A Review on Xerostomia and Its Various Management Strategies: The Role of Advanced Polymeric Materials in the Treatment Approaches
Abstract
1. Introduction
2. Approach of the Review
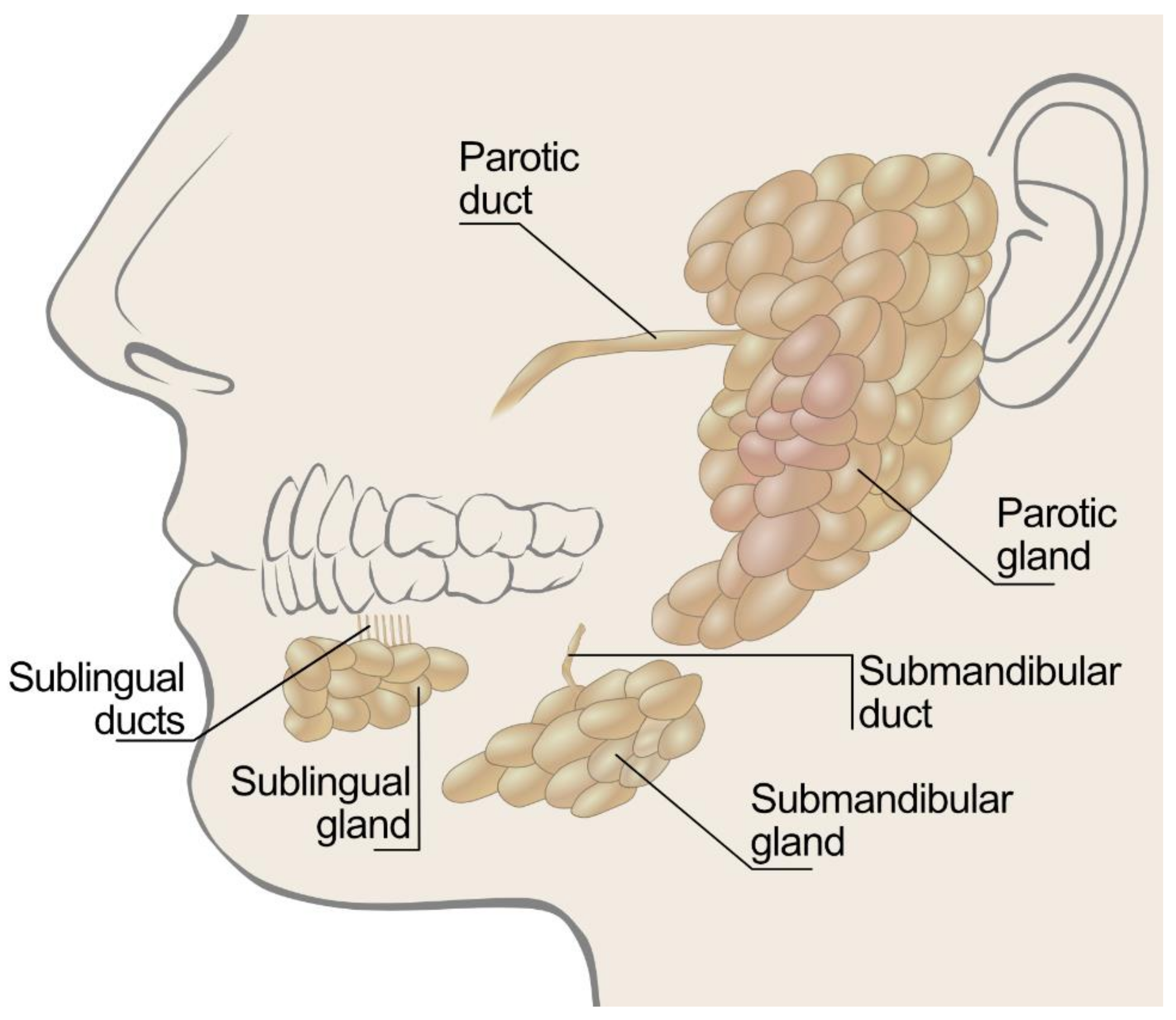
3. Saliva Production in Humans
4. Diagnosis of Xerostomia
5. Causes of Xerostomia
5.1. Local Factors
5.2. Systematic Diseases
6. Effects of Xerostomia
7. Management of Xerostomia
7.1. Preventive Approaches
7.2. Symptomatic Relief: Salivary Substitutes
7.3. Salivary Stimulation
8. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CMC | Carboxymethyl cellulose |
| DMFT | Decayed missing filled teeth |
| HEC | Hydroxyethyl cellulose |
| HM-EHEC | Hydrophobically modified ethyl hydroxyethyl cellulose |
| HPMC | Hydroxypropylmethyl cellulose |
| LM-pectin | Low-methoxylated pectin |
| MC | Methyl cellulose |
| MNA | Mercaptonicotinic acid |
| OHRQOL | Oral health-related quality of life |
| PEG | Poly(ethylene glycol) |
| PEO | Poly(ethylene) oxide |
| PLGA | Poly(lactic-co-glycolic acid) |
| PPG | Poly(propylene glycol) |
| SCMC | Sodium carboxymethyl cellulose |
| SWS | Stimulated whole saliva |
| UWS | Unstimulated whole saliva |
References
- Cassolato, S.F.; Turnbull, R.S. Xerostomia: Clinical aspects and treatment. Gerodontology 2003, 20, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A.; Pillemer, S.R.; Baum, B.J. Xerostomia and the geriatric patient. J. Am. Geriatr. Soc. 2002, 50, 535–543. [Google Scholar] [CrossRef]
- Dawes, C. How much saliva is enough for avoidance of xerostomia? Caries Res. 2004, 38, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Hopcraft, M.S.; Tan, C. Xerostomia: An update for clinicians. Aust. Dent. J. 2010, 55, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Van der Putten, G.J.; Brand, H.S.; Schols, J.M.; de Baat, C. The diagnostic suitability of a xerostomia questionnaire and the association between xerostomia, hyposalivation and medication use in a group of nursing home residents. Clin. Oral Investig. 2011, 15, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.C.; van der Ven, P.F.; Sonies, B.C.; Weiffenbach, J.M.; Baum, B.J. Xerostomia: Evaluation of a symptom with increasing significance. J. Am. Dent. Assoc. 1985, 110, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C. Physiological factors affecting salivary flow rate, oral sugar clearance, and the sensation of dry mouth in man. J. Dent. Res. 1987, 66, 648–653. [Google Scholar] [CrossRef]
- Guggenheimer, J.; Moore, P.A. Xerostomia: Etiology, recognition and treatment. J. Am. Dent. Assoc. 2003, 134, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Tanasiewicz, M.; Hildebrandt, T.; Obersztyn, I. Xerostomia of Various Etiologies: A Review of the Literature. Adv. Clin. Exp. Med. 2016, 25, 199–206. [Google Scholar] [CrossRef]
- Hanning, S.M.; Yu, T.; Jones, D.S.; Andrews, G.P.; Kieser, J.A.; Medlicott, N.J. Lecithin-based emulsions for potential use as saliva substitutes in patients with xerostomia—Viscoelastic properties. Int. J. Pharm. 2013, 456, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Neyraud, E.; Palicki, O.; Schwartz, C.; Nicklaus, S.; Feron, G. Variability of human saliva composition: Possible relationships with fat perception and liking. Arch. Oral Biol. 2012, 57, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Amerongen, A.V.; Veerman, E.C. Saliva—The defender of the oral cavity. Oral Dis. 2002, 8, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C.; Wood, C.M. The contribution of oral minor mucous gland secretions to the volume of whole saliva in man. Arch. Oral Biol. 1973, 18, 337–342. [Google Scholar] [CrossRef]
- Proctor, G.B.; Carpenter, G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. Basic Clin. 2007, 133, 3–18. [Google Scholar] [CrossRef]
- Rao, R.S.; Akula, R.; Satyanarayana, T.S.V.; Indugu, V. Recent Advances of Pacemakers in Treatment of Xerostomia: A Systematic Review. J. Int. Soc. Prev. Community Dent. 2019, 9, 311–315. [Google Scholar] [PubMed]
- Culp, D.J.; Graham, L.A.; Latchney, L.R.; Hand, A.R. Rat sublingual gland as a model to study glandular mucous cell secretion. Am. J. Physiol. 1991, 260, C1233–C1244. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Felix, D.H. Oral medicine—Update for the dental practitioner: Dry mouth and disorders of salivation. Br. Dent. J. 2005, 199, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C. Salivary flow patterns and the health of hard and soft oral tissues. J. Am. Dent. Assoc. 2008, 139, 18s–24s. [Google Scholar] [CrossRef] [PubMed]
- Sreebny, L.M.; Valdini, A. Xerostomia: A Neglected Symptom. Arch. Intern. Med. 1987, 147, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.M.; Bardow, A.; Jensen, S.B.; Nauntofte, B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. 2002, 8, 117–129. [Google Scholar] [CrossRef]
- Sreebny, L.M. Saliva in health and disease: An appraisal and update. Int. Dent. J. 2000, 50, 140–161. [Google Scholar] [CrossRef] [PubMed]
- Thomson, W.M.; Chalmers, J.M.; Spencer, A.J.; Williams, S.M. The Xerostomia Inventory: A multi-item approach to measuring dry mouth. Community Dent. Health 1999, 16, 12–17. [Google Scholar] [PubMed]
- López-Pintor, R.M.; Casañas, E.; González-Serrano, J.; Serrano, J.; Ramírez, L.; de Arriba, L.; Hernández, G. Xerostomia, Hyposalivation, and Salivary Flow in Diabetes Patients. J. Diabetes Res. 2016, 2016, 4372852. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, V.; Nix, P. Managing the patient presenting with xerostomia: A review. Int. J. Clin. Pract. 2010, 64, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Valdez, I.H.; Fox, P.C. Diagnosis and management of salivary dysfunction. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 1993, 4, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Connell, C.L.; Abati, S. Diagnosis and management of xerostomia and hyposalivation. Ther. Clin. Risk Manag. 2014, 11, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.C.; Busch, K.A.; Baum, B.J. Subjective reports of xerostomia and objective measures of salivary gland performance. J. Am. Dent. Assoc. 1987, 115, 581–584. [Google Scholar] [CrossRef]
- Ship, J.A.; Fox, P.C.; Baum, B.J. How Much Saliva is Enough? J. Am. Dent. Assoc. 1991, 122, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Ghezzi, E.M.; Ship, J.A. Development of a Visual Analogue Scale questionnaire for subjective assessment of salivary dysfunction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001, 91, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Navazesh, M.; Christensen, C.; Brightman, V. Clinical criteria for the diagnosis of salivary gland hypofunction. J. Dent. Res. 1992, 71, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Bustillos, H.; Indorf, A.; Alwan, L.; Thompson, J.; Jung, L. Xerostomia: An immunotherapy-related adverse effect in cancer patients. Supportive Care Cancer 2022, 30, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Napeñas, J.J.; Brennan, M.T.; Fox, P.C. Diagnosis and treatment of xerostomia (dry mouth). Odontology 2009, 97, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Gil-Montoya, J.A.; Guardia-López, I.; González-Moles, M.A. Evaluation of the clinical efficacy of a mouthwash and oral gel containing the antimicrobial proteins lactoperoxidase, lysozyme and lactoferrin in elderly patients with dry mouth—A pilot study. Gerodontology 2008, 25, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Jensen, S.B. Management of Hyposalivation and Xerostomia: Criteria for Treatment Strategies. Compend. Contin. Educ. Dent. 2015, 36, 600–603. [Google Scholar] [PubMed]
- Fantozzi, P.J.; Pampena, E.; di Vanna, D.; Pellegrino, E.; Corbi, D.; Mammucari, S.; Alessi, F.; Pampena, R.; Bertazzoni, G.; Minisola, S.; et al. Xerostomia, gustatory and olfactory dysfunctions in patients with COVID-19. Am. J. Otolaryngol. 2020, 41, 102721. [Google Scholar] [CrossRef]
- Nederfors, T.; Isaksson, R.; Mörnstad, H.; Dahlöf, C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population—Relation to age, sex and pharmacotherapy. Community Dent. Oral Epidemiol. 1997, 25, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, J.; van Campen, J.P.C.M.; van der Jagt, H.; Beijnen, J.H.; Tulner, L.R. Prevalence of Xerostomia and Its Relationship with Underlying Diseases, Medication, and Nutrition: A Descriptive Observational Study. J. Am. Geriatr. Soc. 2013, 61, 1836–1837. [Google Scholar] [CrossRef] [PubMed]
- Marcott, S.; Dewan, K.; Kwan, M.; Baik, F.; Lee, Y.-J.; Sirjani, D. Where Dysphagia Begins: Polypharmacy and Xerostomia. Fed Pract. 2020, 37, 234–241. [Google Scholar]
- Tsibouklis, J.; Middleton, A.M.; Patel, N.; Pratten, J. Toward mucoadhesive hydrogel formulations for the management of xerostomia: The physicochemical, biological, and pharmacological considerations. J. Biomed. Mater. Res. 2013, 101, 3327–3338. [Google Scholar] [CrossRef]
- Peker, I.; Alkurt, M.T.; Usalan, G. Clinical evaluation of medications on oral and dental health. Int. Dent. J. 2008, 58, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Scully, C. Drug effects on salivary glands: Dry mouth. Oral Dis. 2003, 9, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.C.K.; Lexomboon, D.; Sandborgh-Englund, G.; Haasum, Y.; Johnell, K. Medications That Cause Dry Mouth As an Adverse Effect in Older People: A Systematic Review and Metaanalysis. J. Am. Geriatr. Soc. 2018, 66, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A. Diagnosing, managing, and preventing salivary gland disorders. Oral Dis. 2002, 8, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Arany, S.; Kopycka-Kedzierawski, D.T.; Caprio, T.V.; Watson, G.E. Anticholinergic medication: Related dry mouth and effects on the salivary glands. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D. Hyposalivation and Xerostomia: Etiology, Complications, and Medical Management. Dent. Clin. N. Am. 2016, 60, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Dirix, P.; Nuyts, S.; van den Bogaert, W. Radiation-induced xerostomia in patients with head and neck cancer: A literature review. Cancer 2006, 107, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Messmer, M.B.; Thomsen, A.; Kirste, S.; Becker, G.; Momm, F. Xerostomia after radiotherapy in the head & neck area: Long-term observations. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2011, 98, 48–50. [Google Scholar]
- Wu, V.W.C.; Leung, K.Y. A Review on the Assessment of Radiation Induced Salivary Gland Damage After Radiotherapy. Front. Oncol. 2019, 9, 1090. [Google Scholar] [CrossRef] [PubMed]
- Logemann, J.A.; Smith, C.H.; Pauloski, B.R.; Rademaker, A.W.; Lazarus, C.L.; Colangelo, L.A.; Mittal, B.; MacCracken, E.; Gaziano, J.; Stachowiak, L.; et al. Effects of xerostomia on perception and performance of swallow function. Head Neck 2001, 23, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Tribius, S.; Sommer, J.; Prosch, C.; Bajrovic, A.; Muenscher, A.; Blessmann, M.; Kruell, A.; Petersen, C.; Todorovic, M.; Tennstedt, P. Xerostomia after radiotherapy. Strahlenther. Und Onkol. 2013, 189, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Mignogna, M.D.; Fedele, S.; Russo, L.L.; Muzio, L.L.; Wolff, A. Sjögren’s syndrome: The diagnostic potential of early oral manifestations preceding hyposalivation/xerostomia. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2005, 34, 1–6. [Google Scholar] [CrossRef]
- Stankeviciene, I.; Puriene, A.; Mieliauskaite, D.; Stangvaltaite-Mouhat, L.; Aleksejuniene, J. Detection of xerostomia, Sicca, and Sjogren’s syndromes in a national sample of adults. BMC Oral Health 2021, 21, 552. [Google Scholar] [CrossRef] [PubMed]
- Van der Reijden, W.A.; Vissink, A.; Veerman, E.C.I.; Amerongen, A.V.N. Treatment of oral dryness related complaints (xerostomia) in Sjögren’s syndrome. Ann. Rheum. Dis. 1999, 58, 465–474. [Google Scholar] [CrossRef]
- Abadi, P.A.; Koopaie, M.; Montazeri, R. Comparison of salivary nitric oxide and oral health in diabetic patients with and without xerostomia. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 11–15. [Google Scholar] [CrossRef]
- Al-Maskari, A.Y.; Al-Maskari, M.Y.; Al-Sudairy, S. Oral Manifestations and Complications of Diabetes Mellitus: A review. Sultan Qaboos Univ. Med. J. 2011, 11, 179–186. [Google Scholar]
- Sandberg, G.E.; Sundberg, H.E.; Fjellstrom, C.A.; Wikblad, K.F. Type 2 diabetes and oral health: A comparison between diabetic and non-diabetic subjects. Diabetes Res. Clin. Pract. 2000, 50, 27–34. [Google Scholar] [CrossRef]
- Guggenheimer, J.; Moore, P.A.; Rossie, K.; Myers, D.; Mongelluzzo, M.B.; Block, H.M.; Weyant, R.; Orchard, T. Insulin-dependent diabetes mellitus and oral soft tissue pathologies: I. Prevalence and characteristics of non-candidal lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2000, 89, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Valerio, M.A.; Kanjirath, P.P.; Klausner, C.P.; Peters, M.C. A qualitative examination of patient awareness and understanding of type 2 diabetes and oral health care needs. Diabetes Res. Clin. Pract. 2011, 93, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Åstrøm, A.N.; Lie, S.A.; Ekback, G.; Gülcan, F.; Ordell, S. Self-reported dry mouth among ageing people: A longitudinal, cross-national study. Eur. J. Oral Sci. 2019, 127, 130–138. [Google Scholar] [CrossRef]
- Johansson, A.-K.; Johansson, A.; Unell, L.; Ekbäck, G.; Ordell, S.; Carlsson, G.E. Self-reported dry mouth in 50- to 80-year-old Swedes: Longitudinal and cross-sectional population studies. J. Oral Rehabil. 2020, 47, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J.; Bromberg, J.; Terpenning, M.S.; Bretz, W.A.; Dominguez, B.L.; Grossman, N.S.; Langmore, S.E. Xerostomia, xerogenic medications and food avoidances in selected geriatric groups. J. Am. Geriatr. Soc. 1995, 43, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Fife, R.S.; Chase, W.F.; Dore, R.K.; Wiesenhutter, C.W.; Lockhart, P.B.; Tindall, E.; Suen, J.Y. Cevimeline for the treatment of xerostomia in patients with Sjögren syndrome: A randomized trial. Arch. Intern Med. 2002, 162, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Dodds, M.W.J.; Johnson, D.A.; Yeh, C.-K. Health benefits of saliva: A review. J. Dent. 2005, 33, 223–233. [Google Scholar] [CrossRef]
- Nadig, S.D.; Ashwathappa, D.T.; Manjunath, M.; Krishna, S.; Annaji, A.G.; Shivaprakash, P.K. A relationship between salivary flow rates and Candida counts in patients with xerostomia. J. Oral Maxillofac. Pathol. JOMFP 2017, 21, 316. [Google Scholar] [CrossRef] [PubMed]
- Koshimune, S.; Awano, S.; Gohara, K.; Kurihara, E.; Ansai, T.; Takehara, T. Low salivary flow and volatile sulfur compounds in mouth air. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 38–41. [Google Scholar] [CrossRef]
- Dormenval, V.; Budtz-Jørgensen, E.; Mojon, P.; Bruyère, A.; Rapin, C.H. Associations between malnutrition, poor general health and oral dryness in hospitalized elderly patients. Age Ageing 1998, 27, 123–128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iwasaki, M.; Borgnakke, W.S.; Yoshihara, A.; Ito, K.; Ogawa, H.; Nohno, K.; Sato, M.; Minagawa, K.; Ansai, T.; Miyazaki, H. Hyposalivation and 10-year all-cause mortality in an elderly Japanese population. Gerodontology 2018, 35, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.C.; Grisius, M.; Massey, W. Salivary hypofunction and xerostomia: Diagnosis and treatment. Dent. Clin. N. Am. 2005, 49, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Alhejoury, H.; Mogharbel, L.; Al-Qadhi, M.; Shamlan, S.; Alturki, A.; Babatin, W.; Alaishan, R.M.; Pullishery, F. Artificial saliva for therapeutic management of xerostomia: A narrative review. J. Pharm. Bioallied Sci. 2021, 13, 903–907. [Google Scholar]
- Newbrun, E. Current treatment modalities of oral problems of patients with Sjögren’s syndrome: Caries prevention. Adv. Dent. Res. 1996, 10, 29–34. [Google Scholar] [CrossRef]
- Jansma, J.; Vissink, A.; Spijkervet, F.K.; Roodenburg, J.L.; Panders, A.K.; Vermey, A.; Szabó, B.G.; Gravenmade, E.J. Protocol for the prevention and treatment of oral sequelae resulting from head and neck radiation therapy. Cancer 1992, 70, 2171–2180. [Google Scholar] [CrossRef]
- Barbe, A.G. Medication-Induced Xerostomia and Hyposalivation in the Elderly: Culprits, Complications, and Management. Drugs Aging 2018, 35, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Zero, D.T.; Brennan, M.T.; Daniels, T.E.; Papas, A.; Stewart, C.; Pinto, A.; Al-Hashimi, I.; Navazesh, M.; Rhodus, N.; Sciubba, J.; et al. Clinical practice guidelines for oral management of Sjögren disease: Dental caries prevention. J. Am. Dent. Assoc. 2016, 147, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.E. Efficacy and economic evaluation of pilocarpine in treating radiation-induced xerostomia. Expert Opin. Pharmacother. 2003, 4, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Mese, H.; Matsuo, R. Salivary secretion, taste and hyposalivation. J. Oral Rehabil. 2007, 34, 711–723. [Google Scholar] [CrossRef]
- Hu, J.; Andablo-Reyes, E.; Mighell, A.; Pavitt, S.; Sarkar, A. Dry mouth diagnosis and saliva substitutes-A review from a textural perspective. J. Texture Stud. 2021, 52, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Lam-Ubol, A.; Matangkasombut, O.; Trachootham, D.; Tarapan, S.; Sattabanasuk, V.; Talungchit, S.; Paemuang, W.; Phonyiam, T.; Chokchaitam, O.; Mungkung, O.O. Efficacy of gel-based artificial saliva on Candida colonization and saliva properties in xerostomic post-radiotherapy head and neck cancer patients: A randomized controlled trial. Clin. Oral Investig. 2021, 25, 1815–1827. [Google Scholar] [CrossRef]
- Bonda, P.L.F.; Farinone, M.; Pattarino, F.; Bonda, A.F. Artificial saliva substitutes evaluation: The role of some chemical-physical properties. Glob. J. Res. Anal. 2018, 7, 67–70. [Google Scholar]
- Salum, F.G.; Medella-Junior, F.A.C.; Figueiredo, M.A.Z.; Cherubini, K. Salivary hypofunction: An update on therapeutic strategies. Gerodontology 2018, 35, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-S.; Chung, J.-W.; Kim, Y.-K.; Chung, S.-C.; Kho, H.-S. Viscosity and wettability of animal mucin solutions and human saliva. Oral Dis. 2007, 13, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.S. The rheological properties of saliva. Rheol. Acta 1971, 10, 28–35. [Google Scholar] [CrossRef]
- Łysik, D.; Niemirowicz-Laskowska, K.; Bucki, R.; Tokajuk, G.; Mystkowska, J. Artificial Saliva: Challenges and Future Perspectives for the Treatment of Xerostomia. Int. J. Mol. Sci. 2019, 20, 3199. [Google Scholar] [CrossRef] [PubMed]
- Jornet, P.L.; Hernandez, L.; García, F.G.; Molero, F.G.; López, E.P.-F.; Tvarijonaviciute, A. A Clinical Study on the Efficacy and Tolerability of a New Topical Gel and Toothpaste in Patients with Xerostomia: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 5641. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Emerton, S.; Le, N.D.; Stevenson-Moore, P. A double-blind crossover trial of Oral Balance gel and Biotene toothpaste versus placebo in patients with xerostomia following radiation therapy. Oral Oncol. 1999, 35, 132–137. [Google Scholar] [CrossRef]
- Shahdad, S.A.; Taylor, C.; Barclay, S.C.; Steen, I.N.; Preshaw, P.M. A double-blind, crossover study of Biotène Oralbalance and BioXtra systems as salivary substitutes in patients with post-radiotherapy xerostomia. Eur. J. Cancer Care 2005, 14, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.; Fox, P.C.; Porter, S.; Konttinen, Y.T. Established and novel approaches for the management of hyposalivation and xerostomia. Curr. Pharm. Des. 2012, 18, 5515–5521. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vissink, A.; de Jong, H.P.; Busscher, H.J.; Arends, J.; Gravenmade, E.J. Wetting Properties of Human Saliva and Saliva Substitutes. J. Dent. Res. 1986, 65, 1121–1124. [Google Scholar] [CrossRef]
- Amal, A.S.S.; Hussain, S.; Jalaluddin, M.A. Preparation of Artificial Saliva Formulation. In Proceedings of the ICB Pharma II “Current Breakthrough in Pharmacy Materials and Analyses”, Indonesia; 2015. Available online: https://publikasiilmiah.ums.ac.id/xmlui/handle/11617/6203 (accessed on 26 January 2022).
- Jellema, A.P.; Langendijk, H.; Bergenhenegouwen, L.; van der Reijden, W.; Leemans, R.; Smeele, L.; Slotman, B.J. The efficacy of Xialine® in patients with xerostomia resulting from radiotherapy for head and neck cancer: A pilot-study. Radiother. Oncol. 2001, 59, 157–160. [Google Scholar] [CrossRef]
- Hahnel, S.; Behr, M.; Handel, G.; Bürgers, R. Saliva substitutes for the treatment of radiation-induced xerostomia—A review. Supportive Care Cancer 2009, 17, 1331–1343. [Google Scholar] [CrossRef]
- Momm, F.; Volegova-Neher, N.J.; Schulte-Mönting, J.; Guttenberger, R. Different Saliva Substitutes for Treatment of Xerostomia Following Radiotherapy. Strahlenther. Onkol. 2005, 181, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Aykut-Yetkiner, A.; Wiegand, A.; Attin, T. The effect of saliva substitutes on enamel erosion in vitro. J. Dent. 2014, 42, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, A.M.; Shohadai, S.P.; Schulte-Mönting, J. Effect of saliva substitutes on mineral content of demineralized and sound dental enamel. Supportive Care Cancer 2001, 9, 40–47. [Google Scholar] [CrossRef]
- Jaiswal, N.; Patil, P.G.; Gangurde, A.; Parkhedkar, R.D. Wettability of 3 different artificial saliva substitutes on heat-polymerized acrylic resin. J. Prosthet. Dent. 2019, 121, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Salom, M.; Hachulla, E.; Bertolus, C.; Deschaumes, C.; Simoneau, G.; Mouly, S. Efficacy and safety of a new oral saliva equivalent in the management of xerostomia: A national, multicenter, randomized study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 301–309. [Google Scholar] [CrossRef]
- Roy, N.; Tanner, K.; Gray, S.D.; Blomgren, M.; Fisher, K.V. An evaluation of the effects of three laryngeal lubricants on phonation threshold pressure (PTP). J. Voice 2003, 17, 331–342. [Google Scholar] [CrossRef]
- Smith, G.; Smith, A.J.; Shaw, L.; Shaw, M.J. Artificial saliva substitutes and mineral dissolution. J. Oral Rehabil. 2001, 28, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Shirodaria, S.; Kilbourn, T.; Richardson, M. Subjective assessment of a new moisturizing mouth spray for the symptomatic relief of dry mouth. J. Clin. Dent. 2006, 17, 45–51. [Google Scholar] [PubMed]
- Malallah, O.S.; Garcia, C.M.A.; Proctor, G.B.; Forbes, B.; Royall, P.G. Buccal drug delivery technologies for patient-centred treatment of radiation-induced xerostomia (dry mouth). Int. J. Pharm. 2018, 541, 157–166. [Google Scholar] [CrossRef]
- Skrinjar, I.; Boras, V.V.; Bakale, I.; Rogulj, A.A.; Brailo, V.; Juras, D.V.; Alajbeg, I.; Vrdoljak, D.V. Comparison between three different saliva substitutes in patients with hyposalivation. Clin. Oral Investig. 2015, 19, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Fehder, W.P. Nursing care & management of pathological oral conditions among women and children, MCN. Am. J. Matern. Child Nurs. 2008, 33, 38–44. [Google Scholar]
- News and Innovations. J. Pain Palliat. Care Pharmacother. 2006, 20, 83–104. [CrossRef]
- Delgado, A.; Ribeiro, A.P.D.; Aslam, M.; Olafsson, V.G.; Pereira, P.N. Erosive assessment of dry mouth lozenges and tablets on enamel and dentin. J. Dent. 2021, 105, 103496. [Google Scholar] [CrossRef]
- Femiano, F.; Rullo, R.; di Spirito, F.; Lanza, A.; Festa, V.M.; Cirillo, N. A comparison of salivary substitutes versus a natural sialogogue (citric acid) in patients complaining of dry mouth as an adverse drug reaction: A clinical, randomized controlled study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, e15–e20. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Moreno, G.; Guardia, J.; Aguilar-Salvatierra, A.; Cabrera-Ayala, M.; Maté-Sánchez de-Val, J.-E.; Calvo-Guirado, J.L. Effectiveness of malic acid 1% in patients with xerostomia induced by antihypertensive drugs. Med. Oral Patol. Oral Y Cir. Bucal 2013, 18, e49–e55. [Google Scholar] [CrossRef]
- Ozen, N.; Sayilan, A.A.; Mut, D.; Sayilan, S.; Avcioglu, Z.; Kulakac, N.; Ecder, T.; Akyolcu, N. The effect of chewing gum on dry mouth, interdialytic weight gain, and intradialytic symptoms: A prospective, randomized controlled trial, Hemodialysis international. Int. Symp. Home Hemodial. 2021, 25, 94–103. [Google Scholar] [CrossRef]
- Bots, C.P.; Brand, H.S.; Veerman, E.C.; Korevaar, J.C.; Valentijn-Benz, M.; Bezemer, P.D.; Valentijn, R.M.; Vos, P.F.; Bijlsma, J.A.; Wee, P.M.T.; et al. Chewing gum and a saliva substitute alleviate thirst and xerostomia in patients on haemodialysis. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2005, 20, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Tulek, A.; Mulic, A.; Hogset, M.; Utheim, T.P.; Sehic, A. Therapeutic Strategies for Dry Mouth Management with Emphasis on Electrostimulation as a Treatment Option. Int. J. Dent. 2021, 2021, 6043488. [Google Scholar] [CrossRef]
- Kaae, J.K.; Stenfeldt, L.; Eriksen, J.G. Xerostomia after Radiotherapy for Oral and Oropharyngeal Cancer: Increasing Salivary Flow with Tasteless Sugar-free Chewing Gum. Front. Oncol. 2016, 6, 111. [Google Scholar] [CrossRef]
- Bossola, M. Xerostomia in patients on chronic hemodialysis: An update. Semin. Dial. 2019, 32, 467–474. [Google Scholar] [CrossRef]
- Olsson, H.; Spak, C.J.; Axell, T. The effect of a chewing gum on salivary secretion, oral mucosal friction, and the feeling of dry mouth in xerostomic patients. Acta Odontol. Scand. 1991, 49, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Maggi, L.; Segale, L.; Conti, S.; Machiste, E.O.; Salini, A.; Conte, U. Preparation and evaluation of release characteristics of 3TabGum, a novel chewing device. Eur. J. Pharm. Sci. 2005, 24, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Fathima, S.R.; Viswanath, V.; Malleswari, M.; Panitha, M.; Reddy, N.P.; Reddy, R.; Sreedevi, N.S. Medicated chewing gums—An Overview. Int. J. Pharm. Anal. Res. 2019, 8, 138–144. [Google Scholar]
- Davies, A.N. A comparison of artificial saliva and chewing gum in the management of xerostomia in patients with advanced cancer. Palliat. Med. 2000, 14, 197–203. [Google Scholar] [CrossRef]
- Björnström, M.; Axéll, T.; Birkhed, D. Comparison between saliva stimulants and saliva substitutes in patients with symptoms related to dry mouth. A Multi-Centre Study. Swed. Dent. J. 1990, 14, 153–161. [Google Scholar] [PubMed]
- Risheim, H.; Arneberg, P. Salivary stimulation by chewing gum and lozenges in rheumatic patients with xerostomia. Scand. J. Dent. Res. 1993, 101, 40–43. [Google Scholar] [CrossRef]
- Khatun, S.; Sutradhar, B.K. Medicated chewing gum: An unconventional drug delivery system. Int. Curr. Pharm. J. 2012, 1, 86–91. [Google Scholar] [CrossRef]
- Aslani, A.; Rostami, F. Medicated chewing gum, a novel drug delivery system. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2015, 20, 403–411. [Google Scholar]
- Aagaard, A.; Godiksen, S.; Teglers, P.T.; Schiødt, M.; Glenert, U. Comparison between new saliva stimulants in patients with dry mouth: A placebo-controlled double-blind crossover study. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 1992, 21, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Lapiedra, R.C.; Gomez, G.E.; Sanchez, B.P.; Pereda, A.A.; Turner, M.D. The Effect of a Combination Saliva Substitute for the Management of Xerostomia and Hyposalivation. J. Maxillofac. Oral Surg. 2015, 14, 653–658. [Google Scholar] [CrossRef]
- Warde, P.; Kroll, B.; O’Sullivan, B.; Aslanidis, J.; Tew-George, E.; Waldron, J.; Maxymiw, W.; Liu, F.F.; Payne, D.; Cummings, B. A phase II study of Biotene in the treatment of postradiation xerostomia in patients with head and neck cancer. Supportive Care Cancer 2000, 8, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.; Glenny, A.M.; Hua, F.; Worthington, H.V. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database Syst. Rev. 2017, 7, 1–150. [Google Scholar] [CrossRef]
- Berk, L. Systemic pilocarpine for treatment of xerostomia. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Navazesh, M. Ship, II, Xerostomia: Diagnosis and treatment. Am. J. Otolaryngol. 1983, 4, 283–292. [Google Scholar] [CrossRef]
- Bernardi, R.; Perin, C.; Becker, F.L.; Ramos, G.Z.; Gheno, G.Z.; Lopes, L.R.; Pires, M.; Barros, H.M. Effect of pilocarpine mouthwash on salivary flow. Braz. J. Med. Biol. Res. = Rev. Bras. De Pesqui. Med. E Biol. 2002, 35, 105–110. [Google Scholar] [CrossRef]
- Braga, M.A.; Tarzia, O.; Bergamaschi, C.C.; Santos, F.A.; Andrade, E.D.; Groppo, F.C. Comparison of the effects of pilocarpine and cevimeline on salivary flow. Int. J. Dent. Hyg. 2009, 7, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.S.; Posner, M.; Jones, C.U.; Biel, M.A.; Hodge, K.M.; Vitti, R.; Armstrong, I.; Yen, C.; Weber, R.S. Cevimeline for the treatment of postirradiation xerostomia in patients with head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, R.; Singh, T.R.R.; Garland, M.J.; Woolfson, A.D.; Donnelly, R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioallied. Sci. 2011, 3, 89–100. [Google Scholar] [PubMed]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Harris, D.; Robinson, J.R. Drug Delivery via the Mucous Membranes of the Oral Cavity. J. Pharm. Sci. 1992, 81, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Nafee, N.A.; Ismail, F.A.; Boraie, N.A.; Mortada, L.M. Mucoadhesive delivery systems. I. Evaluation of mucoadhesive polymers for buccal tablet formulation. Drug Dev. Ind. Pharm. 2004, 30, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Röttges, S. Buccal adhesive chitosan conjugate comprising pilocarpine for xerostomia. Int. J. Biol. Macromol. 2019, 135, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.R.; Le, D.L.; Sato, H.; Sou, K. Nanocapsule pH Regulator: Sustained Continuous Alkali Release from Thermosensitive Liposomes Reduces Acid Erosion. ACS Appl. Mater. Interfaces 2020, 12, 21463–21469. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, M.; Ansari, M.N.M.; Razak, S.I.A.; Khan, M.U.A. A Comprehensive Review on the Applications of Exosomes and Liposomes in Regenerative Medicine and Tissue Engineering. Polymers 2021, 13, 2529. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, M.I.; Martinsen, Ø.G.; Smistad, G.; Hiorth, M. Polymer coated mucoadhesive liposomes intended for the management of xerostomia. Int. J. Pharm. 2017, 527, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Muthumariappan, S.; Ng, W.C.; Adine, C.; Ng, K.K.; Davoodi, P.; Wang, C.H.; Ferreira, J.N. Localized Delivery of Pilocarpine to Hypofunctional Salivary Glands through Electrospun Nanofiber Mats: An Ex Vivo and In Vivo Study. Int. J. Mol. Sci. 2019, 20, 541. [Google Scholar] [CrossRef] [PubMed]





| Category | Drug Substance |
|---|---|
| Antidepressant agents and antipsychotic agents | citalopram, fluoxetine, paroxetine, sertraline, venlafaxine, amitriptyline, imipramine, reboxetine, bupropion hydrochloride, clozapine, chlorpromazine, haloperidol, olanzapine |
| Anticholinergic agents | dicyclomine, mepenzolate |
| Antihypertensive agents | captopril, clonidine, methyldopa, prazosin |
| Antiparkinsonian agents | biperiden, selegiline |
| Diuretic agents | spirnolactone, chlorothiazide, furosemide, hydrochlorothiazide |
| Opioids | morphine, codeine, methadone, pethidine |
| Immunostimulants | interferon-alpha |
| Dosage Forms | Brand Name | Polymers Used | Product Composition | Characteristics of the Formulation: Advantages or Disadvantages | Manufacturer | Ref. |
|---|---|---|---|---|---|---|
| Oral Sprays | Aldiamed® | CMC | Water, propylene glycol, xylitol, glycerol, microcrystalline cellulose, panthenol, CMC, sodium, sodium benzoate, lactoferrin, disodium EDTA, lysozyme, hydrochloride, aroma, Aloe Barbadensis | Significant improvement of xerostomia and increased life quality. Diminished use frequency, as compared to the other respective saliva substitutes, which may be associated to the improved results on mouth dryness. | Certmedica International | [93] |
| Artisial® | Sodium CMC | Sodium CMC, sorbitol, calcium chloride dihydrate, magnesium chloride, dipotassium phosphate, monopotassium phosphate, potassium chloride, sodium chloride | Only minimal enamel mineral loss was observed in relevant published studies. | Jouveinal Laboratoires | [94] | |
| Aqwet® | CMC | Water, CMC, sorbitol, potassium chloride, sodium chloride, magnesium chloride, calcium chloride | Improved wetting ability as compared to similar commercially available saliva substitutes; comparable properties with human saliva. | Cipla Ltd. (Mumbai, India) | [95] | |
| Biotene® | Xanthan gum | Water, glycerin, xylitol, PEG-60, hydrogenated castor oil, VP/NA copolymer, sodium benzoate, Xanthan gum, methylparaben, propylparaben sodium saccharin, cetylpyridinium chloride, limonene | Effective in reducing mouth dryness, taste alteration, and chewing difficulties. Not well-tolerated and limited acceptance from patients. | GlaxoSmithKline | [96] | |
| EMOFLUOR® | HEC | Water, glycerin, sorbitol, maltitol, ammonium phosphate, HEC, ammonium fluoride, methylparaben, sodium saccharin, sodium chloride, potassium chloride, propylparaben | Erosion-protective potential, which may be connected to the product’s film-forming properties. | Dr. Wild&Co AG | [93] | |
| Entertainer® | CMC | Water, CMC, aloe vera, glycerin, dibasic sodium phosphate, potassium chloride | High popularity among performers and voice clinicians; has gained increased interest as possible laryngeal lubricants due to quick throat comfort and vocal quality improvement. However, it has a relatively short-term effect. | KLI Corporation (Carmel, IN, USA) | [97] | |
| Glandosane® | Sodium CMC | Potassium chloride, sodium chloride, magnesium chloride, Magnesii chloridum, calcium chloride, potassium monohydrogen phosphate, sodium CMC, sorbitol | Preferred by patients due to the good taste and the easy handling. However, it has revealed a high demineralizing potential in several in vitro studies. | Helvepharm | [98] | |
| Oasis® | Copovidone | Cetylpyridinium chloride, copovidone, flavor, methylparaben, PEG-60 hydrogenated castor oil, propylparaben, sodium benzoate, sodium saccharin, water, xanthan gum, xylitol | Significantly reduced enamel loss as compared to a positive control. | Oasis Consumer Healthcare | [99] | |
| Stoppers 4® | HEC | Water, glycerin, xylitol, HEC, lysozyme, lactoferrin, glucose oxidase, spearmint (natural), sodium benzoate | Increased enamel loss as compared to a positive control. | Jocott Brands Inc. (Van Nuys, CA, USA) | [93] | |
| Oral Solutions | Act® | Poloxamer | Provides immediate but not long-lasting effect. | Sanofi | [40] | |
| Orazyme | Poloxamer and Sodium CMC | Gluconate, aloe Barbadensis, sodium CMC, poloxamer, water | Similarly with the abovementioned oral solution, it fails to provide long-lasting effect. | Dr. Fresh | [100] | |
| Xeros® | HEC | HEC, betaine, xylitol, sodium fluoride, water, allantoin | Decreases the patients’ discomfort during night but presents more significant effects in patients whose residual secretory potential was severely compromised. | Dentaid | [101] | |
| Gels | Biotene oralbalance | HEC | Lactoperoxidase, lysozyme, glucose oxidase, lactoferrin, hydrogenated starch hydrolysate, xylitol, HEC, glyceryl polymethacrylate beta-D-glucose, aloe vera, potassium thiocyanate | Significant improvement in dryness, swallowing, and taste. Low retention time, which may be attributed to the relatively low viscosity. | GlaxoSmithKline | [87,102,103] |
| OralSeven | HEC | Hydrogenated starch hydrosylate, glycerin, water, xylitol, glyceryl acrylate, acrylic acide copolymer, HEC, aloe barbadenisis, lactoperoxidase, dextrose monohydrate, glucose oxidase, lactoferrin, lysozyme, potassium thiocyanate, cellulose gum | Considerable problems with the application and the handling of the gel were referred by patients. | Oral7 International | [3] | |
| Lozenges | Salese | Ethylcellulose and xanthan gum | Ethyl cellulose, xanthan gum, xylitol, sodium bicarbonate, eucalyptus oil, wintergreen oil, glycerol, zinc gluconate, thymol, calcium sulfate, potassium phosphate dibasic | Significantly low erosive potential on enamel, probably due to formulation’s high pH. However, the efficacy and patients’ acceptance of higher pH products are not yet known. | Nuvora Inc. (Santa Clara, CA, USA) | [56,104] |
| SalivaSure® | CMC | Xylitol, malic acid, dibasic calcium phosphate, CMC, sodium citrate dihydrate, stearic acid, citric acid, magnesium stearate, silica colloidal | Xylitol contained in the formulation reduces plaque formation and minimizes dental caries. Furthermore, no interaction with prescription medications has been reported, and the formulation is regarded as safe for people with diabetes. Main drawback is the short-lasting relief on contact. | Scandinavian Formulas Inc. (Sellersville, PA, USA) | [102,103] |
| Polymer | Examples | Ref. |
|---|---|---|
| Natural polymers | Polymers based on glycerol | [114] |
| Synthetic polymers | Polyisobutylene | [101,115,116,117] |
| Isoprene copolymer | [118] | |
| Styrenebutadiene copolymers | [115,116,117] | |
| Polyvinyl acetate | [118] | |
| Polyvinyl alcohol | [114,119] |
| Product Name | Characteristics | Manufacturer | Ref. |
|---|---|---|---|
| Freedent WhiteTM | As a low-tack chewing gum, it provides a better tolerance in patients with dental prostheses as compared to the normal-tack chewing gums. Nevertheless, several adverse effects (i.e., irritation of mouth, nausea etc.) have been reported. | Wrigley company | [108,115] |
| V6 chewing gum | Acceptable consistency and no reports of mouth irritation. | Gadbury | [116,120] |
| Dentirol chewing gum | Satisfying taste and acceptable consistency. Alleviates the symptoms without increasing the saliva flow rate. | Continental Candy Company, Denmark | [117] |
| Xerostom Chewable Relief Capsules® | Improves speech, swallowing; decreases subjective xerostomia. | Biocosmetics laboratories, Spain | [121] |
| Biotene chewing gum | Xylitol contained in the formulation reduces plaque formation and minimizes dental caries; improved results when combined with the respective oral solution and mouth paste. | GlaxoSmithKline | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapourani, A.; Kontogiannopoulos, K.N.; Manioudaki, A.-E.; Poulopoulos, A.K.; Tsalikis, L.; Assimopoulou, A.N.; Barmpalexis, P. A Review on Xerostomia and Its Various Management Strategies: The Role of Advanced Polymeric Materials in the Treatment Approaches. Polymers 2022, 14, 850. https://doi.org/10.3390/polym14050850
Kapourani A, Kontogiannopoulos KN, Manioudaki A-E, Poulopoulos AK, Tsalikis L, Assimopoulou AN, Barmpalexis P. A Review on Xerostomia and Its Various Management Strategies: The Role of Advanced Polymeric Materials in the Treatment Approaches. Polymers. 2022; 14(5):850. https://doi.org/10.3390/polym14050850
Chicago/Turabian StyleKapourani, Afroditi, Konstantinos N. Kontogiannopoulos, Alexandra-Eleftheria Manioudaki, Athanasios K. Poulopoulos, Lazaros Tsalikis, Andreana N. Assimopoulou, and Panagiotis Barmpalexis. 2022. "A Review on Xerostomia and Its Various Management Strategies: The Role of Advanced Polymeric Materials in the Treatment Approaches" Polymers 14, no. 5: 850. https://doi.org/10.3390/polym14050850
APA StyleKapourani, A., Kontogiannopoulos, K. N., Manioudaki, A.-E., Poulopoulos, A. K., Tsalikis, L., Assimopoulou, A. N., & Barmpalexis, P. (2022). A Review on Xerostomia and Its Various Management Strategies: The Role of Advanced Polymeric Materials in the Treatment Approaches. Polymers, 14(5), 850. https://doi.org/10.3390/polym14050850









