Bacterial Cellulose and Its Applications
Abstract
:1. Introduction
2. Applications
2.1. Food Industry
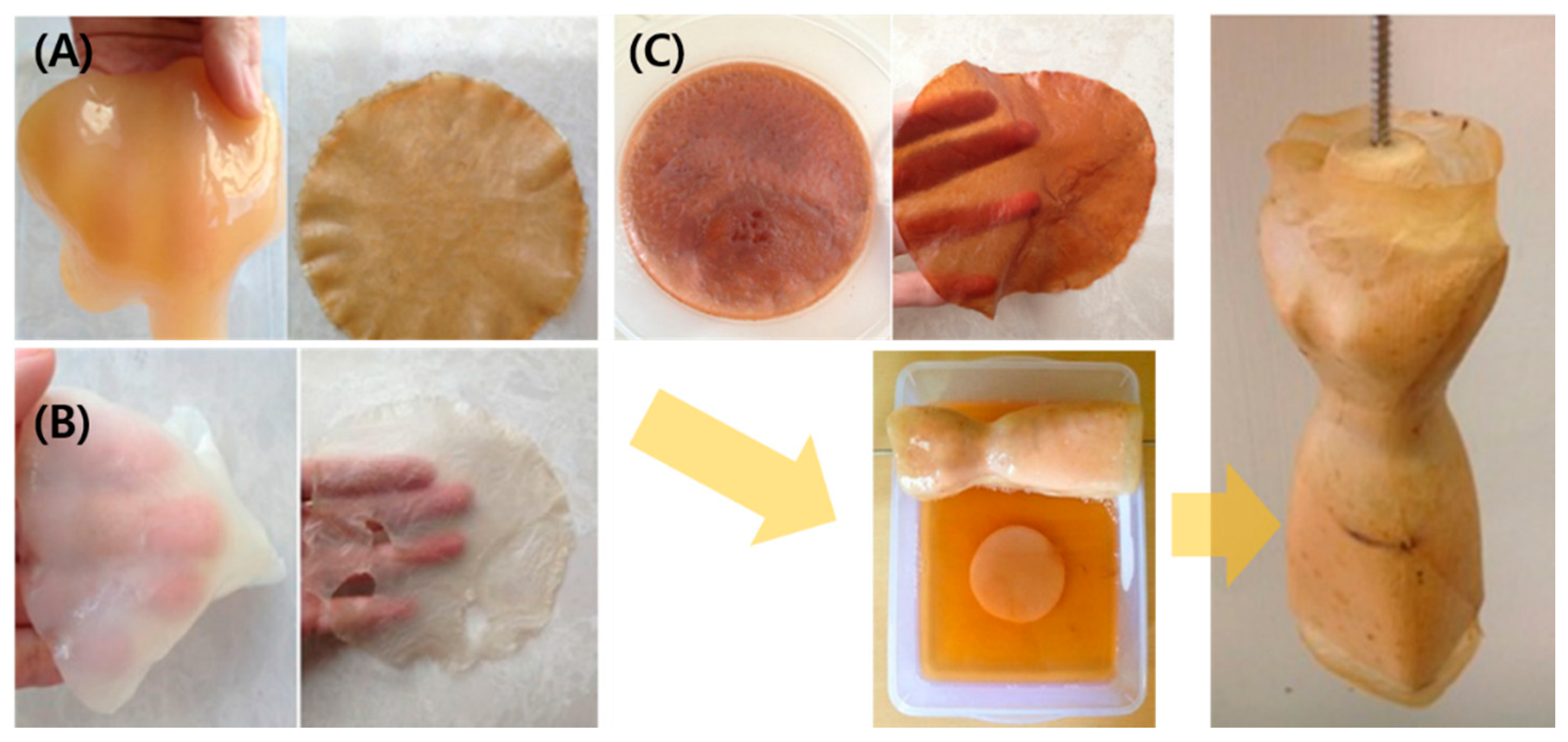
2.1.1. Traditional Dessert
2.1.2. Low Calorie and Low Cholesterol Products
2.1.3. Food Additive and Dietary Aid
2.1.4. Food Packaging Materials
2.2. Biomedical Industry
2.2.1. Wound Dressings
2.2.2. Cartilage Tissue Engineering (CTE)
2.2.3. Bone Tissue Engineering
2.2.4. Dental Implants
2.2.5. Artificial Blood Vessels and Vascular Grafts
2.2.6. Urethral Implants
2.2.7. Artificial Cornea and Retina
2.2.8. Nerve Implants
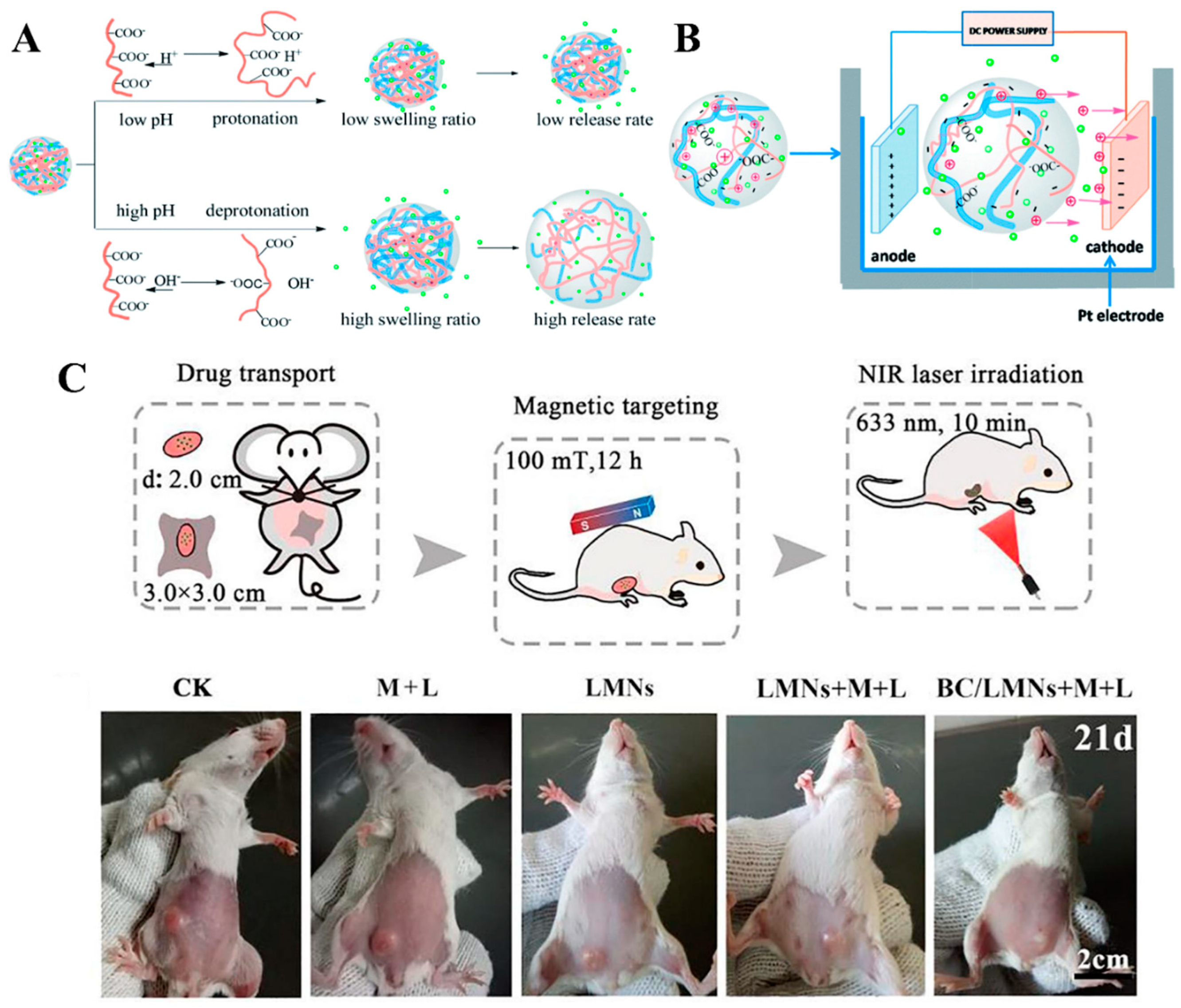
2.2.9. Delivery of Drug and Bioactive Agents
2.2.10. BC Scaffolds for Cell–Enzyme Immobilization
2.3. Paper Industry
2.3.1. Special Functional Paper
2.3.2. High-Retaining Water Paper
2.3.3. Packaging Paper
2.4. Textile Industry
2.5. Electrical and Electronic Industries
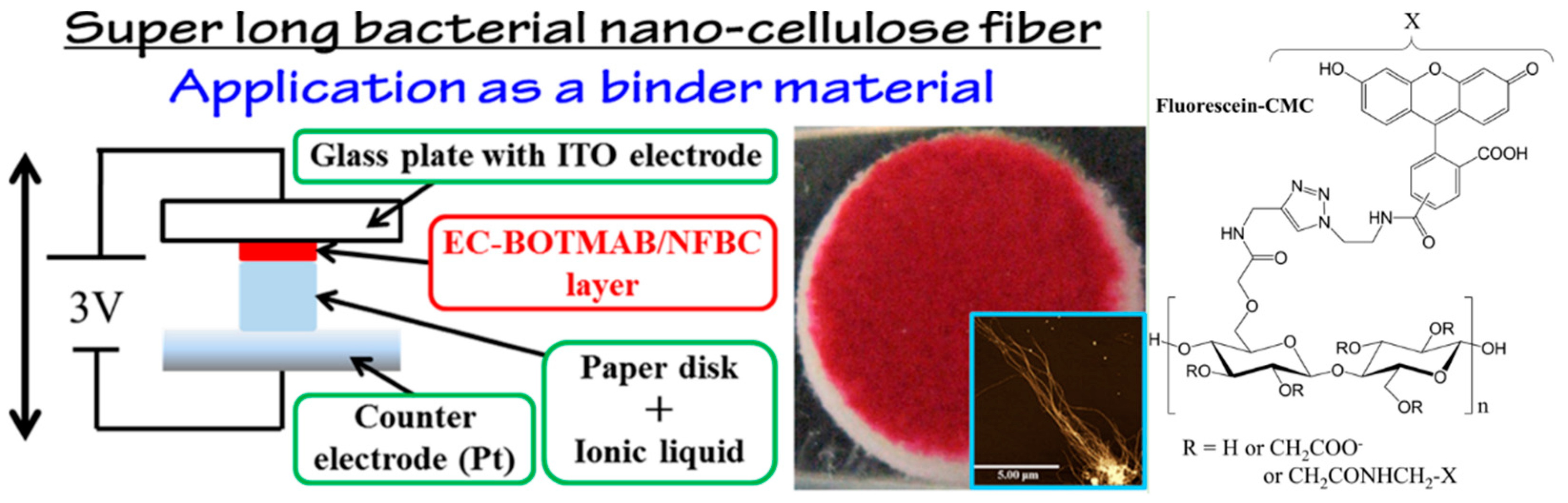
2.5.1. Electrical Display Device
2.5.2. OLED
2.5.3. Fuel Cell
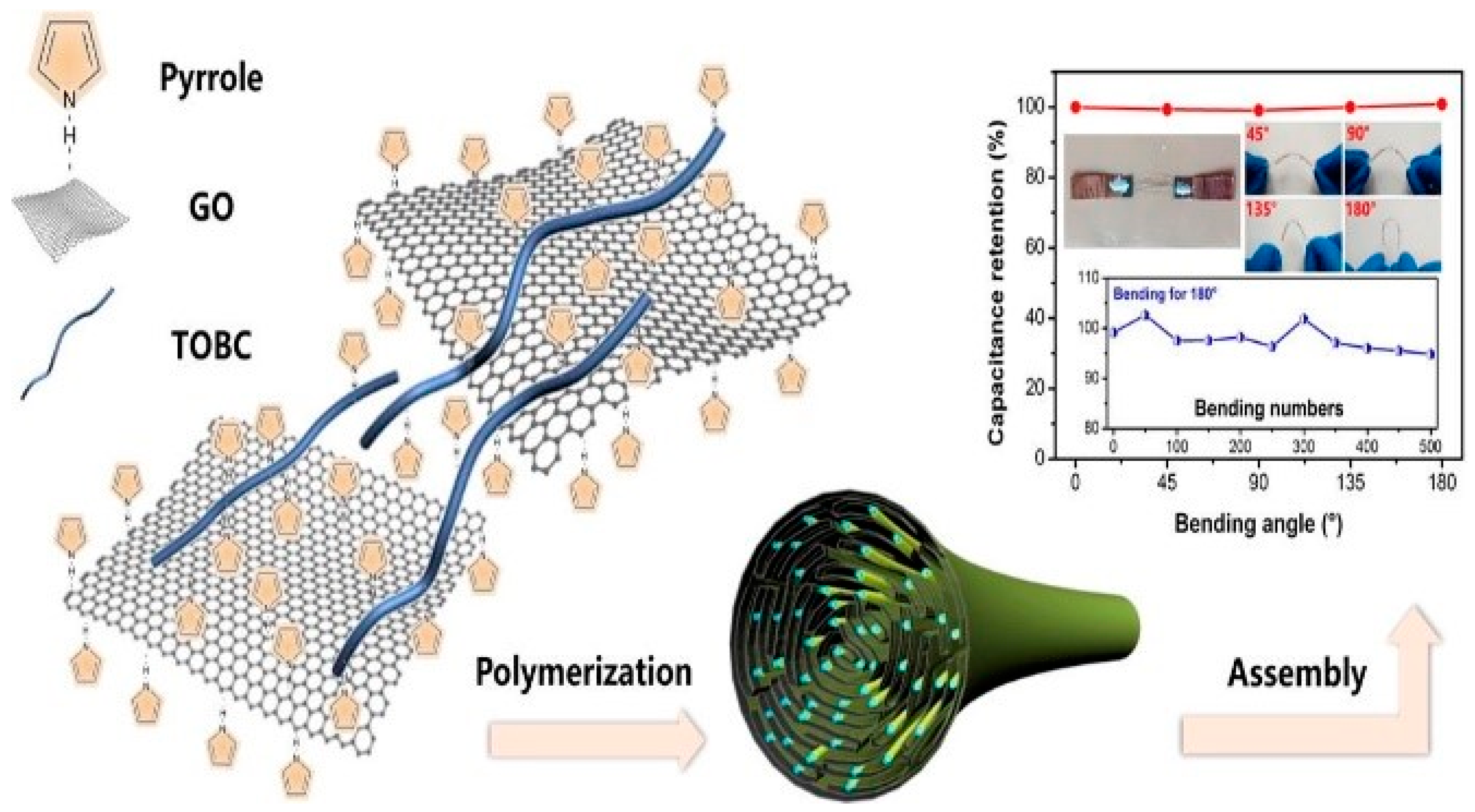
2.5.4. Flexible Supercapacitor
2.5.5. Stereo Headphones and Monitors
2.5.6. Electromagnetic Wave Absorbing Materials
2.6. Etc.
3. Concluding and Future Trends
Funding
Data Availability Statement
Conflicts of Interest
References
- Nechita, P.; Mirela, R.; Ciolacu, F. Xylan Hemicellulose: A renewable material with potential properties for food packaging applications. Sustainability 2021, 13, 13504. [Google Scholar] [CrossRef]
- Iguchi, M.; Yamanaka, S.; Budhiono, A. Bacterial cellulose-a masterpiece of nature’s arts. J. Mater. Sci. 2000, 35, 261–270. [Google Scholar] [CrossRef]
- Abeer, M.M.; Mohd Amin, M.C.; Martin, C. A review of bacterial cellulose-based drug delivery systems: Their biochemistry, current approaches and future prospects. J. Pharm. Pharmacol. 2014, 66, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiyah, M.H.; Joy, J.; Moores, A. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Lupascu, R.E.; Ghica, M.V.; Dinu-Pirvu, C.E.; Popa, L.; Velescu, B.S.; Arsene, A.L. An overview regarding microbial aspects of production and applications of bacterial cellulose. Appl. Microbiol. Mater. 2021, 15, 676. [Google Scholar] [CrossRef]
- Rol, F.; Belgacem, M.N.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2019, 88, 241–264. [Google Scholar] [CrossRef]
- Stanisławska, A.; Staroszczyk, H.; Szkodo, M. The effect of dehydration/rehydration of bacterial nanocellulose on its tensile strength and physicochemical properties. Carbohydr. Polym. 2020, 236, 116023. [Google Scholar] [CrossRef]
- Indriyati, I.; Irmavati, Y.; Puspitasari, T. Comparative study of bacterial cellulose film dried using microwave and air convection heating. J. Eng. Technol. Sci. 2019, 51, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Mani Pujitha, I.; Chandra, S.S.; Mudrika, K. Tuning the physiochemical properties of bacterial cellulose: Effect of drying conditions. J. Mater. Sci. 2019, 54, 12024–12035. [Google Scholar]
- Kiziltas, E.E.; Kiziltas, A.; Gardner, D.J. Synthesis of bacterial cellulose using hot water extracted wood sugars. Carbohydr. Polym. 2015, 124, 131–138. [Google Scholar] [CrossRef]
- Sulaeva, I.; Hettegger, H.; Bergen, A.; Rohrer, C.; Kostic, M.; Konnerth, J.; Rosenau, T.; Rotthast, A. Fabrication of bacterial cellulose-based wound dressings with improved performance by impregnation with alginate. Mater. Sci. Eng. C 2020, 110, 110619. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasat, A.; Jearanai, S.; Moonmangmee, S.; Moonmangmee, D.; Christopher, L.P.; Alam, M.N.; Chaiyasat, P. Novel green hydrogel material using bacterial cellulose. Orient. J. Chem. 2018, 34, 1735–1740. [Google Scholar] [CrossRef]
- Dai, L.; Nan, J.; Tu, X.; He, L.; Wei, B.; Xu, C.; Xu, Y.; Li, S.; Wang, H.; Zhang, J. Improved thermostability and cytocompatibility of bacterial cellulose/collagen composite by collagen fibrillogenesis. Cellulose 2019, 26, 6713–6724. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Ong, K.J.; Ede, J.D.; Pomeroy-Carter, C.A.; Sayes, C.M.; Mulenos, M.R.; Shatkin, J.A. A 90-day dietary study with fibrillated cellulose in Sprague-Dawley rats. Toxicol. Rep. 2020, 7, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Rachatanapum, P.; Klunklin, W.; Jantrawut, P.; Leksawasdi, N.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Chaiyaso, T.; Ruksiriwanich, W.; Phongthai, S.; et al. Effect of monochloroacetic acid on properties of carboxymethyl bacterial cellulose powder and film from nata de coco. Polymers 2021, 13, 488. [Google Scholar] [CrossRef] [PubMed]
- Lapas, M.M.; Gallardo, E.G.; Palo, M.A. The nata organism cultural, characteristics and identity. Philipp. J. Sci. 1967, 96, 91. [Google Scholar]
- Reproduction as Tangerine-Based Biocellulose Diet Beverage. Leisuretimes. Available online: http://www.leisuretimes.co.kr/news/articleView.html?idxno=5294 (accessed on 27 September 2012).
- Czaja, W.; Romanovicz, D.; Brown, R.M., Jr. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 2004, 11, 403–411. [Google Scholar] [CrossRef]
- Siro, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef]
- Chau, C.F.; Yang, P.; Yu, C.M.; Yen, C.G. Investigation on the lipid- and cholesterol lowering abilities of biocellulose. J. Agric. Food Chem. 2008, 56, 2291–2295. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.W.; Lin, H.W. Quality characteristics of Chinese-style meatball containing bacterial cellulose (nata). J. Food Sci. 2004, 69, SNQ107–SNQ111. [Google Scholar] [CrossRef]
- Marchetti, L.; Muzzio, B.; Cerrutti, P.; Andres, S.C.; Califano, A.N. Bacterial nanocellulose as novel additive in low-lipid low-sodium meat sausages. Effect on quality and stability. Food Struct. 2017, 14, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Akoglu, A.; Çakir, İ.; Akoğlu, İ.T.; Karahan, A.G.; Çakmakçi, M.L. Effect of bacterial cellulose as a fat replacer on some quality characteristics of fat reduced sucuk. Gida J. Food 2015, 40, 133–139. [Google Scholar]
- Guo, Y.; Zhang, X.; Hao, W.; Xie, Y.; Chen, L.; Li, Z.; Zhu, B.; Feng, X. Nano-bacterial cellulose/soy protein isolate complex gel as fat substitutes in ice cream model. Carbohydr. Polym. 2018, 198, 620–630. [Google Scholar] [CrossRef]
- Ullah, H.; Santos, H.A.; Khan, T. Applications of bacterial cellulose in food, cosmetics and drug delivery. Cellulose 2016, 23, 2291–2314. [Google Scholar] [CrossRef]
- Dourado, F.; Gama, M.; Rodrigues, A.C. A review on the toxicology and dietetic role of bacterial cellulose. Toxicol. Rep. 2017, 4, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Okiyama, A.; Motoki, M.; Yamanaka, S. Bacterial cellulose II. Processing of the gelationous cellulose for food materials. Food Hydrocoll. 1992, 6, 479–487. [Google Scholar] [CrossRef]
- Khan, S.B.; Kamal, T. Bacterial Cellulose: Synthesis, Production, and Applications; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Coban, E.P.; Biyik, H.H.; Cetin, Ö. Environmentally friendly bacterial cellulose films for food packaging. Eurasian J. Food Sci. Technol. 2021, 5, 127–135. [Google Scholar]
- Amorim, L.F.A.; Mouro, C.; Riool, M.; Gouveia, I.C. Antimicrobial food packaging based on prodigiosin-incorporated double layered bacterial cellulose and chitosan composites. Polymers 2022, 14, 315. [Google Scholar] [CrossRef]
- Salari, M.; Khiabani, M.S.; Mokarram, R.R.; Ghanbarzadeh, B.; Kafil, H.S. Development and evaluation of chitosan based active nanocomposite films containing bacterial cellulose nanocrystals and silver nanoparticles. Food Hydrocoll. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Fabra, M.J.; Lopez-Rubio, A.; Ambrosio-Martin, J.; Lagaron, J.M. Improving the barrier properties of thermoplastic corn starch-based films containing bacterial cellulose nanowhiskers by means of PHA electrospun coatings of interest in food packaging. Food Hydrocoll. 2016, 61, 261–268. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Ramana, K.V.; Bawa, A.S. Bacterial cellulose nanocrystals exhibiting high thermal stability and their polymer nanocomposites. Int. J. Biol. Macromol. 2011, 48, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Abral, H.; Pratama, A.B.; Handayani, D.; Mahardika, M.; Aminah, I.; Sandrawati, N.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. Antimicrobial edible film prepared from bacterial cellulose nanofibers/starch/chitosan for a food packaging alternative. Int. J. Polym. Sci. 2021, 2021, 6641284. [Google Scholar] [CrossRef]
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent advances and applications of bacterial cellulose in biomedicine. Polymers 2021, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Popa, L.; Ghica, M.V.; Tudoroiu, E.E.; Ionescu, D.G.; Dinu-Pirvu, C.E. Bacterial cellulose-A remarkable polymer as a source for biomaterials tailoring. Materials 2022, 15, 1054. [Google Scholar] [CrossRef]
- Aditya, T.; Allain, J.P.; Jaramillo, C.; Restrepo, A.M. Surface modification of bacterial cellulose for biomedical applications. Int. J. Mol. Sci. 2022, 23, 610. [Google Scholar] [CrossRef]
- Kushwaha, A.; Goswami, L.; Kim, B.S. Nanomaterial-based therapy for wound healing. Nanomaterials 2022, 12, 618. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. An up-to-date review of biomaterials application in wound management. Polymers 2022, 14, 421. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Portal, O.; Clark, W.A.; Levinson, D.J. Microbial cellulose wound dressing in the treatment of nonhealing lower extremity ulcers. Wounds 2009, 21, 1–3. [Google Scholar] [PubMed]
- Cielecka, I.; Szustak, M.; Kalinowska, H.; Gendaszewska-Darmach, E.; Ryngajłło, M.; Maniukiewicz, W.; Bielecki, S. Glycerol-plasticized bacterial nanocellulose-based composites with enhanced flexibility and liquid sorption capacity. Cellulose 2019, 26, 5409–5426. [Google Scholar] [CrossRef] [Green Version]
- Cacicedo, M.L.; Pacheco, G.; Islan, G.A.; Alvarez, V.A.; Barud, H.S.; Castro, G.R. Chitosan-bacterial cellulose patch of ciprofloxacin for wound dressing: Preparation and characterization studies. Int. J. Biol. Macromol. 2020, 147, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Piasecka-Zelga, J.; Zelga, P.; Szulc, J.; Wietecha, J.; Ciecha’nska, D. An in vivo biocompatibility study of surgical meshes made from bacterial cellulose modified with chitosan. Int. J. Biol. Macromol. 2018, 116, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Velazquez, G.; Vázquez, M. Characterization of bacterial cellulose films combined with chitosan and polyvinyl alcohol: Evaluation of mechanical and barrier properties. Carbohydr. Polym. 2019, 216, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Meng, C.; Xie, Y.; He, W.; Wang, Y.; Qiao, K.; Yue, L. The effects of two biocompatible plasticizers on the performance of dry bacterial cellulose membrane: A comparative study. Cellulose 2018, 25, 5893–5908. [Google Scholar] [CrossRef]
- Mohamad, N.; Mohd Amin, M.C.; Pandey, M.; Ahmad, N.; Rajab, N.F. Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: Accelerated burn wound healing in an animal model. Carbohydr. Polym. 2014, 114, 312–320. [Google Scholar] [CrossRef]
- Mohamad, N.; Loh, E.Y.X.; Fauzi, M.B.; Ng, M.H.; Amin, M.C.I.M. In vivo evaluation of bacterial cellulose/acrylic acid wound dressing hydrogel containing keratinocytes and fibroblasts for burn wounds. Drug Deliv. Transl. Res. 2019, 9, 444–452. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Nisi, R.; Stoppa, M.; Licciulli, A. Silver-functionalized bacterial cellulose as antibacterial membrane for wound-healing applications. ACS Omega 2017, 2, 3632–3639. [Google Scholar] [CrossRef] [Green Version]
- Jiji, S.; Udhayakumar, S.; Maharajan, K.; Rose, C.; Muralidharan, C.; Kadirvelu, K. Bacterial cellulose matrix with in situ impregnation of silver nanoparticles via catecholic redox chemistry for third degree burn wound healing. Carbohydr. Polym. 2020, 245, 116573. [Google Scholar] [CrossRef] [PubMed]
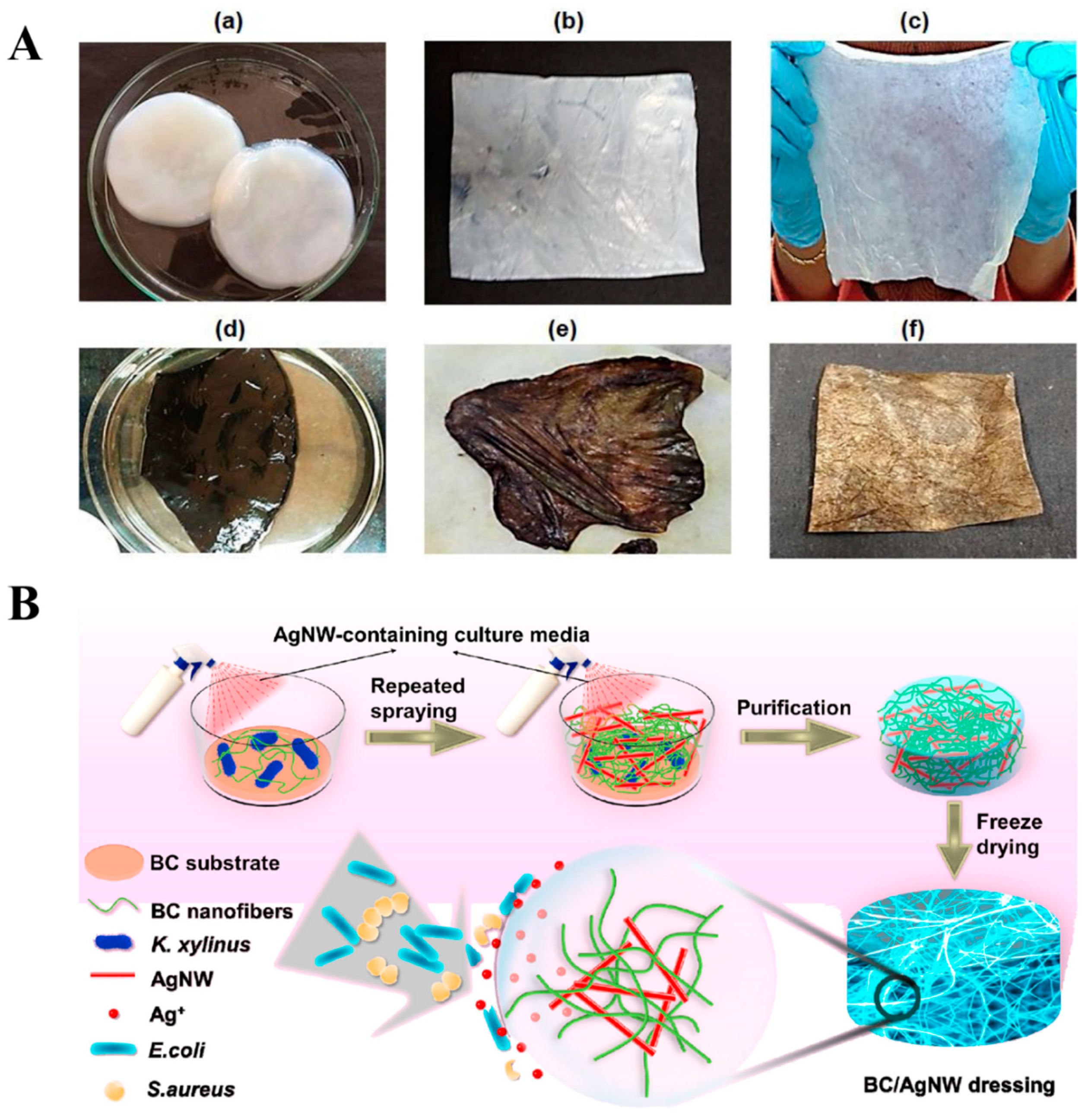
- Wan, Y.; Yang, S.; Wang, J.; Gan, D.; Gama, M.; Yang, Z.; Zhu, Y.; Yao, F.; Luo, H. Scalable synthesis of robust and stretchable composite wound dressings by dispersing silver nanowires in continuous bacterial cellulose. Compos. B Eng. 2020, 199, 108259. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Khattak, A.; Ullah, M.W.; Park, J.K. Bacterial cellulose-titanium dioxide nanocomposites: Nanostructural characteristics, antibacterial mechanism, and biocompatibility. Cellulose 2015, 22, 565–579. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khattak, W.A.; Ullah, M.W.; Khan, S.; Park, J.K. Synthesis of regenerated bacterial cellulose-zinc oxide nanocomposite films for biomedical applications. Cellulose 2014, 21, 433–447. [Google Scholar] [CrossRef]
- Khalid, A.; Khan, R.; Ul-Islam, M.; Khan, T.; Wahid, F. Bacterial cellulose-zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydr. Polym. 2017, 164, 214–221. [Google Scholar] [CrossRef]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef]
- Yadav, V.; Paniliatis, B.J.; Shi, H.; Lee, K.; Cebe, P.; Kaplan, D.L. Novel in vivo-degradable cellulose-chitin copolymer from metabolically engineered Gluconacetobacter xylinus. Appl. Environ. Microbiol. 2020, 76, 6257–6265. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.; Sun, L.; Panilaitis, B.; Kaplan, D.L. In vitro chondrogenesis with lysozyme susceptible bacterial cellulose as a scaffold. J. Tissue Eng. Regen. Med. 2015, 9, E276–E288. [Google Scholar] [CrossRef]
- Yin, N.; Stilwell, M.D.; Santos, T.M.; Wang, H.; Weibel, D.B. Agarose particle-templated porous bacterial cellulose and its application in cartilage growth in vitro. Acta Biomater. 2015, 12, 129–138. [Google Scholar] [CrossRef]
- Wu, J.; Yin, N.; Chen, S.; Weibel, D.B.; Wang, H. Simultaneous 3D cell distribution and bioactivity enhancement of bacterial cellulose (BC) scaffold for articular cartilage tissue engineering. Cellulose 2019, 26, 2513–2528. [Google Scholar] [CrossRef]
- Andersson, J.; Stenhamre, H.; Bäckdahl, H.; Gatenholm, P. Behavior of human chondrocytes in engineered porous bacterial cellulose scaffolds. J. Biomed. Mater. Res. A 2010, 94, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Akaraonye, E.; Filip, J.; Safarikova, M.; Salih, V.; Keshavarz, T.; Knowles, J.C.; Roy, L. Composite scaffolds for cartilage tissue engineering based on natural polymers of bacterial origin, thermoplastic poly (3-hydroxybutyrate) and micro-fibrillated bacterial cellulose. Polym. Int. 2016, 65, 780–791. [Google Scholar] [CrossRef]
- Xun, X.; Li, Y.; Zhu, X.; Zhang, Q.; Lu, Y.; Yang, Z.; Wan, Y.; Yao, F.; Deng, X.; Luo, H. Fabrication of Robust, Shape Recoverable, Macroporous Bacterial Cellulose Scaffolds for Cartilage Tissue Engineering. Macromol. Biosci. 2021, 2021, 2100167. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Li, T.; Song, X.; Yang, X.; Li, S.; Chen, L.; Liu, P.; Gong, X.; Chen, C.; Sun, L. Preparation and characterization of methacrylated gelatin/bacterial cellulose composite hydrogels for cartilage tissue engineering. Regen. Biomater. 2020, 7, 195–202. [Google Scholar] [CrossRef]
- Nürnberger, S.; Schneider, C.; van Osch, G.V.M.; Keibl, C.; Rieder, B.; Monforte, X.; Teuschl, A.H.; Mühleder, S.; Holnthoner, W.; Schädl, B.; et al. Repopulation of an auricular cartilage scaffold, AuriScaff, perforated with an enzyme combination. Acta Biomater. 2019, 86, 207–222. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Y.; Yan, S.; Liu, Z.; He, S.; Cui, L.; Yin, J. Poly(L-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater. 2014, 10, 276–288. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, X.; Yu, K.; Meng, H.; Zheng, Y.; Peng, J.; Lu, S.; Liu, X.; Xie, Y.; Qiao, K. Fabrication of nanofibrous microcarriers mimicking extracellular matrix for functional microtissue formation and cartilage regeneration. Biomaterials 2018, 171, 118–132. [Google Scholar] [CrossRef]
- Horbert, V.; Boettcher, J.; Foehr, P.; Kramer, F.; Udhardt, U.; Bungartz, M.; Brinkmann, O.; Burgkart, R.H.; Klemm, D.O.; Kinne, R.W. Laser perforation and cell seeding improve bacterial nanocellulose as a potential cartilage implant in the in vitro cartilage punch model. Cellulose 2019, 26, 647–664. [Google Scholar] [CrossRef]
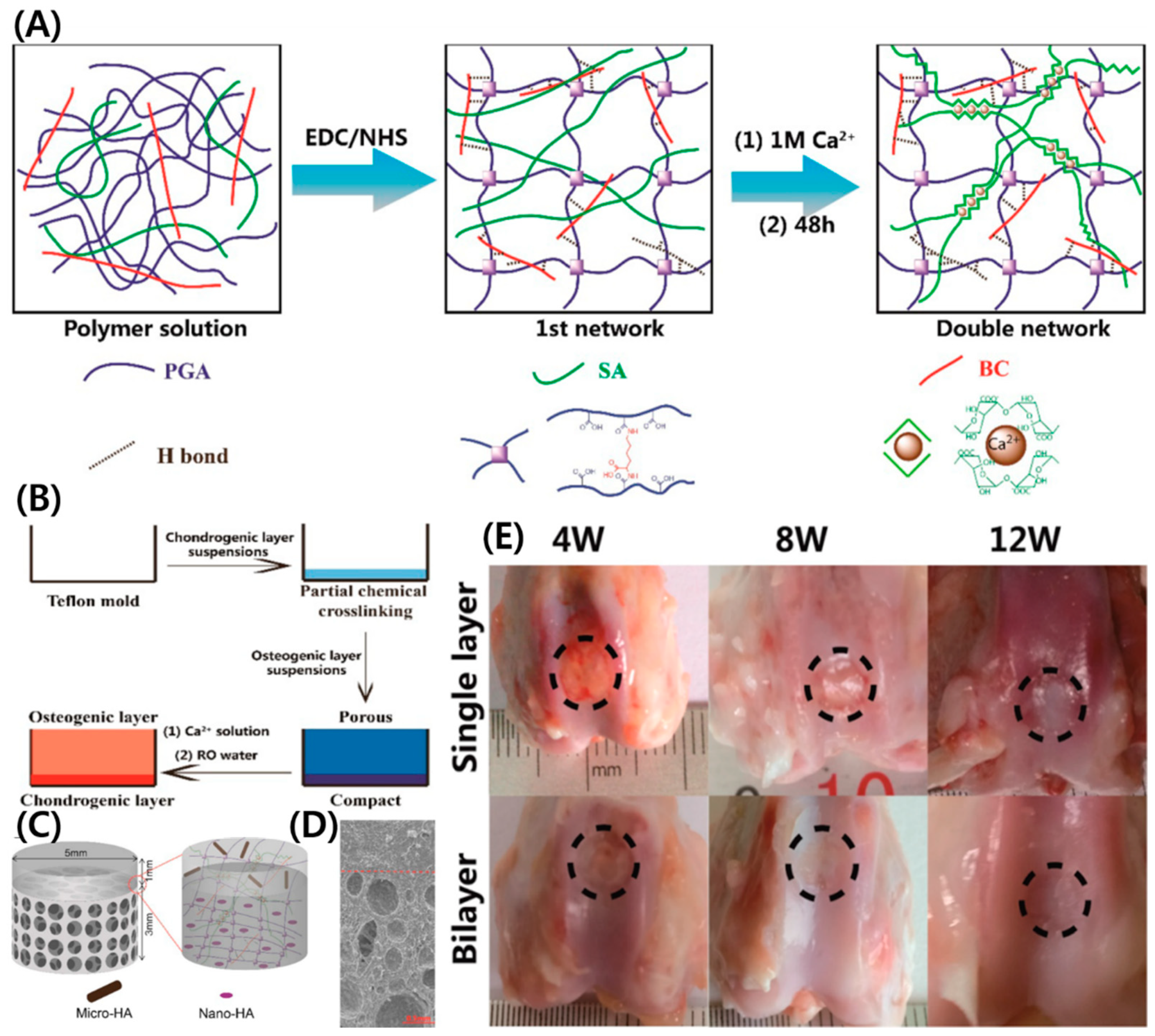
- Zhu, X.; Chen, T.; Feng, B.; Weng, J.; Duan, K.; Wang, J.; Lu, X. Biomimetic bacterial cellulose-enhanced double-network hydrogel with excellent mechanical properties applied for the osteochondral defect repair. ACS Biomater. Sci. Eng. 2018, 4, 3534–3544. [Google Scholar] [CrossRef]
- Kumbhar, J.V.; Jadhav, S.H.; Bodas, D.S.; Barhanpurkar-Naik, A.; Wani, M.R.; Paknikar, K.M.; Rajwade, J.M. In vitro and in vivo studies of a novel bacterial cellulose-based acellular bilayer nanocomposite scaffold for the repair of osteochondral defects. Int. J. Nanomed. 2017, 12, 6437–6459. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wang, L.; Zhang, W.; Sun, Z.; Li, Y.; Yang, M.; Zeng, D.; Peng, B.; Zheng, W.; Jiang, X.; et al. Reverse reconstruction and bioprinting of bacterial cellulose-based functional total intervertebral disc for therapeutic implantation. Small 2018, 14, 1702582. [Google Scholar] [CrossRef] [PubMed]
- Favi, P.M.; Benson, R.S.; Neilsen, N.R.; Hammonds, R.L.; Bates, C.C.; Stephens, C.P.; Dhar, M.S. Cell proliferation, viability, and in vitro differentiation of equine mesenchymal stem cells seeded on bacterial cellulose hydrogel scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
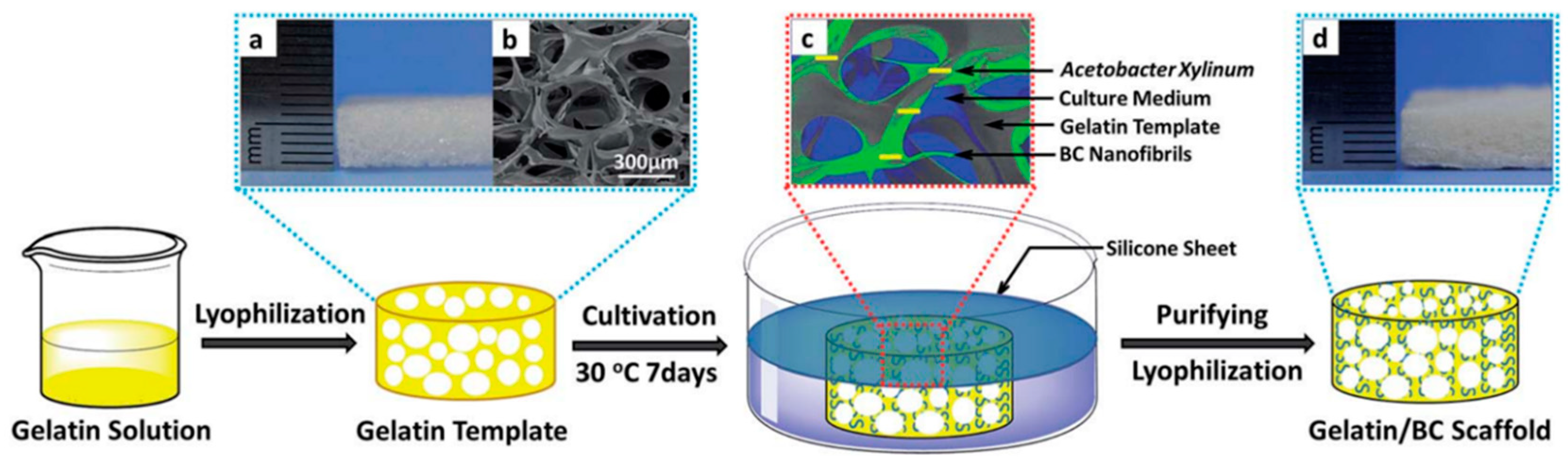
- Sundberg, J.; Götherström, C.; Gatenholm, P. Biosynthesis and in vitro evaluation of macroporous mineralized bacterial nanocellulose scaffolds for bone tissue engineering. Biomed. Mater. Eng. 2015, 25, 39–52. [Google Scholar] [CrossRef]
- Bab, I.; Gazit, D.; Chorev, M.; Muhlrad, A.; Shteyer, A.; Greenberg, Z.; Namdar, M.; Kahn, A. Histone H4-related osteogenic growth peptide (OGP): A novel circulating stimulator of osteoblastic activity. EMBO J. 1992, 11, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Saska, S.; Scarel-Caminaga, R.M.; Teixeira, L.N.; Franchi, L.P.; Dos Santos, R.A.; Gaspar, A.M.M.; de Oliveira, P.T.; Rosa, A.L.; Takahashi, C.S.; Messaddeq, Y.; et al. Characterization and in vitro evaluation of bacterial cellulose membranes functionalized with osteogenic growth peptide for bone tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2253–2266. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Li, Y.; Sun, J.; Zhang, H.; Chen, L.; Chen, B.; Yang, H.; Wang, Z. The osteogenesis of bacterial cellulose scaffold loaded with bone morphogenetic protein-2. Biomaterials 2012, 33, 6644–6649. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Mishra, R.; Roy, P.; Singh, R.P. 3-D macro/microporous-nanofibrous bacterial cellulose scaffolds seeded with BMP-2 preconditioned mesenchymal stem cells exhibit remarkable potential for bone tissue engineering. Int. J. Biol. Macromol. 2021, 167, 934–946. [Google Scholar] [CrossRef]
- Tazi, N.; Zhang, Z.; Messaddeq, Y.; Almeida-Lopes, L.; Zanardi, L.M.; Levinson, D.; Rouabhia, M. Hydroxyapatite bioactivated bacterial cellulose promotes osteoblast growth and the formation of bone nodules. AMB Express 2012, 2, 61. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, A.M.; Unal, S.; Sahin, A.; Oktar, F.N.; Sengor, M.; Ekren, N.; Gunduz, O.; Kalaskar, D.M. 3D printed polycaprolactone/gelatin/bacterial cellulose/hydroxyapatite composite scaffold for bone tissue engineering. Polymers 2020, 12, 1962. [Google Scholar] [CrossRef]
- Gutiérrez-Hernández, J.M.; Escobar-García, D.M.; Escalante, A.; Flores, H.; González, F.J.; Gatenholm, P.; Toriz, G. In vitro evaluation of osteoblastic cells on bacterial cellulose modified with multi-walled carbon nanotubes as scaffold for bone regeneration, Mater. Sci. Eng. C 2017, 75, 445–453. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Yang, E.; Shao, Y.; Zhang, X.; Dai, K. Modification and evaluation of micro-nano structured porous bacterial cellulose scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xiong, G.; Huang, Y.; He, F.; Wang, Y.; Wan, Y. Preparation and characterization of a novel COL/BC composite for potential tissue engineering scaffolds. Mater. Chem. Phys. 2008, 110, 193–196. [Google Scholar] [CrossRef]
- Delgado, L.M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng. Part B Rev. 2015, 21, 298–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Wang, X.C.; Li, X.Y.; Jiang, F. A 3D porous microsphere with multistage structure and component based on bacterial cellulose and collagen for bone tissue engineering. Carbohydr. Polym. 2020, 236, 116043. [Google Scholar] [CrossRef]
- Saska, S.; Teixeira, L.N.; de Castro Raucci, L.M.S.; Scarel-Caminaga, R.M.; Franchi, L.P.; Dos Santos, R.A.; Santagneli, S.H.; Capela, M.V.; de Oliveira, P.T.; Takahashi, C.S.; et al. Nanocellulose-collagen-apatite composite associated with osteogenic growth peptide for bone regeneration. Int. J. Biol. 2017, 103, 467–476. [Google Scholar] [CrossRef] [Green Version]
- Ran, J.; Jiang, P.; Liu, S.; Sun, G.; Yan, P.; Shen, X.; Tong, H. Constructing multi-component organic/inorganic composite bacterial cellulose-gelatin/hydroxyapatite double-network scaffold platform for stem cell-mediated bone tissue engineering. Mater. Sci. Eng. C 2017, 78, 130–140. [Google Scholar] [CrossRef]
- Coelho, F.; Cavicchioli, M.; Specian, S.S.; Scarel-Caminaga, R.M.; de Aquino Penteado, L.; de Medeiros, A.I.; de Lima Ribeiro, S.J.; de Oliveira Capote, T.S. Bacterial cellulose membrane functionalized with hydroxiapatite and anti-bone morphogenetic protein 2: A promising material for bone regeneration. PLoS ONE 2019, 14, e0221286. [Google Scholar] [CrossRef]
- Rattanapisit, K.; Abdulheem, S.; Chaikeawkaew, D.; Kubera, A.; Mason, H.S.; Ma, J.K.; Pavasant, P.; Phoolcharoen, W. Recombinant human osteopontin expressed in Nicotiana benthamiana stimulates osteogenesis related genes in human periodontal ligament cells. Sci. Rep. 2017, 7, 17358. [Google Scholar] [CrossRef] [Green Version]
- Klinthoopthamrong, N.; Chaikiawkeaw, D.; Phoolcharoen, W.; Rattanapisit, K.; Kaewpungsup, P.; Pavasant, P.; Hoven, V.P. Bacterial cellulose membrane conjugated with plant-derived osteopontin: Preparation and its potential for bone tissue regeneration. Int. J. Bio. Macromol. 2020, 149, 51–59. [Google Scholar] [CrossRef]
- Codreanu, A.; Balta, C.; Herman, H.; Cotoraci, C.; Mihali, C.V.; Zurbau, N.; Zaharia, C.; Rapa, M.; Stanescu, P.; Radu, I.C.; et al. Bacterial cellulose-modified polyhydroxyalkanoates scaffolds promotes bone formation in critical size calvarial defects in mice. Materials 2020, 13, 1433. [Google Scholar] [CrossRef] [Green Version]
- Mensah, A.; Chen, Y.; Christopher, N.; Wei, Q. Membrane technological pathways and inherent structure of bacterial cellulose composites for drug delivery. Bioengineering 2022, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- An, S.J.; Lee, S.H.; Huh, J.B.; Jeong, S.I.; Park, J.S.; Gwon, H.J.; Kang, E.S.; Jeong, C.M.; Lim, Y.M. Preparation and characterization of resorbable bacterial cellulose membranes treated by electron beam irradiation for guided bone regeneration. Int. J. Mol. Sci. 2017, 18, 2236. [Google Scholar] [CrossRef] [PubMed]
- Pigossi, S.C.; de Oliveira, G.J.; Finoti, L.S.; Nepomuceno, R.; Spolidorio, L.C.; Rossa, C., Jr.; Ribeiro, S.J.; Saska, S.; Scarel-Caminaga, R.M. Bacterial cellulose-hydroxyapatite composites with osteogenic growth peptide (OGP) or pentapeptide OGP on bone regeneration in critical-size calvarial defect model. J. Biomed. Mater. Res. A 2015, 103, 3397–3406. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Sha, J.; Bai, Y.; Matsuda, Y.; Hideshima, K.; Yamada, T.; Kanno, T. Efficacy of bacterial cellulose as a carrier of BMP-2 for bone regeneration in a rabbit frontal sinus model. Materials 2019, 12, 2489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiaoprakobkij, N.; Sanchavanakit, N.; Subbalekha, K.; Pavasant, P.; Phisalaphong, M. Characterization and biocompatibility of bacterial cellulose/alginate composite sponges with human keratinocytes and gingival fibroblasts. Carbohydr. Polym. 2011, 85, 548–553. [Google Scholar] [CrossRef]
- Jinga, S.I.; Voicu, G.; Stoica-Guzun, A.; Stroescu, M.; Grumezescu, A.M.; Bleotu, C. Biocellulose nanowhiskers cement composites for endodontic use. Dig. J. Nanomater. Biostruct. 2014, 9, 543–550. [Google Scholar]
- Gorgieva, S. Bacterial cellulose as a versatile platform for research and development of biomedical materials. Processes 2020, 8, 624. [Google Scholar] [CrossRef]
- Zahedmanesh, H.; Mackle, J.N.; Sellborn, A.; Drotz, K.; Bodin, A.; Gatenholm, P.; Lally, C. Bacterial cellulose as a potential vascular graft: Mechanical characterization and constitutive model development. J. Biomed. Mater. Res. Part B Appl. Biomater. J. 2011, 97, 105–113. [Google Scholar] [CrossRef]
- Wan, Y.; Gao, C.; Han, M.; Liang, H.; Ren, K.; Wang, Y.; Luo, H. Preparation and characterization of bacterial cellulose/heparin hybrid nanofiber for potential vascular tissue engineering scaffolds. Polym. Adv. Technol. 2011, 22, 2643–2648. [Google Scholar] [CrossRef]
- Li, Z.; Lv, X.; Chen, S.; Wang, B.; Feng, C.; Xu, Y.; Wang, H. Improved cell infiltration and vascularization of three-dimensional bacterial cellulose nanofibrous scaffolds by template biosynthesis. RSC Adv. 2016, 6, 42229–42239. [Google Scholar] [CrossRef]
- Sämfors, S.; Karlsson, K.; Sundberg, J.; Markstedt, K.; Gatenholm, P. Biofabrication of bacterial nanocellulose scaffolds with complex vascular structure. Biofabrication 2019, 11, 045010. [Google Scholar] [CrossRef] [PubMed]
- Schumann, D.A.; Wippermann, J.; Klemm, D.O.; Kramer, F.; Koth, D.; Kosmehl, H.; Wahlers, T.; Salehi-Gelani, S. Artificial vascular implants from bacterial cellulose: Preliminary results of small arterial substitutes. Cellulose 2009, 16, 877–885. [Google Scholar] [CrossRef]
- Scherner, M.; Reutter, S.; Klemm, D.; Sterner-Kock, A.; Guschlbauer, M.; Richter, T.; Langebartels, G.; Madershahian, N.; Wahlers, T.; Wippermann, J. In vivo application of tissue-engineered blood vessels of bacterial cellulose as small arterial substitutes: Proof of concept? J. Surg. Res. 2014, 189, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, K.; Feng, J.; Liu, J.; Huang, R.; Chen, Z.; Yang, J.; Dai, Z.; Chen, Y.; Wang, N.; et al. Construction of Small-Diameter Vascular Graft by Shape-Memory and Self-Rolling Bacterial Cellulose Membrane. Adv. Healthc. Mater. 2017, 6, 1601343. [Google Scholar] [CrossRef] [PubMed]
- Leitao, A.F.; Faria, M.A.; Faustino, A.M.; Moreira, R.; Mela, P.; Loureiro, L.; Silva, I.; Gama, M. A novel small-caliber bacterial cellulose vascular prosthesis: Production, characterization, and preliminary In vivo testing. Macromol. Biosci. 2016, 16, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Bao, L.; Li, X.; Chen, L.; Hong, F.F. Potential of PVA-doped bacterial nano-cellulose tubular composites for artificial blood vessels. J. Mater. Chem. B 2015, 3, 8537–8547. [Google Scholar] [CrossRef]
- Zang, S.; Zhang, R.; Chen, H.; Lu, Y.; Zhou, J.; Chang, X.; Qiu, G.; Wu, Z.; Yang, G. Investigation on artificial blood vessels prepared from bacterial cellulose. Mater. Sci. Eng. C 2015, 46, 111–117. [Google Scholar] [CrossRef]
- Culenova, M.; Bakos, D.; Ziaran, S.; Bodnarova, S.; Varga, I.; Danisovic, L. Bioengineered scaffolds as substitutes for grafts for urethra reconstruction. Materials 2019, 12, 3449. [Google Scholar] [CrossRef] [Green Version]
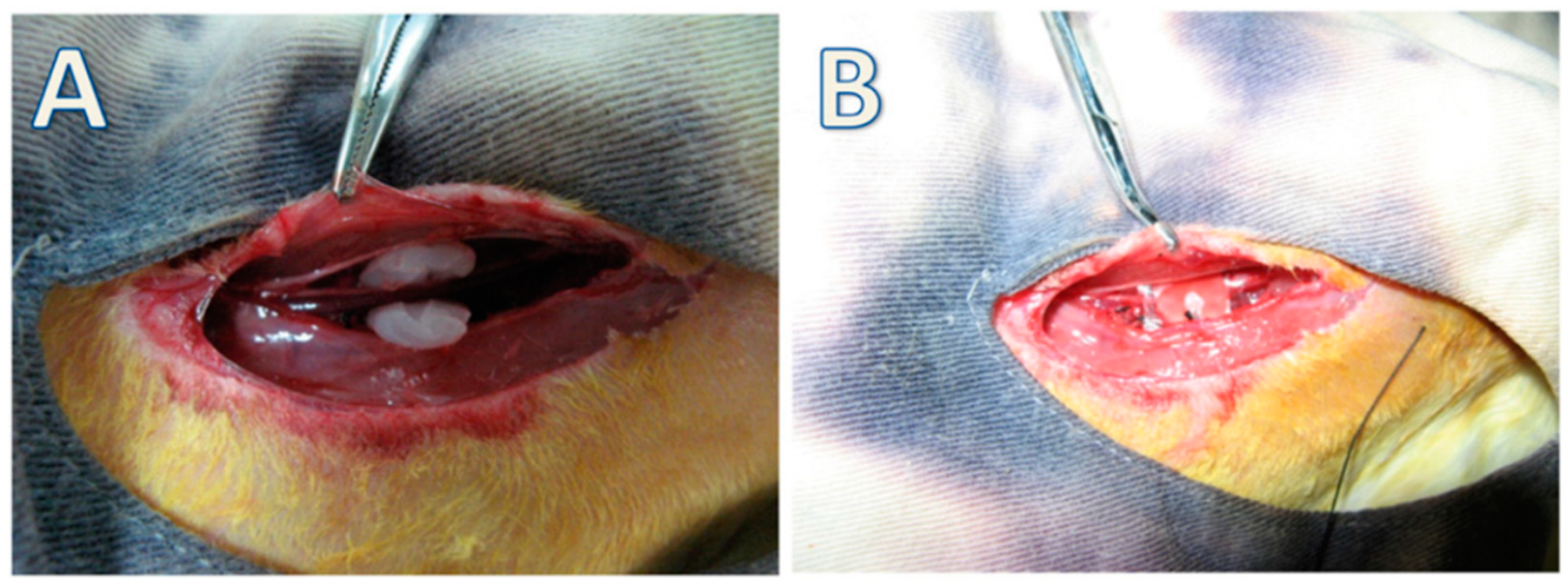
- Lima, S.V.C.; Machado, M.R.; Pinto, F.C.M.; Lira, M.M.D.M.; Albuquerque, A.V.D.; Lustosa, E.S.; Silva, J.G.M.D.; Campos, O. A new material to prevent urethral damage after implantation of artificial devices: An experimental study. Int. Braz. J. Urol. 2017, 43, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Maia, G.T.D.S.; Albuquerque, A.V.D.; Martins, E.D.; Lira, F.T.D.; Souza, V.S.B.D.; Silva, A.A.D.; Lira, M.M.D.M.; Lima, S.V.C. Bacterial cellulose to reinforce urethrovesical anastomosis, A translational study. Acta Cir. Bras. 2018, 33, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Yang, J.; Feng, C.; Li, Z.; Chen, S.; Xie, M.; Huang, J.; Li, H.; Wang, H.; Xu, Y. Bacterial cellulose-based biomimetic nanofibrous scaffold with muscle cells for hollow organ tissue engineering. ACS Biomater. Sci. Eng. 2016, 2, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Feng, C.; Liu, Y.; Peng, X.; Chen, S.; Xiao, D.; Wang, H.; Li, Z.; Xu, Y.; Lu, M. A smart bilayered scaffold supporting keratinocytes and muscle cells in micro/nano-scale for urethral reconstruction. Theranostics 2018, 8, 3153–3163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepúlveda, R.V.; Valente, F.L.; Reis, E.C.C.; Araújo, F.R.; Eleotério, R.B.; Queiroz, P.V.S.; Borges, A.P.B. Bacterial cellulose and bacterial cellulose/polycaprolactone composite as tissue substitutes in rabbits’ cornea. Pesqui. Vet. Bras. 2016, 36, 986–992. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Cao, J.; Zhao, S.; Luo, H.; Yang, Z.; Gama, M.; Zhang, Q.; Su, D.; Wan, Y. Biocompatibility evaluation of bacterial cellulose as a scaffold material for tissue-engineered corneal stroma. Cellulose 2020, 27, 2775–2784. [Google Scholar] [CrossRef] [Green Version]
- Anton-Sales, I.; D’Antin, J.C.; Fernández-Engroba, J.; Charoenrook, V.; Laromaine, A.; Roig, A.; Michael, R. Bacterial nanocellulose as a corneal bandage material: A comparison with amniotic membrane. Biomater. Sci. 2020, 8, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Binder, S.; Stanzel, B.V.; Krebs, I.; Glittenberg, C. Transplantation of the RPE in AMD. Prog. Retin. Eye Res. 2007, 26, 516–554. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Goncalves, S.; Rodrigues, I.P.; Padrão, J.; Silva, J.P.; Sencadas, V.; Lanceros-Mendez, S.; Girão, H.; Gama, F.M.; Dourado, F.; Rodrigues, L.R. Acetylated bacterial cellulose coated with urinary bladder matrix as a substrate for retinal pigment epithelium. Colloids Surf. B Biointerfaces 2016, 139, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Heo, D.N.; Kim, H.J.; Lee, Y.J.; Heo, M.; Lee, S.J.; Lee, D.H.; Do, S.H.; Lee, S.H.; Kwon, I.K. Flexible and highly biocompatible nanofiber-based electrodes for neural surface interfacing. ACS Nano 2017, 11, 2961–2971. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Du, M.; Wang, L.; Li, S.; Wang, G.; Yang, X.; Zhang, L.; Fang, Y.; Zheng, W.; Yang, G.; et al. Bacterial cellulose as a supersoft neural interfacing substrate. ACS Appl. Mater. Interfaces 2018, 10, 33049–33059. [Google Scholar] [CrossRef] [PubMed]
- Pértile, R.; Moreira, S.; Andrade, F.; Domingues, L.; Gama, M. Bacterial cellulose modified using recombinant proteins to improve neuronal and mesenchymal cell adhesion. Biotechnol. Prog. 2012, 28, 526–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.Y.; Park, S.E.; Jo, I.S.; Kim, S.M.; Kang, D.H.; Cho, S.P.; Park, J.B.; Hong, B.H.; Yoon, M.H. Multiscale modulation of nanocrystalline cellulose hydrogel via nanocarbon hybridization for 3D neuronal bilayer formation. Small 2017, 13, 1700331. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.; Yang, J.; Zhu, R.; Zhang, Z.; Li, Y. Development and biocompatibility evaluation of biodegradable bacterial cellulose as a novel peripheral nerve scaffold. J. Biomed. Mater. Res. Part A 2018, 106, 1288–1298. [Google Scholar] [CrossRef]
- Wang, B.; Li, P.; Shangguan, L.; Ma, J.; Mao, K.; Zhang, Q.; Wang, Y.; Liu, Z.; Mao, K. A novel bacterial cellulose membrane immobilized with human umbilical cord mesenchymal stem cells-derived exosome prevents epidural fibrosis. Int. J. Nanomed. 2018, 13, 5257–5273. [Google Scholar] [CrossRef] [Green Version]
- Jing, Y.; Ma, X.; Xu, C.; Tian, H.L.; Chen, S.W. Repair of dural defects with electrospun bacterial cellulose membranes in a rabbit experimental model. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 117, 111246. [Google Scholar] [CrossRef]
- Deng, W.; Tan, Y.; Rajoka, M.S.R.; Xue, Q.; Zhao, L.; Wu, Y. A new type of bilayer dural substitute candidate made up of modified chitin and bacterial cellulose. Carbohydr. Polym. 2021, 256, 117577. [Google Scholar] [CrossRef]
- Stumpf, T.R.; Sandarage, R.V.; Galuta, A.; Fournier, P.; Li, T.; Kirkwood, K.; Yi, X.; Tsai, E.C.; Cao, X. Design and evaluation of a biosynthesized cellulose drug releasing duraplasty. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 110, 110677. [Google Scholar] [CrossRef]
- Dasari, S.; Nijiki, S.; Mbemi, A.; Yedjou, C.G.; Tchounwou, P.B. Pharmacological effects of cisplatin combination with natural products in cancer chemotherapy. Int. J. Mol. Sci. 2022, 23, 1532. [Google Scholar] [CrossRef]
- Treesuppharat, W.; Rojanapanthu, P.; Siangsanoh, C.; Manuspiya, H.; Ummartyotin, S. Synthesis and characterization of bacterial cellulose and gelatin-based hydrogel composites for drug-delivery systems. Biotechnol. Rep. 2017, 15, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Arikibe, J.E.; Lata, R.; Kuboyama, K.; Ougizawa, T.; Rohindra, D. pH-responsive studies of bacterial cellulose/chitosan hydrogels crosslinked with Genipin: Swelling and drug release behavior. ChemistrySelect 2019, 4, 9915–9926. [Google Scholar] [CrossRef]
- Luo, H.; Ao, H.; Li, G.; Li, W.; Xiong, G.; Zhu, Y.; Wan, Y. Bacterial cellulose/graphene oxide nanocomposite as a novel drug delivery system. Curr. Appl. Phys. 2017, 17, 249–254. [Google Scholar] [CrossRef]
- Pandey, M.; Mohamad, N.; Amin, M.C.I.M. Bacterial cellulose/acrylamide pH-sensitive smart hydrogel: Development, characterization, and toxicity studies in ICR mice model. Mol. Pharm. 2014, 11, 3596–3608. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Amin, M.C.I.M.; Mahali, S.M.; Ismail, I.; Chuang, V.T.G. Biocompatible and mucoadhesive bacterial cellulose-g-poly (acrylic acid) hydrogels for oral protein delivery. Mol. Pharm. 2014, 11, 4130–4142. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, Y.; Wang, G.; Lin, Q.; Fan, J. pH-and electro-response characteristics of bacterial cellulose nanofiber/sodium alginate hybrid hydrogels for dual controlled drug delivery. RSC Adv. 2014, 4, 47056–47065. [Google Scholar] [CrossRef]
- Zhang, L.K.; Du, S.; Wang, X.; Jiao, Y.; Yin, L.; Zhang, Y.; Guan, Y.Q. Bacterial cellulose based composites enhanced transdermal drug targeting for breast cancer treatment. Chem. Eng. J. 2019, 370, 749–759. [Google Scholar] [CrossRef]
- Vasic-Racki, D. Industrial biotransformations. In History of Industrial Biotransformations-Dreams and Realities, 2nd ed.; Liese, A., Seelbach, K., Wandrey, C., Eds.; WileyVCH: Weinheim, Germany, 2006; pp. 1–35. [Google Scholar]
- Poulsen, P.B.; Buchholz, K. History of enzymology with emphasis on food production. In Handbook of Food Enzymology, 1st ed.; Whitaker, J.R., Voragen, A.G.J., Wong, D.W.S., Eds.; Marcel Dekker: New York, NY, USA, 2003; pp. 11–20. [Google Scholar]
- Schafer, T.; Kirk, W.; Borchert, T.V.; Fuglsang, C.C.; Pedersen, S.; Salmon, S.; Olsen, H.S.; Deinhammer, R.; Lund, H. Enzymes for technical application. In Biopolymers; Fahnestock, S.R., Steinbuchel, S.R., Eds.; WileyVCH: Weinheim, Germany, 2022; pp. 377–437. [Google Scholar]
- Fernandes, P. Enzymes in food processing: A condensed overview on strategies for better biocatalysts. Enzyme Res. 2010, 2010, 862537. [Google Scholar] [CrossRef] [Green Version]
- Kilara, A.; Shahani, K.M.; Shukla, T.P. The use of immobilized enzymes in the food industry: A review. CRC Crit. Rev. Food Sci. Nutr. 1979, 12, 161–198. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Wang, W.; Jing, Y.; Su, J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: Advances and perspectives. Bioact. Mater. 2022, 14, 169–181. [Google Scholar] [CrossRef]
- Wu, S.C.; Lia, Y.K. Application of bacterial cellulose pellets in enzyme immobilization. J. Mol. Catal. B-Enzym. 2008, 54, 103–108. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Ton, N.M.N.; Le, V.V.M. Optimization of Saccharomyces cerevisiae immobilization in bacterial cellulose by ‘adsorption-incubation’method. Int. Food Res. J. 2009, 16, 59–64. [Google Scholar]
- Ton, M.N.; Le, V.V.M. Application of immobilized yeast in bacterial cellulose to the repeated batch fermentation in wine-making. Int. Food Res. J. 2011, 18, 983–987. [Google Scholar]
- Montealegre, C.M.; Dionisio, E.R.; Sumera, L.V.; Adolacion, J.R.T.; De Leon, R.L. A comparison between the performance of S. Cerevisiae cells immobilized in Nata de Coco biocellulose and calcium alginate during continuous bioethanol production. Int. J. Chem. Eng. Appl. 2012, 3, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Kirdponpattara, S.; Phisalaphong, M. Bacterial cellulose-alginate composite sponge as a yeast cell carrier for ethanol production. Biochem. Eng. J. 2013, 77, 103–109. [Google Scholar] [CrossRef]
- Wu, S.C.; Lia, Y.K.; Ho, C.Y. Glucoamylase immobilization on bacterial cellulose using periodate oxidation method. Int. J. Sci. Eng. 2013, 3, 102086488. [Google Scholar]
- Tam, T.T.M.; Huong, N.T. Optimization of Corynebacterium glutamicum immobilization process on bacterial cellulose carrier and its application for lysine fermentation. IOSR J. Eng. 2014, 4, 33–38. [Google Scholar]
- Chávarri, M.; Maranon, I.; Villaran, M.C. Encapsulation technology to protect probiotic bacteria. In Probiotics; Rigobelo, E.C., Ed.; InTech: London, UK, 2012; pp. 501–540. [Google Scholar]
- Fijałkowski, K.; Peitler, D.; Rakoczy, R.; Zywicka, A. Survival of probiotic lactic acid bacteria immobilized in different forms of bacterial cellulose in simulated gastric juices and bile salt solution. LWT-Food Sci. Technol. 2016, 68, 322–328. [Google Scholar] [CrossRef]
- Khorasani, A.C.; Shojaosadati, S.A. Bacterial nanocellulose-pectin bionanocomposites as prebiotics against drying and gastrointestinal condition. Int. J. Biol. Macromol. 2016, 83, 9–18. [Google Scholar] [CrossRef]
- Chen, L.; Zou, M.; Hong, F.F. Evaluation of fungal laccase immobilized on natural nanostructured bacterial cellulose. Front. Microbiol. 2015, 6, 1245. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.C.; Wu, S.M.; Su, F.M. Novel process for immobilizing an enzyme on a bacterial cellulose membrane through repeated absorption. J. Chem. Technol. Biotechnol. 2017, 92, 109–114. [Google Scholar] [CrossRef]
- Bayazidi, P.; Almasi, H.; Asl, A.K. Immobilization of lysozyme on bacterial cellulose nanofibers: Characteristics, antimicrobial activity and morphological properties. Int. J. Biol. Macromol. 2018, 107, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J. XLIII.-On an acetic ferment which forms cellulose. J. Chem. Soc. Trans. 1886, 49, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.J. The chemical action of pure cultivations of bacterium aceti. J. Chem. Soc. Trans. 1886, 49, 172–187. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, S.; Watanabe, K.; Kitamura, N.; Iguchi, M.; Mitusuhashi, S.; Nishi, Y.; Uryu, M. The structure and mechanical properties of sheets prepared from bacterial cellulose. J. Mater. Sci. 1989, 24, 3141–3145. [Google Scholar] [CrossRef]
- Shibazaki, H.; Kuga, S.; Onabe, F. Mechanical properties of papersheet containing bacterial cellulose. Japan Tappi 1994, 48, 1621–1630. [Google Scholar] [CrossRef]
- Abral, H.; Mahardika, M. Tensile properties of bacterial cellulose nanofibers—Polyester composites. Mater. Sci. Eng. C 2016, 137, 012019–012025. [Google Scholar] [CrossRef] [Green Version]
- Arjmandi, R.; Suib, N.; Hassan, A.; Muhamad, I.I.; Pa’e, N.; Zakaria, Z. Tensile and morphological properties of bacterial cellulose nanowhiskers reinforced polylactic acid nanocomposites. Chem. Eng. Trans. 2017, 56, 1327–1332. [Google Scholar]
- Fei, G.; Wang, Y.; Wang, H.; Ma, Y.; Guo, Q.; Huang, W.; Yang, D.; Shao, Y.; Ni, Y. Fabrication of bacterial cellulose/polyaniline nanocomposite paper with excellent conductivity, strength and flexibility. ACS Sustain. Chem. Eng. 2019, 7, 8215–8225. [Google Scholar] [CrossRef]
- Santos, S.M.; Carbajo, J.M.; Go’mez, N.; Ladero, M.; Villar, J.C. Paper reinforcing by in situ growth of bacterial Cellulose. J. Mater. Sci. 2017, 52, 5882–5893. [Google Scholar] [CrossRef]
- Xiang, Z.; Jin, X.; Liu, Q.; Chen, Y.; Li, J.; Lu, F. The reinforcement mechanism of bacterial cellulose on paper made from woody and non-woody fiber sources. Cellulose 2017, 24, 5147–5156. [Google Scholar] [CrossRef]
- Kamin´ski, K.; Jarosz, M.; Grudzien, J.; Pawlik, J.; Zastawnik, F.; Pandyra, P.; Kołodziejczyk, A.M. Hydrogel bacterial cellulose: A path to improved materials for new eco-friendly textiles. Cellulose 2020, 27, 5353–5365. [Google Scholar] [CrossRef] [Green Version]
- Domskiene, J.; Sederaviciute, F.; Simonaityte, J. Kombucha bacterial cellulose for sustainable fashion. Int. J. Cloth Sci. Tech. 2019, 31, 644–652. [Google Scholar] [CrossRef]
- Johnson, D.C. Bacterial cellulose has potential application as new paper coating. Pulp Pap. 1990, 64, 105–107. [Google Scholar]
- Yuan, J.; Wang, T.; Huang, X.; Wei, W. Dispersion and beating of bacterial cellulose and their influence on paper properties. Bioresources 2016, 11, 9290–9301. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.H.; Chen, K.F.; Yang, R.D.; Yang, F.; Han, W.J. Properties of bacterial cellulose and its influence on the physical properties of paper. BioResources 2010, 6, 144–153. [Google Scholar] [CrossRef]
- Martinez-Sanz, M.; Lopez-Rubio, A.; Lagaron, J.M. Lagaron, Optimization of the Dispersion of Unmodified Bacterial Cellulose Nanowhiskers into Polylactide via Melt Compounding to Significantly Enhance Barrier and Mechanical Properties. Biomacromolecules 2012, 13, 3887–3899. [Google Scholar] [CrossRef]
- Campano, C.; Merayo, N.; Negro, C.; Blanco, A. Low-fibrillated Bacterial Cellulose Nanofibers as a sustainable additive to enhance recycled paper quality. Int. J. Biol. Macromol. 2018, 114, 1077–1083. [Google Scholar] [CrossRef]
- Kang, J.H.; Park, S. Printability Improvement of Hanji using Microbial Cellulose from Saprolegnia ferax. J. Korea TAPPI 2008, 40, 23–29. [Google Scholar]
- Zhang, D.; Qi, L. Synthesis of mesoporous titania networks consisting of anatase nanowires by templating of bacterial cellulose membranes. Chem. Commun. 2005, 21, 2735–2737. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymers and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Effects of physical and chemical structures of bacterial cellulose on its enhancement to paper physical properties. Cellulose 2017, 24, 3513–3523. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, X.; Hu, Z.; Xiang, Z.; Song, T.; Lu, F. A Highly Efficient and Durable Fluorescent Paper Produced from Bacterial Cellulose/Eu Complex and Cellulosic Fibers. Nanomaterials 2019, 9, 1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, R.J.B.; Marques, P.A.A.P.; Martins, M.A.; Neto, C.P.; Trindade, T. Electrostatic assembly and growth of gold nanoparticles in cellulosic fibres. J. Colloid Interface Sci. 2007, 312, 506–512. [Google Scholar] [CrossRef]
- Kang, J.H.; Park, S.C. Production and Characteristics of Cellulose from Saprolegnia ferax. J. Korea TAPPI 2007, 39, 77–83. [Google Scholar]
- Tome´, L.C.; Branda˜o, L.; Mendes, A.M.; Silvestre, A.J.; Neto, C.P.; Gandini, A.; Freire, C.S.; Marrucho, I.M. Preparation and characterization of bacterial cellulose membranes with tailored surface and barrier properties. Cellulose 2010, 17, 1203–1211. [Google Scholar] [CrossRef]
- CLOT Magazine. Available online: http://www.clotmag.com/biomedia/simon-park (accessed on 13 December 2016).
- Suzanne Lee: Biocouture. Available online: https://trendland.com/suzanne-lee-and-her-celluloid-clothing (accessed on 15 December 2021).
- Material Distric. Available online: https://materialdistrict.com/article/handbag-bacterial-cellulose/ (accessed on 26 September 2019).
- Nullarbor FibreTM. Available online: https://nanollose.com/products/nullarbor-fibre/ (accessed on 15 December 2021).
- Ng, F.M.C.; Wang, P.W. Natural self-grown fashion from bacterial cellulose: A paradigm shift design approach in fashion creation. Des. J. 2016, 19, 837–855. [Google Scholar] [CrossRef]
- Kim, B.J.; Sohng, J.K.; Liou, K.K.; Lee, H.C. Preparation of carbone nanomaterial from the microbial cellulose. Korean J. Biotechnol. Bioeng. 2005, 20, 50–54. [Google Scholar]
- Lee, J.G.; Ryu, J.H.; Youn, H.J. Conductive paper through LbL multilayering with conductive polymer: Dominant factors to increase electrical conductivity. Cellulose 2012, 19, 2153–2164. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, H.J.; Yang, H.S. Polymerization of Aniline on Bacterial Cellulose and Characterization of Bacterial Cellulose/Polyaniline Nanocomposite Films. Curr. Appl. Phys. 2012, 12, 75–80. [Google Scholar] [CrossRef]
- Lee, H.J.; Chung, T.J.; Kwon, H.J.; Kim, H.J.; Yin Tze, W.T. Fabrication and evaluation of bacterial cellulose-polyaniline composites by interfacial polymerization. Cellulose 2012, 19, 1251–1258. [Google Scholar] [CrossRef]
- Yoon, S.H.; Jin, H.J.; Kook, M.C.; Pyun, Y.R. Electrically conductive bacterial cellulose by incorporation of carbon nanotubes. Biomacromolecules 2006, 7, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Park, S.; Cheng, J.; Park, M.; Hyun, J. Amphiphilic comb-like polymer for harvest of conductive nano-cellulose. Colloids Surf. B 2012, 89, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Cheng, J.; Choi, J.; Kim, J.; Hyun, J. Electromagnetic nanocomposite of bacterial cellulose using magnetite nanoclusters and polyaniline. Colloid Surf. B 2013, 102, 238–242. [Google Scholar] [CrossRef]
- Park, M.; Lee, D.; Shin, S.; Kim, H.; Hyun, J. Flexible conductive nanocellulose combined with silicon nanoparticles and polyaniline. Carbohydr. Polym. 2016, 140, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Song, J.E.; Silva, C.; Kim, H.R. Production of conductive bacterial cellulose-polyaniline membranes in the presence of metal salts. Text. Res. J. 2019, 90, 1517–1526. [Google Scholar] [CrossRef]
- Liang, H.W.; Guan, Q.F.; Zhu, Z.; Song, L.T.; Yao, H.B.; Lei, X.; Yu, S.H. Highly conductive and stretchable conductors fabricated from bacterial cellulose. NPG Asia Mater. 2012, 4, e19. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, F.; Dong, L.; Li, Z.; Chen, L.; He, X.; Gong, J.; Zhang, J.; Li, Q. Design and Optimization of Flexible Polypyrrole/Bacterial Cellulose Conductive Nanocomposites Using Response Surface Methodology. Polymers 2019, 11, 960. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Han, J.; Jiang, Z.; Chen, S.; Wang, H. Flexible conductive polypyrrole nanocomposite membranes based onbacterial cellulose with amphiphobicity. Carbohydr. Polym. 2015, 117, 230–235. [Google Scholar] [CrossRef]
- Wang, H.; Bian, L.; Zhou, P.; Tang, J.; Tang, W. Core–sheath structured bacterial cellulose/polypyrrole nanocomposites with excellent conductivity as supercapacitors. J. Mater. Chem. A 2013, 1, 578–584. [Google Scholar] [CrossRef]
- Mullera, D.; Rambob, C.R.; Portoc, L.M.; Schreinerd, W.H.; Barra, G.M.O. Structure and properties of polypyrrole/bacterial cellulose nanocomposites. Carbohydr. Polym. 2013, 94, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Y.; Zhang, X.Q.; Shen, Y.T.; Yoshino, K.; Feng, W. A mechanically strong, flexible and conductive film based on bacterial cellulose/graphene nanocomposite. Carbohydr. Polym. 2012, 87, 644–649. [Google Scholar] [CrossRef] [PubMed]
- RICOH. Available online: http://jp.ricoh.com/release/by_field/other/2009/0702_2.html (accessed on 2 July 2009).
- Shah, J.; Brown, R.M. Towards electronic paper displays made from microbial cellulose. Appl. Microbiol. Biot. 2005, 66, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Tahara, K.; Ohba, J.; Kusumoto, R.; Kose, R.; Kono, H.; Matsushima, T.; Fushimi, K.; Isono, T.; Yamamoto, T.; et al. Detailed Structural Analyses of Nanofibrillated Bacterial Cellulose and Its Application as Binder Material for a Display Device. Biomacromolecules 2020, 21, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Legnani, C.; Vilani, C.; Calil, V.L.; Barud, H.S.; Quirino, W.G.; Achete, C.A.; Ribeiro, S.J.L.; Cremona, M. Bacterial cellulose membrane as flexible substrate for organic light emitting devices. Thin Solid Films 2008, 517, 1016–1020. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Juntaro, J.; Sain, M.; Manuspiya, H. Development of transparent bacterial cellulose nanocomposite film as substrate for flexible organic light emitting diode (OLED) display. Ind. Crops. Prod. 2012, 35, 92–97. [Google Scholar] [CrossRef]
- Pinto, E.R.P.; Barud, H.S.; Silva, R.R.; Palmieri, M.; Polito, W.L.; Calil, V.L.; Cremona, M.; Ribeiro, S.J.L.; Messaddeq, Y. Transparent composites prepared from bacterial cellulose and castor oil based polyurethane as substrates for flexible OLEDs. J. Mater. Chem. C 2015, 3, 11581–11588. [Google Scholar] [CrossRef]
- Legnani, C.; Barud, H.S.; Caiut, J.M.A.; Calil, V.L.; Maciel, I.O.; Quirino, W.G.; Ribeiro, S.J.; Cremona, L.M. Transparent bacterial cellulose nanocomposites used as substrate for organic light-emitting diodes. J. Mater. Sci. Mater. Electron. 2019, 30, 16718–16723. [Google Scholar] [CrossRef]
- Evans, B.R.; O’Neill, H.M.; Malyvanh, V.P.; Lee, I.; Woodward, J. Palladium-bacterial cellulose membranes for fuel cells. Biosens. Bioelectron. 2003, 18, 917–923. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Wang, Y.; Ray, U.; Zhu, S.; Dai, J.; Chen, C.; Fu, K.; Jang, S.H.; Henderson, D.; et al. Cellulose-nanofiber-enabled 3D printing of a carbon-nanotube microfiber network. Small Methods 2017, 1, 1700222–1700230. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Shen, F.; Wan, J.; Lacey, S.; Fang, Z.; Dai, H.; Hu, L. Nanocellulose as green dispersant for two-dimensional energy materials. Nano Energy 2015, 13, 346–354. [Google Scholar] [CrossRef]
- Gao, K.; Shao, Z.; Li, J.; Wang, X.; Peng, X.; Wang, W.; Wang, F. Cellulose nanofiber-graphene all solid-state flexible supercapacitors. J. Mater. Chem. A 2013, 1, 63–67. [Google Scholar] [CrossRef]
- Li, S.; Huang, D.; Zhang, B.; Xu, X.; Wang, M.; Yang, G.; Shen, Y. Flexible supercapacitors based on bacterial cellulose paper electrodes. Adv. Energy Mater. 2014, 4, 1301655–1301658. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Edo, M.G.; Alemán, C. Powering the future: Application of cellulose-based materials for supercapacitors. Green Chem. 2016, 18, 5930–5956. [Google Scholar] [CrossRef]
- Zheng, Q.; Cai, Z.; Ma, Z.; Gong, S. Cellulose nanofibril/reduced graphene oxide/carbon nanotube hybrid aerogels for highly flexible and all-solid-state supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 3263–3271. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Z.; Chen, Y.; Weadock, N.; Wan, J.; Vaaland, O.; Han, X.; Li, T.; Hu, L. Tin anode for sodium-ion batteries using natural wood fiber as a mechanical buffer and electrolyte reservoir. Nano Lett. 2013, 13, 3093–3100. [Google Scholar] [CrossRef]
- Gui, Z.; Zhu, H.; Gillette, E.; Han, X.; Rubloff, G.W.; Hu, L.; Lee, S.B. Natural cellulose fiber as substrate for supercapacitor. ACS Nano 2017, 7, 6037–6046. [Google Scholar] [CrossRef]
- Sheng, N.; Chen, S.; Yao, J.; Guan, F.; Zhang, M.; Wang, B.; PengJi, Z.; Wang, H. Polypyrrole@TEMPO-oxidized bacterial cellulose/reduced graphene oxide macrofibers for flexible all-solid-state supercapacitors. Chem. Eng. J. 2019, 368, 1022–1032. [Google Scholar] [CrossRef]
- Jeon, S.R.; Park, J.W.; Kim, H.J. Manufacturing and applications of bacterial cellulose. KIC News 2013, 16, 37–45. [Google Scholar]
- Niyazbekova, Z.T.; Nagmetova, G.; Kurmanbayev, A. An Overview of Bacterial Cellulose Applications. Biotecnol. Theory Pract. 2018, 2, 139648317. [Google Scholar] [CrossRef]
- Barud, H.S.; Souza, J.L.; Santos, D.B.; Crespi, M.S.; Ribeiro, C.A.; Messaddeq, Y.; Ribeiro, S.J.L. Bacterial cellulose/poly(3-hydroxybutyrate) composite membranes. Carbohydr. Polym. 2011, 83, 1279–1284. [Google Scholar] [CrossRef]
- Huang, H.C.; Chen, L.C.; Lin, S.B.; Chen, H.H. Nano-biomaterials application: In situ modification of bacterial cellulose structure by adding HPMC during fermentation. Carbohydr. Polym. 2011, 83, 979–987. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Olsson, R.T.; Lopez-Rubio, A.; Lagaron, J.M. Development of electrospun EVOH fibres reinforced with bacterial cellulose nanowhiskers. Part I: Characterization and method optimization. Cellulose 2011, 18, 335–347. [Google Scholar] [CrossRef]
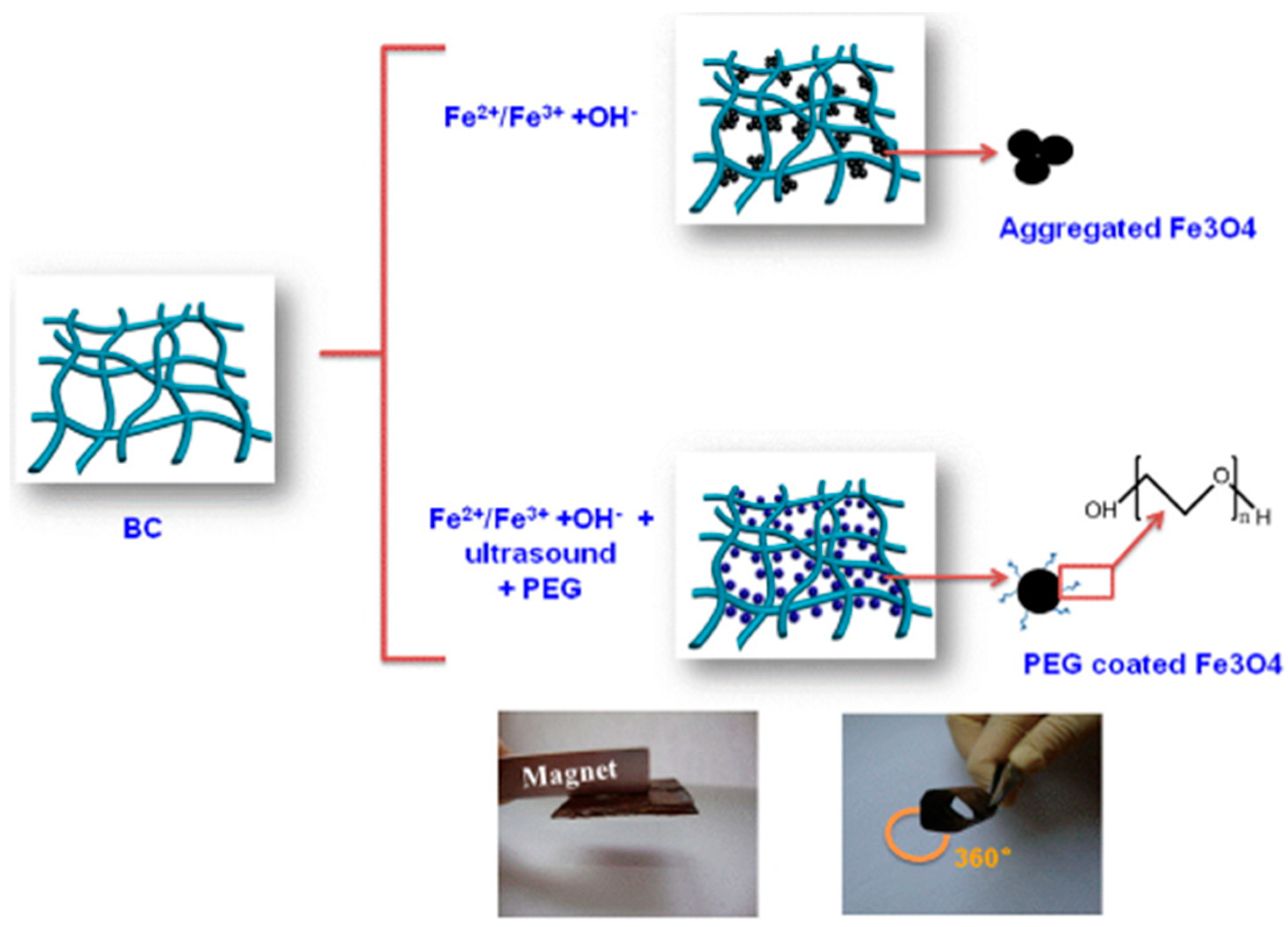
- Zheng, Y.; Yang, J.; Zheng, W.; Wang, X.; Xiang, C.; Tang, L.; Zhang, W.; Chen, S.; Wang, H. Synthesis of flexible magnetic nanohybrid based on bacterial cellulose under ultrasonic irradiation. Mater. Sci. Eng. C 2013, 33, 2407–2412. [Google Scholar] [CrossRef]
- Marins, J.A.; Soares, B.G.; Barud, H.S.; Ribeiro, S.J.L. Flexible magnetic membranes based on bacterial cellulose and its evaluation as electromagnetic interference shielding material. Mater. Sci. Eng. C 2013, 33, 3994–4001. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Zhou, B.; Wang, H. Facile synthesis of ZnO nanoparticles based on bacterial cellulose. Mat. Sci. Eng. B 2010, 170, 88–92. [Google Scholar] [CrossRef]
- Sun, D.; Yang, J.; Wang, X. Bacterial cellulose/TiO2 hybrid nanofibers prepared by the surface hydrolysis method with molecular precision. Nanoscale 2010, 2, 287–292. [Google Scholar] [CrossRef]
- Xiong, C.R.; Kim, M.J.; Balkus, K.J. TiO2 Nanofibers and Core–Shell Structures Prepared Using Mesoporous Molecular Sieves as Templates. Small 2006, 2, 52–56. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Ni, W.H.; Kou, X.S.; Yeung, M.H.; Sun, L.D.; Wang, J.F.; Yan, C.H. Formation of gold and silver nanoparticle arrays and thin shells on mesostructured silica nanofibers. Adv. Funct. Mater. 2007, 17, 3258–3266. [Google Scholar] [CrossRef]
- Martins, D.; Estevinho, B.; Rocha, F.; Dourado, F.; Gama, M. A dry and fully dispersible bacterial cellulose formulation as a stabilizer for oil-in-water emulsions. Carbohydr. Polym. 2020, 230, 115657. [Google Scholar] [CrossRef] [Green Version]











| Method | Tensile Strength (MPa) | Young’s Modulus (GPa) | Folding Endurance (Times) | Ref |
|---|---|---|---|---|
| Mixture of cladophora and fragmented BC | 35~50 | 2.3~4.0 | 10~58 | [162] |
| BC nanofibers–polyester composites | 18~22 | 0.58~0.72 | - | [163] |
| BC nanowhisker-reinforced polylactic acid | 25~27 | 1.1~1.3 | - | [164] |
| BC–polyaniline nanocomposite paper | - | - | 13~50 | [165] |
| Pulp-reinforced BC | 0.1~0.15 (burst strength) | - | - | [166] |
| BC addition to pulp paper | >79.9 N m/g (tensile index) >5 kPa m2/g (burst index) | - | - | [167] |
| Raw BC | 5 | 0.04 | [168] | |
| Hydrogel BC with glycerol | 8 | 0.28 | [168] | |
| BC film obtained through Kombucha | 0.4~12.8 (different to dry states) | 0.02~2.65 | [169] | |
| Mixture of cotton lint and fragmented BC | 1~100 | 0.1~4.9 | - | [164] |
| Method | Electrical Conductivity (S/cm) | Properties | Ref |
|---|---|---|---|
| LBL multilayering of polyethylene imine(PEI) and poly(3,4-ethylene dioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) | 10−5~10−4 | Without loss of paper strength | [189] |
| multiwalled carbon nanotubes (MWCNTs) into BC | 0.14 | Purity, high crystallinity, ultrafine network | [192] |
| BC is cultured in a carbon nanotube (CNT) | 0.104 | High CNT stability by the one-step biosynthesizing | [193] |
| BC is cultured in a medium containing magnetite nanoparticle (MNP) clusters | 0.43 | BC fibers was fully coated with polyaniline, forming hydrogen bonds. | [194] |
| BC composite with silicon nanoparticles(SiNPs) and polyaniline. | 0.017 | Anode material for Li-ion rechargeable batteries | [195] |
| BC–polyaniline (PANI) membrane by the addition of metal salt. | 0.075 | washing durability is improved | [196] |
| Pyrolyzed BC–polydimethylsiloxane | 0.2~0.41 | High tensile and bending strain | [197] |
| polypyrrole nanocomposite membranes based on BC | 0.32 | good electromagnetic shielding effectiveness | [199] |
| wrapping a homogenous layer of polypyrrole (PPy) around BC nanofibers | 77 | for supercapacitors, with a highest mass-specific capacitance hitting 316 F/g at 0.2 A/g current density. | [200] |
| in situ oxidative polymerization of pyrrole (Py) in the presence of BC membrane | 0.01–1.2 | good mechanical properties (40 MPa) | [201] |
| in situ chemical polymerization of polypyrrole–BC | 7.34 | a core-sheath structure exhibited higher thermal stability and flexible | [198] |
| BC–GO nanocomposite using vacuum-assisted self-assembly technique | 1.1 | Well-dispersed GO nanosheets in the BC matrix, flexible and approved mechanical film | [202] |
| BC–polyaniline nanocomposite film by chemical oxidative polymerization | 1.3 | BC fibers were fully encapsulated by polyaniline spherical spheres | [190] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.M.; Rao, K.M.; Zo, S.M.; Shin, E.J.; Han, S.S. Bacterial Cellulose and Its Applications. Polymers 2022, 14, 1080. https://doi.org/10.3390/polym14061080
Choi SM, Rao KM, Zo SM, Shin EJ, Han SS. Bacterial Cellulose and Its Applications. Polymers. 2022; 14(6):1080. https://doi.org/10.3390/polym14061080
Chicago/Turabian StyleChoi, Soon Mo, Kummara Madhusudana Rao, Sun Mi Zo, Eun Joo Shin, and Sung Soo Han. 2022. "Bacterial Cellulose and Its Applications" Polymers 14, no. 6: 1080. https://doi.org/10.3390/polym14061080
APA StyleChoi, S. M., Rao, K. M., Zo, S. M., Shin, E. J., & Han, S. S. (2022). Bacterial Cellulose and Its Applications. Polymers, 14(6), 1080. https://doi.org/10.3390/polym14061080








