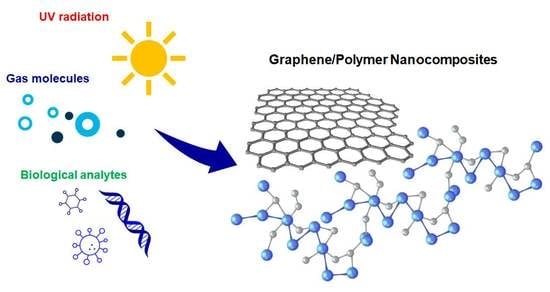
Recent Trends in Graphene/Polymer Nanocomposites for Sensing Devices: Synthesis and Applications in Environmental and Human Health Monitoring
Abstract
:1. Introduction
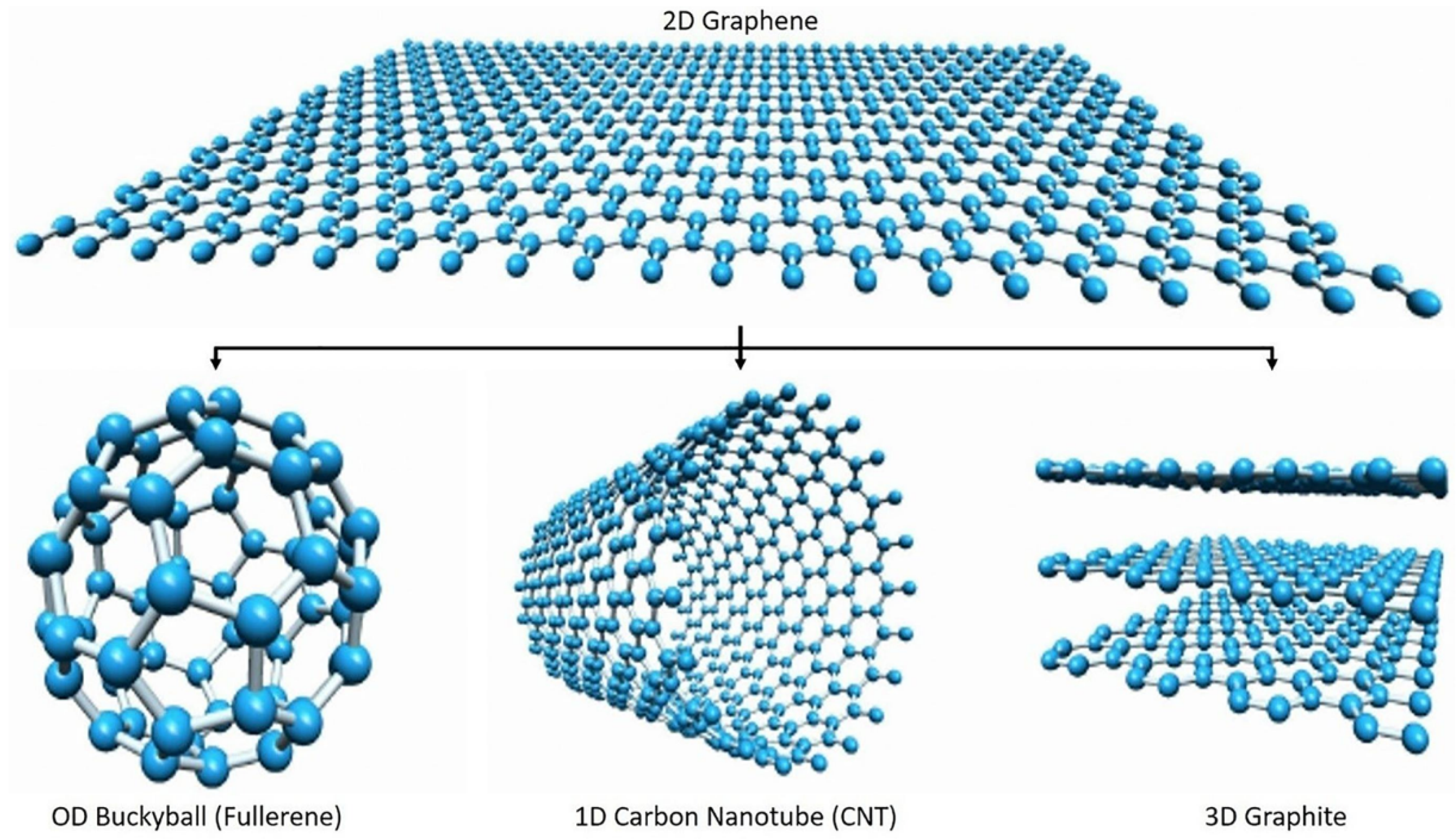
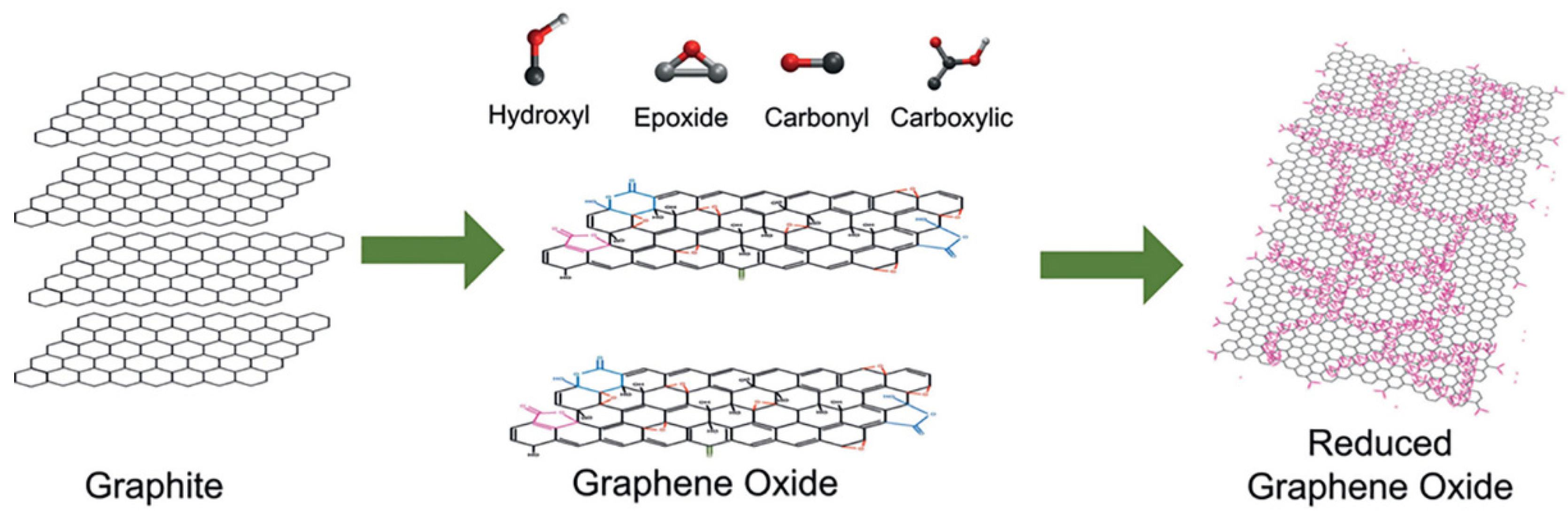
2. The Graphene Nanomaterial: Synthesis and General Properties
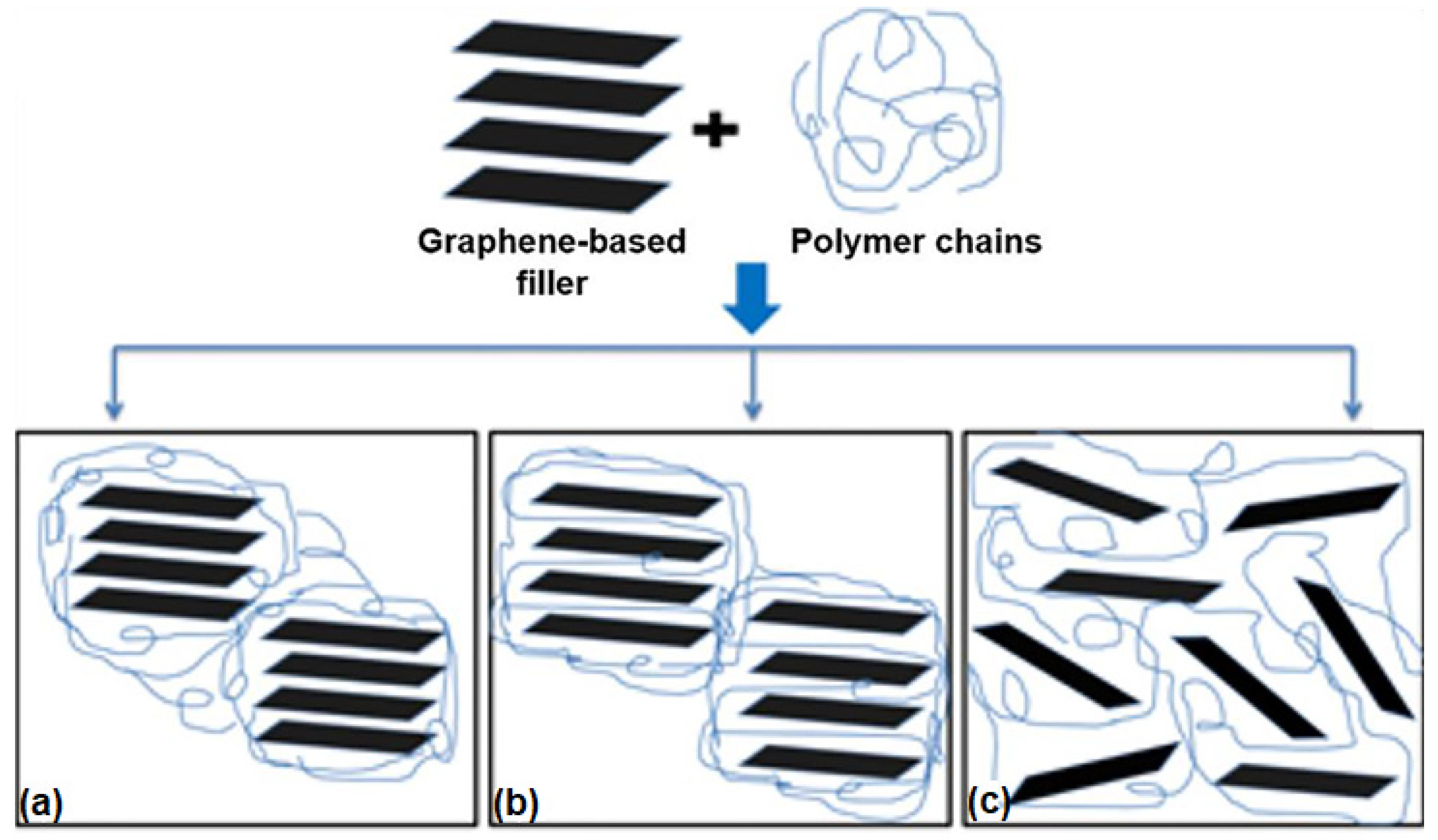
3. Graphene/Polymer Nanocomposites: Fabrication and Properties
4. Applications of Graphene/Polymer Nanocomposites in Sensing Devices
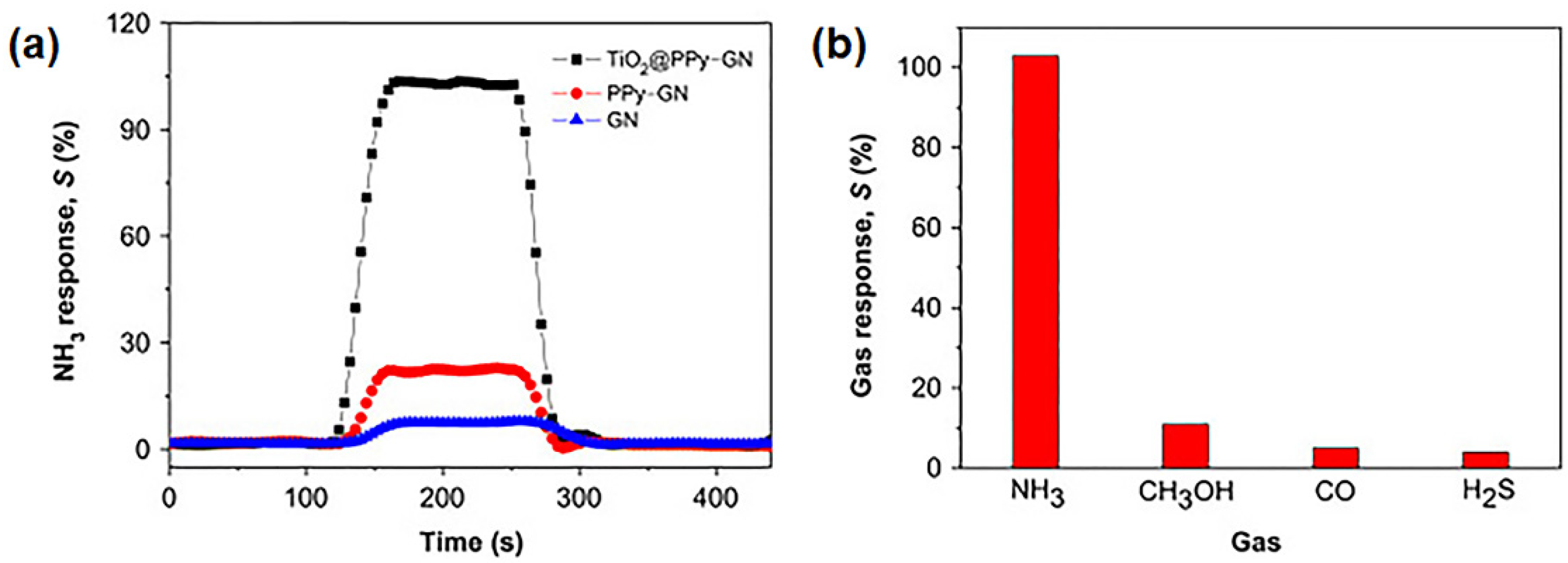
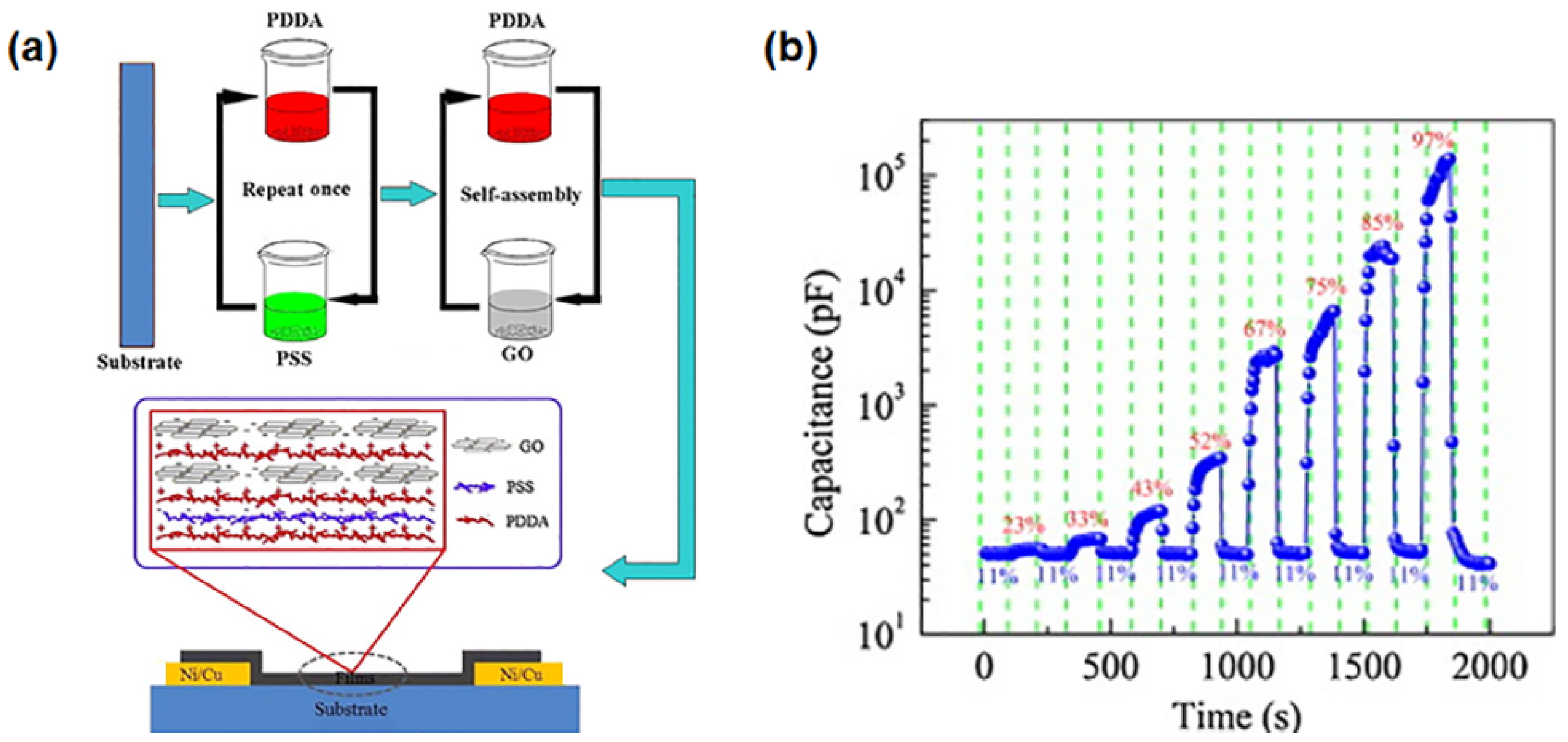
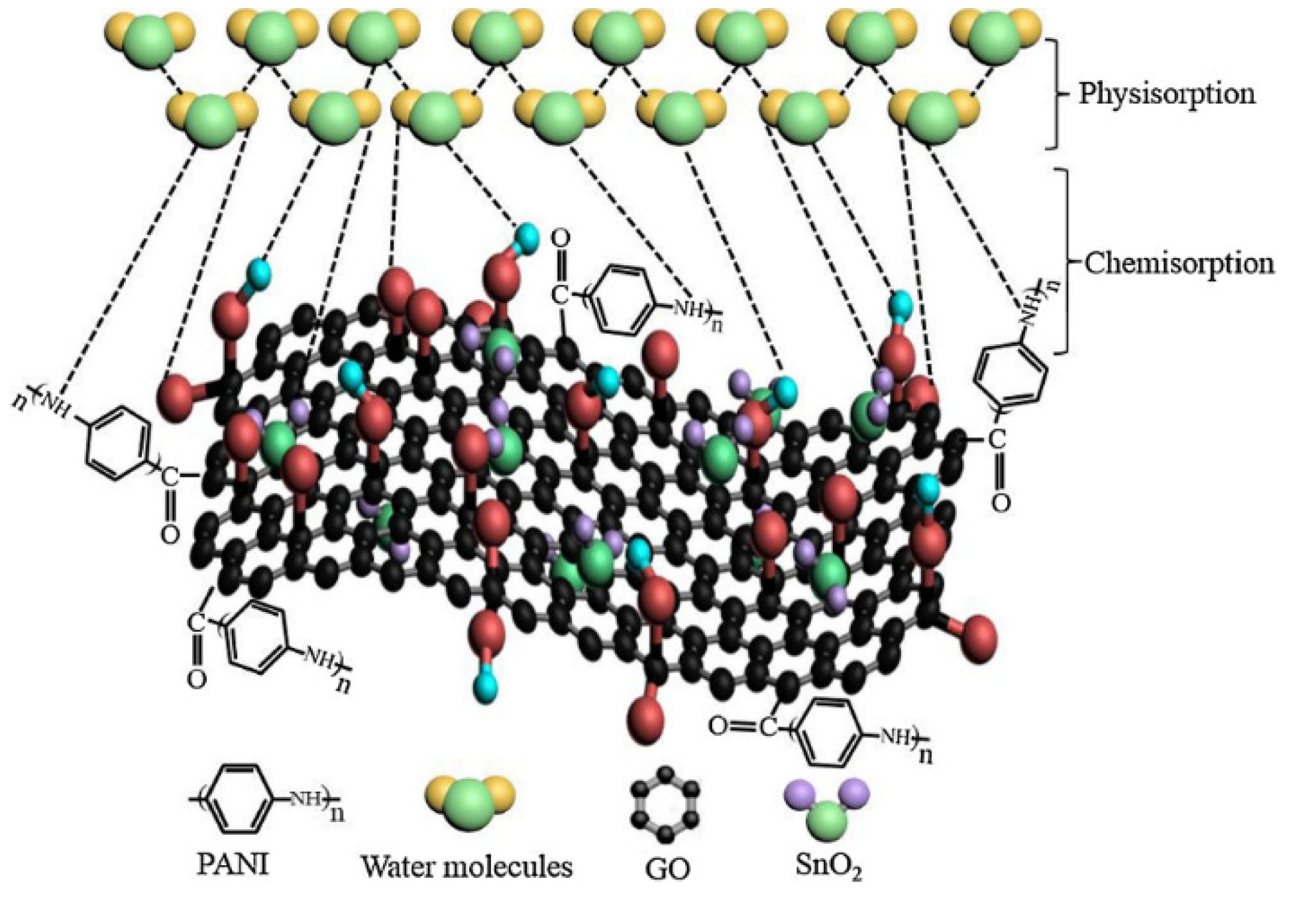
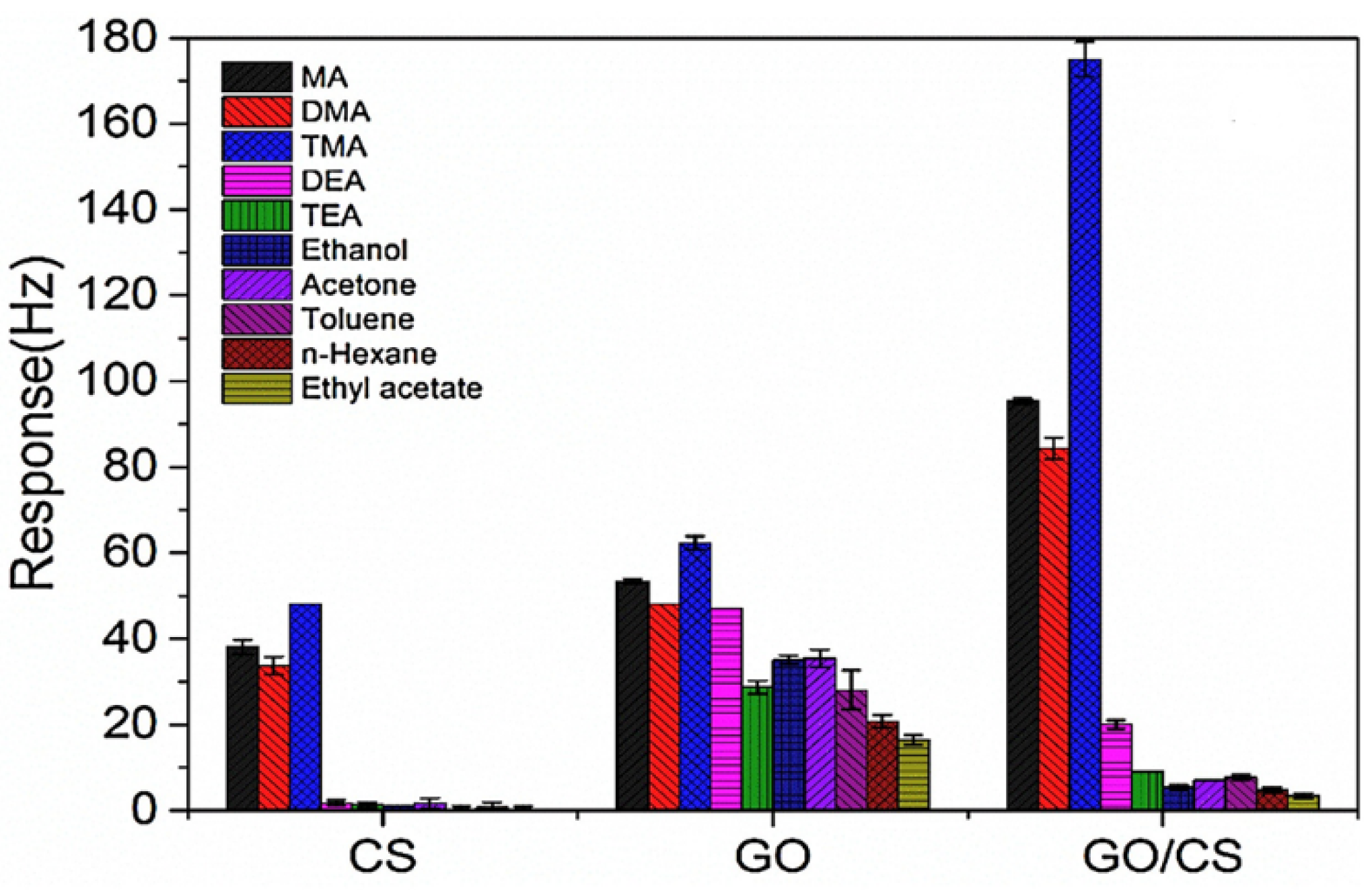
4.1. Applications of GPNs in Environmental Monitoring: Gas and Humidity Sensors
4.2. Applications of GPNs in Environmental Monitoring: UV Radiation Sensors
4.3. Applications of GPNs in Human Health Monitoring
5. Summary and Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeon, I.-Y.; Baek, J.-B. Nanocomposites Derived from Polymers and Inorganic Nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar] [CrossRef] [Green Version]
- Crosby, A.J.; Lee, J.Y. Polymer Nanocomposites: The “Nano” Effect on Mechanical Properties. Polym. Rev. 2007, 47, 217–229. [Google Scholar] [CrossRef]
- Chan, J.X.; Wong, J.F.; Petrů, M.; Hassan, A.; Nirmal, U.; Othman, N.; Ilyas, R.A. Effect of Nanofillers on Tribological Properties of Polymer Nanocomposites: A Review on Recent Development. Polymers 2021, 13, 2867. [Google Scholar] [CrossRef]
- Schodek, D.L.; Ferreira, P.; Ashby, M.F. Nanomaterials, Nanotechnologies and Design: An Introduction for Engineers and Architects; Butterworth-Heinemann: Oxford, UK, 2009. [Google Scholar]
- Vaia, R.A.; Wagner, H.D. Framework for nanocomposites. Mater. Today 2004, 7, 32–37. [Google Scholar] [CrossRef]
- De, A.; Sen, P.; Poddar, A.; Das, A. Synthesis, characterization, electrical transport and magnetic properties of PEDOT–DBSA–Fe3O4 conducting nanocomposite. Synth. Met. 2009, 159, 1002–1007. [Google Scholar] [CrossRef]
- Nihmath, A.; Ramesan, M.T. Fabrication, Characterization and Dielectric Studies of NBR/Hydroxyapatite Nanocomposites. J. Inorg. Organomet. Polym. 2017, 27, 481–489. [Google Scholar] [CrossRef]
- Ng, C.; Ash, B.; Schadler, L.; Siegel, R. A study of the mechanical and permeability properties of nano-and micron-TiO2 filled epoxy composites. Adv. Compos. Lett. 2001, 10, 096369350101000301. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.S.; Youn, J.R. Influence of dispersion states of carbon nanotubes on physical properties of epoxy nanocomposites. Carbon 2005, 43, 1378–1385. [Google Scholar] [CrossRef]
- Vahedi, F.; Shahverdi, H.; Shokrieh, M.; Esmkhani, M. Effects of carbon nanotube content on the mechanical and electrical properties of epoxy-based composites. New Carbon Mater. 2014, 29, 419–425. [Google Scholar] [CrossRef]
- Zaccardi, F.; Santonicola, M.G.; Laurenzi, S. Quantitative assessment of nanofiller dispersion based on grayscale image analysis: A case study on epoxy/carbon nanocomposites. Compos. A Appl. Sci. Manuf. 2018, 115, 302–310. [Google Scholar] [CrossRef]
- Gudarzi, M.M.; Sharif, F. Enhancement of dispersion and bonding of graphene-polymer through wet transfer of functionalized graphene oxide. Express Polym. Lett. 2012, 6, 1017–1031. [Google Scholar] [CrossRef]
- Goda, E.S.; Singu, B.S.; Hong, S.E.; Yoon, K.R. Good dispersion of poly(δ-gluconolactone)-grafted graphene in poly(vinyl alcohol) for significantly enhanced mechanical strength. Mater. Chem. Phys. 2020, 254, 123465. [Google Scholar] [CrossRef]
- Du, X.; Skachko, I.; Barker, A.; Andrei, E.Y. Approaching ballistic transport in suspended graphene. Nat. Nanotechnol. 2008, 3, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Gao, R.; Hu, N.; Chai, J.; Cheng, Y.; Zhang, L.; Wei, H.; Kong, E.S.-W.; Zhang, Y. The Prospective Two-Dimensional Graphene Nanosheets: Preparation, Functionalization and Applications. Nano-Micro Lett. 2012, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Wan, C.; Chen, B. Reinforcement and interphase of polymer/graphene oxide nanocomposites. J. Mater. Chem. 2012, 22, 3637–3646. [Google Scholar] [CrossRef]
- Terrones, M.; Martin, O.; Gonzalez, M.; Pozuelo, J.; Serrano, B.; Cabanelas, J.C.; Vega-Diaz, S.M.; Baselga, J. Interphases in graphene polymer-based nanocomposites: Achievements and challenges. Adv. Mater. 2011, 23, 5302–5310. [Google Scholar] [CrossRef] [Green Version]
- Gong, Q.; Han, H.; Yang, H.; Zhang, M.; Sun, X.; Liang, Y.; Liu, Z.; Zhang, W.; Qiao, J. Sensitive electrochemical DNA sensor for the detection of HIV based on a polyaniline/graphene nanocomposite. J. Mater. 2019, 5, 313–319. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, A.; Du, D.; Lin, Y. Graphene-based immunosensor for electrochemical quantification of phosphorylated p53 (S15). Anal. Chim. Acta 2011, 699, 44–48. [Google Scholar] [CrossRef]
- Ioniţă, M.; Vlăsceanu, G.M.; Watzlawek, A.A.; Voicu, S.I.; Burns, J.S.; Iovu, H. Graphene and functionalized graphene: Extraordinary prospects for nanobiocomposite materials. Compos. B Eng. 2017, 121, 34–57. [Google Scholar] [CrossRef]
- Ponnamma, D.; Sadasivuni, K.K. Graphene/polymer nanocomposites: Role in electronics. In Graphene-Based Polymer Nanocomposites in Electronics; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Rumyantsev, S.; Liu, G.; Shur, M.S.; Potyrailo, R.A.; Balandin, A.A. Selective gas sensing with a single pristine graphene transistor. Nano Lett. 2012, 12, 2294–2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, C.; Jiang, D.; Zou, Y.; Chu, H.; Qiu, S.; Zhang, H.; Xu, F.; Sun, L.; Zheng, L. Ammonia sensor based on polypyrrole–graphene nanocomposite decorated with titania nanoparticles. Ceram. Int. 2015, 41, 6432–6438. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Yang, W.; Yuan, W.; Xu, J.; Jiang, Y. In Situ Polymerization Deposition of Porous Conducting Polymer on Reduced Graphene Oxide for Gas Sensor. ACS Appl. Mater. Interfaces 2014, 6, 13807–13814. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, D.; Zong, X.; Dong, G.; Zhang, Y. High-performance QCM humidity sensor based on graphene oxide/tin oxide/polyaniline ternary nanocomposite prepared by in-situ oxidative polymerization method. Sens. Actuators B Chem. 2018, 262, 531–541. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, J.; Xia, B.; Xue, Q. Ultrahigh performance humidity sensor based on layer-by-layer self-assembly of graphene oxide/polyelectrolyte nanocomposite film. Sens. Actuators B Chem. 2014, 203, 263–270. [Google Scholar] [CrossRef]
- Clausi, M.; Toto, E.; Botti, S.; Laurenzi, S.; La Saponara, V.; Santonicola, M.G. Direct effects of UV irradiation on graphene-based nanocomposite films revealed by electrical resistance tomography. Compos. Sci. Technol. 2019, 183, 107823. [Google Scholar] [CrossRef]
- Santonicola, M.; Toto, E.; Palombi, M.; Paris, C.; Laurenzi, S. Experimental study of solar radiation effects on carbon nanocomposite sensors in simulated space environment. In Proceedings of the International Astronautical Congress (IAC), Bremen, Germany, 1–5 October 2018. [Google Scholar]
- Toto, E.; Santonicola, M.G.; Mancini, M.C.; Laurenzi, S. Ultraviolet-sensing surfaces based on hybrid nanocomposites for radiation monitoring systems. In Proceedings of the 2017 IEEE International Workshop on Metrology for AeroSpace (MetroAeroSpace), Padua, Italy, 21–23 June 2017; pp. 369–373. [Google Scholar] [CrossRef]
- Toto, E.; Botti, S.; Laurenzi, S.; Santonicola, M.G. UV-induced modification of PEDOT:PSS-based nanocomposite films investigated by Raman microscopy mapping. Appl. Surf. Sci. 2020, 513, 145839. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Graphene based sensors and biosensors. Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Ray, T.R.; Choi, J.; Bandodkar, A.J.; Krishnan, S.; Gutruf, P.; Tian, L.; Ghaffari, R.; Rogers, J.A. Bio-integrated wearable systems: A comprehensive review. Chem. Rev. 2019, 119, 5461–5533. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical Applications of Graphene and Graphene Oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, X.; Liu, Y.; Li, L.-J.; Chen, P. Graphene-based biosensors for detection of bacteria and their metabolic activities. J. Mater. Chem. 2011, 21, 12358–12362. [Google Scholar] [CrossRef]
- Syama, S.; Mohanan, P. Comprehensive application of graphene: Emphasis on biomedical concerns. Nano-Micro Lett. 2019, 11, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nambiar, S.; Yeow, J.T.W. Conductive polymer-based sensors for biomedical applications. Biosens. Bioelectron. 2011, 26, 1825–1832. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Huang, J.; Zhang, J.; Pan, D.; Zhou, J.; Ryu, J.E.; Umar, A.; Guo, Z. Advances in Responsively Conductive Polymer Composites and Sensing Applications. Polym. Rev. 2021, 61, 157–193. [Google Scholar] [CrossRef]
- Xie, D.; Jiang, Y.; Pan, W.; Li, D.; Wu, Z.; Li, Y. Fabrication and characterization of polyaniline-based gas sensor by ultra-thin film technology. Sens. Actuators B Chem. 2002, 81, 158–164. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Sireesha, M.; Jagadeesh Babu, V.; Kranthi Kiran, A.S.; Ramakrishna, S. A review on carbon nanotubes in biosensor devices and their applications in medicine. Nanocomposites 2018, 4, 36–57. [Google Scholar] [CrossRef]
- Gao, M.; Dai, L.; Wallace, G. Glucose sensors based on glucose-oxidase-containing polypyrrole/aligned carbon nanotube coaxial nanowire electrodes. Synth. Met. 2003, 137, 1393–1394. [Google Scholar] [CrossRef]
- Wang, J.; Musameh, M. Carbon-nanotubes doped polypyrrole glucose biosensor. Anal. Chim. Acta 2005, 539, 209–213. [Google Scholar] [CrossRef]
- Kanan, S.M.; El-Kadri, O.M.; Abu-Yousef, I.A.; Kanan, M.C. Semiconducting Metal Oxide Based Sensors for Selective Gas Pollutant Detection. Sensors 2009, 9, 8158–8196. [Google Scholar] [CrossRef] [Green Version]
- Dakshayini, B.; Reddy, K.R.; Mishra, A.; Shetti, N.P.; Malode, S.J.; Basu, S.; Naveen, S.; Raghu, A.V. Role of conducting polymer and metal oxide-based hybrids for applications in ampereometric sensors and biosensors. Microchem. J. 2019, 147, 7–24. [Google Scholar] [CrossRef]
- Patil, U.V.; Ramgir, N.S.; Karmakar, N.; Bhogale, A.; Debnath, A.K.; Aswal, D.K.; Gupta, S.K.; Kothari, D.C. Room temperature ammonia sensor based on copper nanoparticle intercalated polyaniline nanocomposite thin films. Appl. Surf. Sci. 2015, 339, 69–74. [Google Scholar] [CrossRef]
- Singh, M.; Kathuroju, P.K.; Jampana, N. Polypyrrole based amperometric glucose biosensors. Sens. Actuators B Chem. 2009, 143, 430–443. [Google Scholar] [CrossRef]
- Krishna, R.S.; Mishra, J.; Nanda, B.; Patro, S.K.; Adetayo, A.; Qureshi, T.S. The role of graphene and its derivatives in modifying different phases of geopolymer composites: A review. Constr. Build. Mater. 2021, 306, 124774. [Google Scholar] [CrossRef]
- Du, J.; Cheng, H.-M. The Fabrication, Properties, and Uses of Graphene/Polymer Composites. Macromol. Chem. Phys. 2012, 213, 1060–1077. [Google Scholar] [CrossRef]
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y. Recent progress in graphene/polymer nanocomposites. Adv. Mater. 2021, 33, 2001105. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, Z.; Zhang, Y.; Morozov, S.; Stormer, H.L.; Zeitler, U.; Maan, J.; Boebinger, G.; Kim, P.; Geim, A.K. Room-temperature quantum Hall effect in graphene. Science 2007, 315, 1379. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tan, Y.-W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Bendali, A.; Hess, L.H.; Seifert, M.; Forster, V.; Stephan, A.F.; Garrido, J.A.; Picaud, S. Purified neurons can survive on peptide-free graphene layers. Adv. Healthc. Mater. 2013, 2, 929–933. [Google Scholar] [CrossRef]
- Li, N.; Zhang, X.; Song, Q.; Su, R.; Zhang, Q.; Kong, T.; Liu, L.; Jin, G.; Tang, M.; Cheng, G. The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomaterials 2011, 32, 9374–9382. [Google Scholar] [CrossRef] [Green Version]
- Sahni, D.; Jea, A.; Mata, J.A.; Marcano, D.C.; Sivaganesan, A.; Berlin, J.M.; Tatsui, C.E.; Sun, Z.; Luerssen, T.G.; Meng, S. Biocompatibility of pristine graphene for neuronal interface. J. Neurosurg. Pediatr. 2013, 11, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fraser, A.; Ye, S.; Merle, G.; Barralet, J. Top-down bottom-up graphene synthesis. Nano Futures 2019, 3, 042003. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, J.; Tao, C.A.; Wu, Y.; Li, Z.; Ma, L.; Wen, Y.; Li, G. A strategy for producing pure single-layer graphene sheets based on a confined self-assembly approach. Angew. Chem. 2009, 121, 5978–5982. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Zhao, K.; Shi, Z.; Gu, Z.; Xu, S. Large scale synthesis of N-doped multi-layered graphene sheets by simple arc-discharge method. Carbon 2010, 48, 255–259. [Google Scholar] [CrossRef]
- Huang, H.; Chen, S.; Wee, A.T.S.; Chen, W. 9—Epitaxial growth of graphene on silicon carbide (SiC). In Graphene, 2nd ed.; Skakalova, V., Kaiser, A.B., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 177–198. [Google Scholar] [CrossRef]
- Rollings, E.; Gweon, G.H.; Zhou, S.Y.; Mun, B.S.; McChesney, J.L.; Hussain, B.S.; Fedorov, A.V.; First, P.N.; de Heer, W.A.; Lanzara, A. Synthesis and characterization of atomically thin graphite films on a silicon carbide substrate. J. Phys. Chem. Solids 2006, 67, 2172–2177. [Google Scholar] [CrossRef] [Green Version]
- Yazdi, G.R.; Iakimov, T.; Yakimova, R. Epitaxial Graphene on SiC: A Review of Growth and Characterization. Crystals 2016, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Munoz, R.; Gomez-Aleixandre, C. Review of CVD synthesis of graphene. Chem. Vap. Depos. 2013, 19, 297–322. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.-D.; Min, B.-K.; Jung, W.-S. Preparation of graphene sheets by the reduction of carbon monoxide. Carbon 2009, 47, 1610–1612. [Google Scholar] [CrossRef]
- Kosynkin, D.V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J.M. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 2009, 458, 872–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, P.; Lowe, S.E.; Simon, G.P.; Zhong, Y.L. Electrochemical exfoliation of graphite and production of functional graphene. Curr. Opin. Colloid. Interface Sci. 2015, 20, 329–338. [Google Scholar] [CrossRef]
- Liu, F.; Wang, C.; Sui, X.; Riaz, M.A.; Xu, M.; Wei, L.; Chen, Y. Synthesis of graphene materials by electrochemical exfoliation: Recent progress and future potential. Carbon Energy 2019, 1, 173–199. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Bourlinos, A.B.; Georgakilas, V.; Zboril, R.; Steriotis, T.A.; Stubos, A.K. Liquid-phase exfoliation of graphite towards solubilized graphenes. Small 2009, 5, 1841–1845. [Google Scholar] [CrossRef]
- Phiri, J.; Gane, P.; Maloney, T.C. General overview of graphene: Production, properties and application in polymer composites. Mater. Sci. Eng. B 2017, 215, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Clancy, A.J.; Bayazit, M.K.; Hodge, S.A.; Skipper, N.T.; Howard, C.A.; Shaffer, M.S. Charged carbon nanomaterials: Redox chemistries of fullerenes, carbon nanotubes, and graphenes. Chem. Rev. 2018, 118, 7363–7408. [Google Scholar] [CrossRef] [Green Version]
- Dao, T.D.; Jeong, H.M. Graphene prepared by thermal reduction–exfoliation of graphite oxide: Effect of raw graphite particle size on the properties of graphite oxide and graphene. Mater. Res. Bull. 2015, 70, 651–657. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Synthesis of Water Soluble Graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Worsley, K.A.; Ramesh, P.; Mandal, S.K.; Niyogi, S.; Itkis, M.E.; Haddon, R.C. Soluble graphene derived from graphite fluoride. Chem. Phys. Lett. 2007, 445, 51–56. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217. [Google Scholar] [CrossRef]
- Chanda, M. Plastics Technology Handbook; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Lombardo, A.; Hasan, T.; Sun, Z.; Colombo, L.; Ferrari, A.C. Production and processing of graphene and 2d crystals. Mater. Today 2012, 15, 564–589. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Li, J.-L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef] [Green Version]
- Dao, T.D.; Lee, H.-I.; Jeong, H.M. Alumina-coated graphene nanosheet and its composite of acrylic rubber. J. Colloid Interface Sci. 2014, 416, 38–43. [Google Scholar] [CrossRef]
- Punckt, C.; Muckel, F.; Wolff, S.; Aksay, I.A.; Chavarin, C.A.; Bacher, G.; Mertin, W. The effect of degree of reduction on the electrical properties of functionalized graphene sheets. Appl. Phys. Lett. 2013, 102, 023114. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Lv, W.; Xie, X.; Tang, D.; Liu, C.; Yang, Q.-H. Towards low temperature thermal exfoliation of graphite oxide for graphene production. Carbon 2013, 62, 11–24. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Wang, J.-W.; Yan, Q.; Zheng, W.-G.; Chen, C.; Yu, Z.-Z. Vacuum-assisted synthesis of graphene from thermal exfoliation and reduction of graphite oxide. J. Mater. Chem. 2011, 21, 5392–5397. [Google Scholar] [CrossRef]
- Kim, J.; Yim, B.-S.; Kim, J.-M.; Kim, J. The effects of functionalized graphene nanosheets on the thermal and mechanical properties of epoxy composites for anisotropic conductive adhesives (ACAs). Microelectron. Reliabil. 2012, 52, 595–602. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, C.; Wang, Y.; Liu, C.; Chen, X.; Xie, H.; Huang, Y.; Cheng, R. Effects of graphene oxides on the cure behaviors of a tetrafunctional epoxy resin. Express Polym. Lett. 2011, 5, 809–818. [Google Scholar] [CrossRef]
- Lee, J.K.; Song, S.; Kim, B. Functionalized graphene sheets-epoxy based nanocomposite for cryotank composite application. Polym. Compos. 2012, 33, 1263–1273. [Google Scholar] [CrossRef]
- Kim, J.; Im, H.; Kim, J.-M.; Kim, J. Thermal and electrical conductivity of Al(OH)3 covered graphene oxide nanosheet/epoxy composites. J. Mater. Sci. 2012, 47, 1418–1426. [Google Scholar] [CrossRef]
- Jiang, T.; Kuila, T.; Kim, N.H.; Ku, B.-C.; Lee, J.H. Enhanced mechanical properties of silanized silica nanoparticle attached graphene oxide/epoxy composites. Compos. Sci. Technol. 2013, 79, 115–125. [Google Scholar] [CrossRef]
- Layek, R.K.; Nandi, A.K. A review on synthesis and properties of polymer functionalized graphene. Polymer 2013, 54, 5087–5103. [Google Scholar] [CrossRef] [Green Version]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Bao, C.; Guo, Y.; Song, L.; Kan, Y.; Qian, X.; Hu, Y. In situ preparation of functionalized graphene oxide/epoxy nanocomposites with effective reinforcements. J. Mater. Chem. 2011, 21, 13290–13298. [Google Scholar] [CrossRef]
- Muradyan, V.; Arbuzov, A.; Sokolov, E.; Babenko, S.; Bondarenko, G. The effect of addition of functionalized graphene oxide on the dielectric properties of epoxy composite. Tech. Phys. Lett. 2013, 39, 798–800. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Feng, X.; Yu, B.; Song, L.; Hu, Y. Functionalization of graphene with grafted polyphosphamide for flame retardant epoxy composites: Synthesis, flammability and mechanism. Polym. Chem. 2014, 5, 1145–1154. [Google Scholar] [CrossRef]
- Fan, J.; Shi, Z.; Wang, J.; Yin, J. Glycidyl methacrylate-modified gum arabic mediated graphene exfoliation and its use for enhancing mechanical performance of hydrogel. Polymer 2013, 54, 3921–3930. [Google Scholar] [CrossRef]
- Cai, D.; Song, M. Recent advance in functionalized graphene/polymer nanocomposites. J. Mater. Chem. 2010, 20, 7906–7915. [Google Scholar] [CrossRef]
- Wang, M.; Yan, C.; Ma, L. Graphene nanocomposites. In Composites and Their Properties; Hu, N., Ed.; InTech: Rijeka, Croatia, 2012; Volume 17. [Google Scholar]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Graphene/elastomer nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Zhu, H.; Liu, T. In situ thermal preparation of polyimide nanocomposite films containing functionalized graphene sheets. ACS Appl. Mater. Interfaces 2010, 2, 3702–3708. [Google Scholar] [CrossRef]
- Higginbotham, A.L.; Lomeda, J.R.; Morgan, A.B.; Tour, J.M. Graphite oxide flame-retardant polymer nanocomposites. ACS Appl. Mater. Interfaces 2009, 1, 2256–2261. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.; Stankovich, S.; Dikin, D.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.; Schniepp, H.; Chen, X.; Ruoff, R. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-L.; Zhang, Y.; Hu, Q.-H.; He, S.; Li, X.; Zhai, M.; Yu, Z.-Z. Enhanced mechanical properties of poly(vinyl alcohol) nanocomposites with glucose-reduced graphene oxide. Mater. Lett. 2013, 102–103, 15–18. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, J.; Yuan, W. Preparation of a poly(methyl methacrylate)-reduced graphene oxide composite with enhanced properties by a solution blending method. Eur. Polym. J. 2012, 48, 1674–1682. [Google Scholar] [CrossRef]
- Wan, C.; Chen, B. Reinforcement of biodegradable poly(butylene succinate) with low loadings of graphene oxide. J. Appl. Polym. Sci. 2013, 127, 5094–5099. [Google Scholar] [CrossRef]
- Lee, J.H.; Marroquin, J.; Rhee, K.Y.; Park, S.J.; Hui, D. Cryomilling application of graphene to improve material properties of graphene/chitosan nanocomposites. Compos. B Eng. 2013, 45, 682–687. [Google Scholar] [CrossRef]
- Kumar, S.K.; Castro, M.; Saiter, A.; Delbreilh, L.; Feller, J.F.; Thomas, S.; Grohens, Y. Development of poly(isobutylene-co-isoprene)/reduced graphene oxide nanocomposites for barrier, dielectric and sensingapplications. Mater. Lett. 2013, 96, 109–112. [Google Scholar] [CrossRef]
- Swain, S. Synthesis and characterization of graphene based unsaturated polyester resin composites. Trans. Electr. Electron. Mater. 2013, 14, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Wang, X.; Wang, J.; Wan, L.; Liu, F.; Zheng, H.; Chen, R.; Xu, C. Preparation and properties of graphene nanosheets–polystyrene nanocomposites via in situ emulsion polymerization. Chem. Phys. Lett. 2010, 484, 247–253. [Google Scholar] [CrossRef]
- Mohamadzadeh Moghadam, M.H.; Sabury, S.; Gudarzi, M.M.; Sharif, F. Graphene oxide-induced polymerization and crystallization to produce highly conductive polyaniline/graphene oxide composite. J. Polym. Sci. A Polym. Chem. 2014, 52, 1545–1554. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, X.; Wong, S.C. Electrical and mechanical properties of expanded graphite-reinforced high-density polyethylene. J. Appl. Polym. Sci. 2004, 91, 2781–2788. [Google Scholar] [CrossRef]
- Kim, H.; Macosko, C.W. Processing-property relationships of polycarbonate/graphene composites. Polymer 2009, 50, 3797–3809. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Fukushima, H.; Drzal, L.T. A new compounding method for exfoliated graphite–polypropylene nanocomposites with enhanced flexural properties and lower percolation threshold. Compos. Sci. Technol. 2007, 67, 2045–2051. [Google Scholar] [CrossRef]
- Mashhadzadeh, A.H.; Fereidoon, A.; Ahangari, M.G. Experimental and multiscale quantum mechanics modeling of the mechanical properties of PVC/graphene nanocomposite. J. Compos. Mater. 2020, 54, 4575–4590. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Zheng, W.-G.; Yan, Q.; Yang, Y.; Wang, J.-W.; Lu, Z.-H.; Ji, G.-Y.; Yu, Z.-Z. Electrically conductive polyethylene terephthalate/graphene nanocomposites prepared by melt compounding. Polymer 2010, 51, 1191–1196. [Google Scholar] [CrossRef]
- Bansal, A.; Yang, H.; Li, C.; Cho, K.; Benicewicz, B.C.; Kumar, S.K.; Schadler, L.S. Quantitative equivalence between polymer nanocomposites and thin polymer films. Nat. Mater. 2005, 4, 693. [Google Scholar] [CrossRef]
- Priestley, R.D.; Ellison, C.J.; Broadbelt, L.J.; Torkelson, J.M. Structural relaxation of polymer glasses at surfaces, interfaces, and in between. Science 2005, 309, 456–459. [Google Scholar] [CrossRef]
- Zakiyan, S.E.; Azizi, H.; Ghasemi, I. Influence of chain mobility on rheological, dielectric and electromagnetic interference shielding properties of poly methyl-methacrylate composites filled with graphene and carbon nanotube. Compos. Sci. Technol. 2017, 142, 10–19. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, B.; Jia, N.; Yu, Y.; Xu, X.; Wang, Y.; Wu, B.; Qian, J.; Xia, R.; Wang, C.; et al. Enhanced thermally conductive and thermomechanical properties of polymethyl methacrylate (PMMA)/graphene nanoplatelets (GNPs) nanocomposites for radiator of electronic components. Polym. Test. 2021, 101, 107237. [Google Scholar] [CrossRef]
- Yu, A.; Ramesh, P.; Itkis, M.E.; Bekyarova, E.; Haddon, R.C. Graphite Nanoplatelet−Epoxy Composite Thermal Interface Materials. J. Phys. Chem. C 2007, 111, 7565–7569. [Google Scholar] [CrossRef]
- Veca, L.M.; Meziani, M.J.; Wang, W.; Wang, X.; Lu, F.; Zhang, P.; Lin, Y.; Fee, R.; Connell, J.W.; Sun, Y.P. Carbon nanosheets for polymeric nanocomposites with high thermal conductivity. Adv. Mater. 2009, 21, 2088–2092. [Google Scholar] [CrossRef]
- Ganguli, S.; Roy, A.K.; Anderson, D.P. Improved thermal conductivity for chemically functionalized exfoliated graphite/epoxy composites. Carbon 2008, 46, 806–817. [Google Scholar] [CrossRef]
- Kumar, P.; Yu, S.; Shahzad, F.; Hong, S.M.; Kim, Y.-H.; Koo, C.M. Ultrahigh electrically and thermally conductive self-aligned graphene/polymer composites using large-area reduced graphene oxides. Carbon 2016, 101, 120–128. [Google Scholar] [CrossRef]
- Yousefi, N.; Gudarzi, M.M.; Zheng, Q.; Aboutalebi, S.H.; Sharif, F.; Kim, J.-K. Self-alignment and high electrical conductivity of ultralarge graphene oxide–polyurethane nanocomposites. J. Mater. Chem. 2012, 22, 12709–12717. [Google Scholar] [CrossRef]
- Tkalya, E.; Ghislandi, M.; Otten, R.; Lotya, M.; Alekseev, A.; van der Schoot, P.; Coleman, J.; de With, G.; Koning, C. Experimental and Theoretical Study of the Influence of the State of Dispersion of Graphene on the Percolation Threshold of Conductive Graphene/Polystyrene Nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 15113–15121. [Google Scholar] [CrossRef]
- Wang, Y.; Shan, J.W.; Weng, G.J. Percolation threshold and electrical conductivity of graphene-based nanocomposites with filler agglomeration and interfacial tunneling. J. Appl. Phys. 2015, 118, 065101. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Gómez-Fatou, M.N.A.; Ania, F.; Flores, A. Nanoindentation assessment of the interphase in carbon nanotube-based hierarchical composites. J. Phys. Chem. C 2012, 116, 24193–24200. [Google Scholar] [CrossRef]
- Gu, Y.; Li, M.; Wang, J.; Zhang, Z. Characterization of the interphase in carbon fiber/polymer composites using a nanoscale dynamic mechanical imaging technique. Carbon 2010, 48, 3229–3235. [Google Scholar] [CrossRef]
- Downing, T.; Kumar, R.; Cross, W.; Kjerengtroen, L.; Kellar, J. Determining the interphase thickness and properties in polymer matrix composites using phase imaging atomic force microscopy and nanoindentation. J. Adhes. Sci. Technol. 2000, 14, 1801–1812. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of Size and Aggregation/Agglomeration of Nanoparticles on the Interfacial/Interphase Properties and Tensile Strength of Polymer Nanocomposites. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Y.; Huang, L. Evaluation of the mechanical properties of graphene-based nanocomposites incorporating a graded interphase based on isoparametric graded finite element model. Compos. Interfaces 2021, 28, 543–575. [Google Scholar] [CrossRef]
- Bokobza, L.; Bresson, B.; Garnaud, G.; Zhang, J. Mechanical and AFM investigations of elastomers filled with multiwall carbon nanotubes. Compos. Interfaces 2012, 19, 285–295. [Google Scholar] [CrossRef]
- Kai, W.; Hirota, Y.; Hua, L.; Inoue, Y. Thermal and mechanical properties of a poly (ϵ-caprolactone)/graphite oxide composite. J. Appl. Polym. Sci. 2008, 107, 1395–1400. [Google Scholar] [CrossRef]
- Hirata, M.; Gotou, T.; Horiuchi, S.; Fujiwara, M.; Ohba, M. Thin-film particles of graphite oxide 1: High-yield synthesis and flexibility of the particles. Carbon 2004, 42, 2929–2937. [Google Scholar] [CrossRef]
- Kim, H.; Miura, Y.; Macosko, C.W. Graphene/polyurethane nanocomposites for improved gas barrier and electrical conductivity. Chem. Mater. 2010, 22, 3441–3450. [Google Scholar] [CrossRef]
- Tung, T.T.; Nine, M.J.; Krebsz, M.; Pasinszki, T.; Coghlan, C.J.; Tran, D.N.; Losic, D. Recent advances in sensing applications of graphene assemblies and their composites. Adv. Funct. Mater. 2017, 27, 1702891. [Google Scholar] [CrossRef]
- Nag, A.; Mitra, A.; Mukhopadhyay, S.C. Graphene and its sensor-based applications: A review. Sens. Actuators A 2018, 270, 177–194. [Google Scholar] [CrossRef]
- Hernaez, M. Applications of Graphene-Based Materials in Sensors. Sensors 2020, 20, 3196. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Soin, N.; Roy, S.S. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: A review. RSC Adv. 2014, 4, 3823–3851. [Google Scholar] [CrossRef]
- Li, D.; Zhang, W.; Yu, X.; Wang, Z.; Su, Z.; Wei, G. When biomolecules meet graphene: From molecular level interactions to material design and applications. Nanoscale 2016, 8, 19491–19509. [Google Scholar] [CrossRef]
- Zou, X.; Wei, S.; Jasensky, J.; Xiao, M.; Wang, Q.; Brooks III, C.L.; Chen, Z. Molecular interactions between graphene and biological molecules. J. Am. Chem. Soc. 2017, 139, 1928–1936. [Google Scholar] [CrossRef]
- Albaris, H.; Karuppasamy, G. Investigation of NH3 gas sensing behavior of intercalated PPy–GO–WO3 hybrid nanocomposite at room temperature. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2020, 257, 114558. [Google Scholar] [CrossRef]
- Al-Hartomy, O.A.; Khasim, S.; Roy, A.; Pasha, A. Highly conductive polyaniline/graphene nano-platelet composite sensor towards detection of toluene and benzene gases. Appl. Phys. A 2019, 125, 12. [Google Scholar] [CrossRef]
- Tjong, S.C. Polymer composites with graphene nanofillers: Electrical properties and applications. J. Nanosci. Nanotechnol. 2014, 14, 1154–1168. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.K.; Basu, S. Graphene-Oxide Nano Composites for Chemical Sensor Applications. C 2016, 2, 12. [Google Scholar] [CrossRef]
- Toda, K.; Furue, R.; Hayami, S. Recent progress in applications of graphene oxide for gas sensing: A review. Anal. Chim. Acta 2015, 878, 43–53. [Google Scholar] [CrossRef]
- Burkhanov, B.G.S.; Gorina, N.B.; Kolchugina, N.B.; Roshan, N.R.; Slovetsky, D.I.; Chistov, E.M. Palladium-based alloy membranes for separation of high purity hydrogen from hydrogen-containing gas mixtures. Platin. Met. Rev. 2011, 55, 3–12. [Google Scholar] [CrossRef]
- Singh, N.B.; Bhattacharya, B.; Sarkar, U. Nickel decorated single-wall carbon nanotube as CO sensor. J. Nanosci. Lett. 2013, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-T.; Wang, Y.-S. High-performance room temperature NH3 gas sensors based on polyaniline-reduced graphene oxide nanocomposite sensitive membrane. J. Alloys Compd. 2019, 789, 693–696. [Google Scholar] [CrossRef]
- Zhang, K.; Hu, R.; Fan, G.; Li, G. Graphene oxide/chitosan nanocomposite coated quartz crystal microbalance sensor for detection of amine vapors. Sens. Actuators B Chem. 2017, 243, 721–730. [Google Scholar] [CrossRef]
- Grate, J.W.; Abraham, M.H. Solubility interactions and the design of chemically selective sorbent coatings for chemical sensors and arrays. Sens. Actuators B Chem. 1991, 3, 85–111. [Google Scholar] [CrossRef]
- Zheng, Y.; Lee, D.; Koo, H.Y.; Maeng, S. Chemically modified graphene/PEDOT:PSS nanocomposite films for hydrogen gas sensing. Carbon 2015, 81, 54–62. [Google Scholar] [CrossRef]
- Bais, A.; McKenzie, R.; Bernhard, G.; Aucamp, P.; Ilyas, M.; Madronich, S.; Tourpali, K. Ozone depletion and climate change: Impacts on UV radiation. Photochem. Photobiol. Sci. 2015, 14, 19–52. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chalmers, A.N. Review of Wearable and Portable Sensors for Monitoring Personal Solar UV Exposure. Ann. Biomed. Eng. 2021, 49, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Henning, A.; Downs, N.J.; Vanos, J.K. Wearable ultraviolet radiation sensors for research and personal use. Int. J. Biometeorol. 2022, 66, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Sliney, D.H.; Stuck, B.E. A Need to Revise Human Exposure Limits for Ultraviolet UV-C Radiation. Photochem. Photobiol. 2021, 97, 485–492. [Google Scholar] [CrossRef]
- Alamdari, S.; Ghamsari, M.S.; Afarideh, H.; Mohammadi, A.; Geranmayeh, S.; Tafreshi, M.J.; Ehsani, M.H. Preparation and characterization of GO-ZnO nanocomposite for UV detection application. Opt. Mater. 2019, 92, 243–250. [Google Scholar] [CrossRef]
- Wang, H.-C.; Hong, Y.; Chen, Z.; Lao, C.; Lu, Y.; Yang, Z.; Zhu, Y.; Liu, X. ZnO UV photodetectors modified by Ag nanoparticles using all-inkjet-printing. Nanoscale Res. Lett. 2020, 15, 176. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Su, J.; Li, H.; Zhang, Q.; Gao, Y. Ag nanoparticles@ ZnO nanowire composite arrays: An absorption enhanced UV photodetector. Opt. Express 2014, 22, 30148–30155. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, P.; Wang, N.; Ma, Y.; San, H. UV-assisted photochemical synthesis of reduced graphene oxide/ZnO nanowires composite for photoresponse enhancement in UV photodetectors. Nanomaterials 2018, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Zare, M.; Safa, S.; Azimirad, R.; Mokhtari, S. Graphene oxide incorporated ZnO nanostructures as a powerful ultraviolet composite detector. J. Mater. Sci. Mater. Electron. 2017, 28, 6919–6927. [Google Scholar] [CrossRef]
- Shao, D.; Yu, M.; Lian, J.; Sawyer, S. An ultraviolet photodetector fabricated from WO3 nanodiscs/reduced graphene oxide composite material. Nanotechnology 2013, 24, 295701. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, Q.; Yang, F.; He, D. Ultraviolet photoconductance of a single hexagonal WO3 nanowire. Nano Res. 2010, 3, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Santonicola, M.G.; Coscia, M.G.; Botti, S.; Laurenzi, S. Graphene/DNA nanostructured films for bioinspired sensing of UV radiation effects. In Proceedings of the International Astronautical Congress (IAC), Toronto, ON, Canada, 29 September–3 October 2014; pp. 6313–6317. [Google Scholar]
- Santonicola, M.G.; Coscia, M.G.; Sirilli, M.; Laurenzi, S. Nanomaterial-based biosensors for a real-time detection of biological damage by UV light. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milano, Italy, 25–29 August 2015; pp. 4391–4394. [Google Scholar] [CrossRef]
- Toto, E.; Palombi, M.; Laurenzi, S.; Santonicola, M.G. Functional nanocomposites with graphene-DNA hybrid fillers: Synthesis and surface properties under UV irradiation. Ceram. Int. 2019, 45, 9631–9637. [Google Scholar] [CrossRef]
- Patil, A.J.; Vickery, J.L.; Scott, T.B.; Mann, S. Aqueous stabilization and self-assembly of graphene sheets into layered bio-nanocomposites using DNA. Adv. Mater. 2009, 21, 3159–3164. [Google Scholar] [CrossRef]
- Hain, T.C.; Kröker, K.; Stich, D.G.; Hertel, T. Influence of DNA conformation on the dispersion of SWNTs: Single-strand DNA vs. hairpin DNA. Soft Matter 2012, 8, 2820–2823. [Google Scholar] [CrossRef]
- Toto, E.; Laurenzi, S.; Santonicola, M.G. Flexible Nanocomposites Based on Polydimethylsiloxane Matrices with DNA-Modified Graphene Filler: Curing Behavior by Differential Scanning Calorimetry. Polymers 2020, 12, 2301. [Google Scholar] [CrossRef]
- Borysiak, M.D.; Bielawski, K.S.; Sniadecki, N.J.; Jenkel, C.F.; Vogt, B.D.; Posner, J.D. Simple replica micromolding of biocompatible styrenic elastomers. Lab Chip 2013, 13, 2773–2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, B.; Zhao, F.; Ping, J.; Ying, Y. Recent Advances in Nanomaterial-Enabled Wearable Sensors: Material Synthesis, Sensor Design, and Personal Health Monitoring. Small 2020, 16, 2002681. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Zhang, A.; Su, M. Nanomaterials for Biosensing Applications. Nanomaterials 2016, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Abu-Raya, Y.S.; Haick, H. Advanced materials for health monitoring with skin-based wearable devices. Adv. Healthc. Mater. 2017, 6, 1700024. [Google Scholar] [CrossRef] [PubMed]
- Jayathilaka, W.A.D.M.; Qi, K.; Qin, Y.; Chinnappan, A.; Serrano-Garcia, W.; Baskar, C.; Wang, H.; He, J.; Cui, S.; Thomas, S.W. Significance of nanomaterials in wearables: A review on wearable actuators and sensors. Adv. Mater. 2019, 31, 1805921. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, L.; Jiang, K.; Wei, Z.; Shen, G. Reviews of wearable healthcare systems: Materials, devices and system integration. Mater. Sci. Eng. R Rep. 2020, 140, 100523. [Google Scholar] [CrossRef]
- Chang, J.-L.; Chang, K.-H.; Hu, C.-C.; Cheng, W.-L.; Zen, J.-M. Improved voltammetric peak separation and sensitivity of uric acid and ascorbic acid at nanoplatelets of graphitic oxide. Electrochem. Commun. 2010, 12, 596–599. [Google Scholar] [CrossRef]
- Du, H.; Ye, J.; Zhang, J.; Huang, X.; Yu, C. A voltammetric sensor based on graphene-modified electrode for simultaneous determination of catechol and hydroquinone. J. Electroanal. Chem. 2011, 650, 209–213. [Google Scholar] [CrossRef]
- Lim, C.X.; Hoh, H.Y.; Ang, P.K.; Loh, K.P. Direct voltammetric detection of DNA and pH sensing on epitaxial graphene: An insight into the role of oxygenated defects. Anal. Chem. 2010, 82, 7387–7393. [Google Scholar] [CrossRef]
- Zhou, M.; Zhai, Y.; Dong, S. Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal. Chem. 2009, 81, 5603–5613. [Google Scholar] [CrossRef]
- Santonicola, M.G. Label-Free Biosensing Platforms Based on Graphene/DNA Interfaces. In Graphene Bioelectronics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 177–191. [Google Scholar] [CrossRef]
- Botti, S.; Rufoloni, A.; Laurenzi, S.; Gay, S.; Rindzevicius, T.; Schmidt, M.S.; Santonicola, M.G. DNA self-assembly on graphene surface studied by SERS mapping. Carbon 2016, 109, 363–372. [Google Scholar] [CrossRef]
- Bonanni, A.; Ambrosi, A.; Pumera, M. Nucleic acid functionalized graphene for biosensing. Chem. Eur. J. 2012, 18, 1668–1673. [Google Scholar] [CrossRef]
- Silva, M.; Alves, N.M.; Paiva, M.C. Graphene-polymer nanocomposites for biomedical applications. Polym. Adv. Technol. 2018, 29, 687–700. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Wu, H.; Aksay, I.A.; Liu, J.; Lin, Y. Glucose oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009, 25, 901–905. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Kang, X.; Wang, C.; Wang, D.; Liu, J.; Aksay, I.A.; Lin, Y. Glucose biosensor based on immobilization of glucose oxidase in platinum nanoparticles/graphene/chitosan nanocomposite film. Talanta 2009, 80, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Sun, Z.; Sun, X.; Liu, B. Graphene Doped Molecularly Imprinted Electrochemical Sensor for Uric Acid. Anal. Lett. 2012, 45, 2717–2727. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ménard-Moyon, C.; Delogu, L.G.; Bianco, A. Graphene and the immune system: Challenges and potentiality. Adv. Drug Deliv. Rev. 2016, 105, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Kasner, M.L.; Su, S.; Patel, K.; Cuellari, R. Highly Sensitive and Selective Dopamine Biosensor Fabricated with Silanized Graphene. J. Phys. Chem. C 2010, 114, 14915–14921. [Google Scholar] [CrossRef]
- Burrs, S.; Bhargava, M.; Sidhu, R.; Kiernan-Lewis, J.; Gomes, C.; Claussen, J.; McLamore, E. A paper based graphene-nanocauliflower hybrid composite for point of care biosensing. Biosens. Bioelectron. 2016, 85, 479–487. [Google Scholar] [CrossRef] [Green Version]



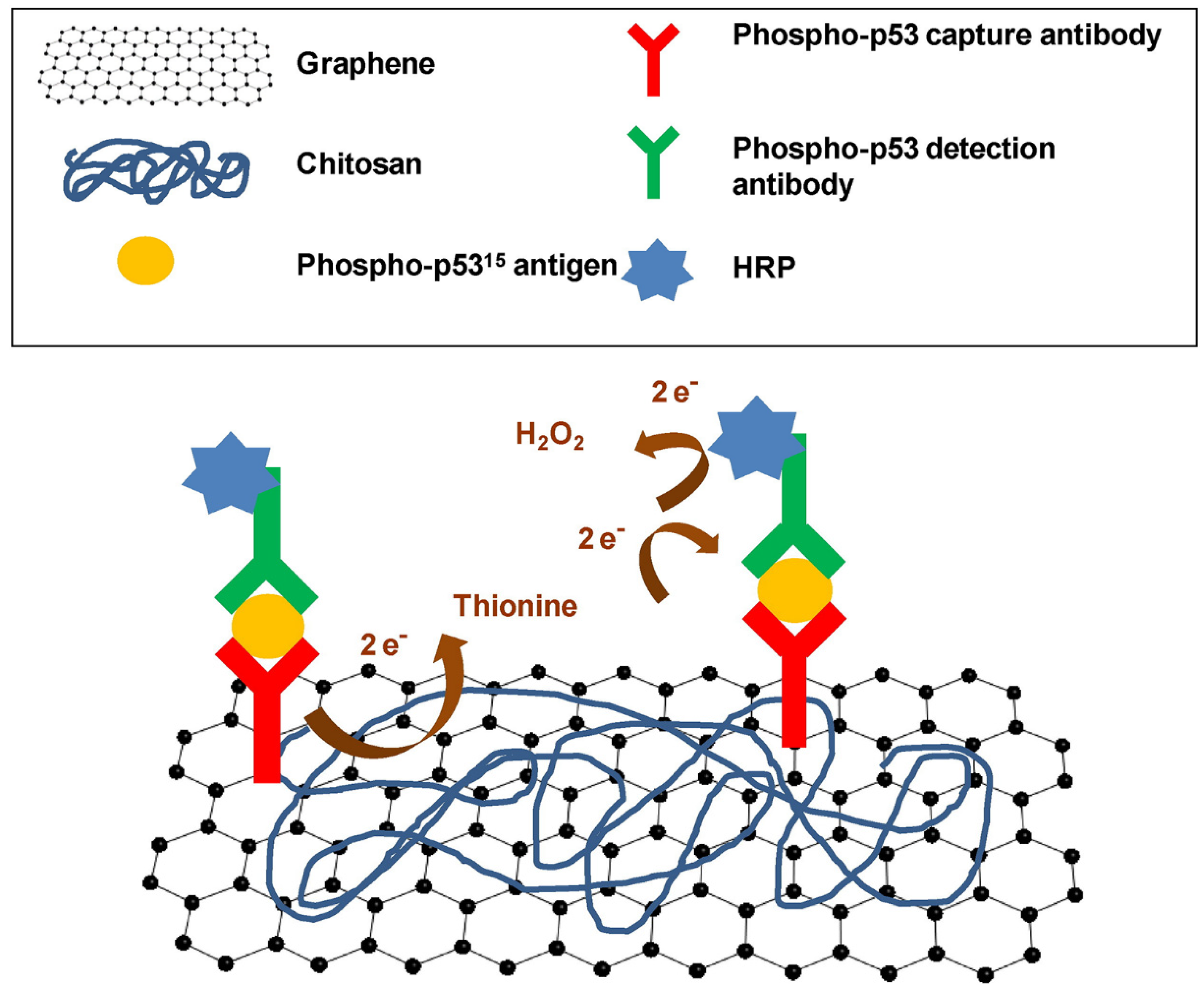
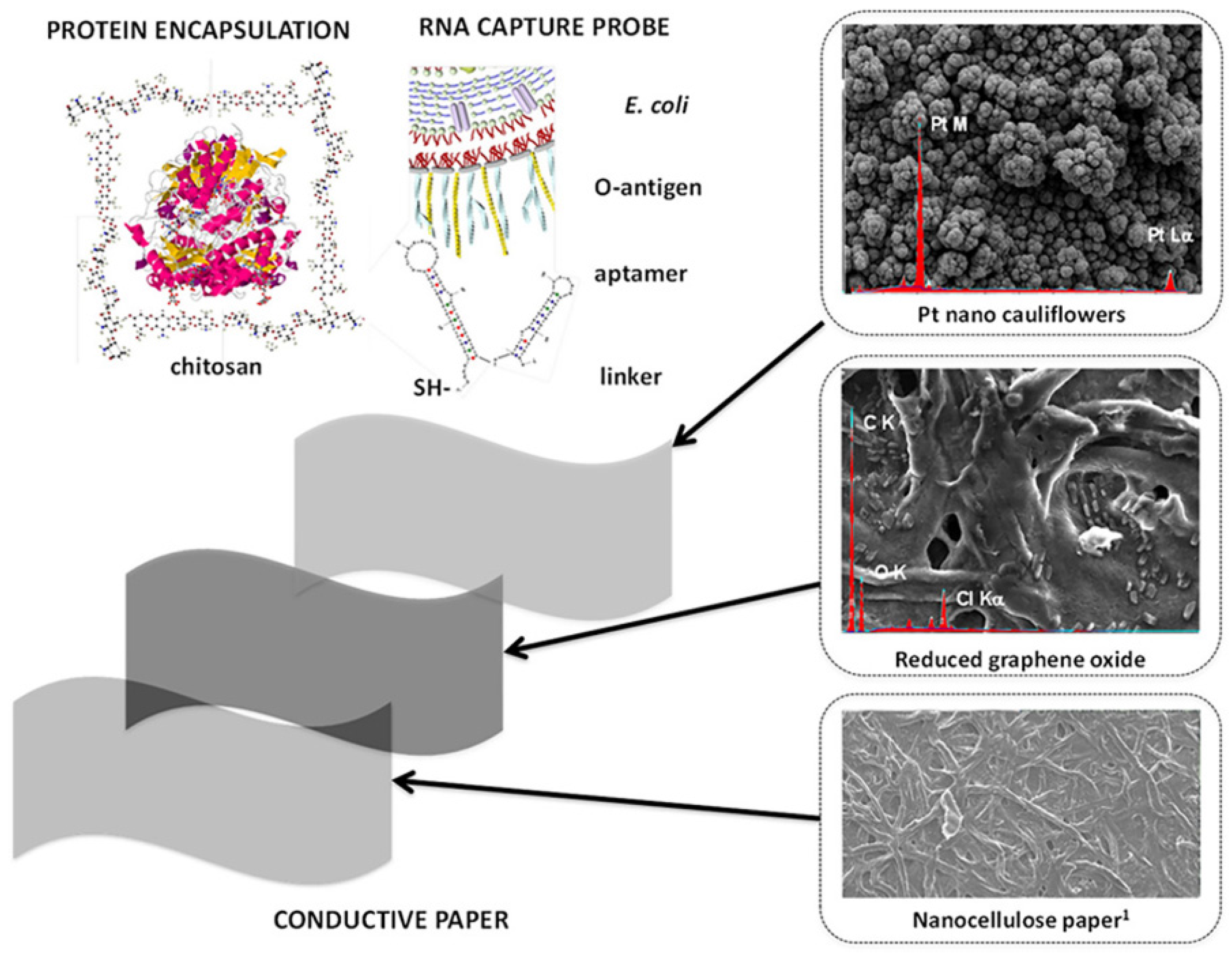
| Technique | Graphene Size | Advantages | Disadvantages | References | |
|---|---|---|---|---|---|
| Thickness | Lateral | ||||
| Confined self-assembly | Single layer | 100 nm | Thickness control | Presence of defects | [62] |
| Arc discharge | Single layer, bilayer, and few layers | Few 100 nm to few µm | Up to 10 g/h of graphene | Low yield of graphene; carbonaceous impurities | [63] |
| Epitaxial growth on SiC | Few layers | Up to cm | Very large area of pure graphene | Very small scale | [64,65,66] |
| CVD | Few layers | Very large (cm) | Large size; high quality | Small production scale | [67] |
| Reduction of carbon monoxide (CO) | Multiple layers | Sub-μm | Unoxidized sheets | Contamination with α-Al2O3 and α-Al2S | [68] |
| Unzipping of carbon nanotubes | Multiple layers | Few μm long nanoribbons | Size controlled by selection of the starting nanotubes | Expensive starting material; oxidized graphene | [69] |
| Technique | Graphene Size | Advantages | Disadvantages | References | |
|---|---|---|---|---|---|
| Thickness | Lateral | ||||
| Electrochemical exfoliation/functionalization of graphite | Single and few layers | 500–700 nm | High electrical conductivity of the functionalized graphene | Cost of ionic liquids | [70,71] |
| Direct sonication of graphite | Single and multiple layers | μm or sub-μm | Unmodified graphene; inexpensive | Low yield; separation | [72,73] |
| Micromechanical exfoliation | Few layers | μm to cm | Large size and unmodified graphene sheets | Very small-scale production | [74] |
| Superacid dissolution of graphite | Mostly single layer | 300–900 nm | Unmodified graphene; scalable | Use of hazardous chlorosulfonic acid; cost of acid removal | [75] |
| Thermal exfoliation/reduction of graphene oxide | Single and few layer | ∼500 nm | 1-step exfoliation/reduction; short heating time; dry basis | High heating temperature; smaller sheet size compared to chemically reduced sheets | [76] |
| Chemical reduction of colloidal graphene oxide in water | Single and multiple layer | μm or sub-μm | Large sheet size; some routes use only water | Some of these methods use hazardous chemicals; only dispersed in hydrophilic polymers | [77] |
| Li alkylation of graphite fluoride | Single layer | μm | Large size; functionalized sheets; no oxygen functionality | Cost of the starting material; restacking after annealing | [78] |
| Polymer Used as Matrix | Type of Graphene Filler | Fabrication Method | Property Enhanced | Reference |
|---|---|---|---|---|
| Poly(vinyl alcohol) (PVA) | Reduced graphene oxide | Solution blending | Mechanical properties (increase in elastic modulus and tensile strength) | [109] |
| Poly(methyl methacrylate) (PMMA) | Reduced graphene oxide | Solution blending | Electrical conductivity | [110] |
| Poly(butylene succinate) (PBS) | Graphene oxide | Solution blending | Mechanical properties (increase in elastic modulus and tensile strength) | [111] |
| Chitosan | Cryomilled graphene | Solution blending | Mechanical properties (increase in tensile strength) | [112] |
| Isobutylene isoprene rubber (IIR) | Reduced graphene oxide | Solution blending | Dielectrical permittivity | [113] |
| Unsaturated polyester resin (UPR) | Graphene nanosheets | Solution blending | Mechanical properties (increase in tensile strength and flexural strength); thermal properties; dielectric strength | [114] |
| Polystyrene (PS) | Graphene nanosheets | In situ polymerization | Electrical conductivity; thermal properties (increase in glass transition temperature and thermal stability) | [115] |
| Polyaniline (PANI) | Graphene oxide | In situ polymerization | Electrical conductivity | [116] |
| High Density Polyethylene (HDPE) | Exfoliated graphene | Melt mixing | Electrical conductivity | [117] |
| Polycarbonate (PC) | Functionalized graphene sheets | Melt mixing | Electrical conductivity | [118] |
| Polypropylene (PP) | Exfoliated graphene | Melt mixing | Mechanical properties (increase in flexural strength) | [119] |
| Poly(vinyl chloride) (PVC) | Graphene nanoplatelets | Melt mixing | Mechanical properties (increase in elastic modulus and tensile strength) | [120] |
| Polyethylene terephthalate (PET) | Graphene nanosheets | Melt mixing | Electrical conductivity | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toto, E.; Laurenzi, S.; Santonicola, M.G. Recent Trends in Graphene/Polymer Nanocomposites for Sensing Devices: Synthesis and Applications in Environmental and Human Health Monitoring. Polymers 2022, 14, 1030. https://doi.org/10.3390/polym14051030
Toto E, Laurenzi S, Santonicola MG. Recent Trends in Graphene/Polymer Nanocomposites for Sensing Devices: Synthesis and Applications in Environmental and Human Health Monitoring. Polymers. 2022; 14(5):1030. https://doi.org/10.3390/polym14051030
Chicago/Turabian StyleToto, Elisa, Susanna Laurenzi, and Maria Gabriella Santonicola. 2022. "Recent Trends in Graphene/Polymer Nanocomposites for Sensing Devices: Synthesis and Applications in Environmental and Human Health Monitoring" Polymers 14, no. 5: 1030. https://doi.org/10.3390/polym14051030
APA StyleToto, E., Laurenzi, S., & Santonicola, M. G. (2022). Recent Trends in Graphene/Polymer Nanocomposites for Sensing Devices: Synthesis and Applications in Environmental and Human Health Monitoring. Polymers, 14(5), 1030. https://doi.org/10.3390/polym14051030