Thermal Properties and Dynamic Characteristics of Electrospun Polylactide/Natural Rubber Fibers during Disintegration in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Analysis of Crystallization
2.3. Electronic Paramagnetic Resonance
2.4. Morphology of the Sample
2.5. FTIR-ATR Spectroscopy
2.6. Thermogravimetric Analysis
2.7. Soil Test
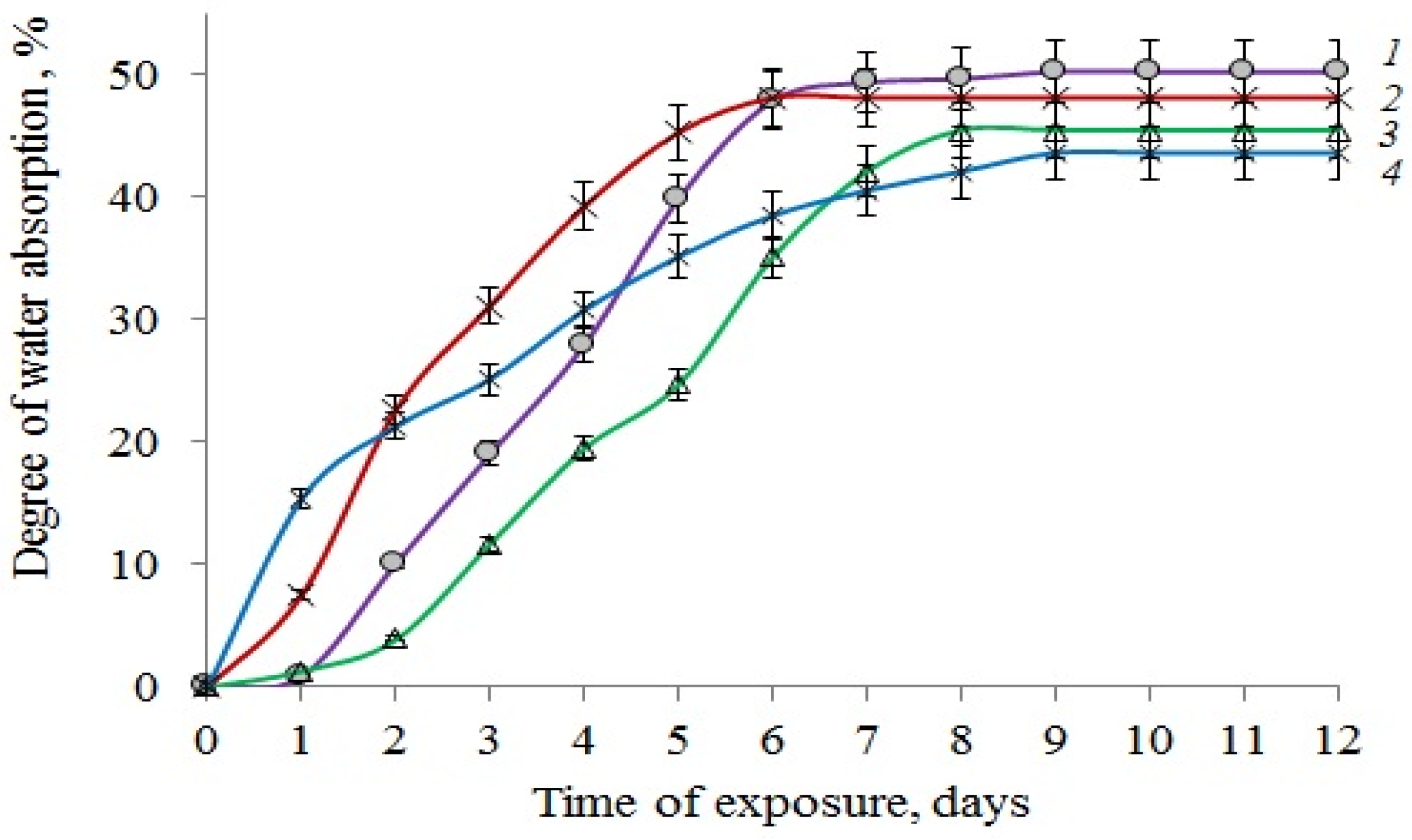
2.8. Water Uptake
2.9. Statistical Processing
3. Results
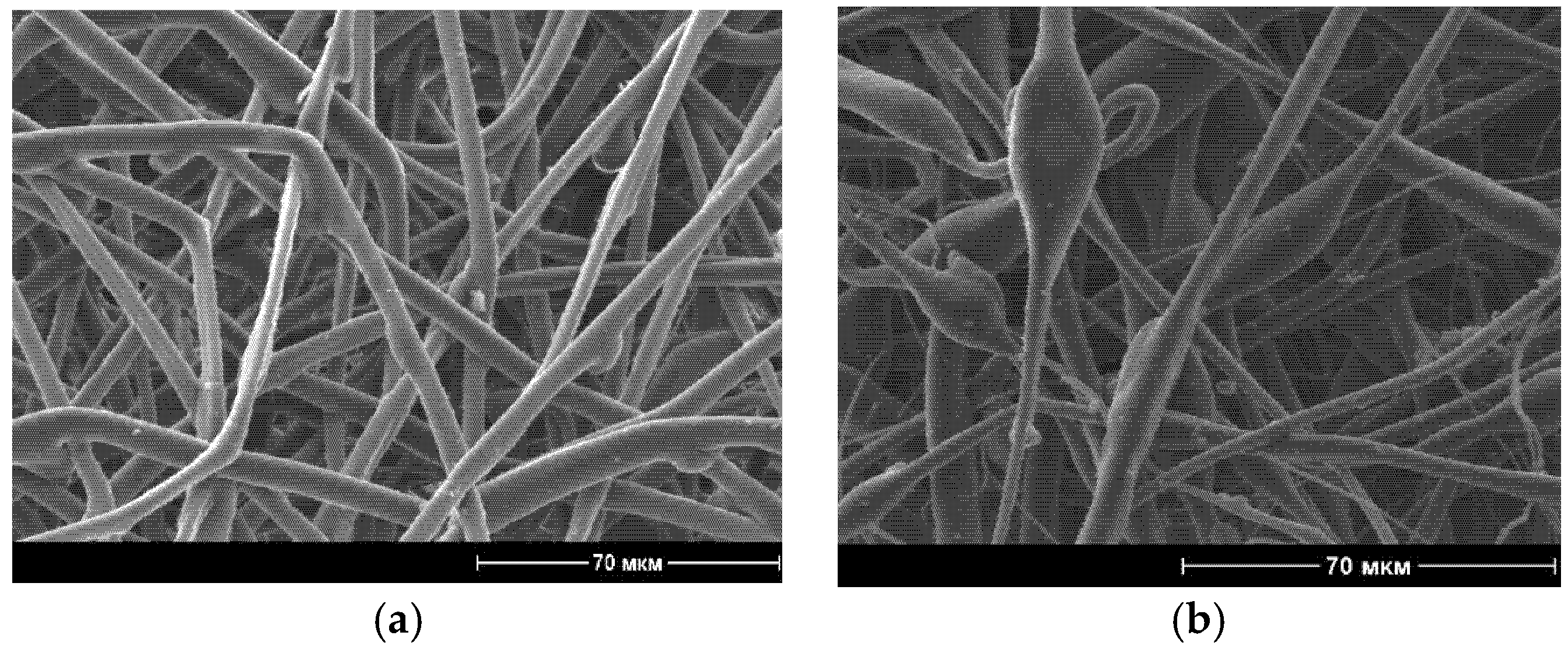
3.1. Morphology
3.2. Crystallization Analysis
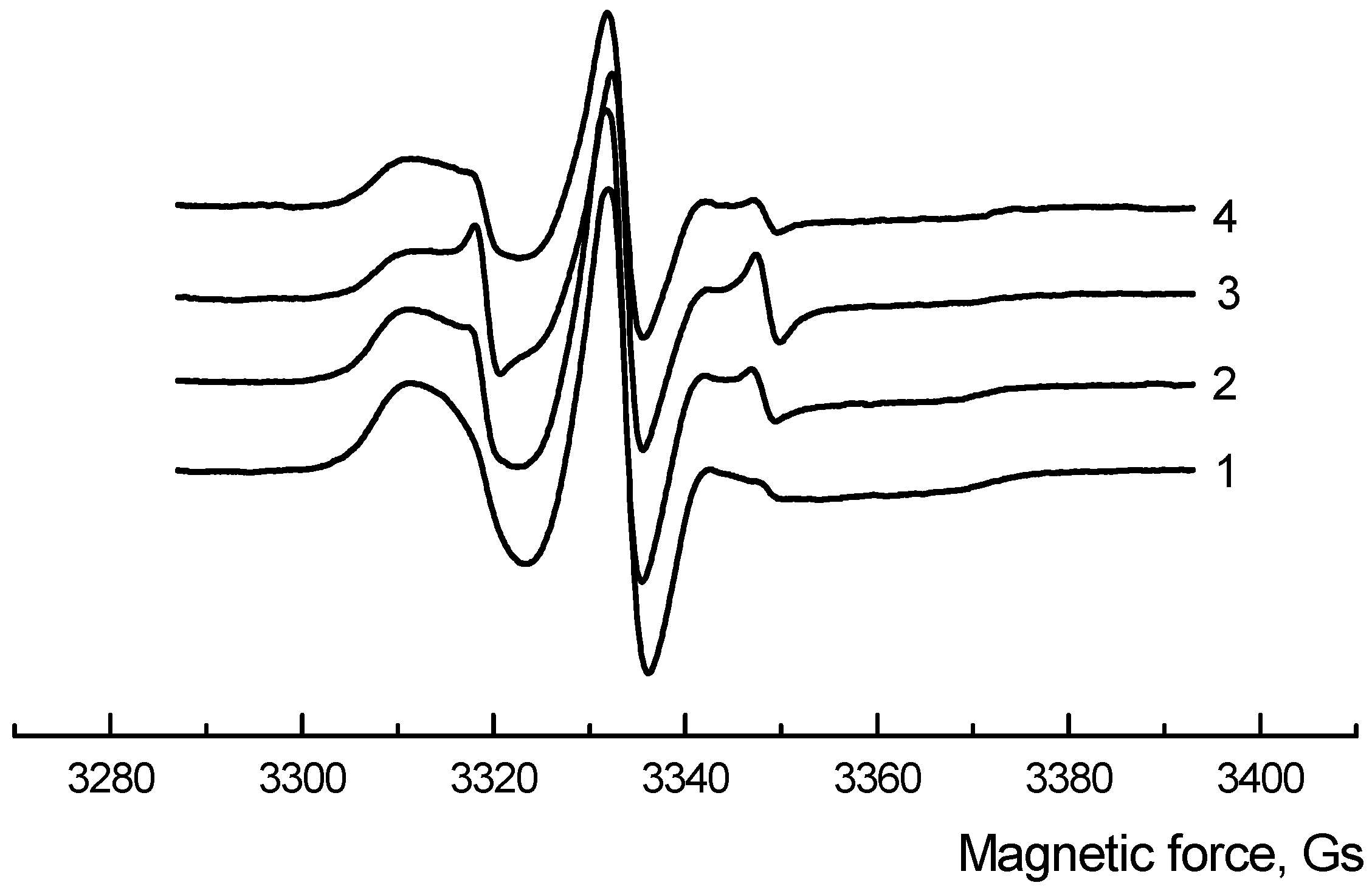
3.3. Macromolecules’ Dynamic
3.4. Thermal Degradation
3.5. FTIR Spectroscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, B.; Chen, C.; Sun, Y.; Yu, M.; Liu, B.; Wangab, X.; Zhou, J. Lignin bio-oil-based electrospun nanofibers with high substitution ratio property for potential carbon nanofibers applications. Polym. Test. 2020, 89, 106591. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Lobanov, A.V.; Karpova, S.G.; Pantyukhov, P.V. Composites based on polylactide and manganese (III) tetraphenylporphyrin. Influence of concentration on the structure and properties. J. Mol. Liq. 2020, 302, 112176. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, H.Y.; Khill, M.S.; Ra, Y.M.; Lee, D.R. Characterization of nano-structured poly(caprolactone) nonwoven mats. Polym. J. 2003, 44, 1287–1294. [Google Scholar] [CrossRef]
- Al-Enizi, A.; Zagho, M.; Elzatahry, A. Polymer-Based Electrospun Nanofibers for Biomedical Applications. Nanomaterials 2018, 8, 259. [Google Scholar] [CrossRef] [Green Version]
- Karpova, S.G.; Ol’khov, A.A.; Popov, A.A.; Zhul’kina, A.L.; Iordanskii, A.L. Analysis of the structure of ultrafine fibers based on poly(3-hydroxybutyrate) and polylactide: Effect of external factors. Polym. Sci. Ser. A 2019, 61, 480–490. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Shibryaeva, L.S. Biodegradable Fiber Composite Based on Polylactide and Its Application for the Plants Growing. RU Patent 2734883 C1, 23 October 2020. [Google Scholar]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, B.; Yang, G.; Gauther, M. Poly(lactic acid)-based biomaterials: Synthesis, modification and applications. Biomed. Sci. Eng. Technol. 2012, 11, 247–282. [Google Scholar]
- Auras, R.; Lim, L.-T.; Selke, S.; Tsuji, H. Poly(Lactic Acid). Synthesis, Structures, Properties, Processing, and Applications: Hydrolytic Degradation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 345–381. [Google Scholar]
- Tertyshnaya, Y.; Jobelius, H.; Olkhov, A.; Shibryaeva, L.; Ivanitskikh, A. Polylactide Fiber Materials and Their Application in Agriculture. Key Eng. Mater. 2022, 910, 623–629. [Google Scholar] [CrossRef]
- Kurtycz, P.; Karwowska, E.; Ciach, T.; Olszyna, A.; Kunicki, A. Biodegradable polylactide (PLA) fiber mats containing Al2O3-Ag nanopowder prepared by electrospinning technique—Antibacterial properties. Fibers Polym. 2013, 14, 1248–1254. [Google Scholar] [CrossRef]
- Holm, V.K.; Ndoni, S.; Risbo, J. The stability of poly(lactic acid) packaging films as influenced by humidity and temperature. J. Food Sci. 2006, 71, 40–44. [Google Scholar] [CrossRef]
- Pongtanayut, K.; Thongpin, C.; Santawitee, O. The effect of rubber on morphology, thermal properties and mechanical properties of PLA/NR and PLA/ENR blends. Energy Proced. 2013, 34, 888–897. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, M.; Piorkowska, E. Mechanisms of plastic deformation in biodegradable polylactide-poly(1,4-cis-isoprene) blends. J. Appl. Polym. Sci. 2012, 124, 4579–4589. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.; Karpova, S.; Moskovskiy, M.; Dorokhov, A. Electrospun Polylactide/Natural Rubber Fibers: Effect Natural Rubber Content on Fiber Morphology and Properties. Polymers 2021, 13, 2232. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, A.; Gieparda, W.; Foksowicz-Flaczyk, J.; Walentowska, J.; Wesolek, D.; Vazquez, B.; Prodi, F.; Belosi, F. Air filtration and antimicrobial capabilities of electrospun PLA/PHB containing ionic liquid. Separ. Purif. Technol. 2015, 154, 154–160. [Google Scholar] [CrossRef]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef]
- Kaur, S.; Sundarrajan, S.; Rana, D.; Matsuura, T.; Ramakrishna, S. Influence of electrospunfiber size on the separation efficiency of thin film nanofiltration composite membrane. J. Membr. Sci. 2012, 392–393, 101–111. [Google Scholar] [CrossRef]
- Casasola, R.; Thomas, N.L.; Trybala, A.; Georgiadou, S. Electrospun poly lactic acid (PLA) fibres: Effect of different solvent systems on fibre morphology and diameter. Polymer 2014, 55, 4728–4737. [Google Scholar] [CrossRef] [Green Version]
- Woo, K.M.; Chen, V.J.; Ma, P.X. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J. Biomed. Mater. 2003, 67, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Patra, S.N.; Easteal, A.J.; Bhattacharyya, D. Parametric study of manufacturing poly(lactic) acid nanofibrous mat by electrospinning. J. Mater. Sci. 2008, 44, 647–654. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Shibryaeva, L.S.; Levina, N.S. Biodestruction of Polylactide and Poly(3-Hydroxybutyrate) Non-Woven Materials by Micromycetes. Fibre Chem. 2020, 52, 43–47. [Google Scholar] [CrossRef]
- Graupner, N.; Herrmann, A.S.; Mussig, J. Natural and man-made cellulose fibre-reinforced poly(lactic acid) (PLA) composites: An overview about mechanical characteristics and application areas. Compos. Part A 2009, 40, 810–821. [Google Scholar] [CrossRef]
- Abdullah, M.F.; Andriyan, A.; Muhamad, F.; Ang, B.C. Effect of core-to-shell flowrate ratio on morphology, crystallinity, mechanical properties and wettability of poly(lactic acid) fibers prepared via modified coaxial electrospinning. Polymer 2021, 237, 124378. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Tan, H. Thermal and thermo-oxidative degradation kinetics and characteristics of poly (lactic acid) and its composites. Waste Manag. 2019, 87, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I. End-life prediction of commercial PLA used for food packaging through short term TGA experiments: Real chance or low reliability? Chin. J. Polym. Sci. 2014, 32, 681–689. [Google Scholar] [CrossRef]
- Carrasco, F.; Pérez-Maqueda, L.A.; Santana, O.O.; Maspoch, M.L. Enhanced general analytical equation for the kinetics of the thermal degradation of poly(lactic acid)/montmorillonite nanocomposites driven by random scission. Polym. Degrad. Stab. 2014, 101, 52–59. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Karpova, S.G.; Tertyshnaya, Y.V.; Podzorova, M.V.; Popov, A.A. Effect of Exposure in Aqueous Medium at Elevated Temperature on the Structure of Nonwoven Materials Based on Polylactide and Natural Rubber. Polym. Sci. Ser. A 2021, 62, 515–525. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.; Podzorova, M.; Moskovskiy, M. Impact of water and UV irradiation on nonwoven polylactide/natural rubber fiber. Polymers 2021, 13, 461. [Google Scholar] [CrossRef]
- Lipsa, R.; Tudorachi, N.; Darie-Nita, R.N.; Oprica, L.; Vasile, C.; Chiriac, C. Biodegradation of poly(lactic acid) and some of its based systems with Trichoderma viride. Int. J. Biol. Macromol. 2016, 88, 515–526. [Google Scholar] [CrossRef]
- Xu, C.; Yuan, D.; Fu, L.; Chen, Y. Physical blend of PLA/NR with co-continuous phase structure: Preparation, rheology property, mechanical properties and morphology. Polym. Test. 2014, 37, 94–101. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Hao, J.; Qiao, Y.; Tian, Y. Thermal degradation of typical plastics under high heating rate conditions by TG-FTIR: Pyrolysis behaviors and kinetic analysis. Energy Convers. Manag. 2018, 171, 1106–1115. [Google Scholar] [CrossRef]
- Molinaro, S.; Romero, M.C.; Boaro, M.; Sensidoni, A.; Lagazio, C.; Morris, M.; Kerry, J. Effect of nanoclay-type and PLA optical purity on the characteristics of PLA-based nanocomposite films. J. Food Eng. 2013, 117, 113–123. [Google Scholar] [CrossRef]
- Copinet, A.; Bertrand, C.; Govindin, S.; Coma, V.; Couturier, Y. Effects of ultraviolet light (315 nm), temperature and relative humidity on the degradation of polylactic acid plastic films. Chemospheres 2004, 55, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Maquelin, K.; Kirschner, C.; Choo-Smith, L.-P.; van den Braak, N.; Endtz, H.P.; Naumann, D.; Puppels, G.J. Identification of medically relevant microorganisms by vibrational spectroscopy. J. Microbiol. Methods 2002, 51, 255. [Google Scholar] [CrossRef]
- Schmitt, J.; Flemming, H.-C. FTIR-spectroscopy in microbial and material analysis. Int. Biodeter. Biodegrad. 1998, 41, 1–11. [Google Scholar] [CrossRef]
- Arrieta, M.R.; Lopez, J.; Rayón, E.; Jiménez, A. Disintegrability under composting conditions of plasticized PLA-PHB blends. Polym. Degrad. Stab. 2014, 108, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Lopez, M.E.; Campo, A.S.; Robledo-Ortíz, J.R.; Arellano, M.; Perez-Fonseca, A.A. Accelerated weathering of poly(lactic acid) and its biocomposites: A review. Polym. Degrad Stab. 2020, 179, 109290. [Google Scholar] [CrossRef]
- Schlegel, I.; Muñoz-Espí, R.; Renz, P.; Lieberwirth, I.; Floudas, G.; Suzuki, Y.; Crespy, D.; Landfester, K. Crystallinity Tunes Permeability of Polymer Nanocapsules. Macromolecules 2017, 50, 4725–4732. [Google Scholar] [CrossRef]
- Rychter, P.; Lewicka, K.; Pastusiak, M.; Domański, M.; Dobrzyński, P. PLGA–PEG terpolymers as a carriers of bioactive agents, influence of PEG blocks content on degradation and release of herbicides into soil. Polym. Degrad. Stabil. 2019, 61, 95–107. [Google Scholar] [CrossRef]
- Lewicka, K.; Dobrzyński, P.; Rychter, P. PLAGA-PEG-PLAGA Terpolymer-Based Carriers of Herbicides for Potential Application in Environment-Friendly, Controlled Release Systems of Agrochemicals. Materials 2020, 13, 2778. [Google Scholar] [CrossRef]
- Deroine, M.; Le Duigou, A.; Corre, Y.-M.; Le Gac, P.-Y.; Davies, P.; Cesar, G.; Bruzaud, S. Accelerated aging of polylactide in aqueous environment: Comparative study between distilled water and seawater. Polym. Degrad. Stab. 2014, 108, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Olewnik-Kruszkowska, E. Influence of the type of buffer solution on thermal and structural properties of polylactide-based composites. Polym. Degrad. Stab. 2016, 129, 87–95. [Google Scholar] [CrossRef]
- Fukusima, K.; Tabuani, D.; Dottori, M.; Armentano, I.; Kenny, J.-M.; Camino, G. Effect of temperature and nanoparticle type on hydrolytic degradation poly(lactic acid) nanocomposites. Polym. Degrad. Stab. 2011, 96, 2120–2129. [Google Scholar] [CrossRef]
- Ohkita, T.; Lee, S.H. Thermal degradation and biodegradability of poly(lactic acid)/corn starch biocomposites. J. Appl. Polym. Sci. 2006, 100, 3009–3017. [Google Scholar] [CrossRef]
- Pranamuda, H.; Tokiwa, Y.; Tanaka, H. Polylactide degradation by an Amycolatopsis sp. Appl. Environ. Microbiol. 1997, 63, 1637–1640. [Google Scholar] [CrossRef] [Green Version]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of polyesters. J. Polym. Environ. 2007, 15, 259–267. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of poly(lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef]
- Bode, H.B.; Kerkho, K.; Jendrossek, D. Bacterial Degradation of Natural and Synthetic Rubber. Biomacromolecules 2001, 2, 295–303. [Google Scholar] [CrossRef]
- Steinbüchel, L.A. Biodegradation of Natural and Synthetic Rubbers; Wiley-VCH Verlag GmbH & Co. KGaA.: Münster, Germany, 2005. [Google Scholar]
- Rose, K.; Tenberge, K.B.; Steinbüchel, A. Identification and characterization of genes from Streptomyces sp. strain K30 responsible for clear zone formation on natural rubber latex and poly(cis-1,4-isoprene) rubber degradation. Biomacromolecules 2005, 6, 180–188. [Google Scholar] [CrossRef]



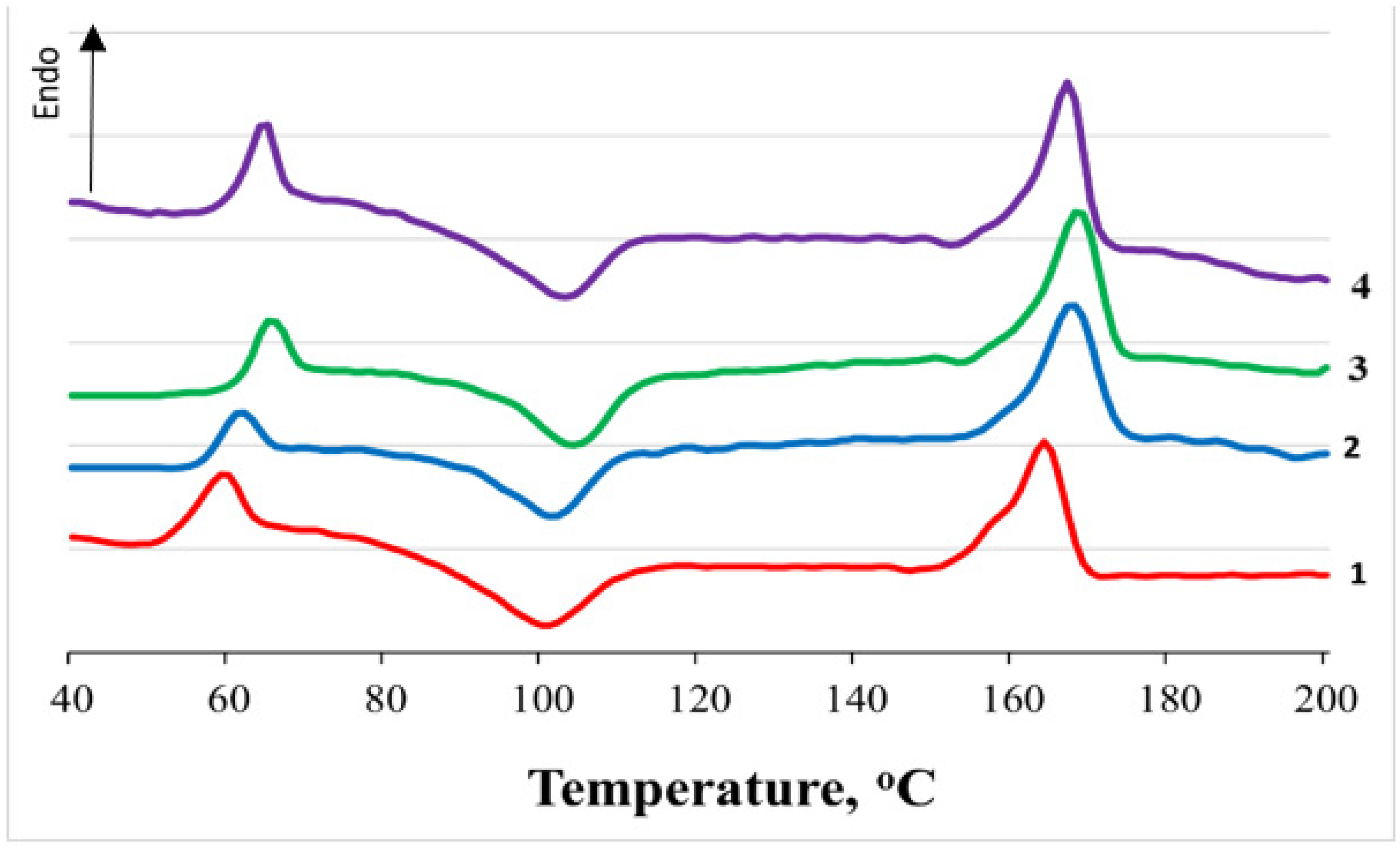




| NR, wt.% | Tg, °C (Δ ± 0.5 °C) | Tm, °C (Δ ± 0.5 °C) | Tcc, °C (Δ ± 0.5 °C) | χcr, % (Δ ± 1%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 d | 60 d | 220 d | 0 d | 60 d | 220 d | 0 d | 60 d | 220 d | 0 d | 60 d | 220 d | |
| 0 | 61 | 62 | 62 | 164 | 166 | 166 | 101 | 104 | 106 | 33 | 38 | 37 |
| 5 | 63 | 63 | 64 | 168 | 167 | 166 | 102 | 104 | 103 | 36 | 35 | 35 |
| 10 | 66 | 61 | 62 | 167 | 167 | 165 | 105 | 102 | 100 | 37 | 36 | 35 |
| 15 | 65 | 62 | 62 | 166 | 165 | 164 | 104 | 100 | 99 | 36 | 34 | 33 |
| NR, wt.% | The Correlation Time | ||
|---|---|---|---|
| τc × 10−10 c−1, initial | τc × 10−10 c−1, 60 days | τc × 10−10 c−1, 220 days | |
| 0 | 72.1 ± 0.19 | 55.2 ± 0.14 | 39.0 ± 0.23 |
| 5 | 28.2 ± 0.15 | 30.7 ± 0.20 | 38.7 ± 0.18 |
| 10 | 19.6 ± 0.12 | 27.8 ± 0.18 | 31.8 ± 0.12 |
| 15 | 17.4 ± 0.18 | 25.0 ± 0.15 | 31.0 ± 0.13 |
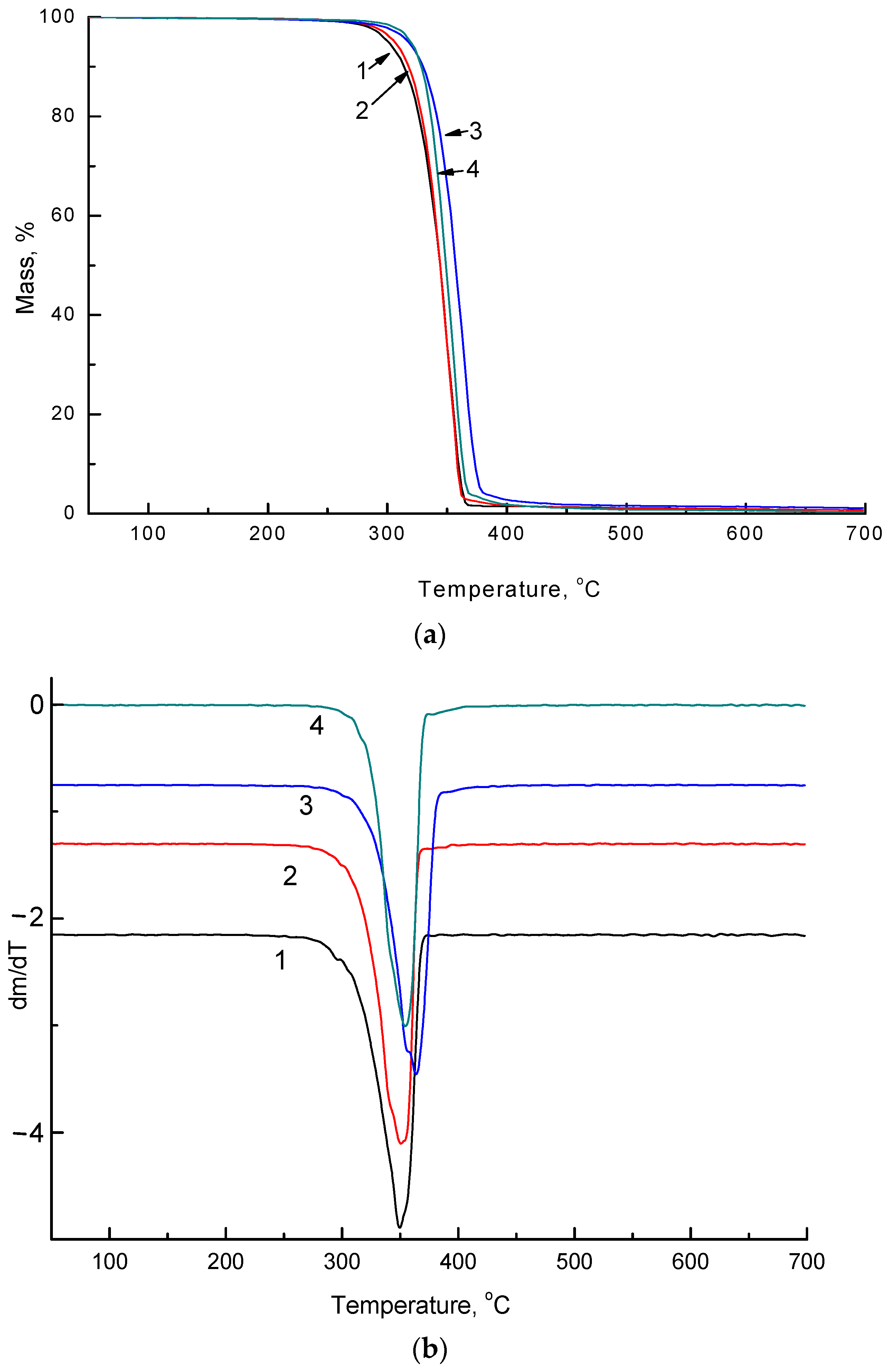
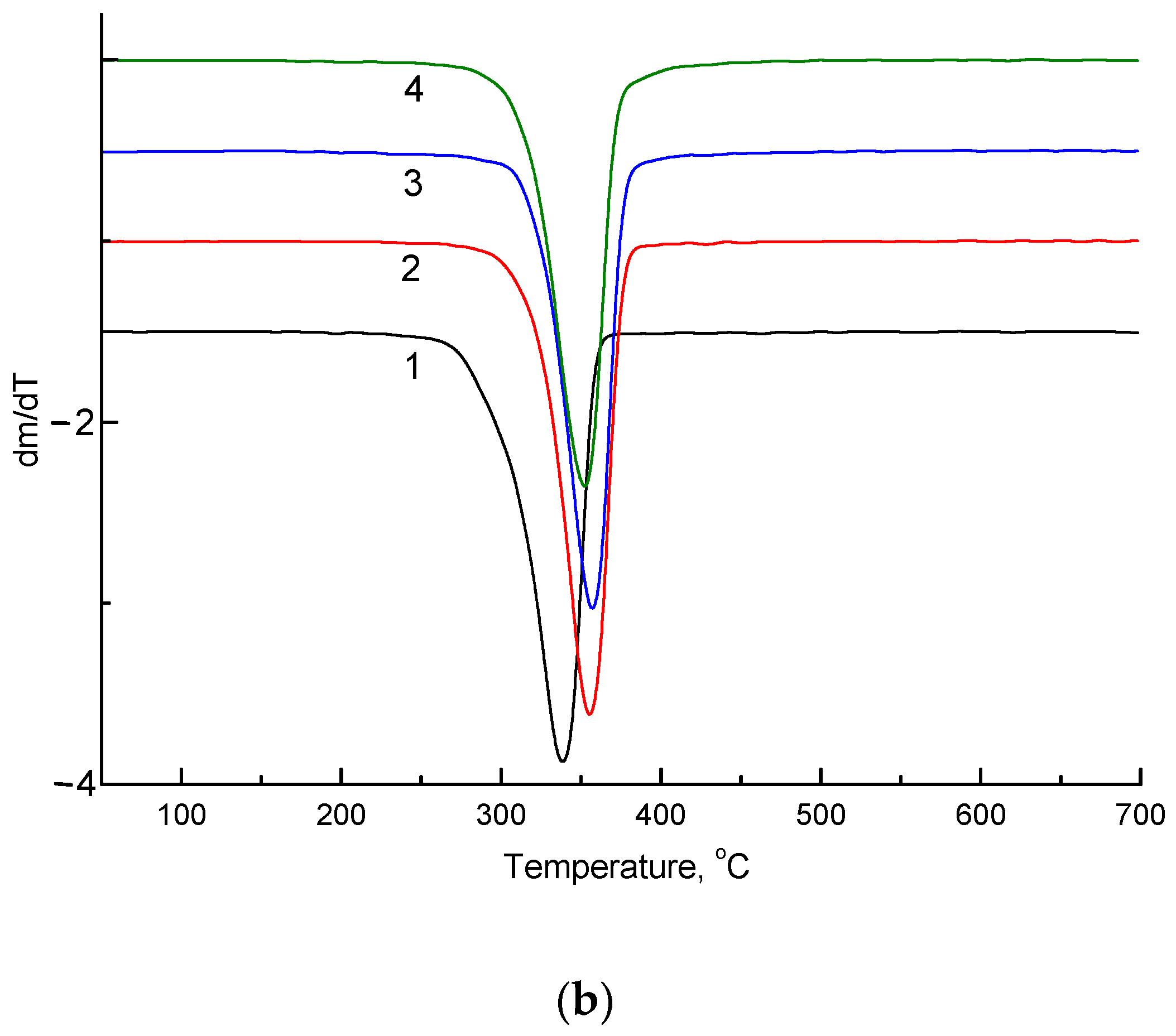
| NR, wt.% | Onset Temperature, °C (Δ ± 0.5 °C) | Tmax, °C (Δ ± 0.5 °C) | ||
|---|---|---|---|---|
| initial | 220 days | initial | 220 days | |
| 0 | 326.3 | 310.9 | 350.1 | 338.9 |
| 5 | 330.0 | 319.1 | 350.7 | 355.7 |
| 10 | 339.0 | 325.7 | 361.5 | 357.0 |
| 15 | 332.4 | 307.3 | 357.3 | 353.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tertyshnaya, Y.V.; Karpova, S.G.; Podzorova, M.V.; Khvatov, A.V.; Moskovskiy, M.N. Thermal Properties and Dynamic Characteristics of Electrospun Polylactide/Natural Rubber Fibers during Disintegration in Soil. Polymers 2022, 14, 1058. https://doi.org/10.3390/polym14051058
Tertyshnaya YV, Karpova SG, Podzorova MV, Khvatov AV, Moskovskiy MN. Thermal Properties and Dynamic Characteristics of Electrospun Polylactide/Natural Rubber Fibers during Disintegration in Soil. Polymers. 2022; 14(5):1058. https://doi.org/10.3390/polym14051058
Chicago/Turabian StyleTertyshnaya, Yulia V., Svetlana G. Karpova, Maria V. Podzorova, Anatoliy V. Khvatov, and Maksim N. Moskovskiy. 2022. "Thermal Properties and Dynamic Characteristics of Electrospun Polylactide/Natural Rubber Fibers during Disintegration in Soil" Polymers 14, no. 5: 1058. https://doi.org/10.3390/polym14051058







