Grape Canes (Vitis vinifera L.) Applications on Packaging and Particleboard Industry: New Bioadhesive Based on Grape Extracts and Citric Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Extraction
2.3. Fourier Transform Infrared Spectroscopy (FTIR) Assay
2.4. Automated Bonding Evaluation System (ABES) Assay
2.5. Particleboard Manufacture and Testing
3. Results and Discussion
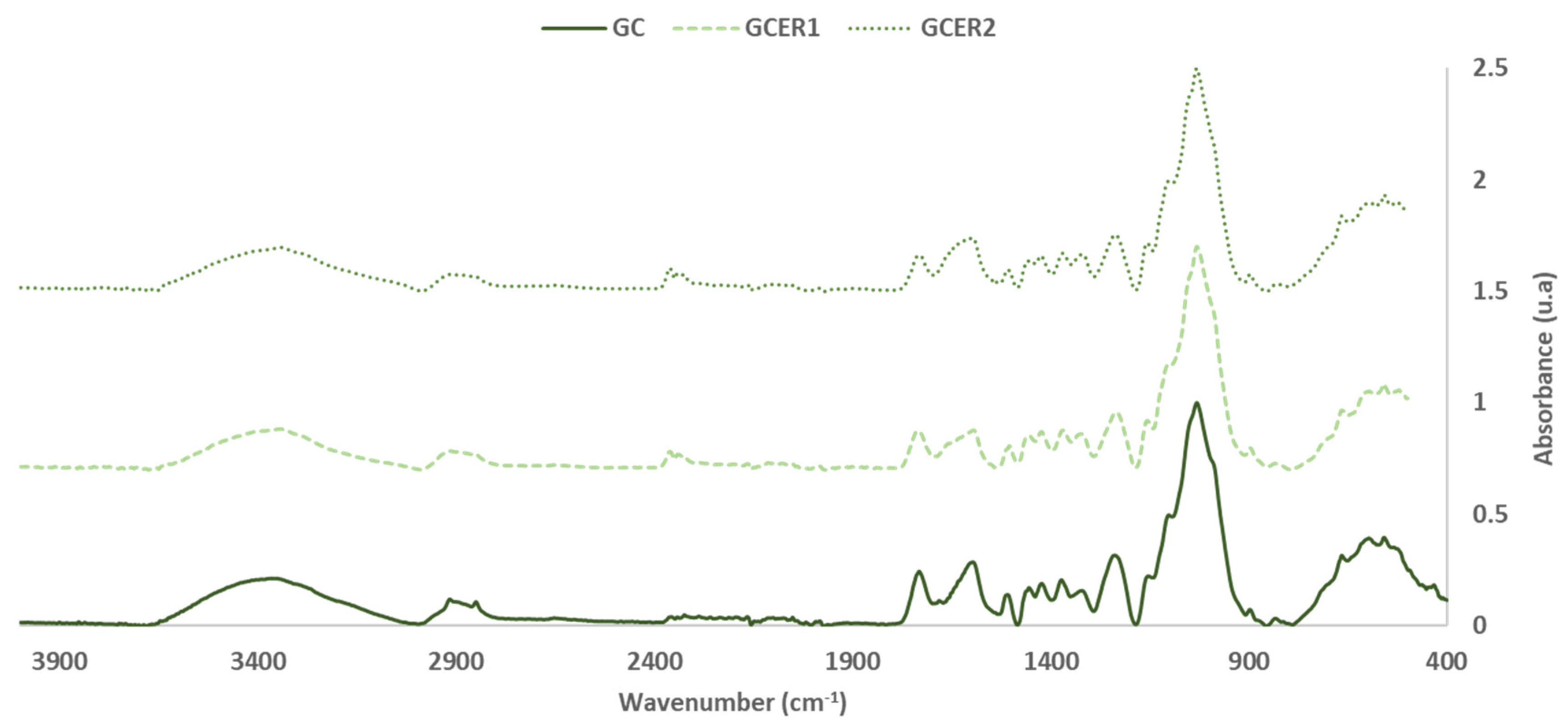
3.1. FTIR-ATR Characterization
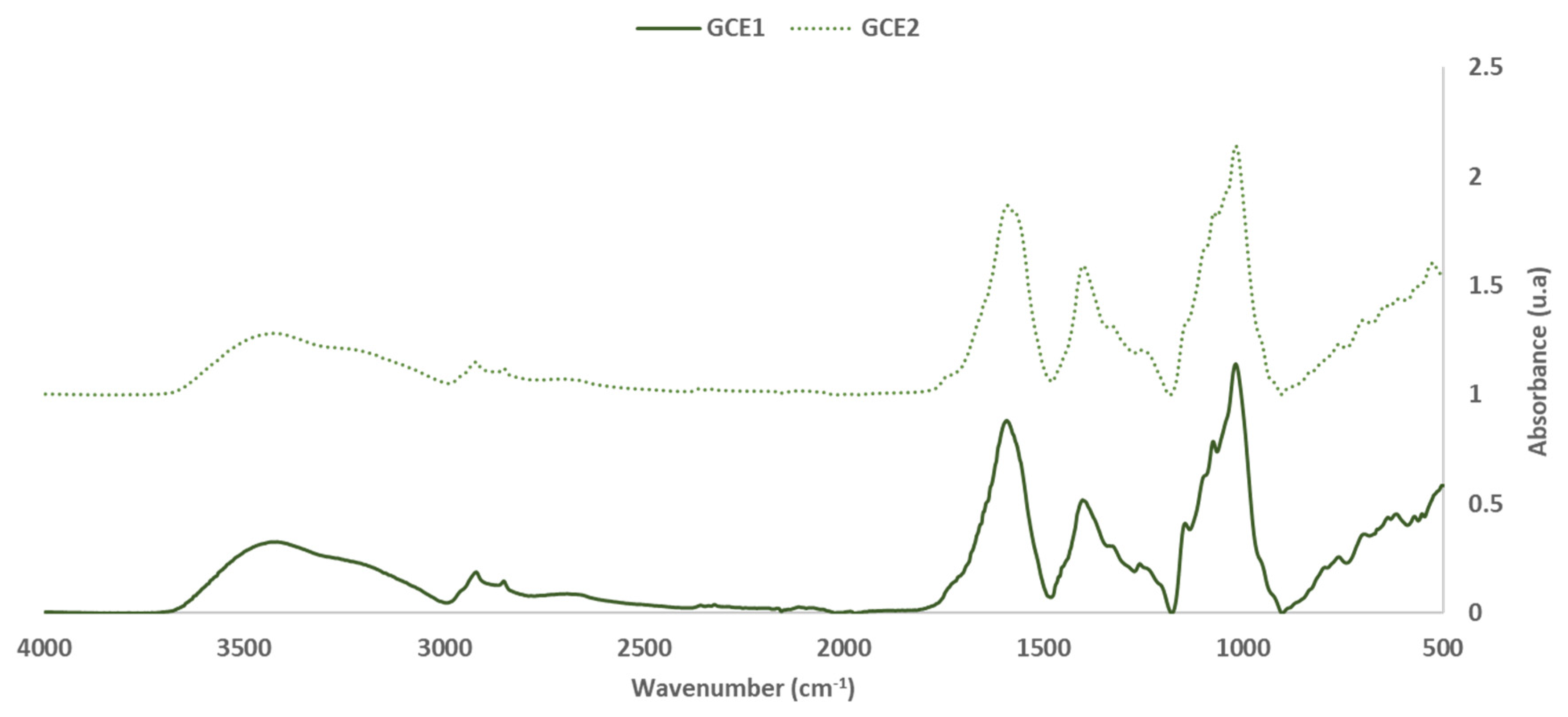
3.2. Extracts Reactivity
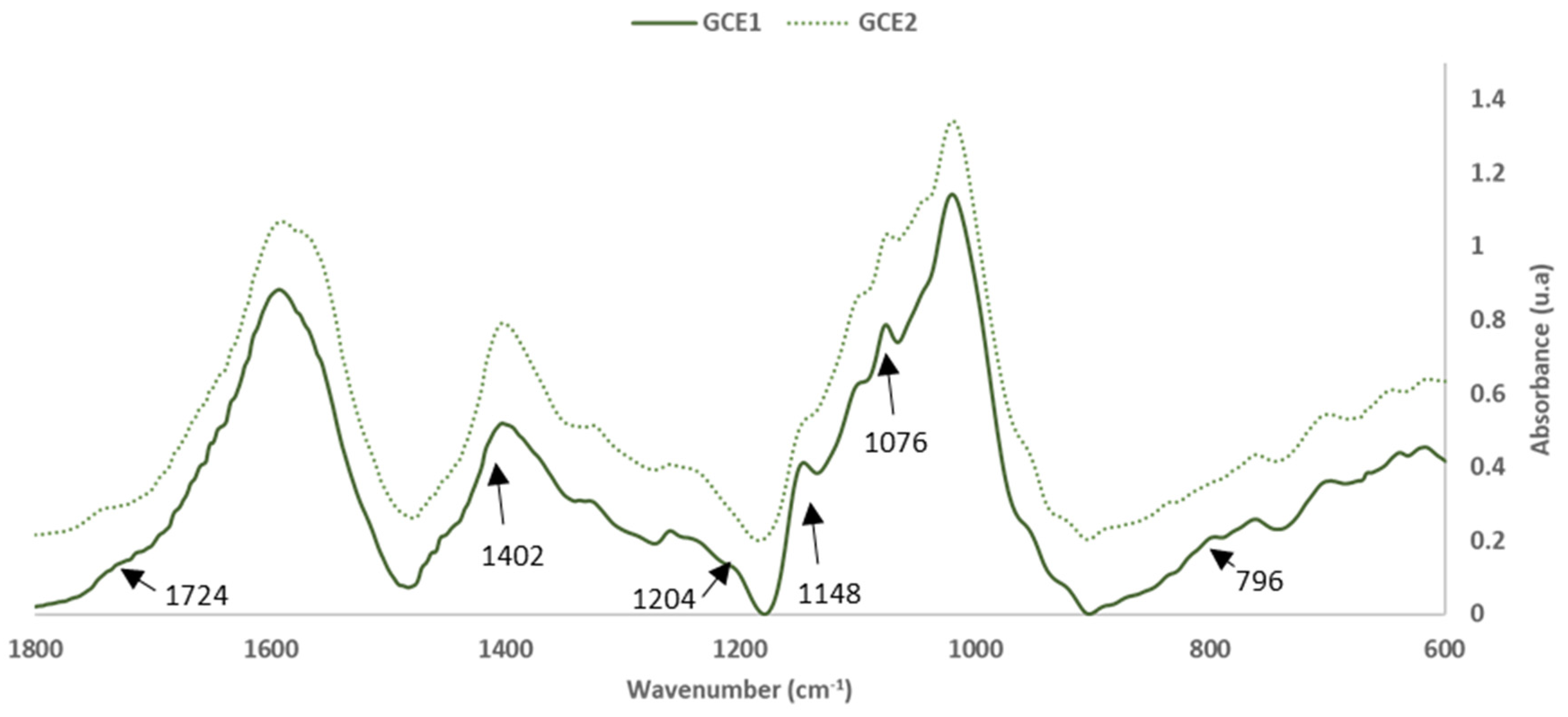
3.2.1. FTIR-ATR Results
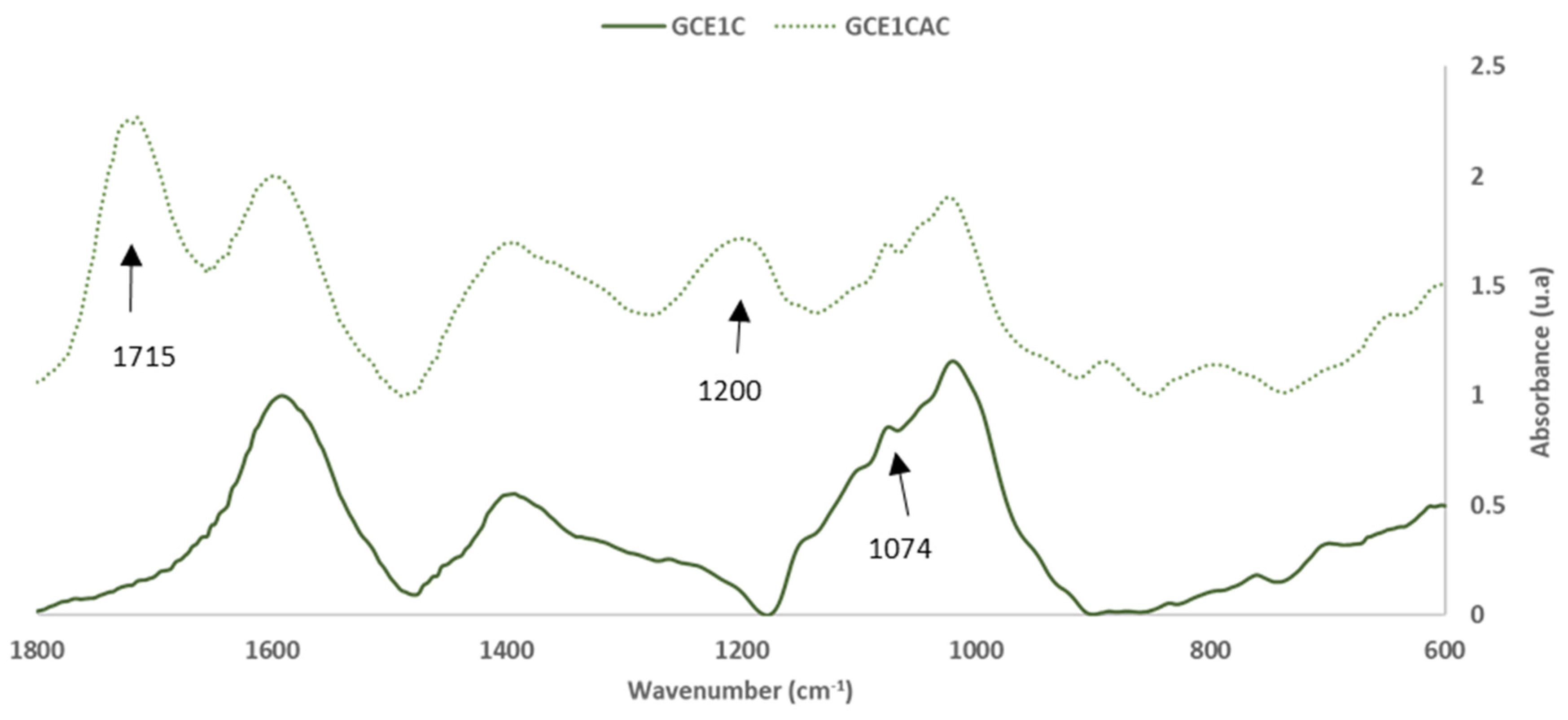
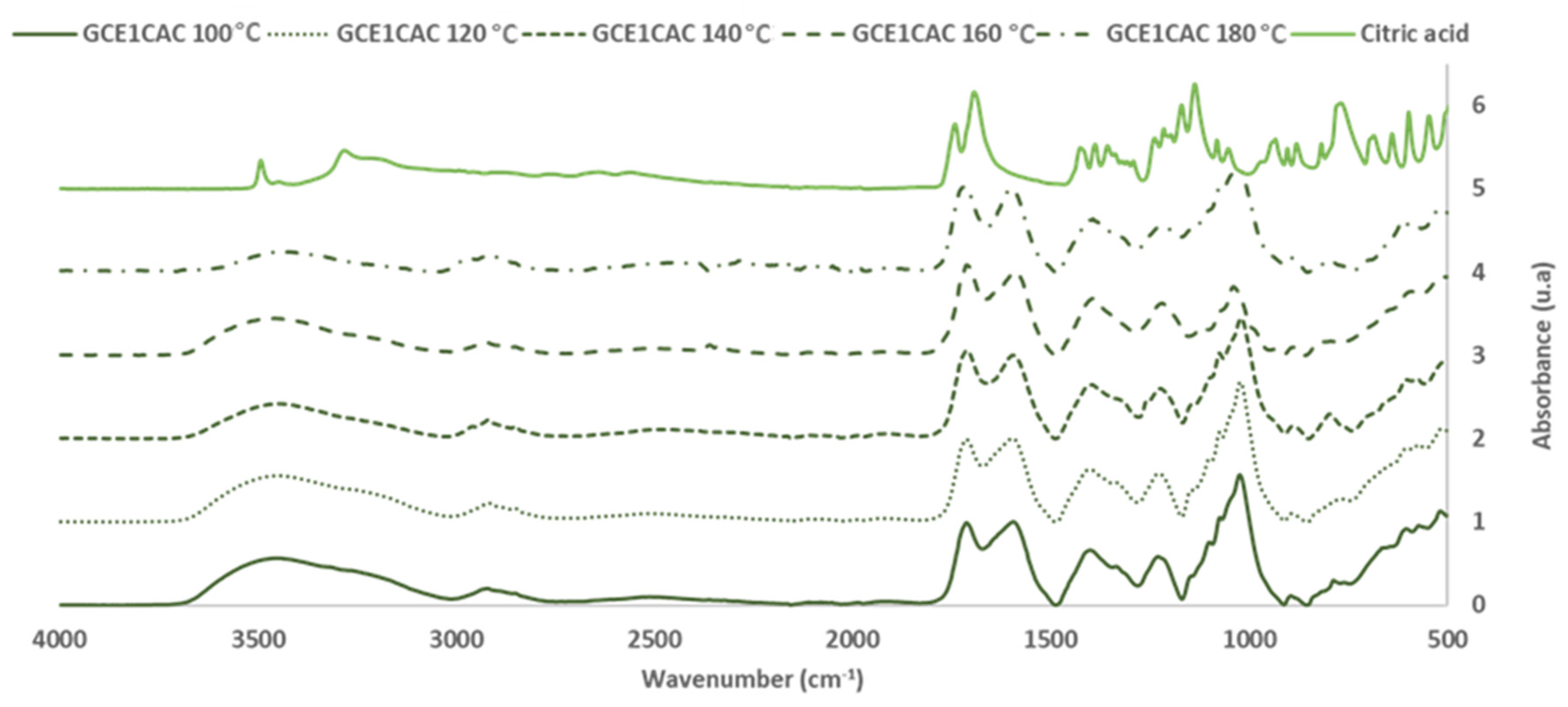
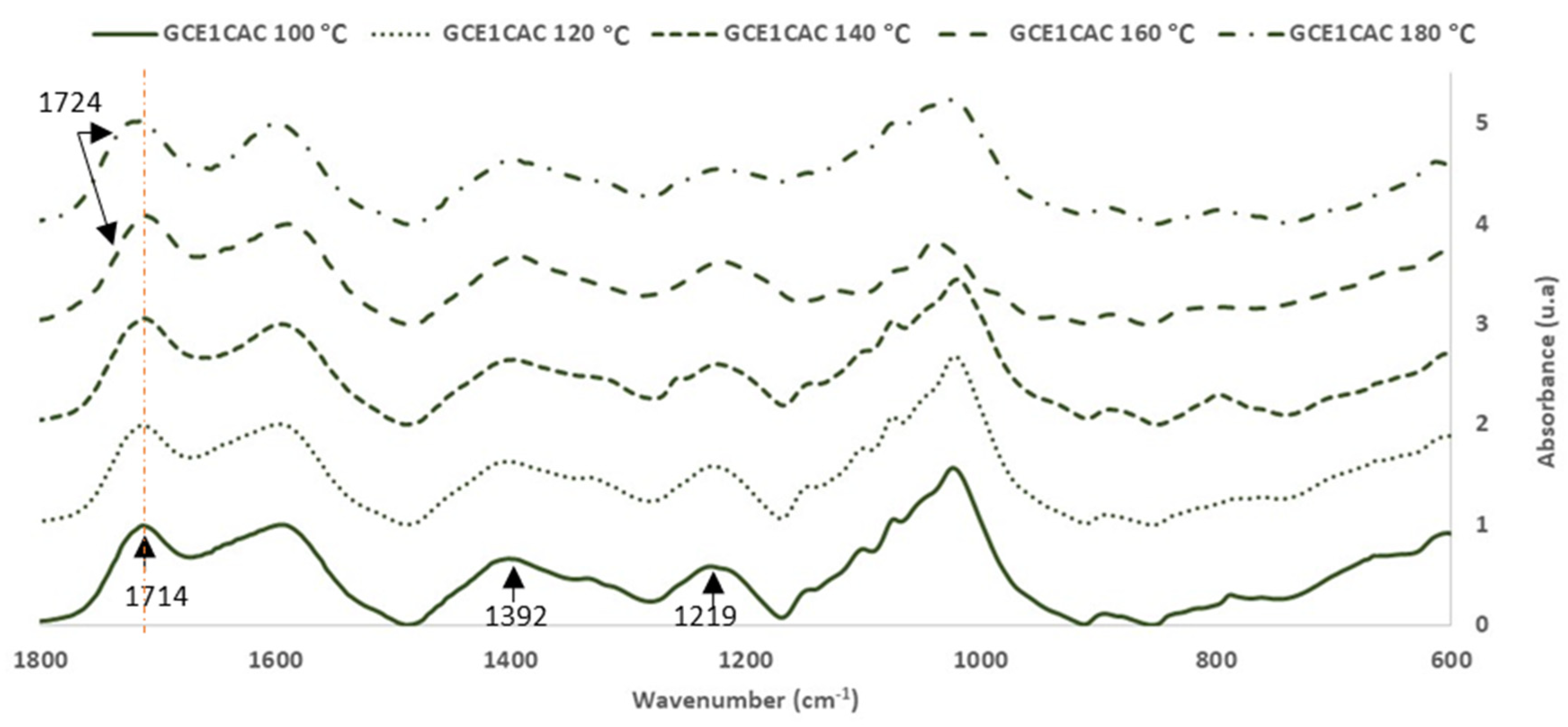
Influence of Temperature in the Curing Reaction of GCE with Citric Acid
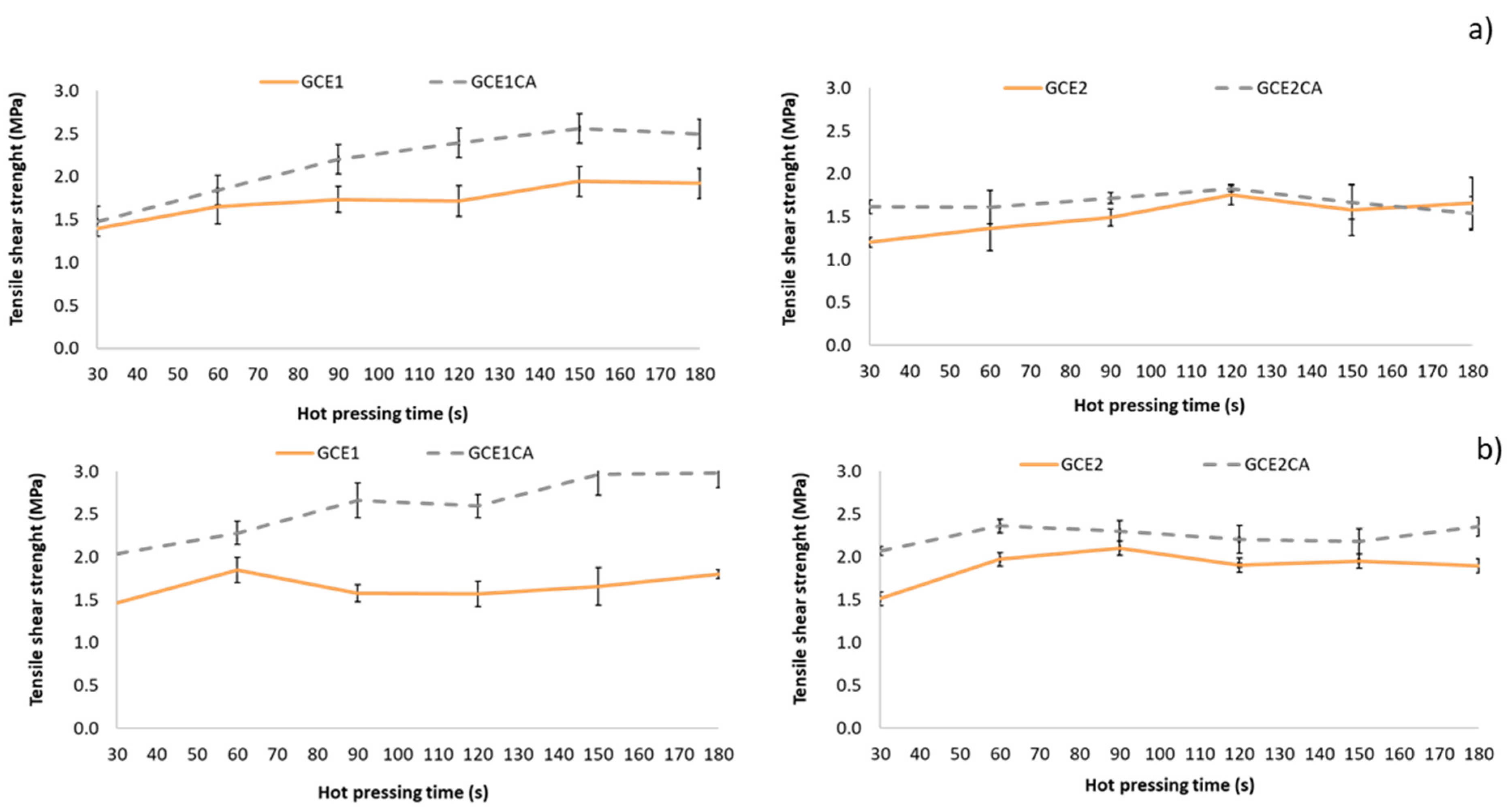
3.2.2. ABES Results
3.3. Particleboard Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cebrián, C.; Sánchez-Gómez, R.; Salinas, M.R.; Alonso, G.L.; Zalacain, A. Effect of post-pruning vine-shoots storage on the evolution of high-value compounds. Ind. Crops Prod. 2017, 109, 730–736. [Google Scholar] [CrossRef]
- Riquelme, S.; Sáez, V.; Escobar, D.; Vergara, C.; Fuentealba, C.; Bustamante, L.; Von-Baer, D.; Jara, P.; Lamperti, L.; Mardones, C. Bench-scale extraction of stilbenoids and other phenolics from stored grape canes (Vitis vinifera): Optimization process, chemical characterization, and potential protection against oxidative damage. J. Chil. Chem. Soc. 2019, 64, 4414–4420. [Google Scholar] [CrossRef]
- Escobar-Avello, D.; Mardones, C.; Saéz, V.; Riquelme, S.; von Baer, D.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A. Pilot-plant scale extraction of phenolic compounds from grape canes: Comprehensive characterization by LC-ESI-LTQ-Orbitrap-MS. Food Res. Int. 2021, 143, 110265. [Google Scholar] [CrossRef]
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. A comparative study of physical pretreatments for the extraction of polyphenols and proteins from vine shoots. Food Res. Int. 2014, 65, 462–468. [Google Scholar] [CrossRef]
- Escobar-Avello, D.; Avendaño-Godoy, J.; Santos, J.; Lozano-Castellón, J.; Mardones, C.; von Baer, D.; Luengo, J.; Lamuela-Raventós, R.; Vallverdú-Queralt, A.; Gómez-Gaete, C. Encapsulation of Phenolic Compounds from a Grape Cane Pilot-Plant Extract in Hydroxypropyl Beta-Cyclodextrin and Maltodextrin by Spray Drying. Antioxidants 2021, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Saéz, V.; Riquelme, S.; Von Baer, D.; Vallverdú-Queralt, A. Phenolic Profile of Grape Canes: Novel Compounds Identified by LC-ESI-LTQ-Orbitrap-MS. Molecules 2019, 24, 3763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, Z.; Guo, Z.; Wang, K.; Zhang, A.; Li, H.; Fang, Y. Antioxidant Effects of Grape Vine Cane Extracts from Different Chinese Grape Varieties on Edible Oils. Molecules 2014, 19, 15213–15223. [Google Scholar] [CrossRef] [Green Version]
- Ntalos, G.A.; Grigoriou, A.H. Characterization and utilisation of vine prunings as a wood substitute for particleboard production. Ind. Crops Prod. 2002, 16, 59–68. [Google Scholar] [CrossRef]
- Yeniocak, M.; Goktas, O.; Erdil, Y.Z.; Ozen, E.; Alma, M.H. Investigating the use of vine pruning stalks (Vitis vinifera L. Cv. Sultani) as raw material for particleboard manufacturing. Wood Res. 2014, 59, 167–176. [Google Scholar]
- Yasar, S.; Guntekin, E.; Cengiz, M.; Tanriverdi, H. The correlation of chemical characteristics and UF-Resin ratios to physical and mechanical properties of particleboard manufactured from vine prunings. Sci. Res. Essays 2010, 5, 737–741. [Google Scholar]
- Mihajlova, J.; Iliev, B.; Popovska, V.J. Impact of Pressing Temperature on Physical and Mechanical Properties of Panels Made from Particles of Raspberry Stems (Rubus idaeus, L.) and Grape Pruning Residues (Vitis vinifera, L.). Int. J. Wood Des. Technol. 2015, 4, 1–9. [Google Scholar]
- Santos, J.; Pereira, J.; Ferreira, N.; Paiva, N.; Ferra, J.; Magalhães, F.; Martins, J.; Dulyanska, Y.; Carvalho, L. Valorisation of non-timber by-products from maritime pine (Pinus pinaster, Ait) for particleboard production. Ind. Crops Prod. 2021, 168, 113581. [Google Scholar] [CrossRef]
- Santos, J.; Pereira, J.; Paiva, N.; Ferra, J.; Magalhães, F.; Martins, J.; de Carvalho, L. Water resistance evaluation of a MFU resins with different molar ratio catalyzed with citric acid. Int. J. Adhes. Adhes. 2021, 103020. [Google Scholar] [CrossRef]
- Santos, J.; Pereira, J.; Ferra, J.; Magalhães, F.D.; Martins, J.M.; Carvalho, L.H. New Cardoon (Cynara cardunculus L.) Particleboards Using Cardoon Leaf Extract and Citric Acid as Bio-adhesive. Mater. Circ. Econ. 2021, 3, 14. [Google Scholar] [CrossRef]
- Santos, J.; Delgado, N.; Fuentes, J.; Fuentealba, C.; Vega-Lara, J.; García, D.E. Exterior grade plywood adhesives based on pine bark polyphenols and hexamine. Ind. Crops Prod. 2018, 122, 340–348. [Google Scholar] [CrossRef]
- Santos, J.; Antorrena, G.; Freire, M.S.; Pizzi, A.; González-Álvarez, J. Environmentally friendly wood adhesives based on chestnut (Castanea sativa) shell tannins. Eur. J. Wood Wood Prod. 2017, 75, 89–100. [Google Scholar] [CrossRef]
- Costa, N.A.; Pereira, J.; Ferra, J.; Cruz, P.; Martins, J.M.; Magalhães, F.; Mendes, A.; Carvalho, L.H. Evaluation of Bonding Performance of Amino Polymers Using ABES. J. Adhes. 2013, 90, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, G.; González-Alvarez, J.; Freire, S.; López-Suevos, F.; Antorrena, G. Characteristics of Pinus pinaster bark extracts obtained under various extraction conditions. Holz Als Roh Werkstoff 2001, 59, 451–456. [Google Scholar] [CrossRef]
- Vázquez, G.; Gonzalez-Alvarez, J.; Freire, M.; Fernandez-Agullo, A.; Santos, J.; Antorrena, G. Chestnut burs as a source of natural antioxidants. Chem. Eng. Trans. 2009, 17, 855–860. [Google Scholar] [CrossRef]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Özgenç, O.; Durmaz, S.; Kustas, S. Chemical Analysis of Tree Barks Using ATR-FTIR Spectroscopy and Conventional Techniques. BioResources 2017, 12, 9143–9151. [Google Scholar] [CrossRef]
- Feng, X.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Traoré, M.; Kaal, J.; Cortizas, A.M. Differentiation between pine woods according to species and growing location using FTIR-ATR. Wood Sci. Technol. 2018, 52, 487–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Kim, H.-J. Curing behavior and viscoelastic properties of pine and wattle tannin-based adhesives studied by dynamic mechanical thermal analysis and FT-IR-ATR spectroscopy. J. Adhes. Sci. Technol. 2003, 17, 1369–1383. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Xu, J.; Xu, N.; He, Y. Quantitative visualization of lignocellulose components in transverse sections of moso bamboo based on FTIR macro- and micro-spectroscopy coupled with chemometrics. Biotechnol. Biofuels 2018, 11, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satapathi, S.; Ravichandran, S.; Mosurkal, R.; Nagarajan, S.; Li, L.; Nagarajan, R.; Samuelson, L.A.; Kumar, J. Biocatalytic Synthesis of Two-Photon Active Resveratrol Oligomer. J. Macromol. Sci. Part A 2011, 48, 1061–1066. [Google Scholar] [CrossRef]
- Gümüfikaya, E.; Usta, M. Crystalline Structure Properties of Bleached and Unbleached Wheat Straw (Triticum aestivum L.) Soda-Oxygen Pulp. Turk. J. Agric. For. 2002, 26, 247–252. [Google Scholar]
- Bertacche, V.; Lorenzi, N.; Nava, D.; Pini, E.; Sinico, C. Host–Guest Interaction Study of Resveratrol with Natural and Modified Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2006, 55, 279–287. [Google Scholar] [CrossRef]
- Teixeira, D.E.; Pereira, D.D.C.; Nakamura, A.P.D.; Brum, S.S. Adhesivity of Bio-Based Anhydrous Citric Acid, Tannin-Citric Acid and Ricinoleic Acid in the Properties of Formaldehyde-Free Medium Density Particleboard (MDP). Drv. Ind. 2020, 71, 235–242. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Kim, H.-J.; Rafailovich, M.; Sokolov, J. Curing monitoring of phenolic resol resins via atomic force microscope and contact angle. Int. J. Adhes. Adhes. 2002, 22, 375–384. [Google Scholar] [CrossRef]
- Huang, H.-K.; Hsu, C.-H.; Hsu, P.-K.; Cho, Y.-M.; Chou, T.-H.; Cheng, Y.-S. Preparation and evaluation of particleboard from insect rearing residue and rice husks using starch/citric acid mixture as a natural binder. Biomass-Convers. Biorefinery 2020, 12, 633–641. [Google Scholar] [CrossRef]
- Chalapud, M.C.; Herdt, M.; Nicolao, E.S.; Ruseckaite, R.A.; Ciannamea, E.M.; Stefani, P.M. Biobased particleboards based on rice husk and soy proteins: Effect of the impregnation with tung oil on the physical and mechanical behavior. Constr. Build. Mater. 2020, 230, 116996. [Google Scholar] [CrossRef]




| Extraction Agent | S/L | Temperature (°C) | Codification | |
|---|---|---|---|---|
| NaOH (%) | NaHCO3 (%) | |||
| 1 | 0 | 1:10 | 90 | CGE1 |
| 0 | 2 | 1:10 | 90 | CGE2 |
| Nomenclature | Extraction Conditions | Extraction Yield (%) | |
|---|---|---|---|
| NaOH (%) | NaHCO3 (%) | ||
| Extract 1 | 1 | 0 | 15.85 ± 3.28 |
| Extract 2 | 0 | 2 | 13.17 ± 2.12 |
| GC | GGER1 | GGER2 | Group | Range | |||
|---|---|---|---|---|---|---|---|
| cm−1 | Intensity | cm−1 | Intensity | cm−1 | Intensity | ||
| 3351 | 20.7 ± 0.8 | 3340 | 19.7 ± 0.9 | 3346 | 18.2 ± 0.7 | -OH stretch | 3336 |
| 2916 | 12.0 ± 0.2 | 2916 | 7.6 ± 0.3 | 2917 | 8.5 ± 0.1 | -CH2- asymmetric stretch | 2916–2936 |
| 2850 | 10.8 ± 0.4 | 2853 | 6.9 ± 0.1 | 2852 | 7.3 ± 0.2 | -CH2- symmetric stretch | 2843–2863 |
| 1732 | 24.5 ± 0.1 | 1730 | 16.2 ± 0.2 | 1737 | 17.4 ± 0.2 | C=O stretch in unconjugated ketones, carbonyls and in ester groups (hemicellulose) | 1738 |
| 1681 | 11.4 ± 0.6 | -- | -- | -- | -- | C=O vibration in carboxylic group of phenolic acids (p-hydroxybenzoic acid) | 1676 |
| 1596 | 28.6 ± 0.2 | 1598 | 24.0 ± 0.2 | 1595 | 17.8 ± 0.5 | Aromatic skeletal vibration and C=O stretch (lignin) | 1595 |
| 1509 | 14.0 ± 0.3 | 1504 | 9.4 ± 0.1 | 1504 | 10.7 ± 0.1 | CAR=CAR (Pp cd.) | 1500–1600 |
| 1455 | 17.1 ± 0.5 | 1453 | 14.0 ± 0.5 | 1453 | 15.1 ± 0.3 | C=C and C-H bond O-H in plane deformation (lignin and hemicellulose) | 1450–1453 |
| 1423 | 19.0 ± 0.2 | 1425 | 15.9 ± 0.2 | 1423 | 17.1 ± 0.1 | CH- deformation; asymmetric in -CH3 and -CH2- (cellulose) | 1430–1485 |
| 1373 | 20.6 ± 0.2 | 1369 | 17.2 ± 0.3 | 1369 | 17.8 ± 0.2 | CH deformation (cellulose and hemicellulose) | 1372 |
| 1322 | 15.8 ± 0.1 | 1320 | 16.7 ± 0.6 | 1323 | 16.1 ± 0.5 | Ph-CHR-OH deformation | 1260–1350 |
| 1239 | 31.7 ± 0.6 | 1237 | 25.4 ± 0.1 | 1235 | 25.8 ± 0.2 | Syringyl ring and C=C stretch in lignin and xylan | 1235 |
| 1154 | 22.4 ± 0.4 | 1157 | 21.7 ± 0.3 | 1156 | 22.0 ± 0.3 | Involves C-O stretching of C-OH/C-O-C (cellulose) | 1160 |
| 1102 | 49.7 ± 0.3 | 1103 | 49.3 ± 0.1 | 1103 | 47.2 ± 0.4 | C-O-C stretch (cellulose and hemicellulose) | 1105 |
| 1072 | 61.9 ± 0.1 | -- | -- | -- | -- | C-O primary alcohol stretching (Pp cd) | 1060–1070 |
| 1051 | 89.9 ± 0.2 | -- | -- | -- | -- | C-O-C aromatic ethers symmetric stretch (pyranose ring) | 1010–1050 |
| 1031 | 100.0 ± 0.0 | 1032 | 100.0 ± 0.0 | 1031 | 100.0 ± 0.0 | C-O, C-C, and C-C-O stretch (cellulose, hemicellulose, and lignin) | 1025–1035 |
| 897 | 7.4 ± 0.9 | 897 | 7.7 ± 0.8 | 897 | 9.8 ± 0.6 | C-O-C aromatic ethers, symmetric stretch | 810–850 |
| GGE1 | GGE2 | Group | Range | ||
|---|---|---|---|---|---|
| cm−1 | Intensity | cm−1 | Intensity | ||
| 3418 | 37.1 ± 0.8 | 3418 | 32.5 ± 1.1 | -OH stretch | 3336 |
| 2920 | 21.4 ± 0.2 | 2920 | 17.5 ± 0.3 | -CH2- asymmetric stretch | 2916–2936 |
| 2851 | 16.6 ± 0.1 | 2851 | 14.0 ± 0.2 | -CH2- symmetric stretch | 2843–2863 |
| 1728 | 15.7 ± 0.1 | 1739 | 10.2 ± 0.2 | C=O stretch in unconjugated ketones, carbonyls and in ester groups | 1738 |
| 1681 | 31.7 ± 0.4 | 1681 | 27.7 ± 0.2 | C=O vibration in carboxylic group of phenolic acids (p-Hydroxybenzoic acid) | 1676 |
| 1593 | 100.0 ± 0.0 | 1591 | 100.0 ± 0.0 | CAR=CAR (Pp) | 1500–1600 |
| 1462 | 18.6 ± 0.1 | 1461 | 14.4 ± 0.2 | CAR= CAR (Benzene skeleton) (SB) [26] | 1460–1465 |
| 1454 | 24.0 ± 0.3 | 1454 | 18.0 ± 0.1 | C-H bending of CH2 groups (Pp cd.) | 1450–1455 |
| 1402 | 58.8 ± 0.2 | 1402 | 68.2 ± 0.3 | C-C stretching vibration (SB) [26] | 1380–1400 |
| 1325 | 34.6 ± 0.1 | 1326 | 36.1 ± 0.1 | Ph-CHR-OH deformation | 1260–1350 |
| 1259 | 25.7 ± 0.1 | 1259 | 23.9 ± 0.2 | ||
| 1204 | 14.1 ± 0.1 | -- | -- | Ph-OH stretching vibration | 1180–1260 |
| 1146 | 46.6 ± 0.3 | 1145 | 37.8 ± 0.4 | CAR-OH linkage due to the presence of oligomer components (SB) [26] | 1155 |
| 1076 | 89.2 ± 0.2 | 1074 | 96.1 ± 0.5 | C-O primary alcohol stretching (Pp cd) | 1060–1070 |
| 1020 | 129.3 ± 0.1 | 1019 | 100.0 ± 0.2 | Aromatic cycle bending (Pp cd) [27] | 1025 |
| 952 | 24.6 ± 0.2 | 953 | 26.4 ± 0.1 | Bending vibration of C=C-H (SB) [28] | 965–988 |
| 796 | 23.8 ± 0.3 | -- | -- | C-O-C aromatic ethers, symmetric stretching | 810–850 |
| 762 | 29.2 ± 0.6 | 760 | 26.9 ± 0.3 | C-C Alkanes skeletal vibrations | 720–750 |
| GGEC1 | GGE1CAC | Group | Range | ||
|---|---|---|---|---|---|
| cm−1 | Intensity | cm−1 | Intensity | ||
| 3409 | 26.2 ± 0.2 | 3426 | 22.4 ± 0.1 | -OH stretch | 3300–3400 |
| 3391 | 26.1 ± 0.3 | 3266 | 9.3 ± 0.1 | ||
| 2920 | 12.8 ± 0.1 | 2921 | 9.1 ± 0.1 | -CH2- asymmetric stretch | 2916–2936 |
| 2851 | 10.8 ± 0.1 | 2851 | 5.9 ± 0.2 | -CH2- symmetric stretch | 2843–2863 |
| 1767 | 7.6 ± 0.0 | -- | -- | C=O stretch in unconjugated ketones, carbonyls and in ester groups | 1738 |
| -- | -- | 1715 | 126.7 ± 0.3 | Axial stretching of carbonyl groups (C=O) in carboxylic acid group (citric acid) | 1700–1750 |
| 1591 | 100.0 ± 0.0 | 1598 | 100.0 ± 0.0 | CAR=CAR (Pp.) | 1500–1600 |
| 1395 | 55.3 ± 0.1 | 1394 | 70.1 ± 0.4 | Ph-CHR-OH deformation | 1260–1350 |
| 1262 | 25.6 ± 0.2 | -- | -- | ||
| -- | -- | 1200 | 71.6 ± 0.6 | C-O bond of -O-(C=O)- stretch [14,29] | 1215 |
| 1074 | 85.7 ± 0.1 | 1075 | 69.3 ± 0.2 | C-O primary alcohol stretching (Pp) | 1060–1070 |
| 1020 | 115.7 ± 0.3 | 1023 | 90.5 ± 0.6 | Aromatic cycle bending (Pp) | 1025 |
| -- | -- | 890 | 15.6 ± 0.3 | C-O-C aromatic ethers, symmetric stretch | 810–850 |
| 760 | 18.3 ± 0.1 | 796 | 14.2 ± 0.2 | C-C Alkanes skeletal vibrations | 720–750 |
| Wavenumber (cm−1) | Type of Bond | Curing Temperature | Band Area |
|---|---|---|---|
| 3000–3300 | -OH stretching | 100 °C | 68.8 ± 1.4 |
| 120 °C | 63.2 ± 0.9 | ||
| 140 °C | 35.7 ± 0.3 | ||
| 160 °C | 15.4 ± 0.6 | ||
| 180 °C | 15.6 ± 0.8 | ||
| 3300–3700 | -OH stretching | 100 °C | 141.5 ± 1.6 |
| 120 °C | 135.2 ± 2.0 | ||
| 140 °C | 101.7 ± 1.3 | ||
| 160 °C | 51.2 ± 0.8 | ||
| 180 °C | 53.6 ± 1.5 | ||
| 2800–3000 | -CH2- stretching | 100 °C | 14.8 ± 0.1 |
| 120 °C | 16.9 ± 0.2 | ||
| 140 °C | 21.9 ± 0.1 | ||
| 160 °C | 27.8 ± 0.3 | ||
| 180 °C | 27.7 ± 0.1 | ||
| 1670–1780 | C=O stretching | 100 °C | 33.0 ± 0.2 |
| 120 °C | 33.3 ± 0.1 | ||
| 140 °C | 41.6 ± 0.3 | ||
| 160 °C | 44.8 ± 0.8 | ||
| 180 °C | 57.3 ± 0.1 | ||
| 1400–1500 | C-C deformation | 100 °C | 64.1 ± 0.2 |
| 120 °C | 63.3 ± 0.5 | ||
| 140 °C | 62.9 ± 0.1 | ||
| 160 °C | 57.1 ± 0.6 | ||
| 180 °C | 60.4 ± 0.4 | ||
| 1170–1280 | C-O stretching | 100 °C | 34.3 ± 0.1 |
| 120 °C | 32.6 ± 0.2 | ||
| 140 °C | 31.5 ± 0.1 | ||
| 160 °C | 20.1 ± 0.9 | ||
| 180 °C | 17.9 ± 1.1 |
| Wavenumber (cm−1) | Type of Bond | Curing Temperature | Band Area |
|---|---|---|---|
| 3000–3300 | -OH stretching | 100 °C | 52.9 ± 2.1 |
| 120 °C | 29.3 ± 0.9 | ||
| 140 °C | 9.1 ± 0.1 | ||
| 160 °C | 7.9 ± 0.2 | ||
| 3300–3700 | -OH stretching | 100 °C | 128.8 ± 0.6 |
| 120 °C | 100.6 ± 1.3 | ||
| 140 °C | 54.3 ± 0.9 | ||
| 160 °C | 43.2 ± 0.2 | ||
| 2800–3000 | -CH2- stretching | 100 °C | 34.2 ± 0.2 |
| 120 °C | 35.9 ± 0.1 | ||
| 140 °C | 35.8 ± 0.3 | ||
| 160 °C | 36.4 ± 0.2 | ||
| 1670–1780 | C=O stretching | 100 °C | 153.3 ± 0.2 |
| 120 °C | 179.2 ± 0.1 | ||
| 140 °C | 182.9 ± 0.3 | ||
| 160 °C | 186.7 ± 0.6 |
| Type of By-Product | Density (kg/m3) | Dry IB Strength (MPa) | 24 h in Water-Thickness Swelling (%) |
|---|---|---|---|
| IW | 686 ± 2 | 0.59 ± 0.02 | 24.9 ± 0.8 |
| GC | 616 ± 9 | 0.62 ± 0.04 | 16.9 ± 0.8 |
| GCER1 | 606 ± 2 | 0.65 ± 0.01 | 23.3 ± 2.1 |
| EN 312 Requirement (type P2) | ≥0.35 | -- | |
| Type of Adhesive | Material Used as Particles | Density (kg/m3) | IB Strength (MPa) | Bending Strength (N/mm2) |
|---|---|---|---|---|
| (67%) GCE1 + (33%) CA | GC | 534 ± 9.2 | 0.16 ± 0.01 | 3.26 ± 0.30 |
| (67%) GCE1 + (33%) CA | GCER1 | 518 ± 17 | 0.19 ± 0.02 | 2.18 ± 0.42 |
| (67%) GCE2 + (33%) CA | GC | 524 ± 1.4 | 0.18 ± 0.02 | 3.18 ± 0.16 |
| (67%) GCE2 + (33%) CA | GCER2 | 526 ± 12 | 0.16 ± 0.01 | 2.15 ± 0.20 |
| CEN/TS 16368 | 0.28 | 4.0 |
| Type of Adhesive | By-Product Used as Particles | Thickness Swelling (2 h in Water) (%) | Water Absorption (2 h in Water) (%) | Thickness Swelling (24 h in Water) (%) | Water Absorption (24 h in Water) (%) | Thickness Swelling (1 Week Dry) (%) |
|---|---|---|---|---|---|---|
| (67%) GCE1 + (33%) CA | GC | 31.6 ± 1.4 | 98.6 ± 12.4 | 39.7 ± 1.0 | 106.5 ± 1.3 | 19.2 ± 1.9 |
| (67%) GCE1 + (33%) CA | GCER1 | 46.3 ± 2.3 | 127.9 ± 2.90 | 48.9 ± 4.5 | 145.2 ± 2.1 | 33.4 ± 9.3 |
| (67%) GCE2 + (33%) CA | GC | 51.6 ± 0.8 | 99.0 ± 13.9 | 55.5 ± 1.8 | 127.6 ± 6.5 | 34.2 ± 1.2 |
| (67%) GCE2 + (33%) CA | GCER2 | 49.7 ± 4.3 | 146.1 ± 11.3 | 57.3 ± 3.0 | 147.0 ± 1.4 | 36.3 ± 9.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.; Pereira, J.; Escobar-Avello, D.; Ferreira, I.; Vieira, C.; Magalhães, F.D.; Martins, J.M.; Carvalho, L.H. Grape Canes (Vitis vinifera L.) Applications on Packaging and Particleboard Industry: New Bioadhesive Based on Grape Extracts and Citric Acid. Polymers 2022, 14, 1137. https://doi.org/10.3390/polym14061137
Santos J, Pereira J, Escobar-Avello D, Ferreira I, Vieira C, Magalhães FD, Martins JM, Carvalho LH. Grape Canes (Vitis vinifera L.) Applications on Packaging and Particleboard Industry: New Bioadhesive Based on Grape Extracts and Citric Acid. Polymers. 2022; 14(6):1137. https://doi.org/10.3390/polym14061137
Chicago/Turabian StyleSantos, Jorge, João Pereira, Danilo Escobar-Avello, Irene Ferreira, Carlos Vieira, Fernão D. Magalhães, Jorge Manuel Martins, and Luísa H. Carvalho. 2022. "Grape Canes (Vitis vinifera L.) Applications on Packaging and Particleboard Industry: New Bioadhesive Based on Grape Extracts and Citric Acid" Polymers 14, no. 6: 1137. https://doi.org/10.3390/polym14061137
APA StyleSantos, J., Pereira, J., Escobar-Avello, D., Ferreira, I., Vieira, C., Magalhães, F. D., Martins, J. M., & Carvalho, L. H. (2022). Grape Canes (Vitis vinifera L.) Applications on Packaging and Particleboard Industry: New Bioadhesive Based on Grape Extracts and Citric Acid. Polymers, 14(6), 1137. https://doi.org/10.3390/polym14061137











