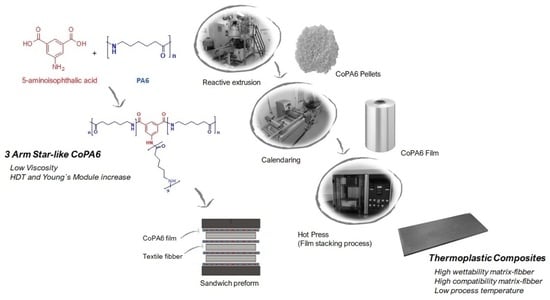
Star-Branched Polyamides as the Matrix in Thermoplastic Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
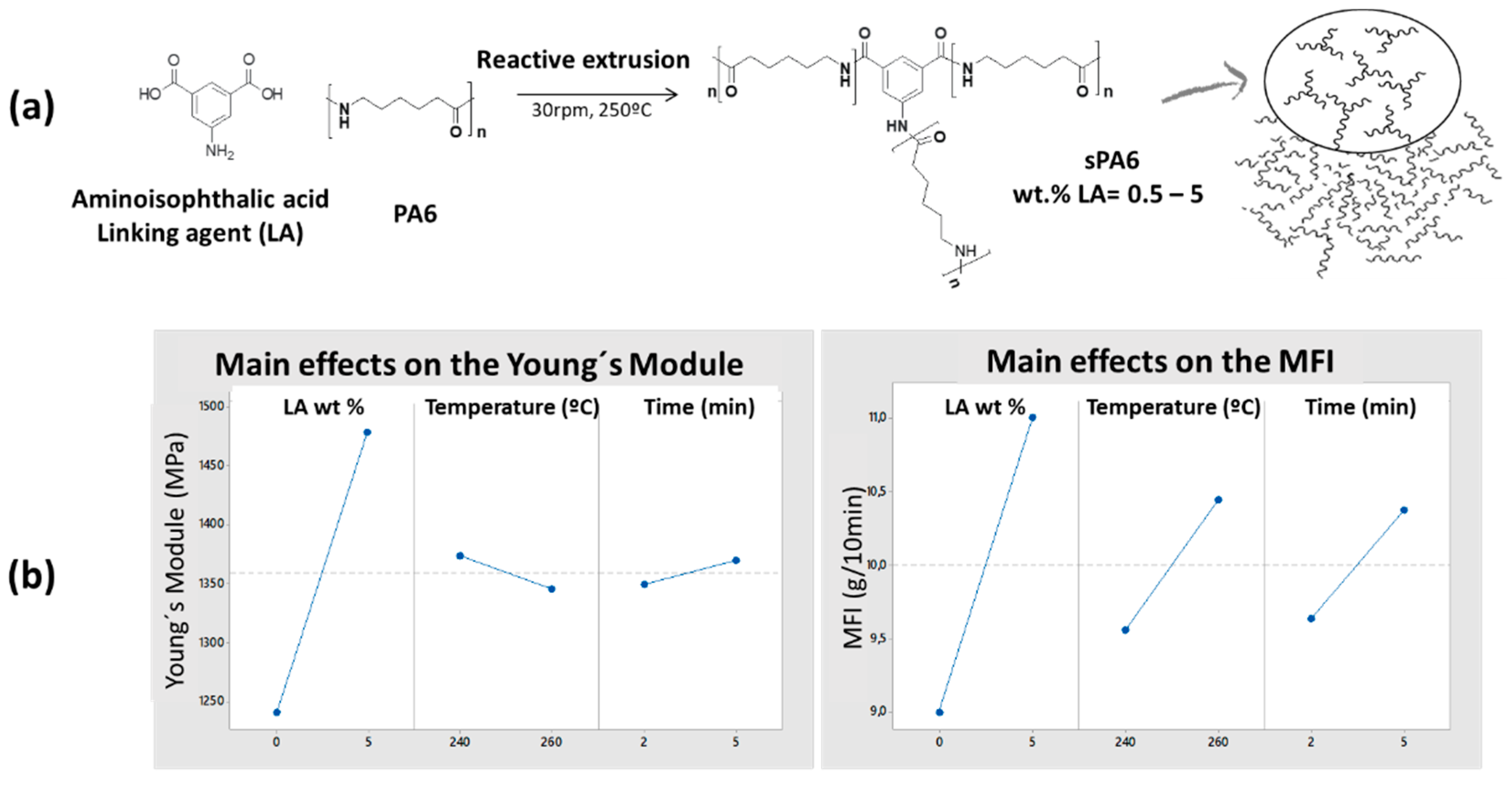
2.2. Reactive Extrusion Process
2.3. Injection Molding Process of sPA6
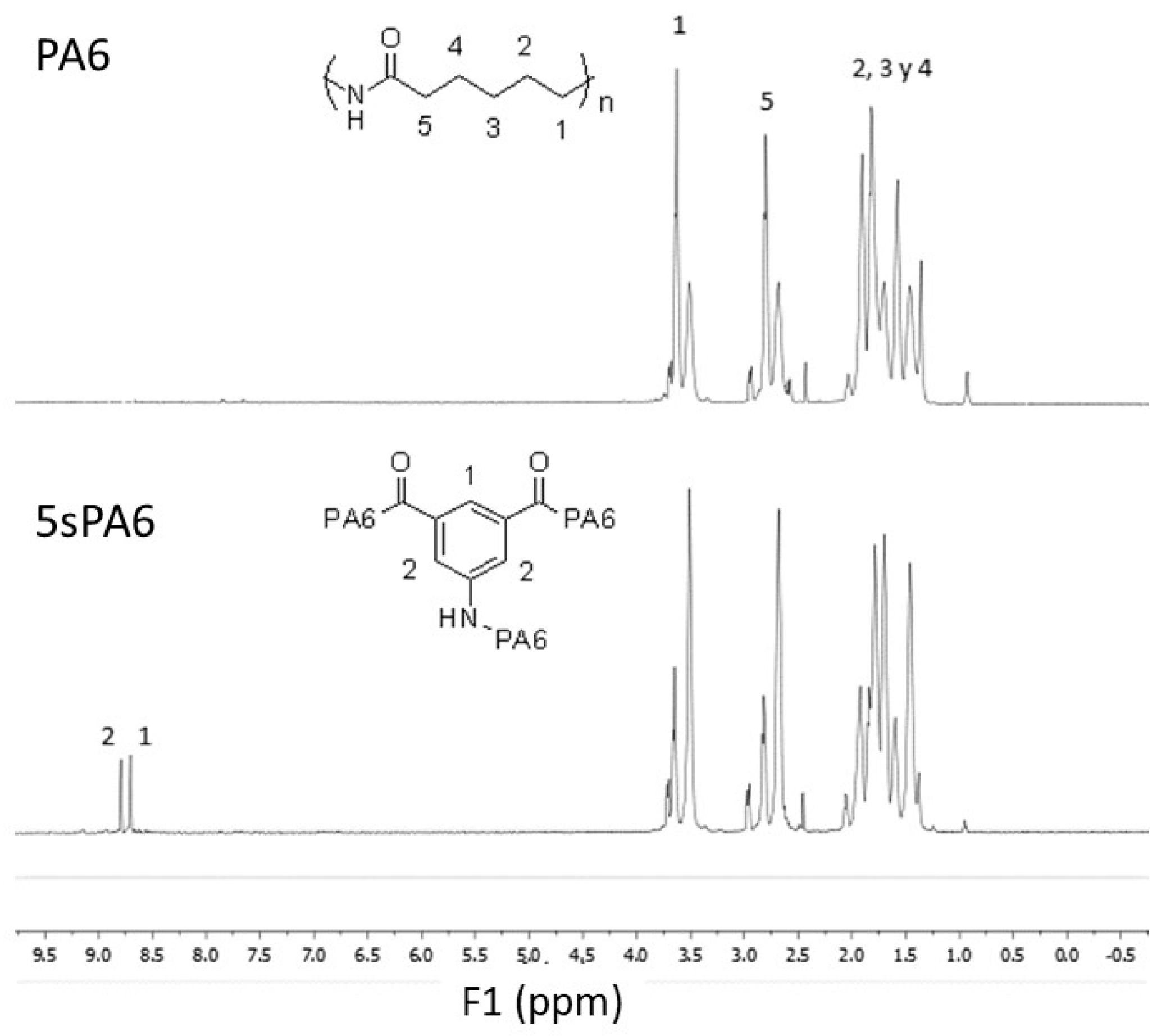
2.4. Characterization of sPA6
2.4.1. Morphological Characterization
2.4.2. Thermal Characterization
2.4.3. Rheological Characterization
2.4.4. Mechanical Characterization
2.5. Obtaining of the Thermoplastic Composite Based on sPA
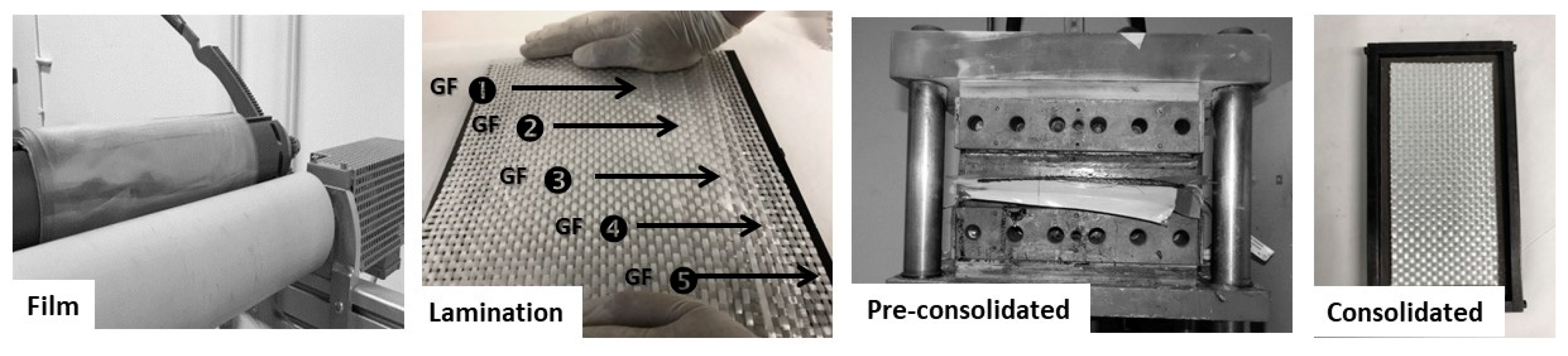
2.5.1. Calendering Process
2.5.2. Compression Molding (Film Stacking)
2.5.3. Thermoplastic Composites’ Characterization
2.5.4. Welding Tests of Composites Obtained with Matrix sPA6
3. Results and Discussion
3.1. Obtaining and Characterization of Star-Branched PA
3.1.1. Morphological Characterization
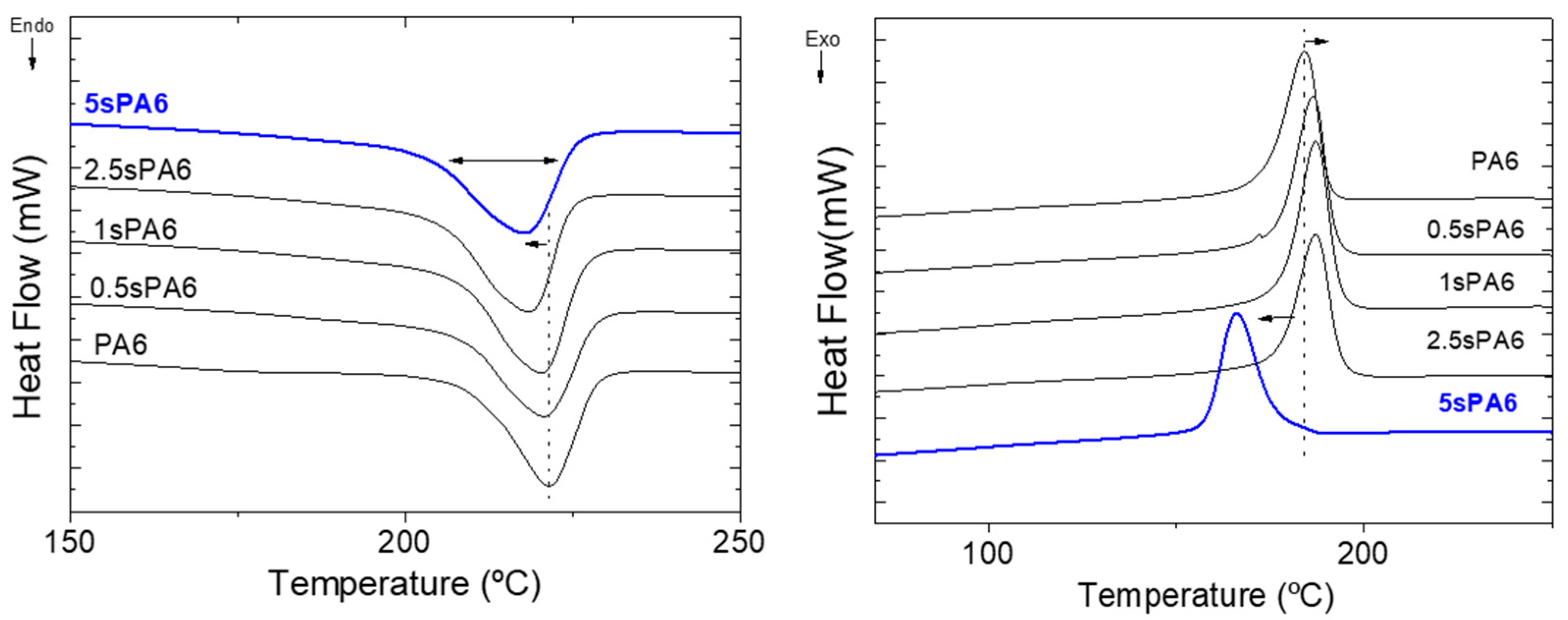
3.1.2. Thermal Properties
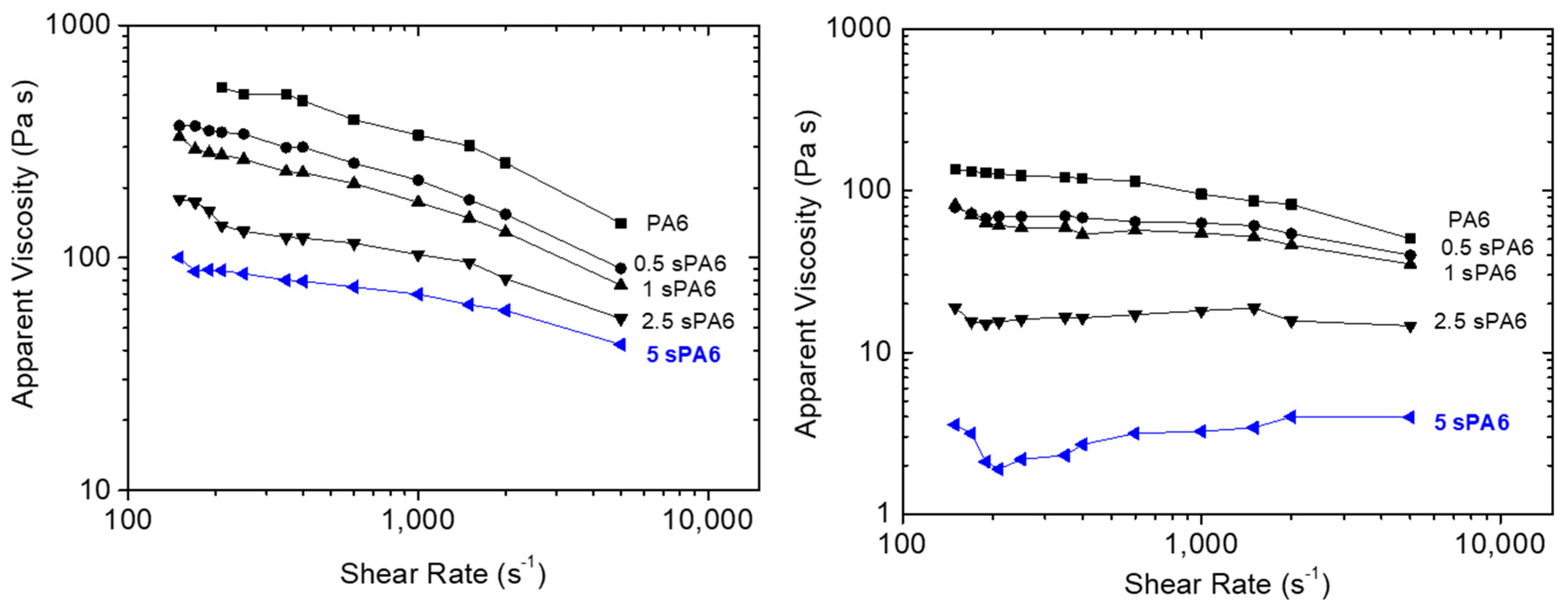
3.1.3. Rheological Characterization
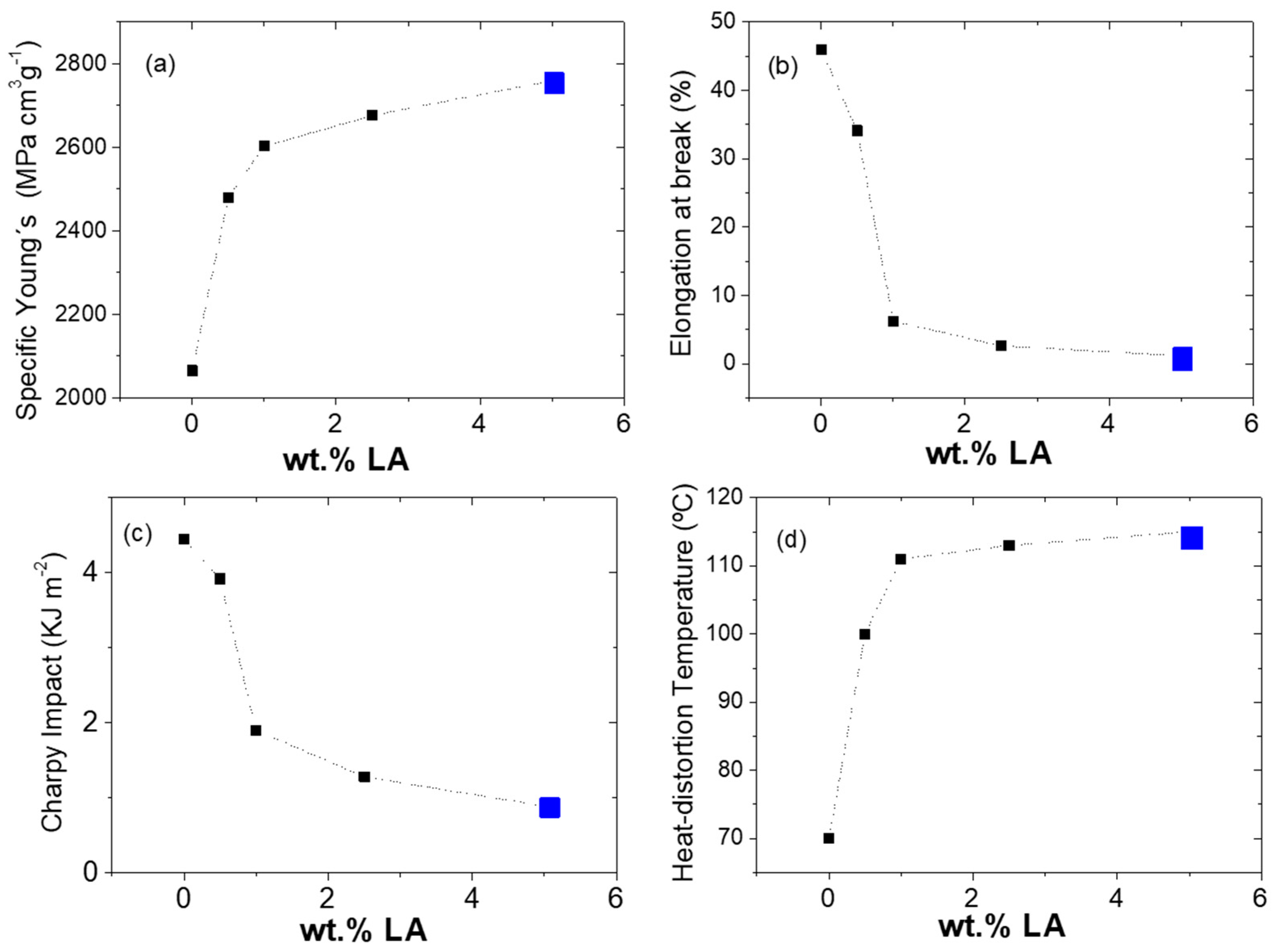
3.1.4. Mechanical Properties
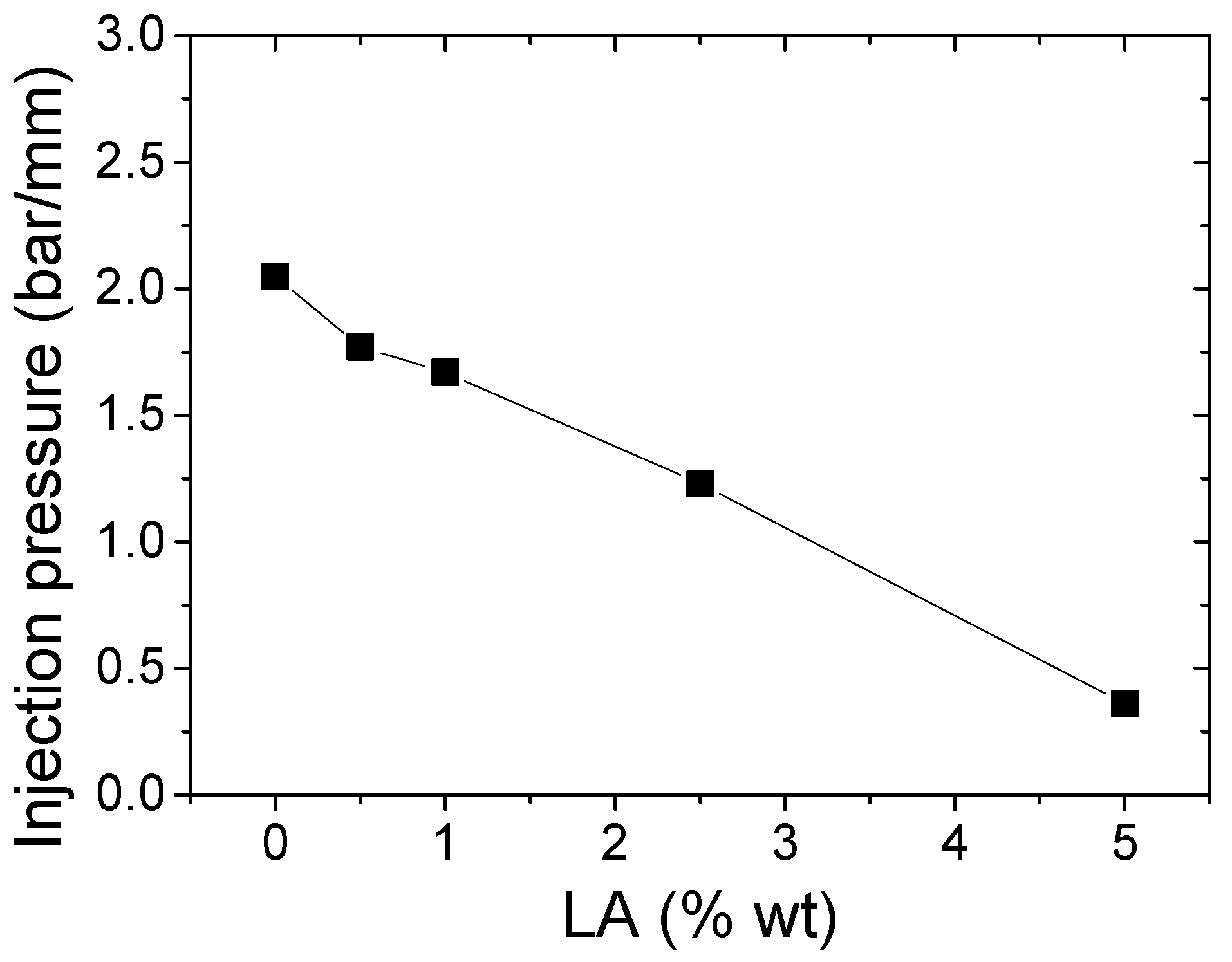
3.2. Characterization of Thermoplastic Composites Based on Star-Branched PA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, S.S.; Jin, F.L.; Rhee, K.Y.; Hui, D.; Park, S.J. Recent advances in carbon-fiber-reinforced thermoplastic composites: A review. Compos. Part B Eng. 2018, 142, 241–250. [Google Scholar] [CrossRef]
- Bhudolia, S.K.; Gohel, G.; Leong, K.F. Advances in Ultrasonic Welding of Thermoplastic Composites: A Review. Materials 2020, 13, 1284. [Google Scholar] [CrossRef] [Green Version]
- Banik, N. A review on the use of thermoplastic composites and their effects in induction welding method. Mater. Today Proc. 2018, 5, 20239–20249. [Google Scholar] [CrossRef]
- Martin, I.; Saenz del Castillo, D.; Fernandez, A.; Güemes, A. Advanced Thermoplastic Composite Manufacturing by In-Situ Consolidation: A Review. J. Compos. Sci. 2020, 4, 149. [Google Scholar] [CrossRef]
- Ageyeva, T.; Sibikin, I.; Kovács, J.G. A Review of Thermoplastic Resin Transfer Molding: Process Modeling and Simulation. Polymers 2019, 11, 1555. [Google Scholar] [CrossRef] [Green Version]
- Boros, R.; Sibikin, I.; Ageyeva, T.; Kovács, J.G. Development and Validation of a Test Mold for Thermoplastic Resin Transfer Molding of Reactive PA-6. Polymers 2020, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Papa, I.; Pagliarulo, V. Polypropylene/Basalt Fabric Laminates: Flexural Properties and Impact Damage Behavior. Polymers 2020, 12, 1079. [Google Scholar] [CrossRef]
- Asensio, M.; Esfandiari, P.; Núñez, K.; Silva, J.F.; Marques, A.; Merino, J.C.; Pastor, J.M. Processing of pre-impregnated thermoplastic towpreg reinforced by continuous glass fibre and recycled PET by pultrusion. Compos. Part B Eng. 2020, 200, 108365. [Google Scholar] [CrossRef]
- Asensio, M.; Nuñez, K.; Guerrero, J.; Herrero, M.; Merino, J.C.; Pastor, J.M. Rheological modification of recycled poly(ethylene terephthalate): Blending and reactive extrusion. Polym. Degrad. Stab. 2020, 179, 109258. [Google Scholar] [CrossRef]
- Seguela, R. Overview and critical survey of polyamide6 structural habits: Misconceptions and controversies. J. Polym. Sci. 2020, 58, 2971–3003. [Google Scholar] [CrossRef]
- Nakajima, H.; Dijkstra, P.; Loos, K. The recent developments in biobased polymers toward general and engineering applications: Polymers that are upgraded from biodegradable polymers, analogous to petroleum-derived polymers, and newly developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- Warakomski, J.M. Synthesis and Properties of Star-Branched Nylon 6. Chem. Mater. 1992, 4, 1000–1004. [Google Scholar] [CrossRef]
- Baumann, F.E.; Haeger, H.; Novikova, O.; Oenbrink, G.; Richter, R.; Finke, M. Synthesis and characterization of star-branched PA12 grafted onto PEi core molecule. J. Appl. Polym. Sci. 2005, 96, 2413–2422. [Google Scholar] [CrossRef]
- Martino, L.; Basilissi, L.; Farina, H.; Ortenzi, M.A.; Zini, E.; Di Silvestro, G.; Scandola, M. Bio-based polyamide 11: Synthesis, rheology and solid-state properties of star structures. Eur. Polym. J. 2014, 59, 69–77. [Google Scholar] [CrossRef]
- Ren, J.M.; McKenzie, T.G.; Fu, Q.; Wong, E.H.H.; Xu, J.; An, Z.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star Polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhou, L.; Liu, Y.; Xu, W.; Xiong, Y. High-Flow Nylon 6 by In Situ Polymerization: Synthesis and Characterization. J. Appl. Polym. Sci. 2008, 108, 2365–2372. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, L.; Xiong, Y.; Liu, G.; Xu, W. Department Isothermal Crystallization Kinetics of High-Flow Nylon 6 by Differential Scanning Calorimetry. J. Appl. Polym. Sci. 2009, 111, 2930–2937. [Google Scholar] [CrossRef]
- Fu, P.; Wang, M.; Liu, M.; Jing, Q.; Cai, Y.; Wang, Y.; Zhao, Q. Preparation and characterization of star-shaped nylon 6 with high flowability. J. Polym. Res. 2011, 18, 651–657. [Google Scholar] [CrossRef]
- Wan, J.; Li, C.; Fan, H.; Bu, Z.Y.; Li, B.G. Elucidating isothermal crystallization behaviors of nylon-11s. Influence of star-chain branching. Thermochim. Acta 2012, 544, 99–104. [Google Scholar] [CrossRef]
- Yuan, C.; Di Silvestro, G.; Speroni, F.; Guaita, C.; Zhang, H. Control of Macromolecular Architecture of Polyamides by Poly-functional Agents. 2. Use of Oligomerization in Polycondensation Study. Macromol. Symp. 2003, 199, 109–124. [Google Scholar] [CrossRef]
- Steeman, P.; Nijenhuis, A. The effect of random branching on the balance between flow and mechanical properties of polyamide-6. Polymer 2010, 51, 2700–2707. [Google Scholar] [CrossRef]
- Wan, J.; Bu, Z.Y.; Li, C.; Fan, H.; Li, B.G. Preparation, melting, glass relaxation and nonisothermal crystallization kinetics of a novel dendritic nylon-11. Thermochim. Acta 2011, 524, 117–127. [Google Scholar] [CrossRef]
- Zhu, N.; Gong, H.; Han, W.; Zeng, W.B.; Wang, H.X.; Fang, Z.; Li, X.; Zhang, K.; Li, Z.J.; Guo, K. Synthesis and characterization of star-branched polyamide 6 via anionic ring-opening polymerization with N,N′,N″-trimesoyltricaprolactam as a multifunctional activator. Chin. Chem. Lett. 2015, 26, 1389–1392. [Google Scholar] [CrossRef]
- Luo, Z.; Xia, B.; Fu, Z. Synthesis of star-shaped polymers by coupling reaction between multifunctional core and terminal functionalized polymers. J. Polym. Res. 2011, 18, 1983–1990. [Google Scholar] [CrossRef]
- Cai, J.; Liu, Z.; Cao, B.; Guan, X.; Liu, S.; Zhao, J. Simultaneous Improvement of the Processability and Mechanical Properties of Polyamide-6 by Chain Extension in Extrusion. Ind. Eng. Chem. Res. 2020, 59, 14334–14343. [Google Scholar] [CrossRef]
- Ozmen, S.C.; Ozkoc, G.; Serhatli, E. Thermal, mechanical and physical properties of chain extended recycled polyamide 6 via reactive extrusion: Effect of chain extender types. Polym. Degrad. Stab. 2019, 162, 76–84. [Google Scholar] [CrossRef]
- Tuna, B.; Benkreira, H. Reactive Extrusion of Polyamide 6 Using a Novel Chain Extender. Polym. Eng. Sci. 2019, 59, E25–E31. [Google Scholar] [CrossRef]
- Tuna, B.; Benkreira, H. Chain Extension of Recycled PA6. Polym. Eng. Sci. 2018, 58, 1037–1042. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.P.; Seo, Y. Effect of Molecular Structure Change on the Melt Rheological Properties of a Polyamide (Nylon 6). ACS Omega 2018, 3, 16549–16555. [Google Scholar] [CrossRef]
- Oh, K.; Kim, H.; Seo, Y. Effect of Diamine Addition on Structural Features and Physical Properties of Polyamide 6 Synthesized by Anionic Ring-Opening Polymerization of ϵ-Caprolactam. ACS Omega 2019, 4, 17117–17124. [Google Scholar] [CrossRef]
- Dencheva, N.; Nunes, T.; Oliveira, M.J.; Denchev, Z. Microfibrillar composites based on polyamide/polyethylene blends. 1. Structure investigations in oriented and isotropic polyamide 6. Polymer 2005, 46, 887–901. [Google Scholar] [CrossRef]
- González-De Los Santos, E.A.; López-Rodríguez, A.S.; Lozano-González, M.J.; Soriano-Corral, F. Starlike nylon 6/polyurethane block copolymers by reaction injection-molding process (RIM). J. Appl. Polym. Sci. 2001, 80, 2483–2494. [Google Scholar] [CrossRef]
- Pepin, J.; Gaucher, V.; Rochas, C.; Lefebvre, J.M. In-situ SAXS/WAXS investigations of the mechanically-induced phase transitions in semi-crystalline polyamides. Polymer 2019, 175, 87–98. [Google Scholar] [CrossRef]
- Michalski, A.; Brzezinski, M.; Lapienis, G.; Biela, T. Star-shaped and branched polylactides: Synthesis, characterization, and properties. Prog. Polym. Sci. 2019, 89, 159–212. [Google Scholar] [CrossRef]
- Yang, D.P.; Oo, M.N.N.L.; Deen, G.R.; Li, Z.; Loh, X.J. Nano-Star-Shaped Polymers for Drug Delivery Applications. Macromol. Rapid Commun. 2017, 38, 1700410. [Google Scholar] [CrossRef]
- Le Gac, P.Y.; Arhant, M.; Le Gall, M.; Davies, P. Yield stress changes induced by water in polyamide 6: Characterization and modeling. Polym. Degrad. Stab. 2017, 137, 272–280. [Google Scholar] [CrossRef] [Green Version]
- McKee, M.G.; Unal, S.; Wilkes, G.L.; Long, T.E. Branched polyesters: Recent advances in synthesis and performance. Prog. Polym. Sci. 2005, 30, 507–539. [Google Scholar] [CrossRef]
- Abdelwahab, M.; Codou, A.; Anstey, A.; Mohanty, A.K.; Misra, M. Studies on the dimensional stability and mechanical properties of nanobiocomposites from polyamide 6-filled with biocarbon and nanoclay hybrid systems. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105695. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Yang, L.; Zhang, F.; Xie, L.; Luo, Z.; Xiang, K. Synergistic effect of polyfunctional silane coupling agent and styrene acrylonitrile copolymer on the water-resistant and mechanical performances of glass fiber–reinforced polyamide 6. Polym. Adv. Technol. 2019, 30, 1951–1958. [Google Scholar] [CrossRef]
- Yan, X.; Imai, Y.; Shimamoto, D.; Hotta, Y. Relationship study between crystal structure and thermal/mechanical properties of polyamide 6 reinforced and unreinforced by carbon fiber from macro and local view. Polymer 2014, 55, 6186–6194. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.; Yan, W.; Qin, J.; He, M.; Qin, S.; Yu, J. Enhanced mechanical and thermal properties of polyamide 6/p (N-(4-F-phenylmaleimide)-alt-styrene) composites based on interfacial complexation inducing crystal transformation. Polymer 2021, 214, 123237. [Google Scholar] [CrossRef]
- Chen, K.; Jia, M.; Sun, H.; Xue, P. Thermoplastic reaction injection pultrusion for continuous glass fiber-reinforced polyamide-6 composites. Materials 2019, 12, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jongbloed, B.; Teuwen, J.; Palardy, G.; Fernandez Villegas, I.; Benedictus, R. Continuous ultrasonic welding of thermoplastic composites: Enhancing the weld uniformity by changing the energy director. J. Compos. Mater. 2020, 54, 2023–2035. [Google Scholar] [CrossRef]
- Villegas, I.F. In situ monitoring of ultrasonic welding of thermoplastic composites through power and displacement data. J. Thermoplast. Compos. Mater. 2015, 28, 66–85. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Luo, Y.; Zhang, Z.; Wang, L. Study on heating process of ultrasonic welding for thermoplastics. J. Thermoplast. Compos. Mater. 2010, 23, 647–664. [Google Scholar] [CrossRef]
- Gutnik, V.G.; Gorbach, N.V.; Dashkov, A.V. Some characteristics of ultrasonic welding of polymers. Fibre Chem. 2002, 34, 426–432. [Google Scholar] [CrossRef]
- Sýkorová, L.; Šuba, O.; Žaludek, M.; Kubišová, M. The strength study of ultrasonically welded thermoplastic. Mater. Sci. Forum 2019, 952, 135–142. [Google Scholar] [CrossRef]
- Ochôa, P.; Fernandez Villegas, I.; Groves, R.M.; Benedictus, R. Experimental assessment of the influence of welding process parameters on Lamb wave transmission across ultrasonically welded thermoplastic composite joints. Mech. Syst. Signal. Process. 2018, 99, 197–218. [Google Scholar] [CrossRef] [Green Version]






| Sample | Mw a (g/mol) | Mn a (g/mol) | Mw/Mn | Xc b ± 1 % | Tc c ± 0.5 °C | Tm d ± 0.5 °C | Td e ± 0.5 °C | MFI f (g/10min) | [ƞ] g (dL/g) | Mz g |
|---|---|---|---|---|---|---|---|---|---|---|
| PA6 | 90,778 | 72,981 | 1.24 | 37.6 | 191.7 | 221.0 | 460.2 | 25 | 0.85 | 24,902 |
| 0.5sPA6 | 85,405 | 74,890 | 1.14 | 36.3 | 193.3 | 219.3 | 459.6 | 26 | 0.80 | 23,304 |
| 1sPA6 | 84,617 | 74,197 | 1.14 | 35.0 | 193.9 | 219.0 | 457.1 | 29 | 0.77 | 22,462 |
| 2.5sPA6 | 81,109 | 71,549 | 1.13 | 34.4 | 192.6 | 218.0 | 450.7 | 48 | 0.70 | 20,330 |
| 5sPA6 | 77,120 | 68,335 | 1.13 | 31.0 | 176.0 | 217.0 | 448.6 | 95 | 0.59 | 16,921 |
| Sample | Young’s Modulus (MPa) | Elongation at Break (%) | Charpy Impact (kJ/m2) | HDT (°C) |
|---|---|---|---|---|
| PA6 | 2084 ± 21 | 46 ± 14 | 4.5 ± 1 | 70 ± 1 |
| 0.5sPA6 | 2815 ± 64 | 34 ± 9 | 3.9 ± 1 | 98 ± 2 |
| 1sPA6 | 2956 ± 44 | 10 ± 3 | 1.9 ± 0.5 | 109 ± 1 |
| 2.5sPA6 | 3061 ± 68 | <1 | 1.3 ± 0.5 | 113 ± 1 |
| 5sPA6 | 3160 ± 88 | <1 | 0.9 ± 0.3 | 115 ± 1 |
| Specimens | Welded Test Specimens | |||
|---|---|---|---|---|
| Thermoplastic Composite | Energy Director Based on 2.5sPA6 | Max. Strength | Displacement | Tensile Strength |
| (N) | (mm) | (MPa) | ||
| 2.5sPA6 matrix | No | 2059 | 0.88 | 12.2 |
| 2.5sPA6 matrix | Yes | 2134 | 0.91 | 14.5 |
| Raw PA6 matrix | No | 1782 | 1.32 | 8.4 |
| Raw PA6 matrix | Yes | 1898 | 1.74 | 10.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez Carrero, K.C.; Herrero, M.; Asensio, M.; Guerrero, J.; Merino, J.C.; Pastor, J.M. Star-Branched Polyamides as the Matrix in Thermoplastic Composites. Polymers 2022, 14, 942. https://doi.org/10.3390/polym14050942
Núñez Carrero KC, Herrero M, Asensio M, Guerrero J, Merino JC, Pastor JM. Star-Branched Polyamides as the Matrix in Thermoplastic Composites. Polymers. 2022; 14(5):942. https://doi.org/10.3390/polym14050942
Chicago/Turabian StyleNúñez Carrero, Karina C., Manuel Herrero, María Asensio, Julia Guerrero, Juan Carlos Merino, and José María Pastor. 2022. "Star-Branched Polyamides as the Matrix in Thermoplastic Composites" Polymers 14, no. 5: 942. https://doi.org/10.3390/polym14050942
APA StyleNúñez Carrero, K. C., Herrero, M., Asensio, M., Guerrero, J., Merino, J. C., & Pastor, J. M. (2022). Star-Branched Polyamides as the Matrix in Thermoplastic Composites. Polymers, 14(5), 942. https://doi.org/10.3390/polym14050942