Enhancement of Ultraviolet Light Resistance of Colorless and Transparent Semi-Alicyclic Polyimide Nanocomposite Films via the Incorporation of Hindered Amine Light Stabilizers for Potential Applications in Flexible Optoelectronics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
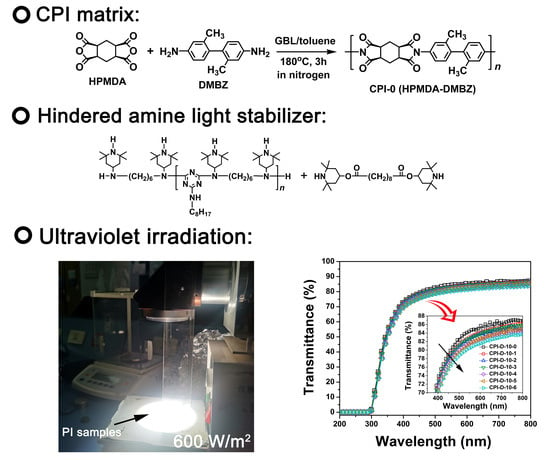
2.3. Preparation of the CPI-0 (HPMDA-DMBZ) Film
2.4. Preparation of CPI/HALS Nanocomposite Films
3. Results and Discussion
3.1. HALS Evaluation
3.2. CPI-0 and CPI/HALS Nanocomposite Film Preparation
3.3. Optical Properties
3.4. Photo-Degradation Behaviors of the CPI-D Nanocomposite Films
3.5. Thermal Properties of the CPI-D Nanocomposite Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, N.; Fujiwara, E.; Nara, M.; Ishige, R.; Ando, S. Colorless copolyimide films ehibbiting large stokes-shifted photoliminescence applicable for spectral conversion. ACS Appl. Polym. Mater. 2021, 3, 3911–3921. [Google Scholar] [CrossRef]
- Miyane, S.; Chen, C.K.; Lin, Y.C.; Ueda, M.; Chen, W.C. Thermally stable colorless copolyimides with a low dielectric constant and dissipation factor and their organic field-effect transistor applications. ACS Appl. Polym. Mater. 2021, 3, 3153–3163. [Google Scholar] [CrossRef]
- Kim, H.; Hwang, S.; Hwang, T.; In, J.B.; Yeo, J. Digitally patterned mesoporous carbon nanostructures of colorless polyimide for transparent and flexible micro-supercapacitor. Energies 2021, 14, 2547. [Google Scholar] [CrossRef]
- Wright, J.S.; Jones, A.; Farmer, B.; Rodman, D.L.; Minton, T.K. POSS-enhanced cololess organic/inorganic nanocomposite (CORIN®) for atomic oxygen resistance in low earth orbit. CEAS Space J. 2021, 13, 399–413. [Google Scholar] [CrossRef]
- Gao, H.; Lan, X.; Liu, L.; Xiao, X.; Liu, Y.; Leng, J. Study on performances of colorless and transparent shape memory polyimide film in space thermal cycling, atomic oxygen and ultraviolet irradiation environments. Smart Mater. Struct. 2017, 26, 095001. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, G.; Dong, B.; Liu, J. Synthesis and properties of polyimide silica nanocomposite film with high transparent and radiation resistance. Nanomaterials 2021, 11, 562. [Google Scholar] [CrossRef]
- Pei, X.; Wang, Q. Different responses of several kinds of copolymerized polyimide films to ultraviolet irradiation. Appl. Surf. Sci. 2007, 253, 5494–5500. [Google Scholar] [CrossRef]
- Plis, E.A.; Engelhart, D.P.; Cooper, R.; Johnston, W.R.; Ferguson, D.; Hoffmann, R. Review of radiation-induced effects in polyimide. Appl. Sci. 2019, 9, 1999. [Google Scholar] [CrossRef] [Green Version]
- Rabek, J.F. Photodegradation and photo-oxidative degradation of heterochain polymers. In Polymer Photodegradation; Springer: Dordrecht, The Netherlands, 1995; pp. 255–352. [Google Scholar]
- Hoyle, C.E.; Anzures, E.T. Photodegradation of polyimides. I. A spectral, viscometric, chromatographic, and weight loss investigation of polyimides based on a hexafluorinated dianhydride. J. Appl. Polym. Sci. 1991, 43, 1–10. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Anzures, E.T. Photodegradation of polyimides. II. Thermal property changes of polyimides based on a hexafluorinated dianhydride. J. Appl. Polym. Sci. 1991, 43, 11–18. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Anzures, E.T. Photodegradation of polyimides. III. The effect of chemical composition, radiation source, atmosphere, and processing. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 1233–1245. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Creed, D.; Nagarajan, R.; Subramanian, P.; Anzures, E.T. Photodegradation of polyimides. 4. Mechanism for the photo-oxidation process based on a model compound. Polymer 1992, 33, 3162–3168. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Anzures, E.T.; Subramanian, P.; Nagarajan, R.; Creed, D. Photodegradation of polyimides. 5. An explanation of the rapid photolytic decomposition of a selected polyimide via anhydride formation. Macromolecules 1992, 25, 6651–6657. [Google Scholar] [CrossRef]
- Creed, D.; Hoyle, C.E.; Subramanian, P.; Nagarajan, R.; Pandey, C.; Anzures, E.T.; Cane, K.M.; Cassidy, P.E. Photodegradation of polyimides. 6. Effect of donor-acceptor groups on the photooxidative stability of polyimides and model compounds. Macromolecules 1994, 27, 832–837. [Google Scholar] [CrossRef]
- Creed, D.; Hoyle, C.E.; Jordan, J.W.; Pandey, C.A.; Nagarajan, R.; Pankasem, S.; Peeler, A.M.; Subramanian, P. Charge transfer states in the photophysics and photochemistry of polyimides and related phthalimides. Macromol. Symp. 1997, 116, 1–13. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- Chung, C.M.; Cho, S.Y.; Lee, J.Y.; Oh, S.Y. Photodegradation behavior of a polyimide containing imidosulfonate groups and cyclobutane rings. Mol. Cryst. Liq. Cryst. 2000, 349, 59–62. [Google Scholar] [CrossRef]
- Mai, A.T.M.; Thakur, A.; Ton, N.N.T.; Nguyen, T.N.; Kaneko, T.; Taniike, T. Photodgradation of a semi-aromatic bio-derived polyimide. Polym. Degrad. Stab. 2021, 184, 109472. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: A review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [Green Version]
- Harito, C.; Bavykin, D.V.; Yuliarto, B.; Dipojono, H.K.; Walsh, F.C. Inhibition of polyimide photodegradation by incorporation of titanate nanotubes into a composite. J. Polym. Environ. 2019, 27, 1505–1515. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Hwang, T.G.; Namgoong, J.W.; Kim, H.M.; Kim, J.P. Effect of linker moiety on linear dimeric benzotrizaole derivatives as highly stable UV absorber for transparent polyimide film. Dyes Pigment. 2020, 18, 108469. [Google Scholar] [CrossRef]
- Gijsman, P. A review on the mechanism of action and applicability of hindered amine stabilizers. Polym. Degrad. Stab. 2017, 145, 2–10. [Google Scholar] [CrossRef]
- Gijsman, P.; Hennekens, J.; Tummers, D. The mechanism of action of hindered amine light stabilizers. Polym. Degrad. Stab. 1993, 39, 225–233. [Google Scholar] [CrossRef]
- Gijsman, P. New synergists for hindered amine light stabilizers. Polymer 2002, 43, 1573–1579. [Google Scholar] [CrossRef]
- Hodgson, J.L.; Coote, M.L. Clarifying the mechanism of the Denisov cycle: How do hindered amine light stabilizers protect polymer coatings from photo-oxidative degradation? Macromolecules 2010, 43, 4573–4583. [Google Scholar] [CrossRef]
- Schaller, C.; Rogez, D.; Braig, A. Hindered amine light stabilizers in pigmented coatings. J. Coat. Technol. Res. 2009, 6, 81–88. [Google Scholar] [CrossRef]
- Klampfl, C.W.; Himmelsbach, M. Advances in the determination of hindered amine light stabilizers—A review. Anal. Chem. Acta 2016, 933, 10–22. [Google Scholar] [CrossRef]
- Kreisberger, G.; Buchberger, W.W. Rapid determination of oligomeric hindered amine light stabilizers in polymeric materials. J. Sep. Sci. 2017, 40, 2366–2373. [Google Scholar] [CrossRef]
- Balabanovich, A.I.; Klimovtsova, I.A.; Prokopovich, V.P.; Prokopchuk, N.R. Thermal stability and thermal decomposition study of hindered amine light stabilizers. Thermochim. Acta 2007, 459, 1–8. [Google Scholar] [CrossRef]
- Pryde, C.A. IR studies of polyimides. I. Effects of chemical and physical changes during cure. J. Polym. Sci. Part A Polym. Chem. 1989, 27, 711–724. [Google Scholar] [CrossRef]














| CPI | CPI-0, DMAc (g, g) | HALS, DMAc (mg, g) | MHALS/MCPI |
|---|---|---|---|
| CPI-1 | 3.9475, 21.0525 | 3.9475, 1.3390 | 0.1% |
| CPI-3 | 3.9475, 21.0525 | 11.8425, 1.3840 | 0.3% |
| CPI-5 | 3.9475, 21.0525 | 19.7375, 1.4285 | 0.5% |
| CPI-10 | 3.9475, 21.0525 | 39.4750, 1.5405 | 1.0% |
| Samples | Description |
|---|---|
| 2020 | Chimassorb® 2020 (BASF, Ludwigshafen, Germany) |
| 944 | Chimassorb® 944 (BASF, Ludwigshafen, Germany) |
| 783 | Tinuvin® 783 (BASF, Ludwigshafen, Germany) |
| 791 | Tinuvin® 791 (BASF, Ludwigshafen, Germany) |
| CPI-0 | CPI (HPMDA-DMBZ) matrix |
| CPI-1 | CPI/HALS nanocomposite film with the MHALS/MCPI = 0.1% |
| CPI-3 | CPI/HALS nanocomposite film with the MHALS/MCPI = 0.3% |
| CPI-5 | CPI/HALS nanocomposite film with the MHALS/MCPI = 0.5% |
| CPI-10 | CPI/HALS nanocomposite film with the MHALS/MCPI = 1.0% |
| CPI-A-X | Series A, CPI-0/2020 composite films; X: contents of 2020 (X = 1, 0.1%; X = 3, 0.3%; X = 5, 0.5%; X = 10, 1.0%) |
| CPI-B-X | Series B, CPI-0/944 composite films; X: contents of 944 (X = 1, 0.1%; X = 3, 0.3%; X = 5, 0.5%; X = 10, 1.0%) |
| CPI-C-X | Series C, CPI-0/783 composite films; X: contents of 783 (X = 1, 0.1%; X = 3, 0.3%; X = 5, 0.5%; X = 10, 1.0%) |
| CPI-D-X | Series D, CPI-0/791 composite films; X: contents of 791 (X = 1, 0.1%; X = 3, 0.3%; X = 5, 0.5%; X = 10, 1.0%) |
| CPI-D-X-Y | CPI-0/791 composite film with different UV exposure time; X: contents of 791 (X = 1, 0.1%; X = 10, 1.0%); Y: Xenon lamp exposure time (Y = 1, 1 h; Y = 1.5, 1.5 h; Y = 2, 2 h; Y = 3, 3 h; Y = 4, 4 h; Y = 5, 5 h; Y = 6, 6 h) |
| HALS | FWHM 1 (°) | T5% 2 (°C) | T10% 2 (°C) | Tmax1 2 (°C) | Tmax2 2 (°C) | Rw500 2 (%) |
|---|---|---|---|---|---|---|
| 783 | 7.164 | 361.9 | 372.8 | 399.7 | 502.3 | 15.3 |
| 791 | 3.678 | 322.4 | 342.3 | 376.3 | 503.7 | 18.2 |
| 944 | 7.599 | 425.5 | 450.3 | NA 3 | 510.8 | 45.2 |
| 2020 | 8.090 | 420.5 | 448.8 | NA | 509.0 | 48.7 |
| Property | CPI-0 | Series A (2020) | Series B (944) | Series C (783) | Series D (791) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A-1 | A-3 | A-5 | A-10 | B-1 | B-3 | B-5 | B-10 | C-1 | C-3 | C-5 | C-10 | D-1 | D-3 | D-5 | D-10 | ||
| λcut 1 (nm) | 292 | 290 | 293 | 298 | 293 | 289 | 293 | 290 | 294 | 297 | 292 | 294 | 300 | 293 | 294 | 292 | 294 |
| T400 2 (%) | 76.9 | 72.7 | 68.1 | 54.8 | 47.0 | 81.7 | 74.8 | 73.5 | 40.9 | 70.9 | 65.6 | 55.5 | 45.1 | 77.5 | 77.5 | 75.3 | 70.2 |
| T450 2 (%) | 81.9 | 80.7 | 77.7 | 68.2 | 60.2 | 84.8 | 80.6 | 79.6 | 52.0 | 79.6 | 74.7 | 64.6 | 57.7 | 82.6 | 83.3 | 80.7 | 79.0 |
| CPI Samples | T350, Xe 1 (%) | L*Xe 2 | b*Xe 2 | hazeXe 2 (%) |
|---|---|---|---|---|
| CPI-0-0 | 55.7 | 95.12 | 3.38 | 1.46 |
| CPI-0-1 | 45.4 | 94.92 | 3.82 | 3.12 |
| CPI-0-2 | 42.7 | 94.55 | 5.65 | 4.97 |
| CPI-0-3 | 33.7 | 94.23 | 8.22 | 7.03 |
| CPI-0-4 | 30.2 | 92.71 | 15.59 | 7.12 |
| CPI-0-5 | 21.2 | 91.91 | 20.60 | 8.81 |
| CPI-0-6 | 17.5 | 91.38 | 21.95 | 9.33 |
| CPI-D-1-0 | 61.4 | 95.46 | 1.84 | 0.69 |
| CPI-D-1-1 | 53.8 | 95.38 | 1.51 | 3.46 |
| CPI-D-1-2 | 55.0 | 95.34 | 1.46 | 3.19 |
| CPI-D-1-3 | 53.4 | 95.33 | 1.46 | 3.87 |
| CPI-D-1-4 | 55.6 | 95.36 | 1.38 | 4.20 |
| CPI-D-1-5 | 55.9 | 95.37 | 1.46 | 3.10 |
| CPI-D-1-6 | 53.8 | 95.36 | 1.51 | 3.34 |
| CPI-D-10-0 | 54.3 | 94.96 | 2.82 | 3.38 |
| CPI-D-10-1 | 53.4 | 94.93 | 2.11 | 5.33 |
| CPI-D-10-2 | 50.2 | 94.93 | 2.07 | 5.95 |
| CPI-D-10-3 | 53.1 | 94.97 | 2.04 | 5.57 |
| CPI-D-10-4 | 50.8 | 94.95 | 2.00 | 5.53 |
| CPI-D-10-5 | 51.8 | 95.25 | 1.97 | 5.61 |
| CPI-D-10-6 | 49.9 | 94.95 | 2.16 | 7.63 |
| PI Samples | T5% 1 (°C) | T10% 1 (°C) | Tmax 1 (°C) | Rw750 1 (%) | CTE 1 (10−6/K) |
|---|---|---|---|---|---|
| CPI-0 | 521.5 | 531.2 | 541.9 | 51.2 | 46.9 |
| CPI-D-1 | 521.6 | 528.8 | 542.7 | 42.9 | 53.8 |
| CPI-D-3 | 526.3 | 535.0 | 550.1 | 41.1 | 53.1 |
| CPI-D-5 | 520.4 | 529.4 | 544.0 | 42.7 | 55.6 |
| CPI-D-10 | 524.1 | 533.6 | 548.1 | 40.3 | 55.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.-Y.; He, Z.-B.; Yuan, S.-Q.; Wu, H.; Zhi, X.-X.; Zhang, Y.; Chen, S.-J.; Liu, J.-G. Enhancement of Ultraviolet Light Resistance of Colorless and Transparent Semi-Alicyclic Polyimide Nanocomposite Films via the Incorporation of Hindered Amine Light Stabilizers for Potential Applications in Flexible Optoelectronics. Polymers 2022, 14, 1091. https://doi.org/10.3390/polym14061091
Wei X-Y, He Z-B, Yuan S-Q, Wu H, Zhi X-X, Zhang Y, Chen S-J, Liu J-G. Enhancement of Ultraviolet Light Resistance of Colorless and Transparent Semi-Alicyclic Polyimide Nanocomposite Films via the Incorporation of Hindered Amine Light Stabilizers for Potential Applications in Flexible Optoelectronics. Polymers. 2022; 14(6):1091. https://doi.org/10.3390/polym14061091
Chicago/Turabian StyleWei, Xin-Ying, Zhi-Bin He, Shun-Qi Yuan, Hao Wu, Xin-Xin Zhi, Yan Zhang, Shu-Jing Chen, and Jin-Gang Liu. 2022. "Enhancement of Ultraviolet Light Resistance of Colorless and Transparent Semi-Alicyclic Polyimide Nanocomposite Films via the Incorporation of Hindered Amine Light Stabilizers for Potential Applications in Flexible Optoelectronics" Polymers 14, no. 6: 1091. https://doi.org/10.3390/polym14061091