Glass Transition Temperature of PLGA Particles and the Influence on Drug Delivery Applications
Abstract
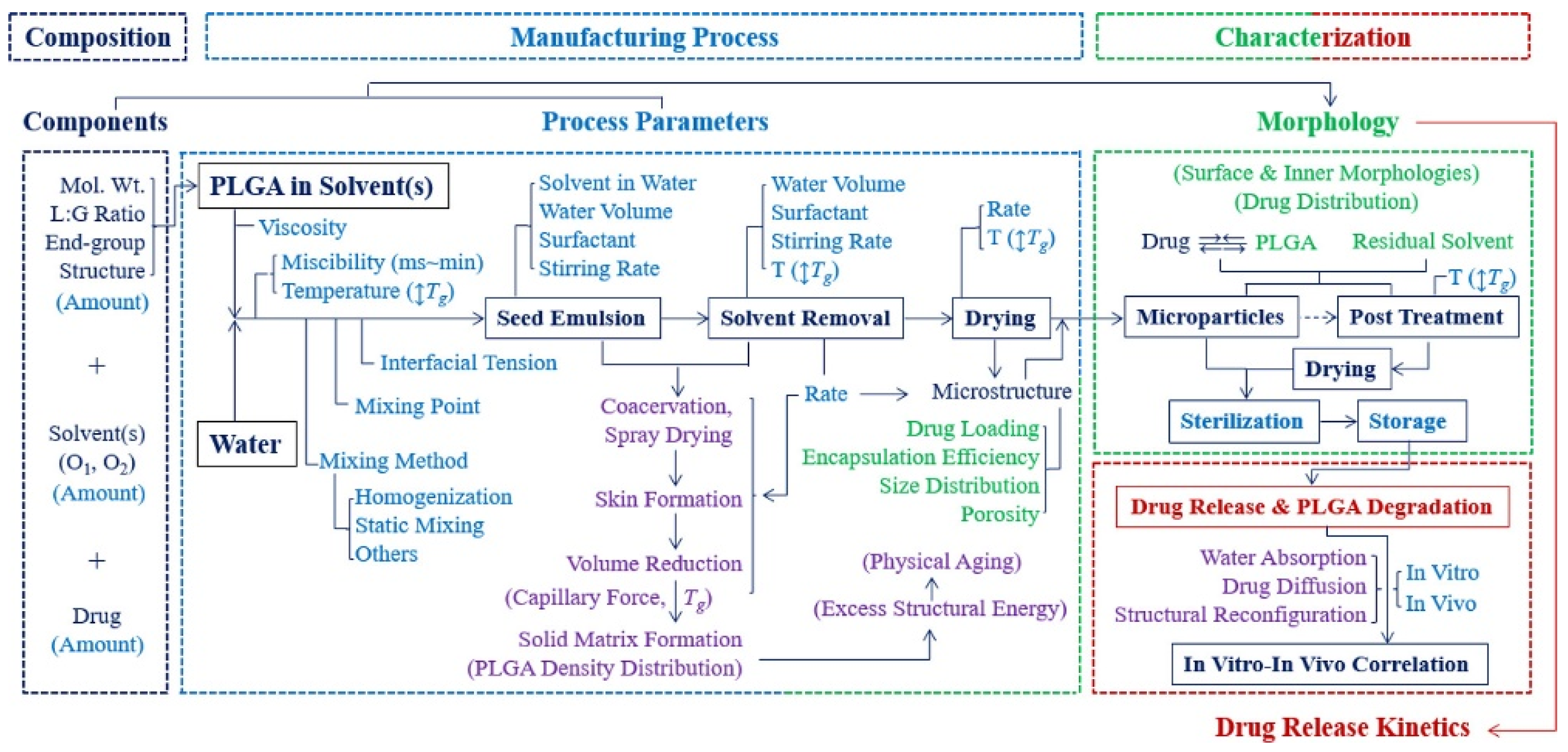
:1. Introduction
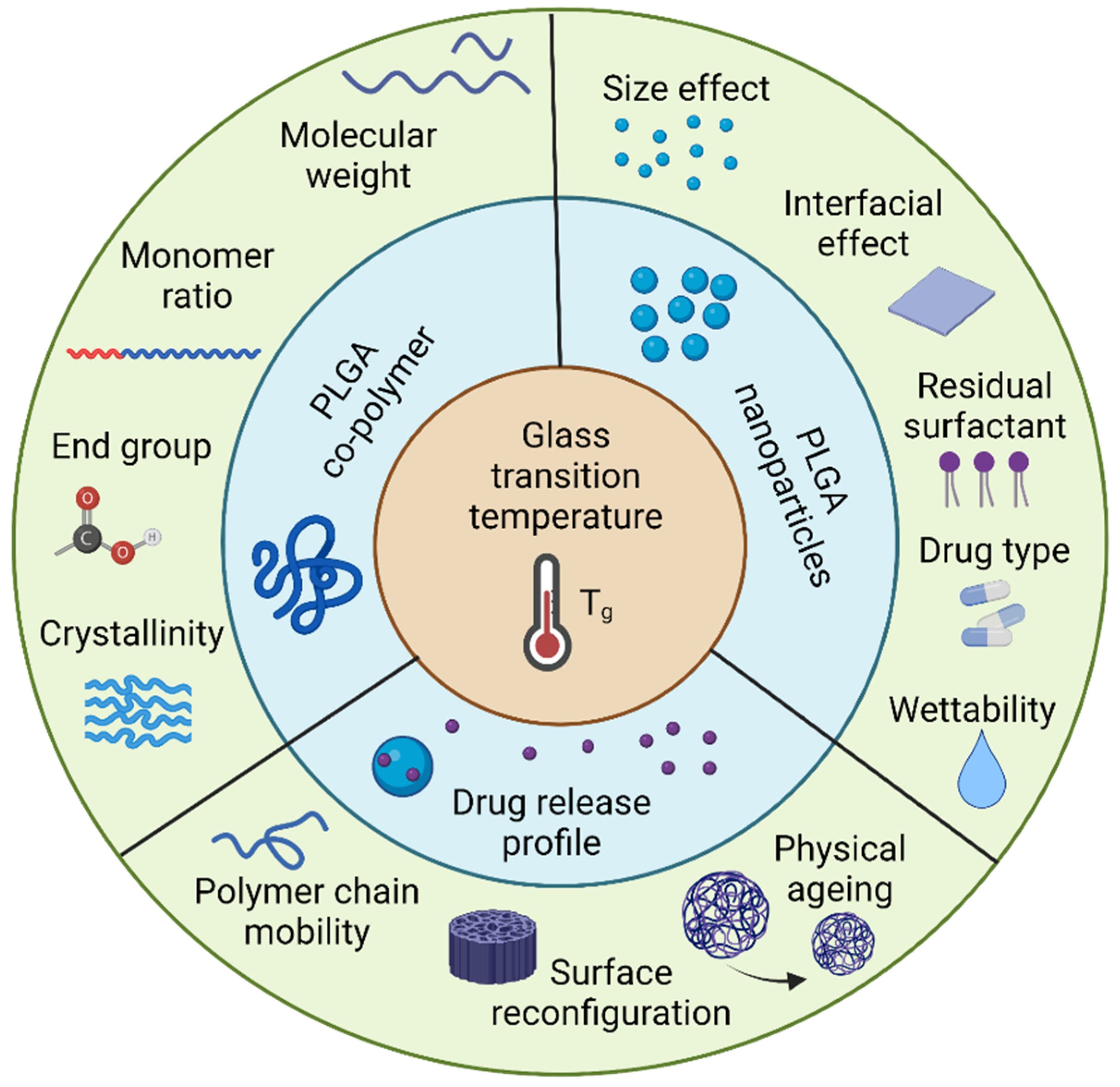
2. Glass Transition Temperature of PLGA Particles
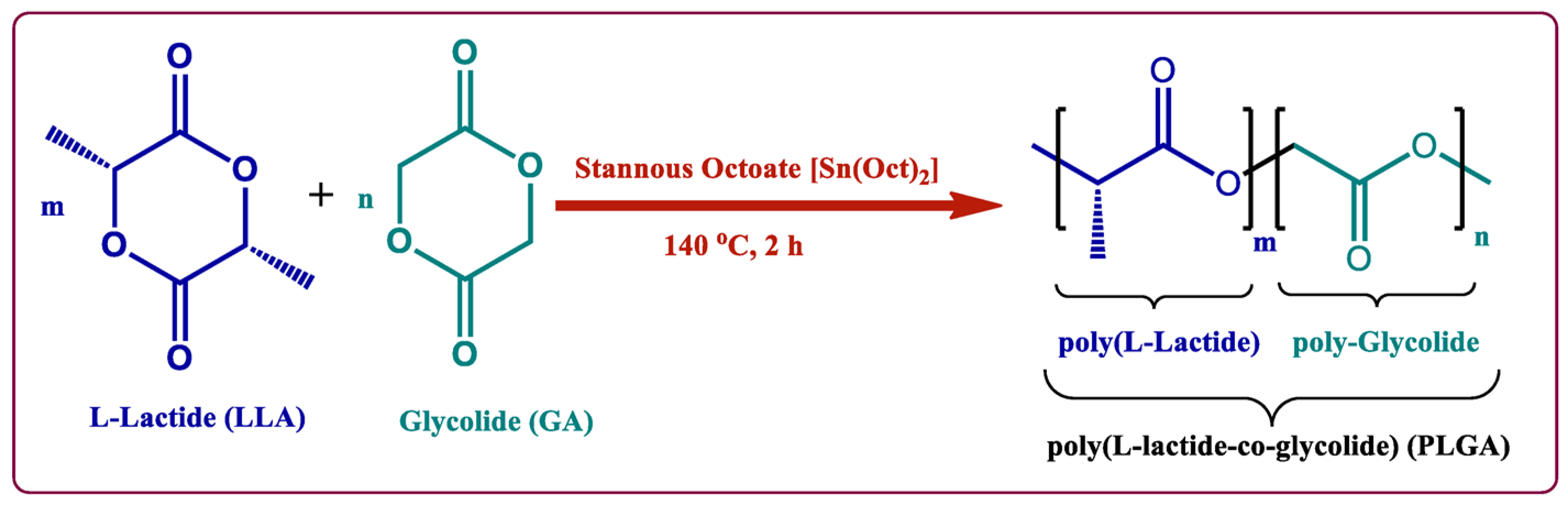
2.1. PLGA Copolymer
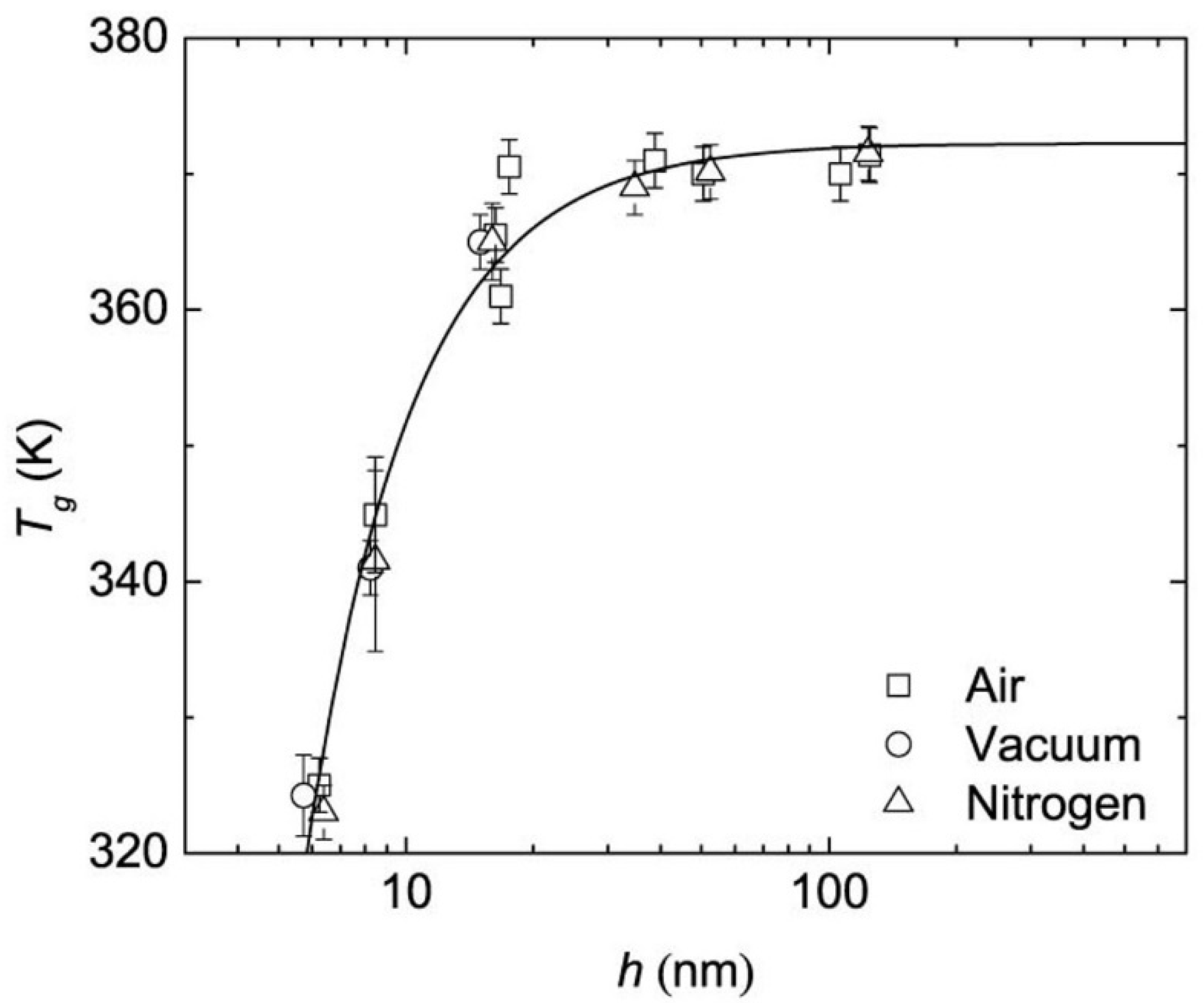
2.2. Glass Transition Temperature of Polymeric Particles
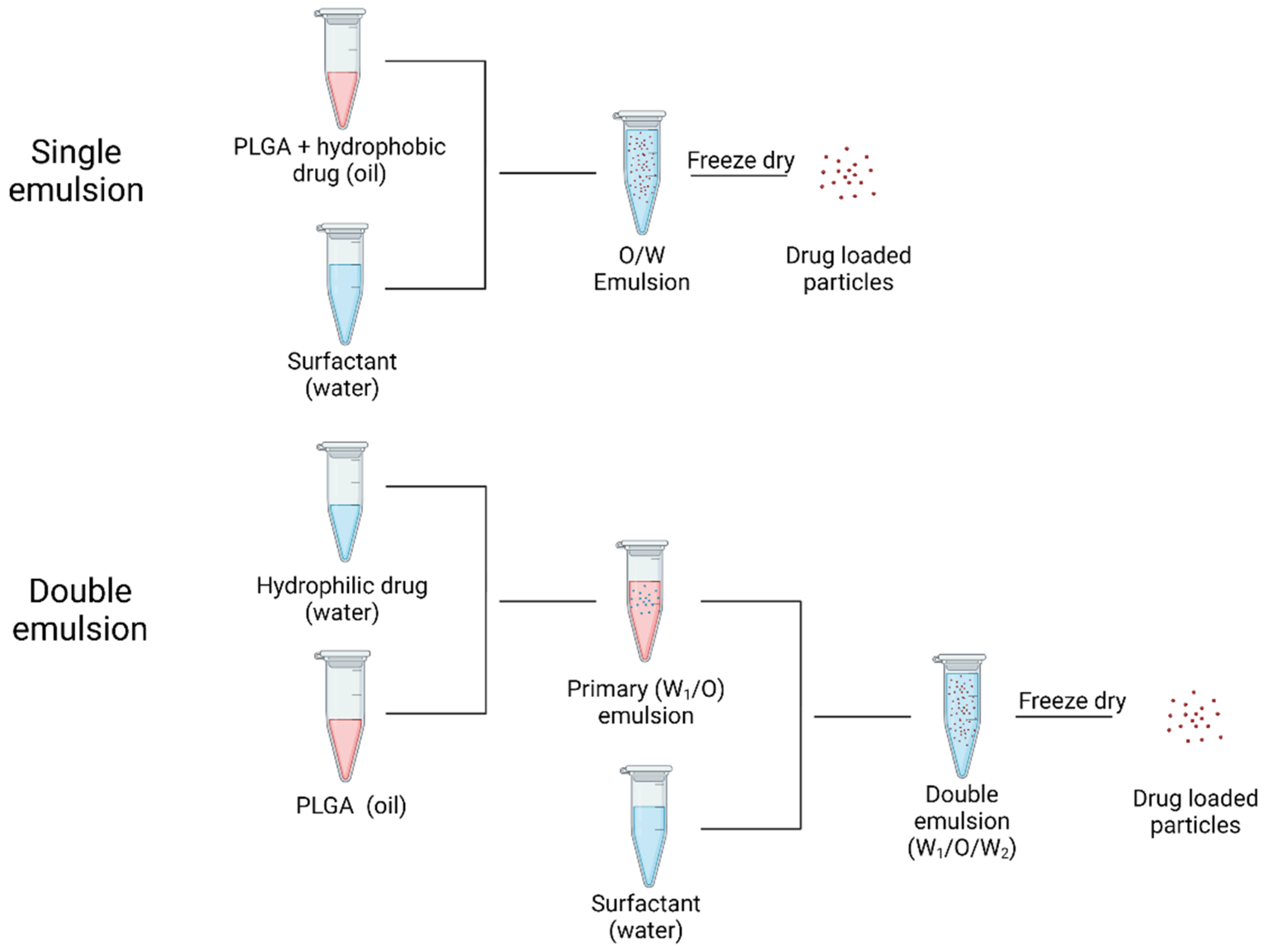
2.3. Drug Effect
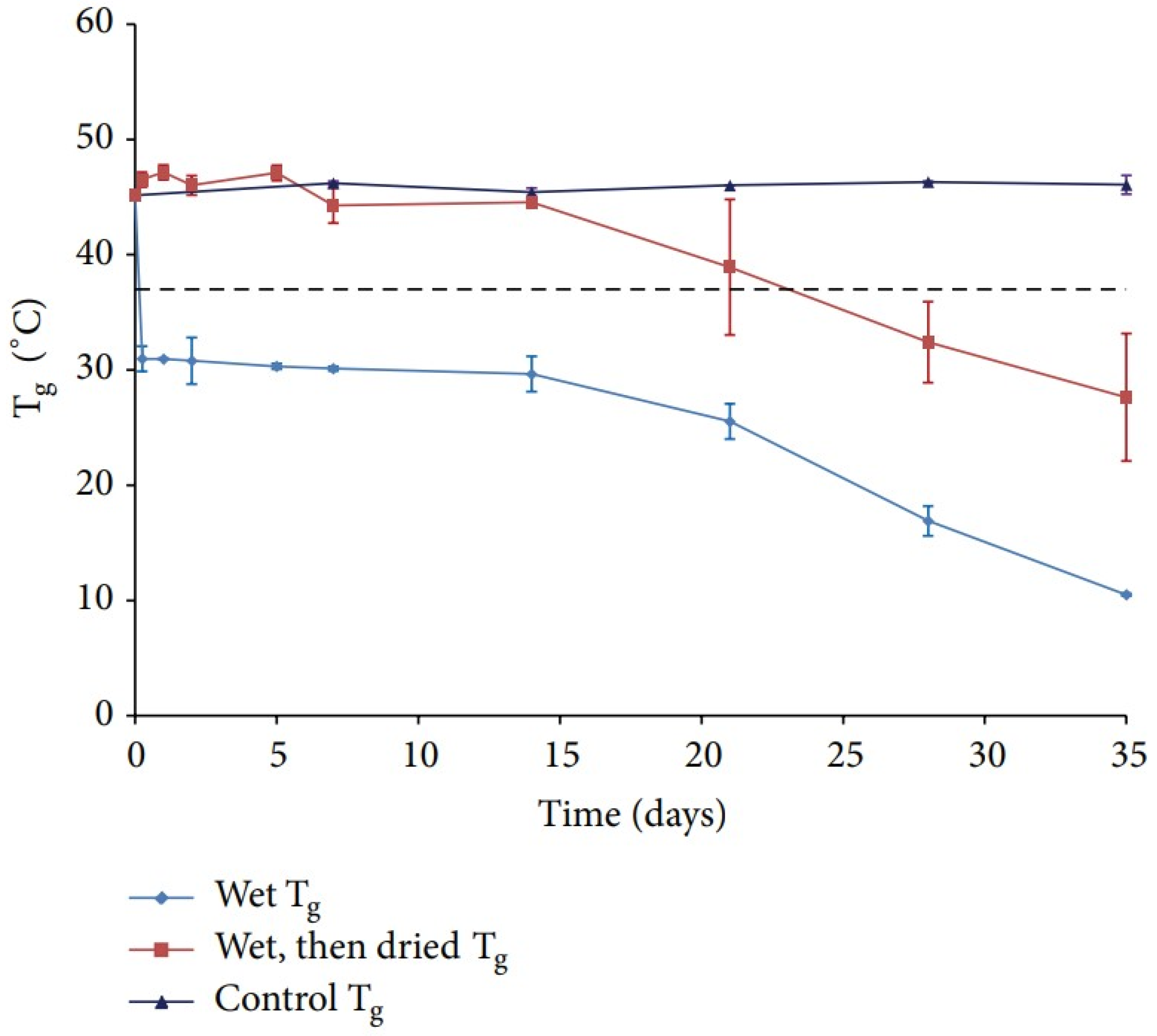
2.4. Water Content
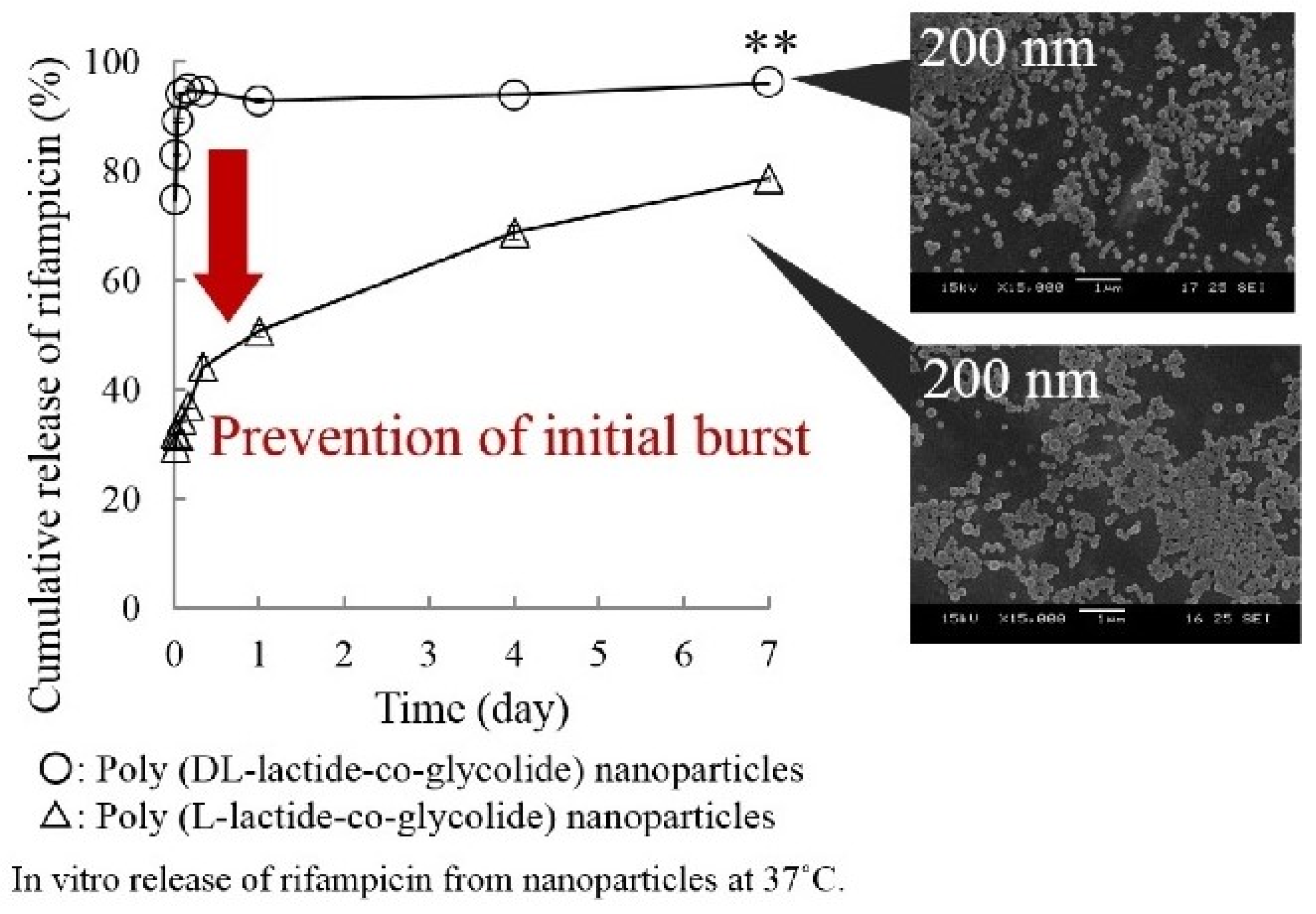
2.5. Residual Surfactant
3. Influence of Tg on Drug Delivery
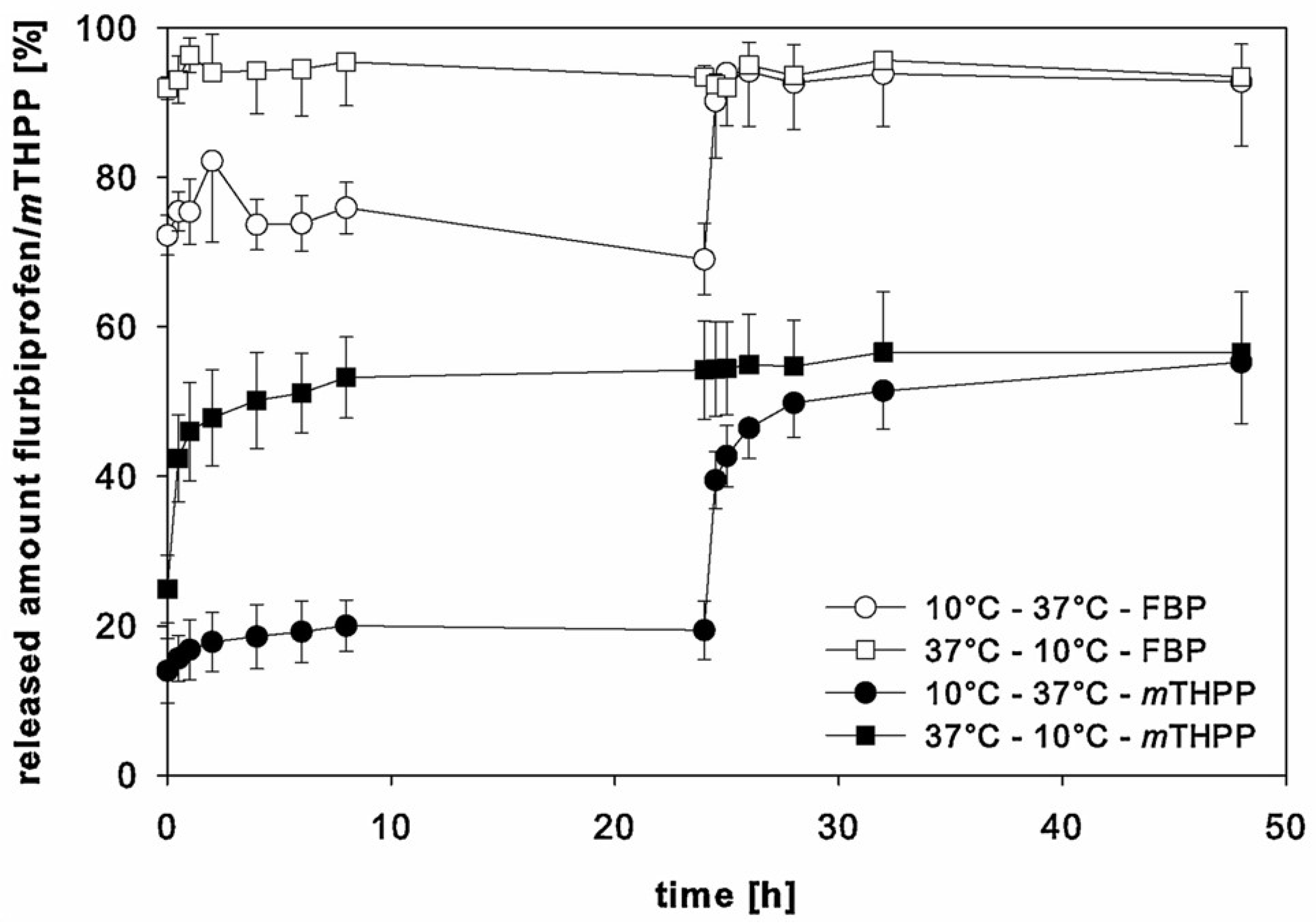
3.1. Particle Mobility
3.2. Physical Ageing of Particles
3.3. Surface Reconfiguration
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zeng, X.; Tao, W.; Meiab, L.; Huangab, L.; Tanab, C.; Fengabc, S.S. Cholic acid-functionalized nanoparticles of star-shaped PLGA-vitamin E TPGS copolymer for docetaxel delivery to cervical cancer. Biomaterials 2013, 34, 6058–6067. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-Y.; Pho, Q.-H.; Wu, X.-Y.; Chin, T.-Y.; Chen, C.-M.; Fang, P.-H.; Lin, Y.-C.; Hsieh, M.-F. PLGA Microspheres Loaded with β-Cyclodextrin Complexes of Epigallocatechin-3-Gallate for the Anti-Inflammatory Properties in Activated Microglial Cells. Polymers 2018, 10, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, N.-Q.; Zhou, J.; Walker, J.; Li, L.; Hong, J.K.; Olsen, K.F.; Tang, J.; Ackermann, R.; Wang, Y.; Qin, B.; et al. Microencapsulation of luteinizing hormone-releasing hormone agonist in poly (lactic-co-glycolic acid) microspheres by spray-drying. J. Control Release 2020, 321, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; An, N.; Zhang, H.; Zhang, Q.; Zhang, K.; Hu, X.; Wu, Y.; Wu, F.; Xiao, J.; Zhang, H.; et al. Sustained-release of PDGF from PLGA microsphere embedded thermo-sensitive hydrogel promoting wound healing by inhibiting autophagy. J. Drug Deliv. Sci. Technol. 2019, 55, 101405. [Google Scholar] [CrossRef]
- Zhai, J.; Wang, Y.-E.; Zhou, X.; Ma, Y.; Guan, S. Long-term sustained release Poly(lactic-co-glycolic acid) microspheres of asenapine maleate with improved bioavailability for chronic neuropsychiatric diseases. Drug Deliv. 2020, 27, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, L.; Wan, F.; Bera, H.; Cun, D.; Rantanen, J.; Yang, M. Quality by design thinking in the development of long-acting injectable PLGA/PLA-based microspheres for peptide and protein drug delivery. Int. J. Pharm. 2020, 585, 119441. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, F.; Duan, W.; Mu, X.; Fang, S.; Lu, N.; Zhou, X.; Kong, W. Engineering a “PEG-g-PEI/DNA nanoparticle-in- PLGA microsphere” hybrid controlled release system to enhance immunogenicity of DNA vaccine. Mater. Sci. Eng. C 2019, 106, 110294. [Google Scholar] [CrossRef]
- Lee, P.W.; Pokorski, J.K. Poly(lactic-co-glycolic acid) devices: Production and applications for sustained protein delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1516. [Google Scholar] [CrossRef]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional Polymeric Nanoplatforms for Brain Diseases Diagnosis, Therapy and Theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Desai, N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef] [Green Version]
- Zhai, P.; Chen, X.; Schreyer, D.J. PLGA/alginate composite microspheres for hydrophilic protein delivery. Mater. Sci. Eng. C 2015, 56, 251–259. [Google Scholar] [CrossRef]
- Ozeki, T.; Kaneko, D.; Hashizawa, K.; Imai, Y.; Tagami, T.; Okada, H. Improvement of survival in C6 rat glioma model by a sustained drug release from localized PLGA microspheres in a thermoreversible hydrogel. Int. J. Pharm. 2012, 427, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, V.L.; Hartmayer, C.; Planz, O.; Groettrup, M. Cytotoxic T cell vaccination with PLGA microspheres interferes with influenza A virus replication in the lung and suppresses the infectious disease. J. Control. Release 2015, 216, 121–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alange, V.V.; Birajdar, R.P.; Kulkarni, R.V. Functionally modified polyacrylamide-graft-gum karaya pH-sensitive spray dried microspheres for colon targeting of an anti-cancer drug. Int. J. Biol. Macromol. 2017, 102, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Terry, T.L.; Givens, B.E.; Rodgers, V.; Salem, A.K. Tunable Properties of Poly-DL-Lactide-Monomethoxypolyethylene Glycol Porous Microparticles for Sustained Release of Polyethylenimine-DNA Polyplexes. AAPS PharmSciTech 2019, 20, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, G.; Yang, G.; He, Y.; Li, X.; Du, T.; Xu, L.; Zhou, S. Uniformly sized hollow microspheres loaded with polydopamine nanoparticles and doxorubicin for local chemo-photothermal combination therapy. Chem. Eng. J. 2020, 379. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Y.; Li, B.-Z.; Adhikari, B.; Wang, Y.; Huang, H.-L.; Chen, D. Production of protein-loaded starch microspheres using water-in-water emulsion method. Carbohydr. Polym. 2019, 231, 115692. [Google Scholar] [CrossRef]
- Zhou, X.; Hou, C.; Chang, T.-L.; Zhang, Q.; Liang, J.F. Controlled released of drug from doubled-walled PVA hydrogel/PCL microspheres prepared by single needle electrospraying method. Colloids Surf. B Biointerfaces 2019, 187, 110645. [Google Scholar] [CrossRef]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef]
- Grigsby, C.; Leong, K.W. Balancing protection and release of DNA: Tools to address a bottleneck of non-viral gene delivery. J. R. Soc. Interface 2009, 7, S67–S82. [Google Scholar] [CrossRef]
- Putney, S.D.; Burke, P.A. Improving protein therapeutics with sustained-release formulations. Nat. Biotechnol. 1998, 16, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Lerch, S.; Dass, M.; Musyanovych, A.; Landfester, K.; Mailänder, V. Polymeric nanoparticles of different sizes overcome the cell membrane barrier. Eur. J. Pharm. Biopharm. 2013, 84, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood–brain barrier by nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef]
- Wang, D.; Wu, L.-P. Nanomaterials for delivery of nucleic acid to the central nervous system (CNS). Mater. Sci. Eng. C 2017, 70, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Moradian, H.; Keshvari, H.; Fasehee, H.; Dinarvand, R.; Faghihi, S. Combining NT3-overexpressing MSCs and PLGA microcarriers for brain tissue engineering: A potential tool for treatment of Parkinson’s disease. Mater. Sci. Eng. C 2017, 76, 934–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Liu, X.; Chen, X.; Chua, M.X.; Wu, Y.-L. Targeted delivery of Bcl-2 conversion gene by MPEG-PCL-PEI-FA cationic copolymer to combat therapeutic resistant cancer. Mater. Sci. Eng. C 2017, 76, 66–72. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, Z.; Wu, C.; Wang, X.; Liow, S.S.; Li, Z.; Wu, Y.-L.; Cheng, H.; Wu, Z.; Wu, C.; et al. Overcoming STC2 mediated drug resistance through drug and gene co -delivery by PHB-PDMAEMA cationic polyester in liver cancer cells. Mater. Sci. Eng. C 2018, 83, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef]
- Peres, C.; de Matos, A.I.N.; Conniot, J.; Sainz, V.; Zupančič, E.; Silva, J.M.; Graca, L.; Gaspar, R.; Préat, V.; Florindo, H.F. Poly(lactic acid)-based particulate systems are promising tools for immune modulation. Acta Biomater. 2017, 48, 41–57. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Karnik, R.; Gu, F.; Basto, P.; Cannizzaro, C.; Dean, L.; Kyei-Manu, W.; Langer, R.; Farokhzad, O.C. Microfluidic Platform for Controlled Synthesis of Polymeric Nanoparticles. Nano Lett. 2008, 8, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.T.; Streck, S.; Hook, S.M.; McDowell, A. Utilization of Microfluidics for the Preparation of Polymeric Nanoparticles for the Antioxidant Rutin: A Comparison with Bulk Production. Pharm. Nanotechnol. 2019, 7, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Lappe, S.; Mulac, D.; Langer, K. Polymeric nanoparticles—Influence of the glass transition temperature on drug release. Int. J. Pharm. 2017, 517, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, I.; Tomoda, K.; Hamano, A.; Makino, K. Effects of physicochemical properties of poly(lactide-co-glycolide) on drug release behavior of hydrophobic drug-loaded nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 771–778. [Google Scholar] [CrossRef]
- Takeuchi, I.; Yamaguchi, S.; Goto, S.; Makino, K. Drug release behavior of hydrophobic drug-loaded poly (lactide-co-glycolide) nanoparticles: Effects of glass transition temperature. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 328–333. [Google Scholar] [CrossRef]
- Park, K.; Otte, A.; Sharifi, F.; Garner, J.; Skidmore, S.; Park, H.; Jhon, Y.K.; Qin, B.; Wang, Y. Formulation composition, manufacturing process, and characterization of poly(lactide-co-glycolide) microparticles. J. Control Release 2020, 329, 1150–1161. [Google Scholar] [CrossRef]
- Djemour, A.; Sanctuary, R.; Baller, J. Mobility restrictions and glass transition behaviour of an epoxy resin under confinement. Soft Matter 2015, 11, 2683–2690. [Google Scholar] [CrossRef]
- Huang, M.; Tunnicliffe, L.B.; Thomas, A.G.; Busfield, J.J. The glass transition, segmental relaxations and viscoelastic behaviour of particulate-reinforced natural rubber. Eur. Polym. J. 2015, 67, 232–241. [Google Scholar] [CrossRef]
- Samith, V.; Ramos-Moore, E. Study of glass transition in functionalized poly(itaconate)s by differential scanning calorimetry, Raman spectroscopy and thermogravimetric analysis. J. Non Cryst. Solids 2015, 408, 37–42. [Google Scholar] [CrossRef]
- Raczkowska, J.; Stetsyshyn, Y.; Awsiuk, K.; Lekka, M.; Marzec, M.; Harhay, K.; Ohar, H.; Ostapiv, D.; Sharan, M.; Yaremchuk, I.; et al. Temperature-responsive grafted polymer brushes obtained from renewable sources with potential application as substrates for tissue engineering. Appl. Surf. Sci. 2017, 407, 546–554. [Google Scholar] [CrossRef]
- Stetsyshyn, Y.; Raczkowska, J.; Lishchynskyi, O.; Awsiuk, K.; Zemla, J.; Dąbczyński, P.; Kostruba, A.; Harhay, K.; Ohar, H.; Orzechowska, B. Glass transition in temperature-responsive poly (butyl methacrylate) grafted polymer brushes. Impact of thickness and temperature on wetting, morphology, and cell growth. J. Mater. Chem. B 2018, 6, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Carballido, A.; Puebla, P.; Herrero-Vanrell, R.; Pastoriza, P. Radiosterilisation of indomethacin PLGA/PEG-derivative microspheres: Protective effects of low temperature during gamma-irradiation. Int. J. Pharm. 2006, 313, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, C.; Espejo, L.; Torrado, S.; Torrado, J.J. Effect of c-Sterilization Process on PLGA Microspheres Loaded with Insulin-Like Growth Factor-I (IGF-I). J. Biomater. Appl. 2003, 18, 95–108. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; Rehman, A.U. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, C.-S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug-polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 1692–1716. [Google Scholar] [CrossRef]
- Ding, D.; Zhu, Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater. Sci. Eng. C 2018, 92, 1041–1060. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [Green Version]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of hybrid PLGA nanoparticles: Future of smart drug delivery and theranostics medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- Cunha, A.; Gaubert, A.; Latxague, L.; Dehay, B. PLGA-Based Nanoparticles for Neuroprotective Drug Delivery in Neurodegenerative Diseases. Pharmaceutics 2021, 13, 1042. [Google Scholar] [CrossRef]
- Erbetta, C.D.C. Synthesis and Characterization of Poly(D,L-Lactide-co-Glycolide) Copolymer. J. Biomater. Nanobiotechnol. 2012, 3, 208–225. [Google Scholar] [CrossRef]
- Butreddy, A.; Gaddam, R.P.; Kommineni, N.; Dudhipala, N.; Voshavar, C. PLGA/PLA-Based Long-Acting Injectable Depot Microspheres in Clinical Use: Production and Characterization Overview for Protein/Peptide Delivery. Int. J. Mol. Sci. 2021, 22, 8884. [Google Scholar] [CrossRef] [PubMed]
- Robin, B.; Albert, C.; Beladjine, M.; Legrand, F.-X.; Geiger, S.; Moine, L.; Nicolas, V.; Canette, A.; Trichet, M.; Tsapis, N.; et al. Tuning morphology of Pickering emulsions stabilised by biodegradable PLGA nanoparticles: How PLGA characteristics influence emulsion properties. J. Colloid Interface Sci. 2021, 595, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Streck, S.; Neumann, H.; Nielsen, H.M.; Rades, T.; McDowell, A. Comparison of bulk and microfluidics methods for the formulation of poly-lactic-co-glycolic acid (PLGA) nanoparticles modified with cell-penetrating peptides of different architectures. Int. J. Pharm. X 2019, 1, 100030. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) As biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wang, S.; Zhu, L.; Wang, F.; Zhou, W. The effect of glycolic acid monomer ratio on the emulsifying activity of PLGA in preparation of protein-loaded SLN. Colloids Surf. B Biointerfaces 2009, 74, 358–361. [Google Scholar] [CrossRef]
- Park, P.I.P.; Jonnalagadda, S. Predictors of glass transition in the biodegradable poly-lactide and poly-lactide-co-glycolide polymers. J. Appl. Polym. Sci. 2006, 100, 1983–1987. [Google Scholar] [CrossRef]
- Brostow, W.; Chiu, R.; Kalogeras, I.M.; Vassilikou-Dova, A. Prediction of glass transition temperatures: Binary blends and copolymers. Mater. Lett. 2008, 62, 3152–3155. [Google Scholar] [CrossRef]
- Koerner, J.; Horvath, D.; Groettrup, M. Harnessing Dendritic Cells for Poly (D,L-lactide-co-glycolide) Microspheres (PLGA MS)—Mediated Anti-tumor Therapy. Front. Immunol. 2019, 10, 707. [Google Scholar] [CrossRef] [Green Version]
- Hsu, M.-Y.; Feng, C.-H.; Liu, Y.-W.; Liu, S.-J. An Orthogonal Model to Study the Effect of Electrospraying Parameters on the Morphology of poly (d,l)-lactide-co-glycolide (PLGA) Particles. Appl. Sci. 2019, 9, 1077. [Google Scholar] [CrossRef] [Green Version]
- Fox, T.G., Jr.; Flory, P.J. Second-order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Zeng, X.M.; Martin, G.P.; Marriott, C. Effects of molecular weight of polyvinylpyrrolidone on the glass transition and crystallization of co-lyophilized sucrose. Int. J. Pharm. 2001, 218, 63–73. [Google Scholar] [CrossRef]
- Lee, J.S.; Chae, G.S.; Khang, G.; Kim, M.S.; Cho, S.H.; Lee, H.B. The effect of gamma irradiation on PLGA and release behavior of BCNU from PLGA wafer. Macromol. Res. 2003, 11, 352–356. [Google Scholar] [CrossRef]
- Mizuno, A.; Mitsuiki, M.; Motoki, M. Effect of Crystallinity on the Glass Transition Temperature of Starch. J. Agric. Food Chem. 1998, 46, 98–103. [Google Scholar] [CrossRef]
- Kawai, K.; Fukami, K.; Thanatuksorn, P.; Viriyarattanasak, C.; Kajiwara, K. Effects of moisture content, molecular weight, and crystallinity on the glass transition temperature of inulin. Carbohydr. Polym. 2011, 83, 934–939. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, C.Z.; Tanaka, K.; Takahara, A.; Kajiyama, T. Effect of chain end group on surface glass transition temperature of thin polymer film. Phys. Lett. A 2001, 281, 363–367. [Google Scholar] [CrossRef]
- Keddie, J.L.; Jones, R.A.; Cory, R.A. Size-Dependent Depression of the Glass Transition Temperature in Polymer Films. Eur. Lett. 1994, 27, 59–64. [Google Scholar] [CrossRef]
- Raegen, A.; Massa, M.V.; Forrest, J.A.; Dalnoki-Veress, K. Effect of atmosphere on reductions in the glass transition of thin polystyrene films. Eur. Phys. J. E 2008, 27, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, Y.; Priestley, R.D. Glass Transition Temperature of Polymer Nanoparticles under Soft and Hard Confinement. Macromolecules 2011, 44, 4001–4006. [Google Scholar] [CrossRef]
- Christie, D.; Zhang, C.; Fu, J.; Koel, B.; Priestley, R.D. Glass transition temperature of colloidal polystyrene dispersed in various liquids. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1776–1783. [Google Scholar] [CrossRef]
- Feng, S.; Li, Z.; Liu, R.; Mai, B.; Wu, Q.; Liang, G.; Gao, H.; Zhu, F. Glass transition of polystyrene nanospheres under different confined environments in aqueous dispersions. Soft Matter 2013, 9, 4614–4620. [Google Scholar] [CrossRef]
- Ramazani, F.; Chen, W.; Van Nostrum, C.F.; Storm, G.; Kiessling, F.; Lammers, T.; Hennink, W.E.; Kok, R.J. Strategies for encapsulation of small hydrophilic and amphiphilic drugs in PLGA microspheres: State-of-the-art and challenges. Int. J. Pharm. 2016, 499, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Walboomers, X.F.; Jansen, J.A.; Yang, F. Influence of formulation parameters on encapsulation of doxycycline in PLGA microspheres prepared by double emulsion technique for the treatment of periodontitis. J. Drug Deliv. Sci. Technol. 2019, 52, 263–271. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Fan, C.; Yu, K.; Liu, X.; Zhao, X.; Wang, D.; Liu, W.; Su, Z.; Sun, F.; et al. Development of near zero-order release PLGA-based microspheres of a novel antipsychotic. Int. J. Pharm. 2017, 516, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, L.; Su, L.; Wu, X.; Wang, Y.; Liu, L.; Lin, X. Design of a zero-order sustained release PLGA microspheres for palonosetron hydrochloride with high encapsulation efficiency. Int. J. Pharm. 2019, 575, 119006. [Google Scholar] [CrossRef] [PubMed]
- Wischke, C.; Schwendeman, S.P. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int. J. Pharm. 2008, 364, 298–327. [Google Scholar] [CrossRef]
- Ansary, R.H.; Awang, M.B.; Rahman, M.M. Biodegradable Poly(D,L-lactic-co-glycolic acid)-Based Micro/Nanoparticles for Sustained Release of Protein Drugs—A Review. Trop. J. Pharm. Res. 2014, 13, 1179. [Google Scholar] [CrossRef] [Green Version]
- Barreras-Urbina, C.G.; Ramírez-Wong, B.; López-Ahumada, G.A.; Ibarra, S.E.B.; Martínez-Cruz, O.; Tapia-Hernández, J.A.; Félix, F.R. Nano- and Micro-Particles by Nanoprecipitation: Possible Application in the Food and Agricultural Industries. Int. J. Food Prop. 2016, 19, 1912–1923. [Google Scholar] [CrossRef]
- Morais, A.S.; Vieira, E.G.; Afewerki, S.; Sousa, R.B.; Honorio, L.; Cambrussi, A.N.C.O.; Santos, J.A.; Bezerra, R.D.S.; Furtini, J.A.O.; Silva-Filho, E.C.; et al. Fabrication of Polymeric Microparticles by Electrospray: The Impact of Experimental Parameters. J. Funct. Biomater. 2020, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Cabezas, L.I.; Gracia, I.; De Lucas, A.; Rodriguez, J.F. Validation of a Mathematical Model for the Description of Hydrophilic and Hydrophobic Drug Delivery from Biodegradable Foams: Experimental and Comparison Using Indomethacin as Released Drug. Ind. Eng. Chem. Res. 2014, 53, 8866–8873. [Google Scholar] [CrossRef]
- Hans, M.; Lowman, A. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Lee, L.Y.; Ranganath, S.H.; Fu, Y.; Zheng, J.L.; Lee, H.S.; Wang, C.-H.; Smith, K.A. Paclitaxel release from micro-porous PLGA disks. Chem. Eng. Sci. 2009, 64, 4341–4349. [Google Scholar] [CrossRef]
- Siegel, S.; Kahn, J.B.; Metzger, K.; Winey, K.I.; Werner, K.; Dan, N. Effect of drug type on the degradation rate of PLGA matrices. Eur. J. Pharm. Biopharm. 2006, 64, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, F.; Le Brun, V.; Siepmann, J. Drugs acting as plasticizers in polymeric systems: A quantitative treatment. J. Control. Release 2006, 115, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Grune, C.; Zens, C.; Czapka, A.; Scheuer, K.; Thamm, J.; Hoeppener, S.; Jandt, K.D.; Werz, O.; Neugebauer, U.; Fischer, D. Sustainable preparation of anti-inflammatory atorvastatin PLGA nanoparticles. Int. J. Pharm. 2021, 599, 120404. [Google Scholar] [CrossRef]
- Holz, J.P.; Bottene, M.K.; Jahno, V.; Einloft, S.; Ligabue, R. Menthol-loaded PLGA Micro and Nanospheres: Synthesis, Characterization and Degradation in Artificial Saliva. Mater. Res. 2018, 21, 488. [Google Scholar] [CrossRef] [Green Version]
- Shakiba, S.; Astete, C.E.; Cueto, R.; Rodrigues, D.F.; Sabliov, C.M.; Louie, S.M. Asymmetric flow field-flow fractionation (AF4) with fluorescence and multi-detector analysis for direct, real-time, size-resolved measurements of drug release from polymeric nanoparticles. J. Control Release 2021, 338, 410–421. [Google Scholar] [CrossRef]
- Farid, E.A.; Davachi, S.M.; Pezeshki-Modaress, M.; Taranejoo, S.; Seyfi, J.; Hejazi, I.; Hakim, M.T.; Najafi, F.; D’Amico, C.; Abbaspourrad, A. Preparation and characterization of polylactic-co-glycolic acid/insulin nanoparticles encapsulated in methacrylate coated gelatin with sustained release for specific medical applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 910–937. [Google Scholar] [CrossRef]
- Sokol, M.B.; Nikolskaya, E.; Yabbarov, N.; Zenin, V.A.; Faustova, M.R.; Belov, A.V.; Zhunina, O.A.; Mollaev, M.D.; Zabolotsky, A.I.; Tereshchenko, O.G.; et al. Development of novel PLGA nanoparticles with co-encapsulation of docetaxel and abiraterone acetate for a highly efficient delivery into tumor cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 1150–1158. [Google Scholar] [CrossRef]
- Sedki, M.; Khalil, I.; El-Sherbiny, I.M. Hybrid nanocarrier system for guiding and augmenting simvastatin cytotoxic activity against prostate cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, S641–S650. [Google Scholar] [CrossRef] [Green Version]
- Levine, H.; Slade, L. Water as a plasticizer: Physico-chemical aspects of low-moisture polymeric systems. In Water Science Reviews 3: Water Dynamics; Cambridge University Press: Cambridge, UK, 1988; pp. 79–185. [Google Scholar] [CrossRef]
- Passerini, N.; Craig, D. An investigation into the effects of residual water on the glass transition temperature of polylactide microspheres using modulated temperature DSC. J. Control Release 2001, 73, 111–115. [Google Scholar] [CrossRef]
- D’Souza, S.; Dorati, R.; DeLuca, P.P. Effect of Hydration on Physicochemical Properties of End-Capped PLGA. Adv. Biomater. 2014, 2014, 834942. [Google Scholar] [CrossRef] [Green Version]
- Anton, N.; Benoit, J.-P.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates—A review. J. Control Release 2008, 128, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Bouissou, C.; Rouse, J.J.; Price, R.; Van Der Walle, C.F. The Influence of Surfactant on PLGA Microsphere Glass Transition and Water Sorption: Remodeling the Surface Morphology to Attenuate the Burst Release. Pharm. Res. 2006, 23, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Panyam, J.; Prabha, S.; Labhasetwar, V. Residual polyvinyl alcohol associated with poly (d,l-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J. Control Release 2002, 82, 105–114. [Google Scholar] [CrossRef]
- Spek, S.; Haeuser, M.; Schaefer, M.; Langer, K. Characterisation of PEGylated PLGA nanoparticles comparing the nanoparticle bulk to the particle surface using UV/vis spectroscopy, SEC, 1H NMR spectroscopy, and X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2015, 347, 378–385. [Google Scholar] [CrossRef]
- Roth, C.B. Polymer Glasses, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 4–5. [Google Scholar]
- Priestley, R.D.; Ellison, C.J.; Broadbelt, L.J.; Torkelson, J.M. Structural Relaxation of Polymer Glasses at Surfaces, Interfaces, and in between. Science 2005, 309, 456–459. [Google Scholar] [CrossRef]
- Struik, L. Physical Aging in Amorphous Polymers and Other Materials; Elsevier: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Allison, S.D. Effect Of Structural Relaxation On The Preparation And Drug Release Behavior Of Poly(lactic-co-glycolic)acid Microparticle Drug Delivery Systems. J. Pharm. Sci. 2008, 97, 2022–2035. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kawazoe, N.; Tateishi, T.; Chen, G. Effects of Structural Change Induced by Physical Aging on the Biodegradation Behavior of PLGA Films at Physiological Temperature. Macromol. Mater. Eng. 2011, 296, 1028–1034. [Google Scholar] [CrossRef]
- Brunacci, A.; Cowie, J.; Ferguson, R.; McEwen, I. Enthalpy relaxation in glassy polystyrenes: 1. Polymer 1997, 38, 865–870. [Google Scholar] [CrossRef]
- Dias, M.H.M.; Jansen, K.M.B.; Luinge, J.W.; Bersee, H.E.N.; Benedictus, R. Effect of fiber-matrix adhesion on the creep behavior of CF/PPS composites: Temperature and physical aging characterization. Mech. Time Depend. Mater. 2016, 20, 245–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blasi, P.; D’Souza, S.S.; Selmin, F.; DeLuca, P.P. Plasticizing effect of water on poly(lactide-co-glycolide). J. Control Release 2005, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Skidmore, S.; Hadar, J.; Garner, J.; Park, H.; Otte, A.; Soh, B.K.; Yoon, G.; Yu, D.; Yun, Y.; et al. Injectable, long-acting PLGA formulations: Analyzing PLGA and understanding microparticle formation. J. Control Release 2019, 304, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Otte, A.; Sharifi, F.; Garner, J.; Skidmore, S.; Park, H.; Jhon, Y.K.; Qin, B.; Wang, Y. Potential Roles of the Glass Transition Temperature of PLGA Microparticles in Drug Release Kinetics. Mol. Pharm. 2020, 18, 18–32. [Google Scholar] [CrossRef] [PubMed]





| PLGA LA:GA Mol wt. (g/mol) | Diameter (nm) | Model Drug | Preparation | Tg (°C) | Measurement Heating Rate | Ref. |
|---|---|---|---|---|---|---|
| 50:50 7000–17,000 | Around 200 | None | STM 1 | 39.35 | DSC 10 °C/min | [85] |
| Around 180 | Atorvastatin | STM | 42.49 | |||
| Around 170 | None | SUM 2 | 30.24 | |||
| Around 190 | Atorvastatin | SUM | 35.02 | |||
| 50:50 54,000–69,000 | Around 240 | None | STM | 47.66 | DSC 10 °C/min | [85] |
| Around 230 | Atorvastatin | STM | 47.62 | |||
| Around 225 | None | SUM | 25.98 | |||
| Around 180 | Atorvastatin | SUM | 28.00 | |||
| 85:15 Unknown | 391+/−160 | Menthol | W/O/W | 48.0 | DSC 10 °C/min | [86] |
| 75:25 14,000 3 | 162+/−3 | None | Emulsion-evaporation | 32.7+/−0.2 | DSC 5 °C/min | [53] |
| 75:25 32,000 3 | 155+/−5 | None | Emulsion-evaporation | 37.6+/−0.2 | DSC 5 °C/min | [53] |
| 75:25 32,000 4 | 213+/−18 | None | Emulsion-evaporation | 37.2+/−0.4 | DSC 5 °C/min | [53] |
| 75:25 14,000 4 | 238+/−18 | None | Emulsion-evaporation | 24.8+/−0.6 | DSC 5 °C/min | [53] |
| 50:50 38,000–54,000 | Unknown | Enrofloxacin | Emulsification-diffusion | 32.9+/−0.8 | MDSC 5 °C/min | [87] |
| Unknown | None | 31.26 | ||||
| 62:38 18,400 | 282+/−43 | Insulin | Emulsification-diffusion | 43.14 | DSC 10 °C/min | [88] |
| 50:50 Unknown | 211.9+/−2 | Abiraterone acetate | Modified single emulsion | 45.64 | DSC 5 °C/min | [89] |
| 170.9+/−2.1 | Docetaxel | 45.93 | ||||
| 256.3+/−9.4 | Abiraterone acetate/Docetaxel | 46.61 | ||||
| 50:50 Unknown | 179+/−13 | Rutin | Single solvent evaporation | 46.19 | DSC 5 °C/min | [33] |
| 123+/−4 | Rutin | Microfluidics | 44.03 | |||
| 75:25 Unknown | Unknown | Simvastatin | Emulsion solvent evaporation | 51.5 | DSC 10 °C/min | [90] |
| Unknown | 226.8+/−6.8 | Flurbiprofen | Emulsion diffusion | 28.8+/−0.6 | DSC 20 °C/min | [34] |
| 224.2+/−5.3 | Flurbiprofen | 26.9+/−0.5 | ||||
| 222.8+/−4.8 | Flurbiprofen | 25.3+/−1.1 | ||||
| 216.0+/−3.8 | Flurbiprofen | 22.4+/−1.5 | ||||
| 223.3+/−11.7 | Flurbiprofen | 19.9+/−1.6 | ||||
| 237.4+/−9.1 | mTHPP | 32.4+/−1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; McEnnis, K. Glass Transition Temperature of PLGA Particles and the Influence on Drug Delivery Applications. Polymers 2022, 14, 993. https://doi.org/10.3390/polym14050993
Liu G, McEnnis K. Glass Transition Temperature of PLGA Particles and the Influence on Drug Delivery Applications. Polymers. 2022; 14(5):993. https://doi.org/10.3390/polym14050993
Chicago/Turabian StyleLiu, Guangliang, and Kathleen McEnnis. 2022. "Glass Transition Temperature of PLGA Particles and the Influence on Drug Delivery Applications" Polymers 14, no. 5: 993. https://doi.org/10.3390/polym14050993
APA StyleLiu, G., & McEnnis, K. (2022). Glass Transition Temperature of PLGA Particles and the Influence on Drug Delivery Applications. Polymers, 14(5), 993. https://doi.org/10.3390/polym14050993






