F.; Du Prez, F.E. Chemistry of crosslinking processes for self-healing polymers. Macromol. Rapid Commun. 2013, 34, 290–309. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Zhang, Y.; Xia, Y. A temperature-sensitive drug release system based on phase-change materials. Angew. Chem. Int. Ed. 2010, 49, 7904–7908. [Google Scholar] [CrossRef]
- Devi, N.; Kakati, D.K. Smart porous microparticles based on gelatin/sodium alginate polyelectrolyte complex. J. Food Eng. 2013, 117, 193–204. [Google Scholar] [CrossRef]
- De La Rica, R.; Aili, D.; Stevens, M.M. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv. Drug Deliv. Rev. 2012, 64, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Kokabi, M.; Sirousazar, M.; Hassan, Z.M. PVA–clay nanocomposite hydrogels for wound dressing. Eur. Polym. J. 2007, 43, 773–781. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Mishra, D.; Das, T.; Maiti, T.K. Wound pH-responsive sustained release of therapeutics from a poly (NIPAAm-co-AAc) hydrogel. J. Biomater. Science. Polym. Ed. 2012, 23, 111–132. [Google Scholar] [CrossRef]
- Francesko, A.; Petkova, P.; Tzanov, T. Hydrogel dressings for advanced wound management. Curr. Med. Chem. 2018, 25, 5782–5797. [Google Scholar] [CrossRef] [PubMed]
- Cutting, K.F. Wound exudate: Composition and functions. Br. J. Community Nurs. 2003, 8, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Chen, Z.; Wang, F.; Liu, L.; Wei, R.; Zhang, M. Preparation of self-regulating/anti-adhesive hydrogels and their ability to promote healing in burn wounds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.D.; Cevher, E. Biopolymers as wound healing materials: Challenges and new strategies. Biomater. Appl. Nanomed. 2011, 383–414. [Google Scholar]
- Xue, M.; Zhao, R.; Lin, H.; Jackson, C. Delivery systems of current biologicals for the treatment of chronic cutaneous wounds and severe burns. Adv. Drug Deliv. Rev. 2018, 129, 219–241. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Moreira, A.F.; Correia, I.J. Chitosan based-asymmetric membranes for wound healing: A review. Int. J. Biol. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Weller, C.; Team, V. Interactive dressings and their role in moist wound management. In Advanced Textiles for Wound Care; Elsevier: Amsterdam, The Netherlands, 2019; pp. 105–134. [Google Scholar]
- Op’t Veld, R.C.; Walboomers, X.F.; Jansen, J.A.; Wagener, F.A. Design considerations for hydrogel wound dressings: Strategic and molecular advances. Tissue Eng. Part B Rev. 2020, 26, 230–248. [Google Scholar] [CrossRef]
- Wiegand, C.; Heinze, T.; Hipler, U.C. Comparative in vitro study on cytotoxicity, antimicrobial activity, and binding capacity for pathophysiological factors in chronic wounds of alginate and silver-containing alginate. Wound Repair Regen. 2009, 17, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Ducheyne, P.; Healy, K.E.; Hutmacher, D.W.; Grainger, D.W.; Kirkpatrick, C.J. Comprehensive Biomaterials; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1. [Google Scholar]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.I.; Dias, A.M.; Leal, E.C.; Carvalho, L.; de Sousa, H.C.; Carvalho, E. Chitosan-based dressings loaded with neurotensin—an efficient strategy to improve early diabetic wound healing. Acta Biomater. 2014, 10, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Yodkhum, K.; Charoenteeraboon, J.; Tabata, Y. Chitosan–aluminum monostearate composite sponge dressing containing asiaticoside for wound healing and angiogenesis promotion in chronic wound. Mater. Sci. Eng. C 2015, 50, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.-Y.; Zhu, J.-F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ågren, M. Wound Healing Biomaterials-Volume 2: Functional Biomaterials; Woodhead Publishing: Oxford, UK, 2016. [Google Scholar]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.I.; Dias, A.M.; Suesca, E.; Casadiegos, S.; Leal, E.C.; Fontanilla, M.R.; Carvalho, L.; de Sousa, H.C.; Carvalho, E. Neurotensin-loaded collagen dressings reduce inflammation and improve wound healing in diabetic mice. Biochim. Biophys. Acta 2014, 1842, 32–43. [Google Scholar] [CrossRef]
- Helary, C.; Abed, A.; Mosser, G.; Louedec, L.; Letourneur, D.; Coradin, T.; Giraud-Guille, M.M.; Meddahi-Pellé, A. Evaluation of dense collagen matrices as medicated wound dressing for the treatment of cutaneous chronic wounds. Biomater. Sci. 2015, 3, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Willard, J.J.; Drexler, J.W.; Das, A.; Roy, S.; Shilo, S.; Shoseyov, O.; Powell, H.M. Plant-derived human collagen scaffolds for skin tissue engineering. Tissue Eng. Part A 2013, 19, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.; Krithica, N.; Madhan, B.; Sehgal, P.K. Preparation and properties of tannic acid cross-linked collagen scaffold and its application in wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 560–567. [Google Scholar] [CrossRef]
- Mayet, N.; Choonara, Y.E.; Kumar, P.; Tomar, L.K.; Tyagi, C.; Du Toit, L.C.; Pillay, V. A comprehensive review of advanced biopolymeric wound healing systems. J. Pharm. Sci. 2014, 103, 2211–2230. [Google Scholar] [CrossRef]
- Arora, G.; Singh, I.; Nagpal, M.; Arora, S. Recent advances in stimuli induced pulsatile drug delivery system: A review. Res. J. Pharm. Technol. 2011, 4, 691–703. [Google Scholar]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Smith, D. Vat Polymerization. In Additive Manufacturing Process; Seifi, D.L.B.W.F.H.K.M., Ed.; ASM International: Novelty, OH, USA, 2020; Volume 24. [Google Scholar]
- Das, N. Preparation methods and properties of hydrogel: A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 112–117. [Google Scholar]
- Joshi, S.; Chang, T.-C. Graph-based heuristics for recognition of machined features from a 3D solid model. Comput. Des. 1988, 20, 58–66. [Google Scholar] [CrossRef]
- Dori, D.; Tombre, K. From engineering drawings to 3D CAD models: Are we ready now? Comput. Des. 1995, 27, 243–254. [Google Scholar] [CrossRef]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Tiller, B.; Reid, A.; Zhu, B.; Guerreiro, J.; Domingo-Roca, R.; Jackson, J.C.; Windmill, J. Piezoelectric microphone via a digital light processing 3D printing process. Mater. Des. 2019, 165, 107593. [Google Scholar] [CrossRef]
- Ge, L.; Dong, L.; Wang, D.; Ge, Q.; Gu, G. A digital light processing 3D printer for fast and high-precision fabrication of soft pneumatic actuators. Sens. Actuators A Phys. 2018, 273, 285–292. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Jones, A.; Rusling, J.F. 3D-printed biosensor arrays for medical diagnostics. Micromachines 2018, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Mannoor, M.S.; Jiang, Z.; James, T.; Kong, Y.L.; Malatesta, K.A.; Soboyejo, W.O.; Verma, N.; Gracias, D.H.; McAlpine, M.C. 3D printed bionic ears. Nano Lett. 2013, 13, 2634–2639. [Google Scholar] [CrossRef]
- Low, Z.-X.; Chua, Y.T.; Ray, B.M.; Mattia, D.; Metcalfe, I.S.; Patterson, D.A. Perspective on 3D printing of separation membranes and comparison to related unconventional fabrication techniques. J. Membr. Sci. 2017, 523, 596–613. [Google Scholar] [CrossRef]
- Zhang, J.X.; Hoshino, K. Molecular Sensors and Nanodevices: Principles, Designs and Applications in Biomedical Engineering; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Jammalamadaka, U.; Tappa, K. Recent advances in biomaterials for 3D printing and tissue engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Rusling, J.F. Developing microfluidic sensing devices using 3D printing. ACS Sens. 2018, 3, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ho, C.-C.; Zhang, C.; Wang, B. A review on the 3D printing of functional structures for medical phantoms and regenerated tissue and organ applications. Engineering 2017, 3, 653–662. [Google Scholar] [CrossRef]
- Valentin, T.M.; Leggett, S.E.; Chen, P.-Y.; Sodhi, J.K.; Stephens, L.H.; McClintock, H.D.; Sim, J.Y.; Wong, I.Y. Stereolithographic printing of ionically-crosslinked alginate hydrogels for degradable biomaterials and microfluidics. Lab. Chip 2017, 17, 3474–3488. [Google Scholar] [CrossRef]
- Hinman, S.S.; McKeating, K.S.; Cheng, Q. Plasmonic sensing with 3D printed optics. Anal. Chem. 2017, 89, 12626–12630. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kumar, H.; Tian, Z.; Jin, X.; Holzman, J.F.; Menard, F.; Kim, K. Visible light photoinitiation of cell-adhesive gelatin methacryloyl hydrogels for stereolithography 3D bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 26859–26869. [Google Scholar] [CrossRef] [PubMed]
- Rumley-ouellette, B.J.; Wahry, J.H.; Baker, A.M.; Bernardin, J.D.; Marchi, A.N.; Todd, M.D. In situ printing of conductive poly lactic acid strain sensors embedded into additively manufactured parts. In Proceedings of the 11th International Workshop on Structural Health Monitoring, Stanford, CA, USA, 12–14 September 2017. [Google Scholar]
- Cevenini, L.; Calabretta, M.M.; Tarantino, G.; Michelini, E.; Roda, A. Smartphone-interfaced 3D printed toxicity biosensor integrating bioluminescent “sentinel cells”. Sens. Actuators B Chem. 2016, 225, 249–257. [Google Scholar] [CrossRef]
- Pranzo, D.; Larizza, P.; Filippini, D.; Percoco, G. Extrusion-based 3D printing of microfluidic devices for chemical and biomedical applications: A topical review. Micromachines 2018, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Loo, A.H.; Chua, C.K.; Pumera, M. DNA biosensing with 3D printing technology. Analyst 2017, 142, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Nesaei, S.; Du, D.; Gozen, A.; Lin, Y. 3D Printed Wearable Glucose Sensors. In Proceedings of the ECS Meeting Abstracts, Washington, DC, USA, 13–17 May 2018; p. 2482. [Google Scholar]
- Connell, J.L.; Kim, J.; Shear, J.B.; Bard, A.J.; Whiteley, M. Real-time monitoring of quorum sensing in 3D-printed bacterial aggregates using scanning electrochemical microscopy. Proc. Natl. Acad. Sci. USA 2014, 111, 18255–18260. [Google Scholar] [CrossRef] [PubMed]
- Cretu, A.; Gattin, R.; Brachais, L.; Barbier-Baudry, D. Synthesis and degradation of poly (2-hydroxyethyl methacrylate)-graft-poly (ε-caprolactone) copolymers. Polym. Degrad. Stab. 2004, 83, 399–404. [Google Scholar] [CrossRef]
- Kim, B.; Peppas, N.A. Poly (ethylene glycol)-containing hydrogels for oral protein delivery applications. Biomed. Microdevices 2003, 5, 333–341. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.-F.; Adler, H.-J.P. Review on hydrogel-based pH sensors and microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, F.; Li, M.; Wang, E. pH switching on-off semi-IPN hydrogel based on cross-linked poly (acrylamide-co-acrylic acid) and linear polyallyamine. Polymer 2005, 46, 7695–7700. [Google Scholar] [CrossRef]
- Jirkovec, R.; Samkova, A.; Kalous, T.; Chaloupek, J.; Chvojka, J. Preparation of a Hydrogel Nanofiber Wound Dressing. Nanomaterials 2021, 11, 2178. [Google Scholar] [CrossRef] [PubMed]
- Bourges, X.; Weiss, P.; Coudreuse, A.; Daculsi, G.; Legeay, G. General properties of silated hydroxyethylcellulose for potential biomedical applications. Biopolym. Orig. Res. Biomol. 2002, 63, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Furukawa, H.; Tanaka, Y.; Kurokawa, T.; Osada, Y.; Gong, J.P. True chemical structure of double network hydrogels. Macromolecules 2009, 42, 2184–2189. [Google Scholar] [CrossRef]
- Greenwood, J. The evolution of acute burn care–retiring the split skin graft. Ann. R. Coll. Surg. Engl. 2017, 99, 432–438. [Google Scholar] [CrossRef]
- Wong, K.V.; Hernandez, A. A review of additive manufacturing. Int. Scholarly Res. Not. 2012, 2012, 208760. [Google Scholar] [CrossRef]
- Vaezi, M.; Seitz, H.; Yang, S. A review on 3D micro-additive manufacturing technologies. Int. J. Adv. Manuf. 2013, 67, 1721–1754. [Google Scholar] [CrossRef]
- McMenamin, P.G.; Quayle, M.R.; McHenry, C.R.; Adams, J.W. The production of anatomical teaching resources using three-dimensional (3D) printing technology. Anat. Sci. Educ. 2014, 7, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Li, D.; Tian, X. Development trends in additive manufacturing and 3D printing. Engineering. Epub Ahead Print 2015, 1, 85–89. [Google Scholar] [CrossRef]
- Ventola, C.L. Medical applications for 3D printing: Current and projected uses. Pharm. Ther. 2014, 39, 704. [Google Scholar]
- Zhang, X.; Li, Y.; He, D.; Ma, Z.; Liu, K.; Xue, K.; Li, H. An effective strategy for preparing macroporous and self-healing bioactive hydrogels for cell delivery and wound healing. Chem. Eng. J. 2021, 425, 130677. [Google Scholar] [CrossRef]
- Martelli, N.; Serrano, C.; van den Brink, H.; Pineau, J.; Prognon, P.; Borget, I.; El Batti, S. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016, 159, 1485–1500. [Google Scholar] [CrossRef] [PubMed]
- Tappa, K.; Jammalamadaka, U. Novel biomaterials used in medical 3D printing techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Taormina, G.; Sciancalepore, C.; Messori, M.; Bondioli, F. 3D printing processes for photocurable polymeric materials: Technologies, materials, and future trends. J. Appl. Biomater. Funct. Mater. 2018, 16, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, A.D.; Abbasi, R.; Owens, M.; Olsen, R.J.; Walsh, D.J.; LeFevre, T.B.; Wilking, J.N. Light-based 3D printing of hydrogels with high-resolution channels. Biomed. Phys. Eng. Express 2019, 5, 025035. [Google Scholar] [CrossRef]
- Alketbi, A.S.; Shi, Y.; Li, H.; Raza, A.; Zhang, T. Impact of PEGDA photopolymerization in micro-stereolithography on 3D printed hydrogel structure and swelling. Soft Matter 2021, 17, 7188–7195. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.L.; Kadioglu, O.; Currier, G.F.; Kierl, J.P.; Li, J. Accuracy of digital light processing printing of 3-dimensional dental models. Am. J. Orthod. 2020, 157, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Steyrer, B.; Busetti, B.; Harakály, G.; Liska, R.; Stampfl, J. Hot Lithography vs. room temperature DLP 3D-printing of a dimethacrylate. Addit. Manuf. 2018, 21, 209–214. [Google Scholar] [CrossRef]
- Ge, Q.; Chen, Z.; Cheng, J.; Zhang, B.; Zhang, Y.-F.; Li, H.; He, X.; Yuan, C.; Liu, J.; Magdassi, S. 3D printing of highly stretchable hydrogel with diverse UV curable polymers. Sci. Adv. 2021, 7, 4261. [Google Scholar] [CrossRef] [PubMed]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Francescon, M.; Drigo, D.; Salloum, G.; Baraziol, R.; Tesei, J.; Fraccalanza, E.; Barbone, F. The use of Integra dermal regeneration template versus flaps for reconstruction of full-thickness scalp defects involving the calvaria: A cost–benefit analysis. Aesthetic Plast. Surg. 2016, 40, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Zarybnicka, L.; Stranska, E. Preparation of cation exchange filament for 3D membrane print. Rapid Prototyp. J. 2020, 26, 1435–1445. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, X.; Li, Z.; Liu, D.; Xue, X. Fabrication of pH-responsive TA-keratin bio-composited hydrogels encapsulated with photoluminescent GO quantum dots for improved bacterial inhibition and healing efficacy in wound care management: In vivo wound evaluations. J. Photochem. Photobiol. B 2020, 202, 111676. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Lake, R.; Park, S.; Edwards, S.; Jones, C.; Jeong, K.J. Injectable macroporous hydrogel formed by enzymatic cross-linking of gelatin microgels. ACS Appl. Bio Mater. 2018, 1, 1430–1439. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Zhou, Y. Novel mechanical models of tensile strength and elastic property of FDM AM PLA materials: Experimental and theoretical analyses. Mater. Des. 2019, 181, 108089. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, B.; Xiong, Y.; Tao, R.; Panayi, A.C.; Chen, L.; Tian, W.; Xue, H.; Shi, L.; Zhang, X. Cryogenic 3D printed hydrogel scaffolds loading exosomes accelerate diabetic wound healing. Chem. Eng. J. 2021, 426, 130634. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, R.; Zhao, X.; Zhang, Y.; Tam, A.; Yan, Y.; Shen, H.; Zhang, Y.S.; Qi, J.; Feng, Y. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019, 11, 1–12. [Google Scholar] [CrossRef]
- Sudheesh Kumar, P.; Lakshmanan, V.-K.; Anilkumar, T.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.; Nair, S.V.; Jayakumar, R. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Lakshmanan, V.-K.; Biswas, R.; Nair, S.V.; Jayakumar, R. Synthesis and biological evaluation of chitin hydrogel/nano ZnO composite bandage as antibacterial wound dressing. J. Biomed. Nanotechnol. 2012, 8, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Leppiniemi, J.; Lahtinen, P.; Paajanen, A.; Mahlberg, R.; Metsä-Kortelainen, S.; Pinomaa, T.; Pajari, H.; Vikholm-Lundin, I.; Pursula, P.; Hytönen, V.P. 3D-printable bioactivated nanocellulose–alginate hydrogels. ACS Appl. Mater. Interfaces 2017, 9, 21959–21970. [Google Scholar] [CrossRef] [PubMed]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Arregui, F.J. Nanomaterials for functional textiles and fibers. Nanoscale Res. Lett. 2015, 10, 1–22. [Google Scholar] [CrossRef]
- Montazer, M.; Seifollahzadeh, S. Enhanced self-cleaning, antibacterial and UV protection properties of nano TiO2 treated textile through enzymatic pretreatment. Photochem. Photobiol. 2011, 87, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gan, H.; Meng, Z.; Gu, R.; Wu, Z.; Zhu, X.; Sun, W.; Li, J.; Zheng, Y.; Sun, T. Evaluation of genipin-crosslinked chitosan hydrogels as a potential carrier for silver sulfadiazine nanocrystals. Colloids Surf. B. Biointerfaces 2016, 148, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Anisha, B.; Biswas, R.; Chennazhi, K.; Jayakumar, R. Chitosan–hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int. J. Biol. Macromol. 2013, 62, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Yang, B.; Sun, Y.; Zu, Y.; Deng, Y. A berberine-loaded electrospun poly-(ε-caprolactone) nanofibrous membrane with hemostatic potential and antimicrobial property for wound dressing. J. Biomed. Nanotechnol. 2013, 9, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P. In vivo evaluation of chitosan–PVP–titanium dioxide nanocomposite as wound dressing material. Carbohydr. Polym. 2013, 95, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Yu, H.; Zhang, C.; Chen, X.; Cheng, Z.; Bai, R.; Wu, X.; Yu, Q.; Wu, C.; Diao, Y. Nano-porous nitrocellulose liquid bandage modulates cell and cytokine response and accelerates cutaneous wound healing in a mouse model. Carbohydr. Polym. 2016, 136, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Hadisi, Z.; Nourmohammadi, J.; Nassiri, S.M. The antibacterial and anti-inflammatory investigation of Lawsonia Inermis-gelatin-starch nano-fibrous dressing in burn wound. Int. J. Biol. Macromol. 2018, 107, 2008–2019. [Google Scholar] [CrossRef] [PubMed]
- Oyejide, L.; Mendes, O.R.; Mikaelian, I. Molecular Pathology: Applications in Nonclinical Drug Development. In A Comprehensive Guide to Toxicology in Nonclinical Drug Development; Elsevier: Cambridge, MA, USA, 2017; pp. 407–445. [Google Scholar]
- Lou, E.; Johnson, M.; Sima, C.; Gonzalez-Espinoza, R.; Fleisher, M.; Kris, M.G.; Azzoli, C.G. Serum biomarkers for assessing histology and outcomes in patients with metastatic lung cancer. Cancer Biomark. 2014, 14, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.S.; Bree, R.L.; Rubin, J.M. Prostate cancer: Diagnosis with color Doppler sonography with histologic correlation of each biopsy site. Radiology 1995, 195, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, L.S.; Srivastava, T.K. Conceptualizing physiology of arterial blood pressure regulation through the logic model. Adv. Physiol. Educ. 2016, 40, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.; Navsaria, H.; Ojeh, N. Skin engineering and keratinocyte stem cell therapy. In Tissue Eng.; Elsevier: Cambridge, MA, USA, 2014; pp. 497–528. [Google Scholar]
- Shakespeare, P.G. The role of skin substitutes in the treatment of burn injuries. Clin. Dermatol. 2005, 23, 413–418. [Google Scholar] [CrossRef]
- Stiefel, D.; Schiestl, C.; Meuli, M. Integra Artificial Skin® for burn scar revision in adolescents and children. Burns 2010, 36, 114–120. [Google Scholar] [CrossRef]
- Broussard, K.C.; Powers, J.G. Wound dressings: Selecting the most appropriate type. Am. J. Clin. Dermatol. 2013, 14, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Attalla, K.; Ren, Y.; French, M.A.; Driver, V.R. Ease of use, safety, and efficacy of integra bilayer wound matrix in the treatment of diabetic foot ulcers in an outpatient clinical setting: A prospective pilot study. J. Am. Podiatr. Med. Assoc. 2013, 103, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Böttcher-Haberzeth, S.; Biedermann, T.; Schiestl, C.; Hartmann-Fritsch, F.; Schneider, J.; Reichmann, E.; Meuli, M. Matriderm® 1 mm versus Integra® Single Layer 1.3 mm for one-step closure of full thickness skin defects: A comparative experimental study in rats. Pediatric Surg. Int. 2012, 28, 171–177. [Google Scholar] [CrossRef]
- Kolokythas, P.; Aust, M.; Vogt, P.; Paulsen, F. Dermal subsitute with the collagen-elastin matrix Matriderm in burn injuries: A comprehensive review. Handchir. Mikrochir. Plast. Chir. Organ Dtsch. Arb. Handchir. Organ Dtsch. Arb. Mikrochir. Peripher. Nerven Gefasse Organ V. 2008, 40, 367–371. [Google Scholar]
- Min, J.H.; Yun, I.S.; Lew, D.H.; Roh, T.S.; Lee, W.J. The use of matriderm and autologous skin graft in the treatment of full thickness skin defects. Arch. Plast. Surg. 2014, 41, 330. [Google Scholar] [CrossRef] [PubMed]
- Haslik, W.; Kamolz, L.-P.; Nathschläger, G.; Andel, H.; Meissl, G.; Frey, M. First experiences with the collagen-elastin matrix Matriderm® as a dermal substitute in severe burn injuries of the hand. Burns 2007, 33, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, V.; Brinci, L.; Spallone, D.; Tati, E.; Palla, L.; Lucarini, L.; De Angelis, B. The use of MatriDerm® and skin grafting in post-traumatic wounds. Int. Wound J. 2011, 8, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Nizioł, M.; Paleczny, J.; Junka, A.; Shavandi, A.; Dawiec-Liśniewska, A.; Podstawczyk, D. 3D Printing of Thermoresponsive Hydrogel Laden with an Antimicrobial Agent towards Wound Healing Applications. Bioengineering 2021, 8, 79. [Google Scholar] [CrossRef]
- De Vries, H.; Zeegelaar, J.; Middelkoop, E.; Gijsbers, G.; Van Marle, J.; Wildevuur, C.; Westerhof, W. Reduced wound contraction and scar formation in punch biopsy wounds. Native collagen dermal substitutes. A clinical study. Br. J. Dermatol. 1995, 132, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Cheshire, P.A.; Herson, M.R.; Cleland, H.; Akbarzadeh, S. Artificial dermal templates: A comparative study of NovoSorb™ biodegradable temporising matrix (BTM) and Integra® dermal regeneration template (DRT). Burns 2016, 42, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Biedermann, T.; Widmer, D.; Montano, I.; Meuli, M.; Reichmann, E.; Schiestl, C. Matriderm® versus Integra®: A comparative experimental study. Burns 2009, 35, 51–57. [Google Scholar] [CrossRef]
- Shahrokhi, S.; Arno, A.; Jeschke, M.G. The use of dermal substitutes in burn surgery: Acute phase. Wound Repair Regen. 2014, 22, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Yoshii, F.; Kume, T.; Hashim, K. Syntheses of PVA/starch grafted hydrogels by irradiation. Carbohydr. Polym. 2002, 50, 295–303. [Google Scholar] [CrossRef]
- Khil, M.S.; Cha, D.I.; Kim, H.Y.; Kim, I.S.; Bhattarai, N. Electrospun nanofibrous polyurethane membrane as wound dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2003, 67, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, X.; Nagao, M.; Elias, A.L.; Narain, R.; Chung, H.-J. A pH-Indicating colorimetric tough hydrogel patch towards applications in a substrate for smart wound dressings. Polymers 2017, 9, 558. [Google Scholar] [CrossRef]
- Navarro, J.; Din, M.; Janes, M.E.; Swayambunathan, J.; Fisher, J.P.; Dreher, M.L. Effect of print orientation on microstructural features and mechanical properties of 3D porous structures printed with continuous digital light processing. Rapid Prototyp. J. 2019, 25, 1017–1029. [Google Scholar] [CrossRef]
- Milojević, M.; Harih, G.; Vihar, B.; Vajda, J.; Gradišnik, L.; Zidarič, T.; Stana Kleinschek, K.; Maver, U.; Maver, T. Hybrid 3D printing of advanced hydrogel-based wound dressings with tailorable properties. Pharmaceutics 2021, 13, 564. [Google Scholar] [CrossRef]
- Danielsson, P.; Fredriksson, C.; Huss, F. A novel concept for treating large necrotizing fasciitis wounds with bilayer dermal matrix, split-thickness skin grafts, and negative pressure wound therapy. Wounds (King of Prussia, Pa.) 2009, 21, 215–220. [Google Scholar]
- Wagstaff, M.J.; Salna, I.M.; Caplash, Y.; Greenwood, J.E. Biodegradable Temporising Matrix (BTM) for the reconstruction of defects following serial debridement for necrotising fasciitis: A case series. Burns Open 2019, 3, 12–30. [Google Scholar] [CrossRef]
- Damkat-Thomas, L.; Greenwood, J.E.; Wagstaff, M.J. A synthetic biodegradable temporising matrix in degloving lower extremity trauma reconstruction: A case report. Plast. Reconstr. Surg. Glob. Open 2019, 7, 2110. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, N.; Li, X.; Li, Y.; Xie, Z.; Ma, Z.; Zhao, J.; Hou, X.; Yuan, X. Bioinspired double-dynamic-bond crosslinked bioadhesive enables post-wound closure care. Adv. Funct. Mater. 2020, 30, 2000130. [Google Scholar] [CrossRef]
- Auger, F.A.; Lacroix, D.; Germain, L. Skin substitutes and wound healing. Ski. Pharmacol. Physiol. 2009, 22, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Du, J.; Li, X.; Yu, J.; Ding, B. Breathable, stretchable and adhesive nanofibrous hydrogels as wound dressing materials. Eng. Regen. 2021, 2, 63–69. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Bacelar, A.H.; Cengiz, I.F.; Silva-Correia, J.; Sousa, R.A.; Oliveira, J.M.; Reisa, R.L. Smart” hydrogels in tissue engineering and regenerative medicine applications. Handb. Intell. Scaffolds Tissue Eng. Regen. Med. 2017, 2, 327–361. [Google Scholar]
- Badawy, A.R.; Hassan, M.U.; Elsherif, M.; Ahmed, Z.; Yetisen, A.K.; Butt, H. Contact lenses for color blindness. Adv. Health Mater. 2018, 7, 1800152. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, F.; Wang, X.; Zhang, J.; Wang, D.; Huang, X. A photocurable hybrid chitosan/acrylamide bioink for DLP based 3D bioprinting. Mater. Des. 2021, 202, 109588. [Google Scholar] [CrossRef]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D printing of a multi-layered polypill containing six drugs using a novel stereolithographic method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Cereceres, S.; Lan, Z.; Bryan, L.; Whitely, M.; Wilems, T.; Greer, H.; Alexander, E.R.; Taylor, R.J.; Bernstein, L.; Cohen, N. Bactericidal activity of 3D-printed hydrogel dressing loaded with gallium maltolate. APL Bioeng. 2019, 3, 026102. [Google Scholar] [CrossRef] [PubMed]
- Song, C.K.; Kim, M.-K.; Lee, J.; Davaa, E.; Baskaran, R.; Yang, S.-G. Dopa-empowered Schiff base forming alginate hydrogel glue for rapid hemostatic control. Macromol. Res. 2019, 27, 119–125. [Google Scholar] [CrossRef]
- Süpple, J.; von Glasenapp, J.; Hofmann, E.; Jost-Brinkmann, P.-G.; Koch, P.J. Accurate bracket placement with an indirect bonding method using digitally designed transfer models printed in different orientations—An in vitro study. J. Clin. Med. 2021, 10, 2002. [Google Scholar] [CrossRef]
- Mueller, J.; Shea, K. The effect of build orientation on the mechanical properties in inkjet 3D printing. In Proceedings of the 2015 International Solid Freeform Fabrication Symposium, Austin, TX, USA, 10–12 August 2015. [Google Scholar]
- Jaiswal, L.; Shankar, S.; Rhim, J.-W. Carrageenan-based functional hydrogel film reinforced with sulfur nanoparticles and grapefruit seed extract for wound healing application. Carbohydr. Polym. 2019, 224, 115191. [Google Scholar] [CrossRef] [PubMed]




| Types of Skin Wounds | Caused by |
|---|---|
| Puncture | Often caused by a sharp or pointed object. It pierces through the skin and can also affect the soft tissue beneath. |
| Laceration | The skin is cut open, torn, or torn off completely (avulsion). Lacerations can vary in size, shape, depth, and the left flap of skin. |
| Pressure injury | Lesions are caused by long periods of pressure over a bony part of the body. The hip and heel are common sites for this wound. |
| Incision | A surgical wound or intentional cut to the skin. |
| Abrasion | The skin is scraped or rubbed off. Minor abrasions affect only the top layer of skin. Deep abrasions affect deeper layers of the skin tissues and are more likely to leave a scar. |
| Thermal | Caused by exposure to extreme hot or cold. |
| Chemical | Caused by exposure to strong acids or bases, such as those found in cleaning products, pool chemicals, or drain cleaners. |
| Types of Skin Closure Strips | Characterized | Ref. |
|---|---|---|
| Skin glue | Helps to hold the wound together and allows it to heal. Most of the time, strips are used on the face, arms, legs, and torso. However, the surface areas are clean and dry. | [53] |
| Sutures | In deep wounds, stitches are applied under the skin to enhance injury closure. The body can absorb these stitches or a physician can remove the stitches from the skin surface. | [54] |
| Skin grafts | Are used when the skin around the wound is too damaged to heal together. This may happen with pressure sores or after the skin is removed in surgery. Skin grafts take healthy skin from another area of the body. This healthy skin is then placed over the wound. | [55] |
| Type of Hydrogel | Cross Linker | Characterization | Properties | Limitation | Commercial Producing Companies | Ref. |
|---|---|---|---|---|---|---|
| Alginate | Natural ionic cross linker | A polysaccharide supports cell production of collagen I, reducing the concentration of proinflammatory cytokines in chronic wounds. Due to the hydrophilic nature, it can absorb a high amount of wound exudate. | Hemostatic effect | - | Nu-Gel® (Systagenix), Tegagel® (3M GmbH)), Algosteril® (4M Medical GmbH), Curasorb® Alginate (Medtronic), Sorbsan® (B. Braun Melsungen AG)) Flexible® (Coloplast AG), Kendall™ Hydrocolloid Dressing (Medtronic)). | [102,105] |
| Chitosan | Natural hydrogel | Hemostatic, bacteriostatic, fungistatic properties. | Accelerates healing rate | Dependent on the molecular weight of the macromolecules low elasticity leading to difficulty in producing fibrous wound dressings. | KytoCel® (MasterCare Medical GmbH), Chitoderm® plus (Trusetal Verb and stoffwerk GmbH), a chitosan-coated dressing. | [106,107,108,109,110,111,112] |
| Collagen protein | Natural hydrogel | It is found in ECM, blood vessels, bones and tendons naturally. Collagens of bovine, porcine and avian derivation are common medical products. | High liquid absorbance capability and good mechanical strength. Enhanced vascularization, granulation tissue formation and collagen deposition via fibroblasts, endothelial cells and keratinocytes. | Rapid loss of stability and shape due to enzymatic degradation. Pathogen transmission risk. | CellerateRX® (Wound Care Innovations LLC), Regenecare® Wound Gel (MPM Medical Inc.), Wun’Dres® (Coloplast AG)), Biobrane® (Smith & Nephew), CollaSorb® (Paul Hartmann AG), Fibracol® (Acelity)) Medifil® (Human Bio Science, Inc.), Stimulen™ (Southwest Technologies, Inc.)) | [113,114,115,116,117,118,119,120] |
| Collagen | Synthetic | Well-defined chemical structure and precise modified desired material properties. | Limited activity wound healing process. | polyacrylamide/polysaccharide based FlexiGel® (Smith & Nephew), Poly(ethylene glycol) (PEG)/oakin based Oakin® hydrogel wound dressing (Amerigel), Polyurethane (PU) based AquaClear® dressing (Paul Hartmann AG). | [121] |
| Double Network (DN) | Characteristics | Ref. |
|---|---|---|
| t-DN | t-DN gels become more robust than the c-DN gels when the second network is loosely cross-linked. t-DN gels have a more simple structure than c-DN gels. | [125] |
| c-DN | Interconnection between the two networks through covalent bonds | [125] |
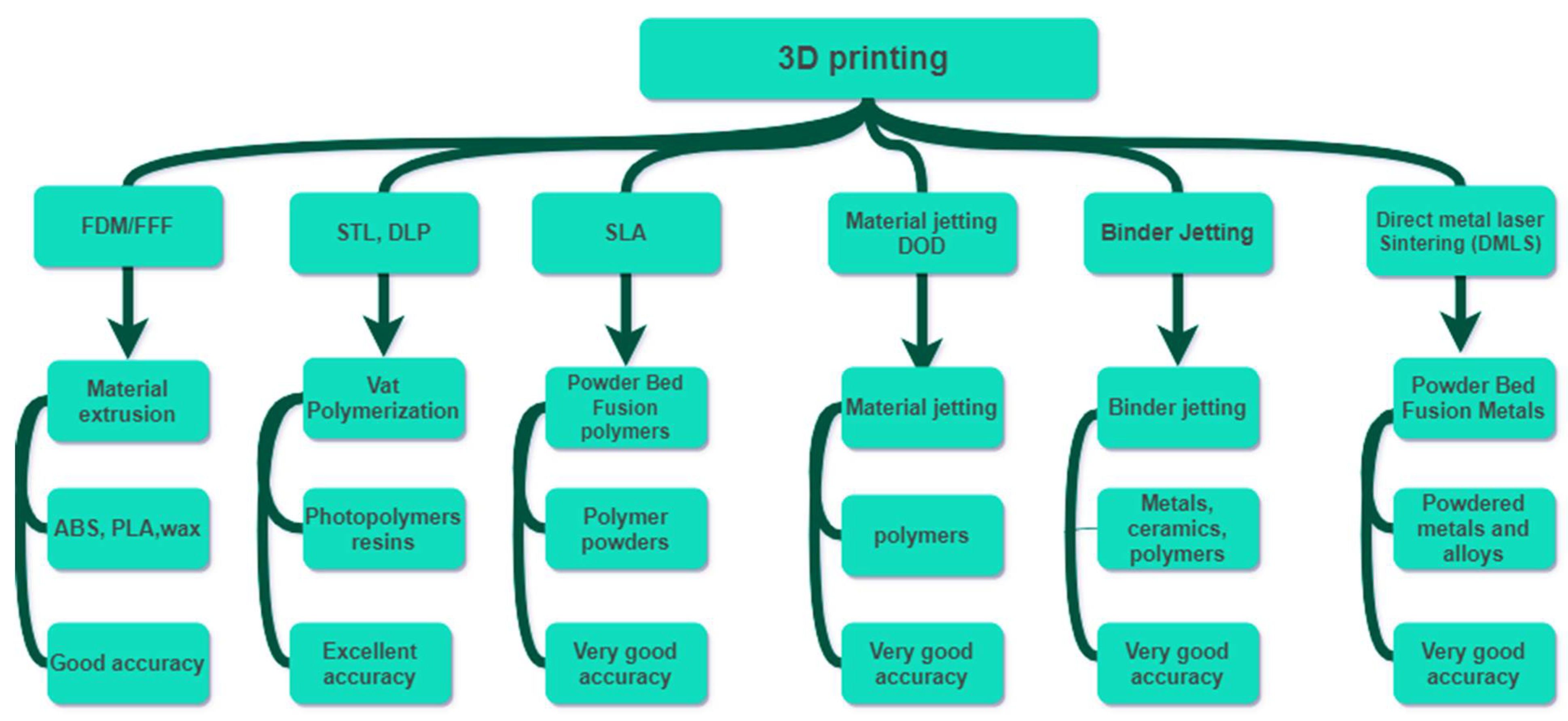
| 3D Printing Methods | Principle | Materials | Accuracy (µm) | Resolution (µm) | Ref. |
|---|---|---|---|---|---|
|
Digital light processing (DLP) | Photo-curing by a digital projector | Photopolymer and photo-resin | 10–25 | x: 25 y: 25 z: 20 | [129,130,131] |
| 3D Inkjet printing | Extrusion of ink and powder liquid binding | Photo-resin or hydrogel | 100 | x: 10 y: 10 z: 50 | [132,133] |
|
Selective laser sintering (SLS) | Laser-induced sintering of powder particles | Metallic powder, polyamide, PVC | 300 | x: 50 y: 50 z: 200 | [134] |
| Polyjet | Deposition of the droplets of the photo-curable liquid material and cured | Polymer | 10–20 | x: 30 y: 30 z: 20 | [135,136,137] |
|
Stereolithography (STL) | UV initiated polymerization cross- section by cross-section | Resin (acrylate or epoxy-based with proprietary photoinitiator) | 25–150 | x: 10 y: 10 z: 15 | [138,139,140] |
|
Fused deposition modeling (FDM) | Extrusion of constant filament | ABS, PLA, wax blend, nylon | 350 | x: 100 y: 100 z: 250 | [141,142,143,144,145,146] |
| 3D Printing Methods | Principle | Materials | Accuracy (µm) | Resolution (µm) | Ref. |
|---|---|---|---|---|---|
| Digital light processing (DLP) | Photo-curing by a digital projector | Photopolymer and photo-resin | 10–25 | x: 25 y: 25 z: 20 | [129,130,131] |
| 3D Inkjet printing | Extrusion of ink and powder liquid binding | Photo-resin or hydrogel | 100 | x: 10 y: 10 z: 50 | [132,133] |
| Selective laser sintering (SLS) | Laser-induced sintering of powder particles | Metallic powder, polyamide, PVC | 300 | x: 50 y: 50 z: 200 | [134] |
| Polyjet | Deposition of the droplets of the photo-curable liquid material and cured | Polymer | 10–20 | x: 30 y: 30 z: 20 | [136,137,162] |
| Stereolithography (STL) | UV initiated polymerization cross- section by cross-section | Resin (acrylate or epoxy based with proprietary photoinitiator) | 25–150 | x: 10 y: 10 z: 15 | [138,139,140] |
| Fused deposition modelling (FDM) | Extrusion of constant filament | ABS, PLA, wax blend, nylon | 350 | x: 100 y: 100 z: 250 | [141,142,143,144,145,146] |
| Nanocomposites | Hydrogel Resin | Wound Types | Advantages | Challenges | Ref. |
|---|---|---|---|---|---|
| Silver nanoparticles (AgNPs) | Chitosan hydrogel | Acute wounds | Self-cleaning and antibacterial properties. | Crosslinking and 3D printing | [180,181,182] |
| AgNPs | Chitosan and hyaluronic acid | Diabetic foot ulcers | Resisting antibiotic bacteria | Crosslinking and fabrication of the nanomaterial | [183] |
| AgNPs | Surface-grafted collagen | Acute wounds | Inhibiting of bacterial growth and increase in membrane water absorption | Agglomeration | [184] |
| TiO2 | Collagen | In vivo and in vitro excision wounds. | Accelerate healing | Crosslinking and fabrication of the nanomaterial | [185] |
| Nano ZnO | Chitin hydrogel | Acute and chronic wounds | Enhanced swelling, blood clotting and antibacterial effect. Absorbing large volumes wound exudate. Controlled degradation, enhanced blood clotting and excellent platelet activation. | Fabrication of the nanomaterial | [178] |
| Nano ZnO | Nitrocellulose | Hard to cover cut wounds | Flexibility, softness, transparency and conformability. | 3D printing | [186] |
| Gelatin oxidized starch nanofibers | Lawsonia inermis (henna) | Treating second degree burn | Enhanced fibroblast attachment, proliferation, collagen secretion and antibacterial activity. | 3D printing | [187] |
| Types of Biomarkers | Characteristics | Application | Examples | Ref. |
|---|---|---|---|---|
| Molecular | They have biophysical properties that allow their measurements in biological samples, such as plasma, serum, cerebrospinal fluid, bronchoalveolar lavage, and biopsy. | Blood glucose | Glucose Hemoglobin A1c levels in diabetes, circulating viral load in viral infections, cholesterol, low-density lipoproteins (LDL), and high-density lipoproteins (HDL) levels in cardiovascular disease. | [188] |
| Histologic | They are obtained from imaging studies. | Grading and staging of cancers | Prostate-specific antigen (PSA) for prostate cancer and fecal occult blood test for colon cancer. | [190] |
| Radiography | They reflect a biochemical or molecular alteration in cells, tissues, or fluids. | Bone mineral density | Nuchal scan for prenatal screening. Assessing lesion load and brain atrophy for patients with multiple sclerosis. | [189] |
| Physiologic | They measures of body processes | Blood pressure | Blood flow Electrocardiogram Functional magnetic resonance imaging. Electroencephalography Metabolism positron emission tomography Spectroscopy. | [191] |
| Type of Sensors | Methodology | Characteristics Ref. | |
|---|---|---|---|
| Temperature | Thermo-responsive | The temperature sensor provides information about the inflammation level. | [211] |
| 3D-printed dual hydrogels with symmetric and alternating segmented tubular structures. | Exhibited spatially programmed swelling behavior in response to temperature in an aqueous environment | [212] | |
| Graphene oxide (GO) to the PNIPAAm-Laponite composite to enhance the temperature responsivity of the hydrogel and to program the shape change. | GO particles are highly responsive to near-infrared light and act as nano-heaters owing to their photothermal properties and their excellent thermal conductivity | [213] | |
| Multi-temperature responsive hydrogel-based structure based on copolymerization level and the dependent group chain length. | 3D printed multi-gel structures with multiple prescribed volume transition temperatures have potential applications in biological systems | [214] | |
| Double network hydrogels were synthesized using a micellar copolymerization process of hydrophobic n-octadecyl acrylate (C18) and N,Ndimethylacrylamide (DMA) in NaCl aqueous solution. | 3D printed thermo-responsive hydrogel film with submillimeter resolution into a capacitor circuit | [154] | |
| pH | pH sensitive dye embedded inside the hydrogel fiber. | Monitor to detect changes in the acidity and basicity of the skin by changing colors. Healing of the skin indicated by acidic color. The potentiometric pH provides information about bacterial infection. | [215] |
| Passive (poly (N-isopropylacrylamide) (PNIPAAm)) to active (poly (2-carboxyethyl acrylate) (PCEA)) layers towards environmental pH changes. | The chemical composition of discrete layers resulted in anisotropic swelling behavior. PCEA (upper layer) swelled in high pH values due to deprotonation of the acid groups while PNIPAAm (lower layer) slightly swelled in an acidic pH. | [216] | |
| Sodium hydrogen carbonate (NaHCO3) vapor as a cross-linker for collagen to provide a homogeneous gelation. | Collagens as a major extracellular matrix protein have several ionizable groups, such as hydroxyl and amine groups in their molecular chains. | [217] | |
| Moisture content | Absorb water due to void imperfections. | Dynamic shape and geometrical expansion, stretching, folding and bending change in response to variations in environmental humidity. | [218,219,220,221] |
| Hydrophilic layer expanded in water and forced a shape change as stretching or folding into the structure | [222] | ||
| Origami-inspired structures including polyurethane hydrogel core and polyurethane elastomer skins. | Discrete localized gaps at elastomeric skin were acting active hinges. During the hydration resulted in different complex structures. | [223] | |
| Composite ink for 3D printing by incorporating cellulose pulp fibers into carboxymethycellulose (CMC) hydrocolloid. | Printed objects underwent reversibly programmed transformation upon hydration and dehydration. | [202] | |
| Upregulation or downregulation of enzyme levels | Modified chitosan functionalized with a fluorogenic substrate | The presence of various types of enzymes can be detected using florigenic or chromogenic substrate. It is highly useful for detection of specific pathogenic bacteria in wound dressing. | [224] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsegay, F.; Elsherif, M.; Butt, H. Smart 3D Printed Hydrogel Skin Wound Bandages: A Review. Polymers 2022, 14, 1012. https://doi.org/10.3390/polym14051012
Tsegay F, Elsherif M, Butt H. Smart 3D Printed Hydrogel Skin Wound Bandages: A Review. Polymers. 2022; 14(5):1012. https://doi.org/10.3390/polym14051012
Chicago/Turabian StyleTsegay, Filmon, Mohamed Elsherif, and Haider Butt. 2022. "Smart 3D Printed Hydrogel Skin Wound Bandages: A Review" Polymers 14, no. 5: 1012. https://doi.org/10.3390/polym14051012
APA StyleTsegay, F., Elsherif, M., & Butt, H. (2022). Smart 3D Printed Hydrogel Skin Wound Bandages: A Review. Polymers, 14(5), 1012. https://doi.org/10.3390/polym14051012









