Effects of Functionalized Kraft Lignin Incorporation on Polypropylene Surface Energy and Practical Adhesion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
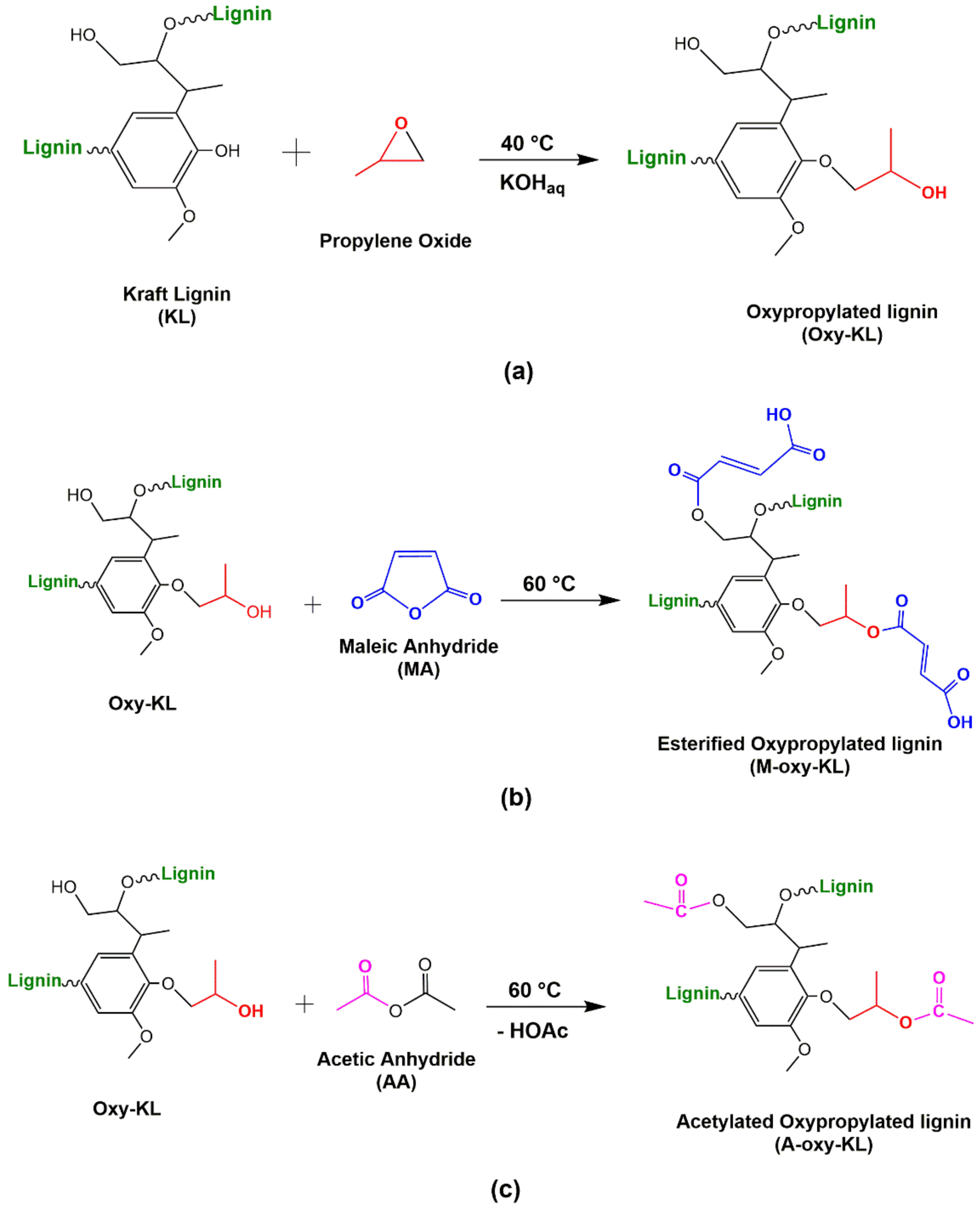
2.2. Modification of KL
2.3. Lignins Characterization
2.3.1. Fourier Transform Infrared Spectroscopy-Attenuated Total Reflectance (FTIR-ATR)
2.3.2. Thermogravimetric Analysis
2.3.3. Differential Scanning Calorimetry (DSC)
2.4. Composites Preparation
2.5. Composites Characterization
2.5.1. Differential Scanning Calorimetry (DSC)
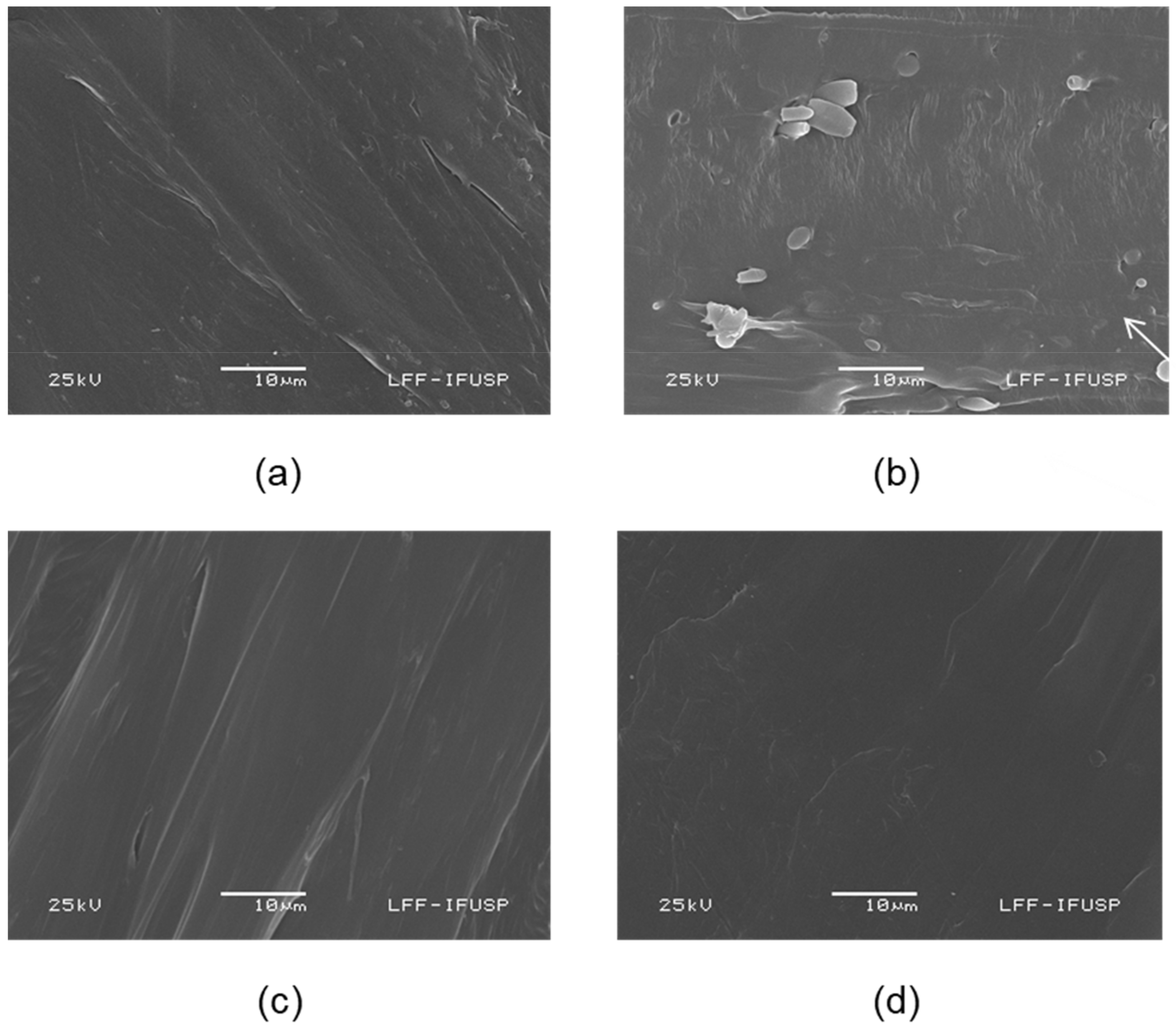
2.5.2. Scanning Electron Microscopy (SEM)
2.5.3. Surface Energy
2.5.4. Practical Adhesion
Lamination Process
T-Peel Test
3. Results and Discussion
3.1. Lignins Modification
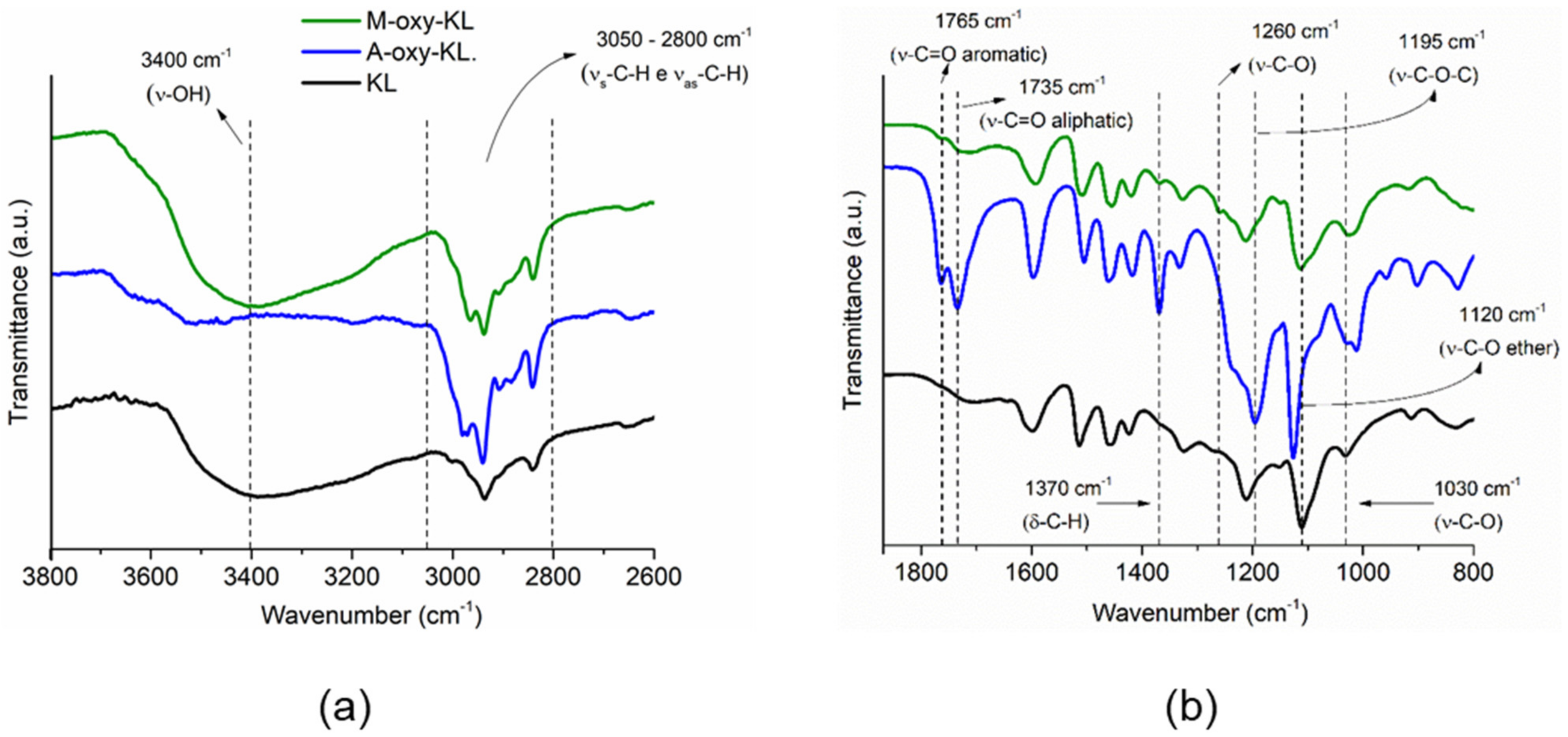
3.1.1. FTI-ATR
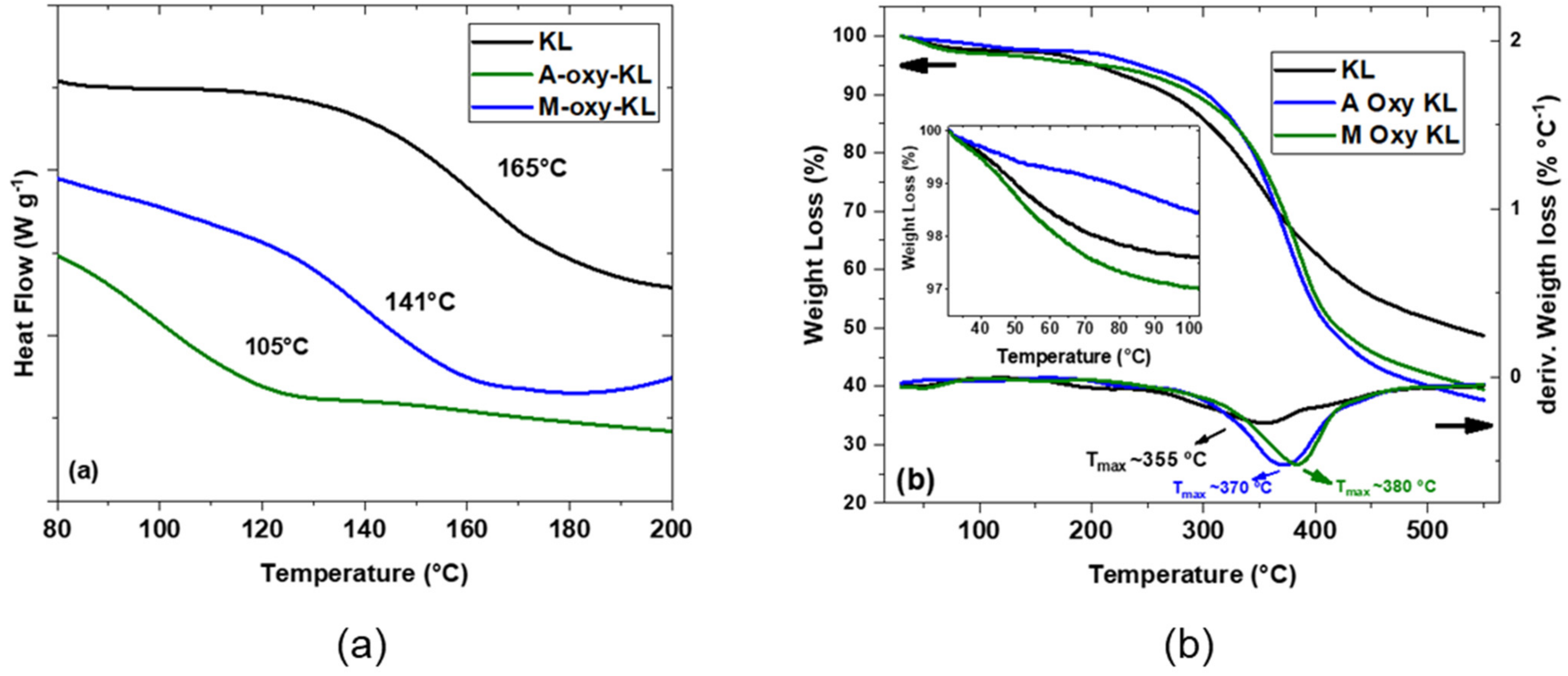
3.1.2. Lignin Thermal Analysis
3.2. Composites Characterization
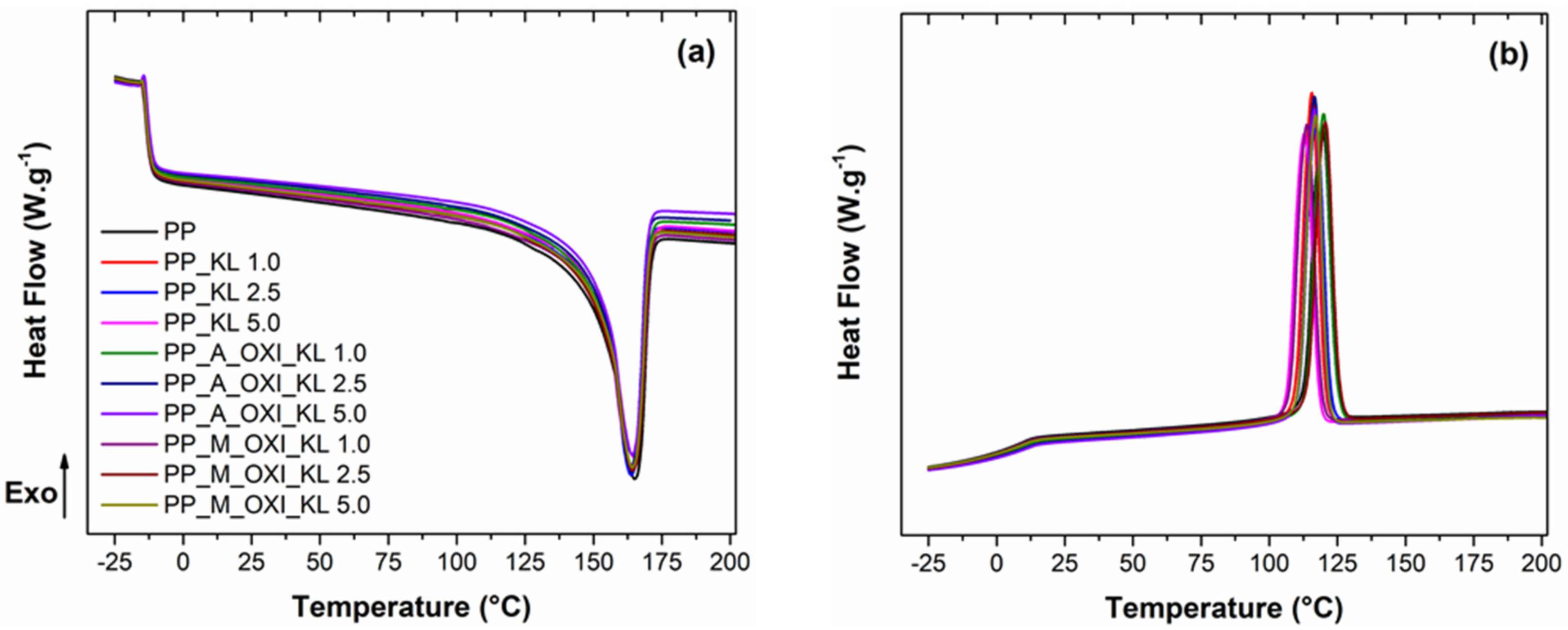
3.2.1. DSC
3.2.2. SEM
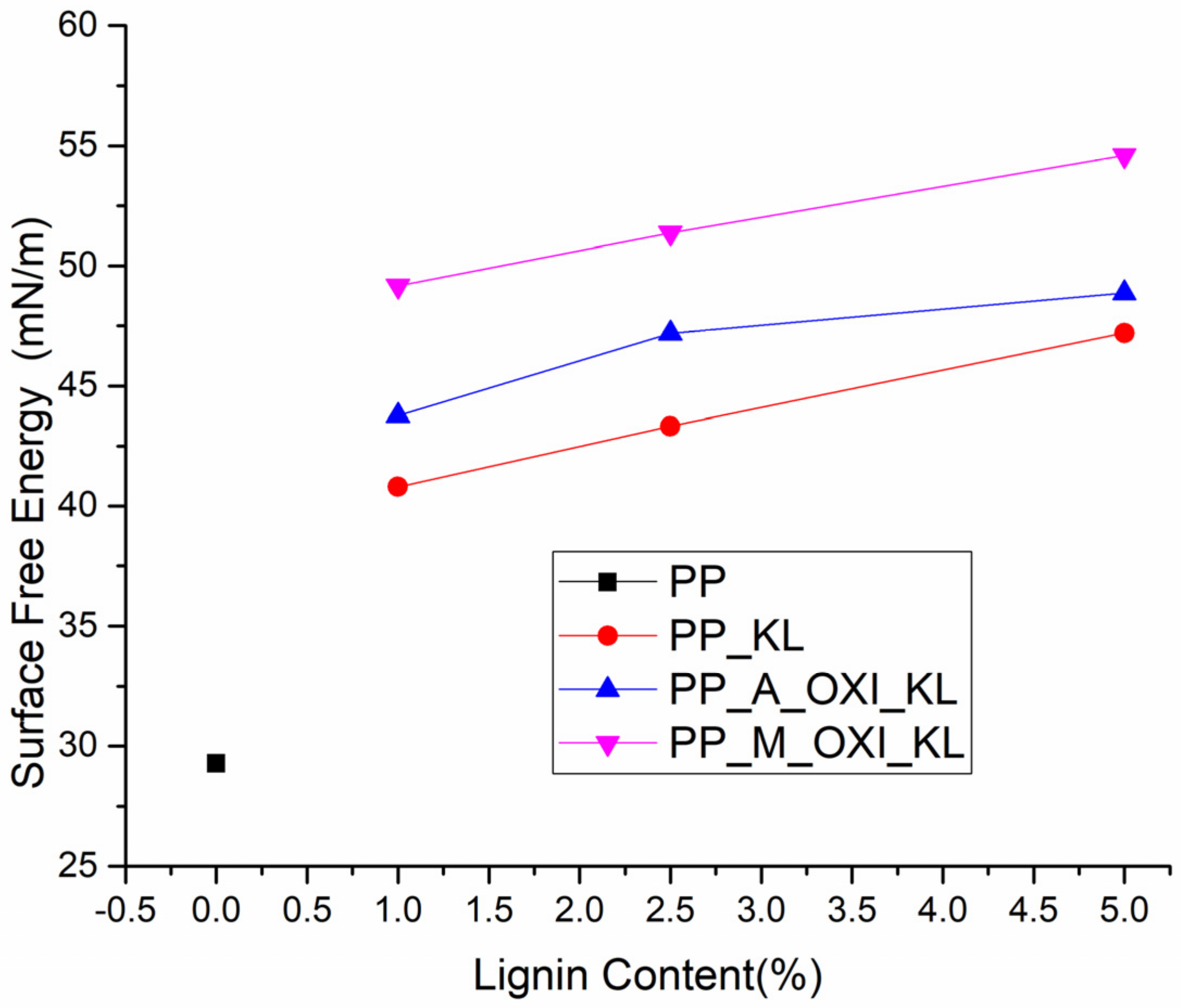
3.2.3. Surface Energy
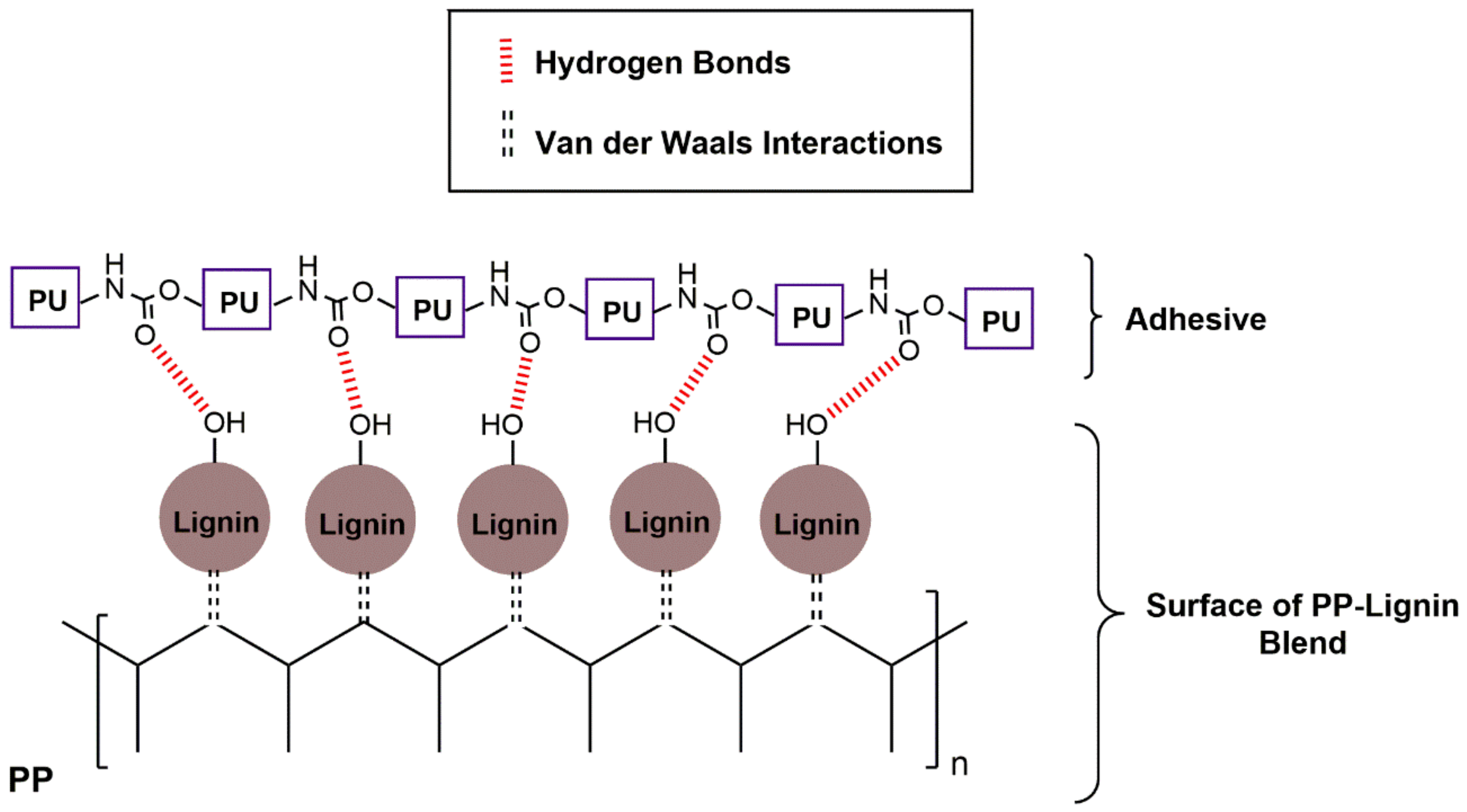
3.2.4. T-Peel Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaymakci, A.; Ayrilmis, N. Surface Roughness and Wettability of Polypropylene Composites Filled with Fast-Growing Biomass: Paulownia Elongata Wood. J. Compos. Mater. 2014, 48, 951–957. [Google Scholar] [CrossRef]
- Hernández-Aguirre, O.A.; Núñez-Pineda, A.; Tapia-Tapia, M.; Espinosa, R.M.G. Surface Modification of Polypropylene Membrane Using Biopolymers with Potential Applications for Metal Ion Removal. J. Chem. 2016, 2016. [Google Scholar] [CrossRef]
- Xia, Z.; Li, J.; Zhang, J.; Zhang, X.; Zheng, X.; Zhang, J. Processing and Valorization of Cellulose, Lignin and Lignocellulose Using Ionic Liquids. J. Bioresour. Bioprod. 2020, 5, 79–95. [Google Scholar] [CrossRef]
- Yu, O.; Kim, K.H. Lignin to Materials: A Focused Review on Recent Novel Lignin Applications. Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.A. Valorization of Lignin in Polymer and Composite Systems for Advanced Engineering Applications—A Review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Yuan, T.Q.; Song, G.Y.; Sun, R.C. Advanced and Versatile Lignin-Derived Biodegradable Composite Film Materials toward a Sustainable World. Green Chem. 2021, 23, 3790–3817. [Google Scholar] [CrossRef]
- De Sousa, R.R.; Gouveia, J.R.; Nacas, A.M.; Tavares, L.B.; Ito, N.M.; de Moura, E.N.; Gaia, F.A.; Pereira, R.F.; dos Santos, D.J. Improvement of Polypropylene Adhesion by Kraft Lignin Incorporation. Mater. Res. 2019, 22. [Google Scholar] [CrossRef]
- Pouteau, C.; Dole, P.; Cathala, B.; Averous, L.; Boquillon, N. Antioxidant Properties of Lignin in Polypropylene. Polym. Degrad. Stab. 2003, 81, 9–18. [Google Scholar] [CrossRef]
- Gadioli, R.; Waldman, W.R.; de Paoli, M.A. Lignin as a Green Primary Antioxidant for Polypropylene. J. Appl. Polym. Sci. 2016, 133, 1–7. [Google Scholar] [CrossRef]
- Toriz, G.; Denes, F.; Young, R.A. Lignin-Polypropylene Composites. Part 1: Composites from Unmodified Lignin and Polypropylene. Polym. Compos. 2002, 23, 806–813. [Google Scholar] [CrossRef]
- Gadioli, R.; Morais, J.A.; Waldman, W.R.; de Paoli, M.A. The Role of Lignin in Polypropylene Composites with Semi-Bleached Cellulose Fibers: Mechanical Properties and Its Activity as Antioxidant. Polym. Degrad. Stab. 2014, 108, 23–34. [Google Scholar] [CrossRef]
- Xu, X.; He, Z.; Lu, S.; Guo, D.; Yu, J. Enhanced Thermal and Mechanical Properties of Lignin/Polypropylene Wood-Plastic Composite by Using Flexible Segment-Containing Reactive Compatibilizer. Macromol. Res. 2014, 22, 1084–1089. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Z.; Xiao, Y.; Pan, Z.; Jia, P.; Feng, G.; Bao, C.; Zhou, Y.; Hua, L. Lignin-Based Phenolic Resin Modified with Whisker Silicon and Its Application. J. Bioresour. Bioprod. 2020, 5, 67–77. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Li, P.; He, C. Conversion of Biomass Lignin to High-Value Polyurethane: A Review. J. Bioresour. Bioprod. 2020, 5, 163–179. [Google Scholar] [CrossRef]
- García, D.E.; Glasser, W.G.; Pizzi, A.; Paczkowski, S.; Laborie, M.-P. Hydroxypropyl Tannin from Pinus Pinaster Bark as Polyol Source in Urethane Chemistry. Eur. Polym. J. 2015, 67, 152–165. [Google Scholar] [CrossRef]
- Chen, Y.; Stark, N.M.; Cai, Z.; Frihart, C.R.; Lorenz, L.F.; Ibach, R.E. Chemical Modification of Kraft Lignin: Effect on Chemical and Thermal Properties. BioResources 2014, 9, 5488–5500. [Google Scholar] [CrossRef] [Green Version]
- Monteil-Rivera, F.; Paquet, L. Solvent-Free Catalyst-Free Microwave-Assisted Acylation of Lignin. Ind. Crops Prod. 2015, 65, 446–453. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. (Eds.) Polymer Handbook, 4th ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Tavares, L.B.; Ito, N.M.; Salvadori, M.C.; dos Santos, D.J.; Rosa, D.S. PBAT/Kraft Lignin Blend in Flexible Laminated Food Packaging: Peeling Resistance and Thermal Degradability. Polym. Test. 2018, 67, 169–176. [Google Scholar] [CrossRef]
- Gouveia, J.R.; Antonino, L.D.; Garcia, G.E.S.; Tavares, L.B.; Santos, A.N.B.; dos Santos, D.J. Kraft Lignin-Containing Polyurethane Adhesives: The Role of Hydroxypropylation on Thermomechanical Properties. J. Adhes. 2021, 97, 1423–1439. [Google Scholar] [CrossRef]
- Kühnel, I.; Saake, B.; Lehnen, R. Oxyalkylation of Lignin with Propylene Carbonate: Influence of Reaction Parameters on the Ensuing Bio-Based Polyols. Ind. Crops Prod. 2017, 101, 75–83. [Google Scholar] [CrossRef]
- Duval, A.; Avérous, L. Cyclic Carbonates as Safe and Versatile Etherifying Reagents for the Functionalization of Lignins and Tannins. ACS Sustain. Chem. Eng. 2017, 5, 7334–7343. [Google Scholar] [CrossRef]
- Jeong, H.; Park, J.; Kim, S.; Lee, J.; Cho, J.W. Use of Acetylated Softwood Kraft Lignin as Filler in Synthetic Polymers. Fibers Polym. 2012, 13, 1310–1318. [Google Scholar] [CrossRef]
- Jeong, H.; Park, J.; Kim, S.; Lee, J.; Ahn, N.; Roh, H. gyoo Preparation and Characterization of Thermoplastic Polyurethanes Using Partially Acetylated Kraft Lignin. Fibers Polym. 2013, 14, 1082–1093. [Google Scholar] [CrossRef]
- Gouveia, J.R.; de Sousa Júnior, R.R.; Ribeiro, A.O.; Saraiva, S.A.; dos Santos, D.J. Effect of Soft Segment Molecular Weight and NCO:OH Ratio on Thermomechanical Properties of Lignin-Based Thermoplastic Polyurethane Adhesive. Eur. Polym. J. 2020, 131, 109690. [Google Scholar] [CrossRef]
- Fodil Cherif, M.; Trache, D.; Brosse, N.; Benaliouche, F.; Tarchoun, A.F. Comparison of the Physicochemical Properties and Thermal Stability of Organosolv and Kraft Lignins from Hardwood and Softwood Biomass for Their Potential Valorization. Waste Biomass Valorization 2020, 11, 6541–6553. [Google Scholar] [CrossRef]
- Saffian, H.A.; Hyun-Joong, K.; Md Tahir, P.; Ibrahim, N.A.; Lee, S.H.; Lee, C.H. Effect of Lignin Modification on Properties of Kenaf core fiber reinforced poly (butylene succinate) biocomposites. Materials 2019, 12, 4043. [Google Scholar]
- Laurichesse, S.; Avérous, L. Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Laurichesse, S.; Huillet, C.; Avérous, L. Original Polyols Based on Organosolv Lignin and Fatty Acids: New Bio-Based Building Blocks for Segmented Polyurethane Synthesis. Green Chem. 2014, 16, 3958–3970. [Google Scholar] [CrossRef]
- Dehne, L.; Vila, C.; Saake, B.; Schwarz, K.U. Esterification of Kraft Lignin as a Method to Improve Structural and Mechanical Properties of Lignin-Polyethylene Blends. J. Appl. Polym. Sci. 2017, 134, 1–8. [Google Scholar] [CrossRef]
- Dehne, L.; Vila Babarro, C.; Saake, B.; Schwarz, K.U. Influence of Lignin Source and Esterification on Properties of Lignin-Polyethylene Blends. Ind. Crops Prod. 2016, 86, 320–328. [Google Scholar] [CrossRef]
- Glasser, W.G.; Jain, R.K. Lignin Derivatives. Holzforschung 1993, 47, 225–233. [Google Scholar] [CrossRef]
- Liu, L.Y.; Hua, Q.; Renneckar, S. A Simple Route to Synthesize Esterified Lignin Derivatives. Green Chem. 2019, 21, 3682–3692. [Google Scholar] [CrossRef]
- Gordobil, O.; Robles, E.; Egüés, I.; Labidi, J. Lignin-Ester Derivatives as Novel Thermoplastic Materials. RSC Adv. 2016, 6, 86909–86917. [Google Scholar] [CrossRef]
- Gordobil, O.; Delucis, R.; Egüés, I.; Labidi, J. Kraft Lignin as Filler in PLA to Improve Ductility and Thermal Properties. Ind. Crops Prod. 2015, 72, 46–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, J.; Fang, X.; Bai, F.; Qiao, K.; Wang, L. Renewable High-Performance Polyurethane Bioplastics Derived from Lignin-Poly(ε-Caprolactone). ACS Sustain. Chem. Eng. 2017, 5, 4276–4284. [Google Scholar] [CrossRef]
- Brodin, I.; Sjöholm, E.; Gellerstedt, G. The Behavior of Kraft Lignin during Thermal Treatment. J. Anal. Appl. Pyrolysis 2010, 87, 70–77. [Google Scholar] [CrossRef]
- Afifi, A.I.; Hindermann, J.P.; Chornet, E.; Overend, R.P. The Cleavage of the ArylOCH3 Bond Using Anisole as a Model Compound. Fuel 1989, 68, 498–504. [Google Scholar] [CrossRef]
- Jia, Z.; Lu, C.; Zhou, P.; Wang, L. Preparation and Characterization of High Boiling Solvent Lignin-Based Polyurethane Film with Lignin as the Only Hydroxyl Group Provider. RSC Adv. 2015, 5, 53949–53955. [Google Scholar] [CrossRef]
- Li, T.; Ma, H.; Wu, S.; Yin, Y. Effect of Highly Selective Oxypropylation of Phenolic Hydroxyl Groups on Subsequent Lignin Pyrolysis: Toward the Lignin Valorization. Energy Convers. Manag. 2020, 207, 112551. [Google Scholar] [CrossRef]
- Saffar, T.; Bouafif, H.; Braghiroli, F.L.; Magdouli, S.; Langlois, A.; Koubaa, A. Production of Bio-Based Polyol from Oxypropylated Pyrolytic Lignin for Rigid Polyurethane Foam Application. Waste Biomass Valorization 2020, 11, 6411–6427. [Google Scholar] [CrossRef]
- Gordobil, O.; Egüés, I.; Llano-Ponte, R.; Labidi, J. Physicochemical Properties of PLA Lignin Blends. Polym. Degrad. Stab. 2014, 108, 330–338. [Google Scholar] [CrossRef]
- Kun, D.; Pukánszky, B. Polymer/Lignin Blends: Interactions, Properties, Applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef] [Green Version]
- Pouteau, C.; Baumberger, S.; Cathala, B.; Dole, P. Lignin-Polymer Blends: Evaluation of Compatibility by Image Analysis. C. R. Biol. 2004, 327, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Bozsódi, B.; Romhányi, V.; Pataki, P.; Kun, D.; Renner, K.; Pukánszky, B. Modification of Interactions in Polypropylene/Lignosulfonate Blends. Mater. Des. 2016, 103, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Dai, H.; Dong, X.; Yang, J.; Zhong, M. Physical Properties of Lignin-based Polypropylene Blends. Polymer Compos. 2011, 32, 1019–1025. [Google Scholar] [CrossRef]
- Szycher, M. Structure–property relations in polyurethanes. In Szycher’s Handbook of Polyurethanes, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 37–86. [Google Scholar]
- Packham, D.E. Surface Energy, Surface Topography and Adhesion. Int. J. Adhes. Adhes. 2003, 23, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Nacas, A.M.; Vidotti, S.E.; Chinellato, A.C.; dos Santos, D.J. The Role of Polyol Reaction Catalysts in the Cure Kinetics and Mechanical Behavior of Polyurethane Adhesives. J. Adhes. 2018, 94, 880–892. [Google Scholar] [CrossRef]
- Luo, S.; Cao, J.; McDonald, A.G. Esterification of Industrial Lignin and Its Effect on the Resulting Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) or Polypropylene Blends. Ind. Crops Prod. 2017, 97, 281–291. [Google Scholar] [CrossRef] [Green Version]
| Name | PP (%) | KL (%) | A-oxy-KL (%) | M-oxy-KL (%) |
|---|---|---|---|---|
| PP | 100 | - | - | - |
| PP_KL_1 | 99 | 1 | - | - |
| PP_KL_2.5 | 97.5 | 2.5 | - | - |
| PP_KL_5 | 95 | 5.0 | - | - |
| PP_A_ Oxi_KL_1 | 99 | - | 1 | - |
| PP_A_Oxi_KL_2.5 | 97.5 | - | 2.5 | - |
| PP_A_Oxi_KL_5 | 95 | - | 5.0 | - |
| PP_M_Oxi_KL_1 | 99 | - | - | 1 |
| PP_M_Oxi_KL_2.5 | 97.5 | - | - | 2.5 |
| PP_M_Oxi_KL_5 | 95 | - | - | 5.0 |
| Surface Tension | Water | Diiodomethane |
|---|---|---|
| Total (mN/m) | 72.8 | 50.8 |
| Polar (mN/m) | 51.0 | 0 |
| Disperse (mN/m) | 21.8 | 50.8 |
| Sample | Tg (°C) | Tc (°C) | Tm (°C) | χ (%) |
|---|---|---|---|---|
| PP | −13.24 | 130.76 | 165.1 | 9.86 |
| PP_KL_1 | −12.94 | 126.53 | 164.1 | 9.73 |
| PP_KL_2.5 | −13.04 | 128.50 | 163.8 | 9.89 |
| PP_KL_5 | −13.13 | 122.91 | 164.6 | 9.59 |
| PP_A_ Oxy_KL_1 | −12.35 | 130.86 | 164.2 | 10.16 |
| PP_A_Oxy_KL_2.5 | −12.45 | 126.22 | 163.5 | 9.71 |
| PP_A_Oxy_KL_5 | −12.15 | 126.22 | 164.0 | 9.96 |
| PP_M_Oxy_KL_1 | −13.13 | 124.10 | 164.2 | 9.54 |
| PP_M_Oxy_KL_2.5 | −13.04 | 131.81 | 164.3 | 10.02 |
| PP_M_Oxy_KL_5 | −12.84 | 126.62 | 164.2 | 9.78 |
| Sample | Water (°) | Diiodomethane (°) |
|---|---|---|
| PP | 78.17 0.61 | 36.87 1.89 |
| PP_KL_1 | 66.12 2.20 | 30.52 2.02 |
| PP_KL_2.5 | 63.25 1.07 | 33.82 0.76 |
| PP_KL_5 | 60.12 1.10 | 38.89 1.32 |
| PP_A_Oxi_KL_1 | 63.21 0.31 | 30.15 2.07 |
| PP_A_Oxi_KL_2.5 | 60.02 0.80 | 35.09 1.87 |
| PP_A_Oxi_KL_5 | 58.36 2.01 | 33.95 1.02 |
| PP_M_Oxi_KL_1 | 58.02 1.87 | 32.71 2.89 |
| PP_M_Oxi_KL_2.5 | 55.89 2.10 | 31.64 2.01 |
| PP_M_Oxi_KL_5 | 52.61 2.85 | 26.78 2.89 |
| Sample | Average Peel Strength (N/m) |
|---|---|
| PP | 27.20 ± 0.84 |
| PP_KL_1 | 28.72 0.32 |
| PP_KL_2.5 | 29.15 0.55 |
| PP_KL_5 | 30.78 0.43 |
| PP_A_ Oxi_KL_1 | 35.26 0.66 |
| PP_A_Oxi_KL_2.5 | 37.68 0.91 |
| PP_A_Oxi_KL_5 | 39.58 0.13 |
| PP_M_Oxi_KL_1 | 39.74 0.89 |
| PP_M_Oxi_KL_2.5 | 42.80 1.02 |
| PP_M_Oxi_KL_5 | 45.15 1.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisneto, M.P.d.S.; Gouveia, J.R.; Antonino, L.D.; Tavares, L.B.; Ito, N.M.; dos Santos, D.J. Effects of Functionalized Kraft Lignin Incorporation on Polypropylene Surface Energy and Practical Adhesion. Polymers 2022, 14, 999. https://doi.org/10.3390/polym14050999
Bisneto MPdS, Gouveia JR, Antonino LD, Tavares LB, Ito NM, dos Santos DJ. Effects of Functionalized Kraft Lignin Incorporation on Polypropylene Surface Energy and Practical Adhesion. Polymers. 2022; 14(5):999. https://doi.org/10.3390/polym14050999
Chicago/Turabian StyleBisneto, Manuel Patricio da Silva, Julia Rocha Gouveia, Leonardo Dalseno Antonino, Lara Basílio Tavares, Nathalie Minako Ito, and Demetrio Jackson dos Santos. 2022. "Effects of Functionalized Kraft Lignin Incorporation on Polypropylene Surface Energy and Practical Adhesion" Polymers 14, no. 5: 999. https://doi.org/10.3390/polym14050999
APA StyleBisneto, M. P. d. S., Gouveia, J. R., Antonino, L. D., Tavares, L. B., Ito, N. M., & dos Santos, D. J. (2022). Effects of Functionalized Kraft Lignin Incorporation on Polypropylene Surface Energy and Practical Adhesion. Polymers, 14(5), 999. https://doi.org/10.3390/polym14050999






