Polyhydroxylated Nanosized Graphite as Multifunctional Building Block for Polyurethanes
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.1.1. Carbon Allotropes
2.1.2. Chemicals
2.2. Preparation of the Dispersions and Stability Evaluation
2.3. Synthesis of Polyurethane
2.4. Characterization of Polyurethane
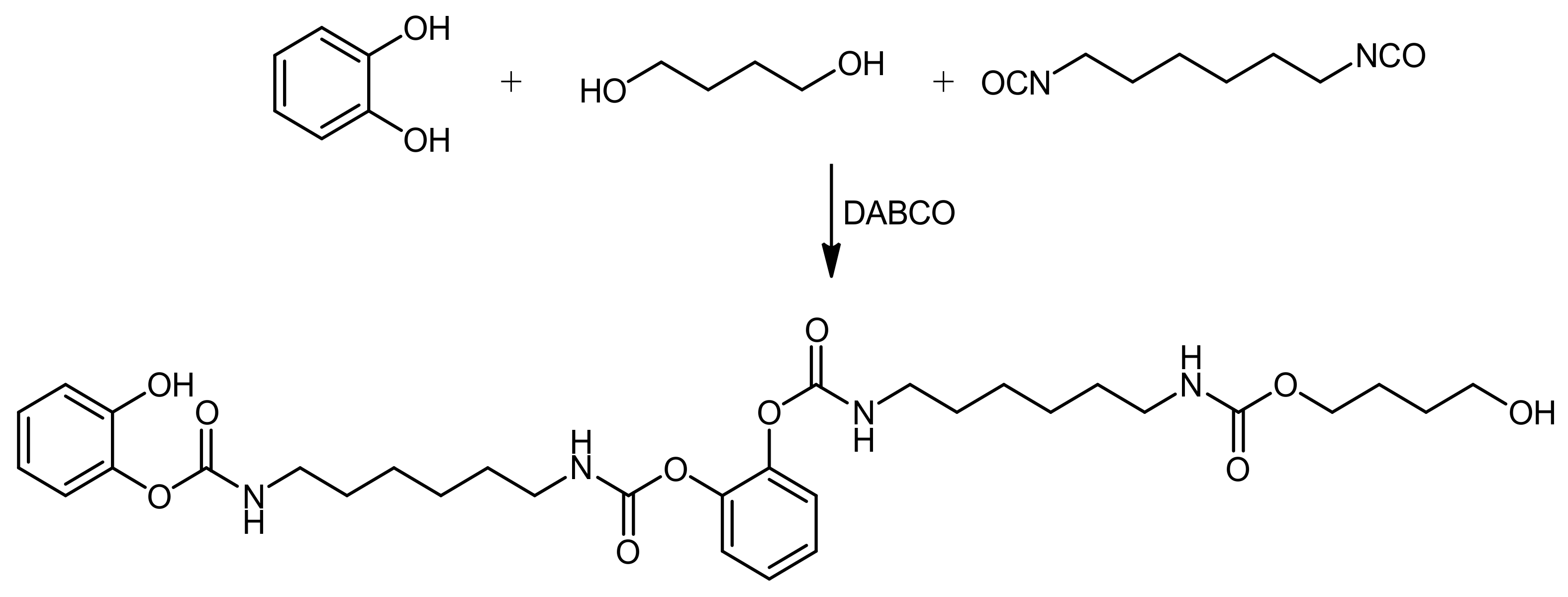
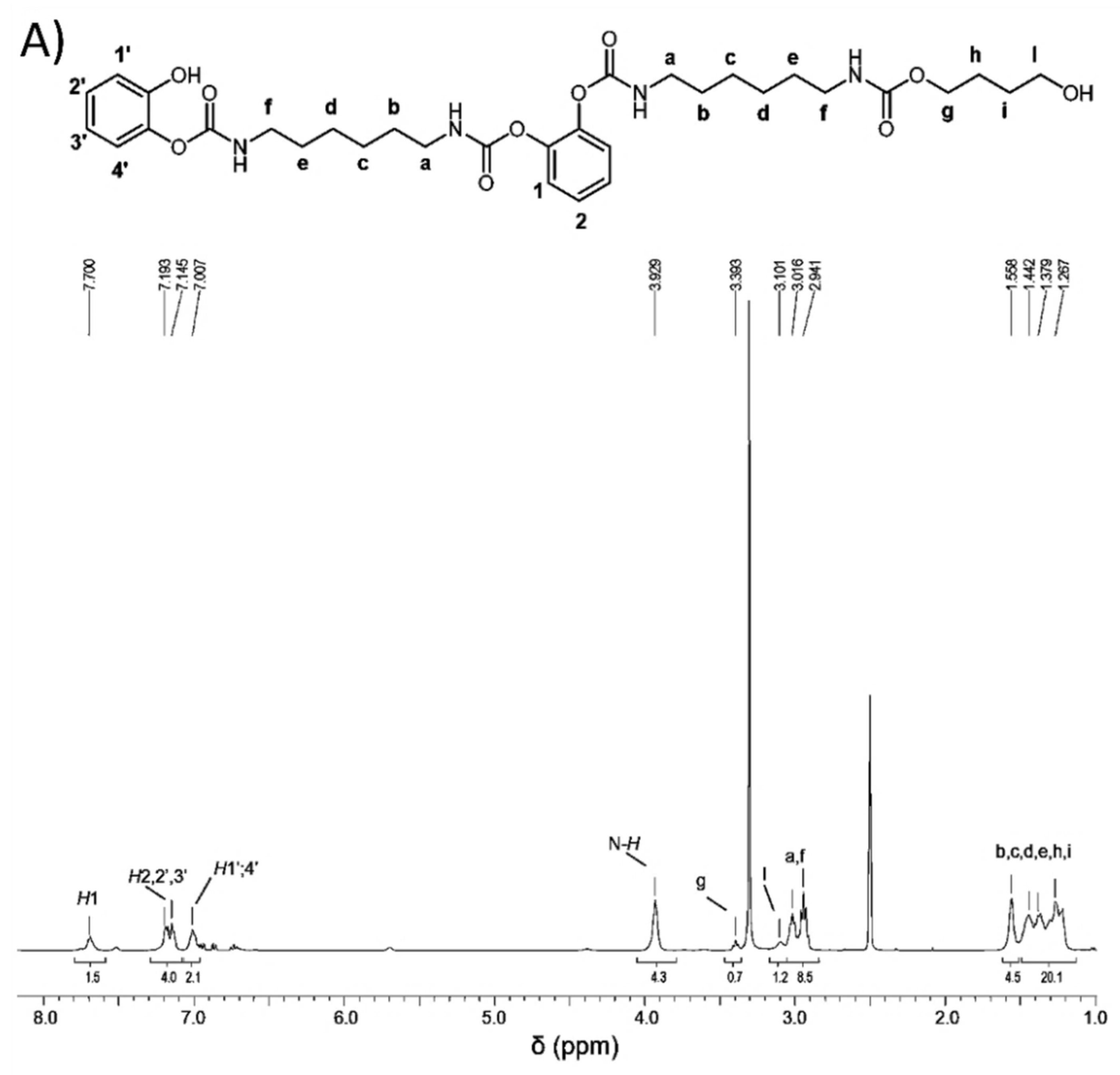
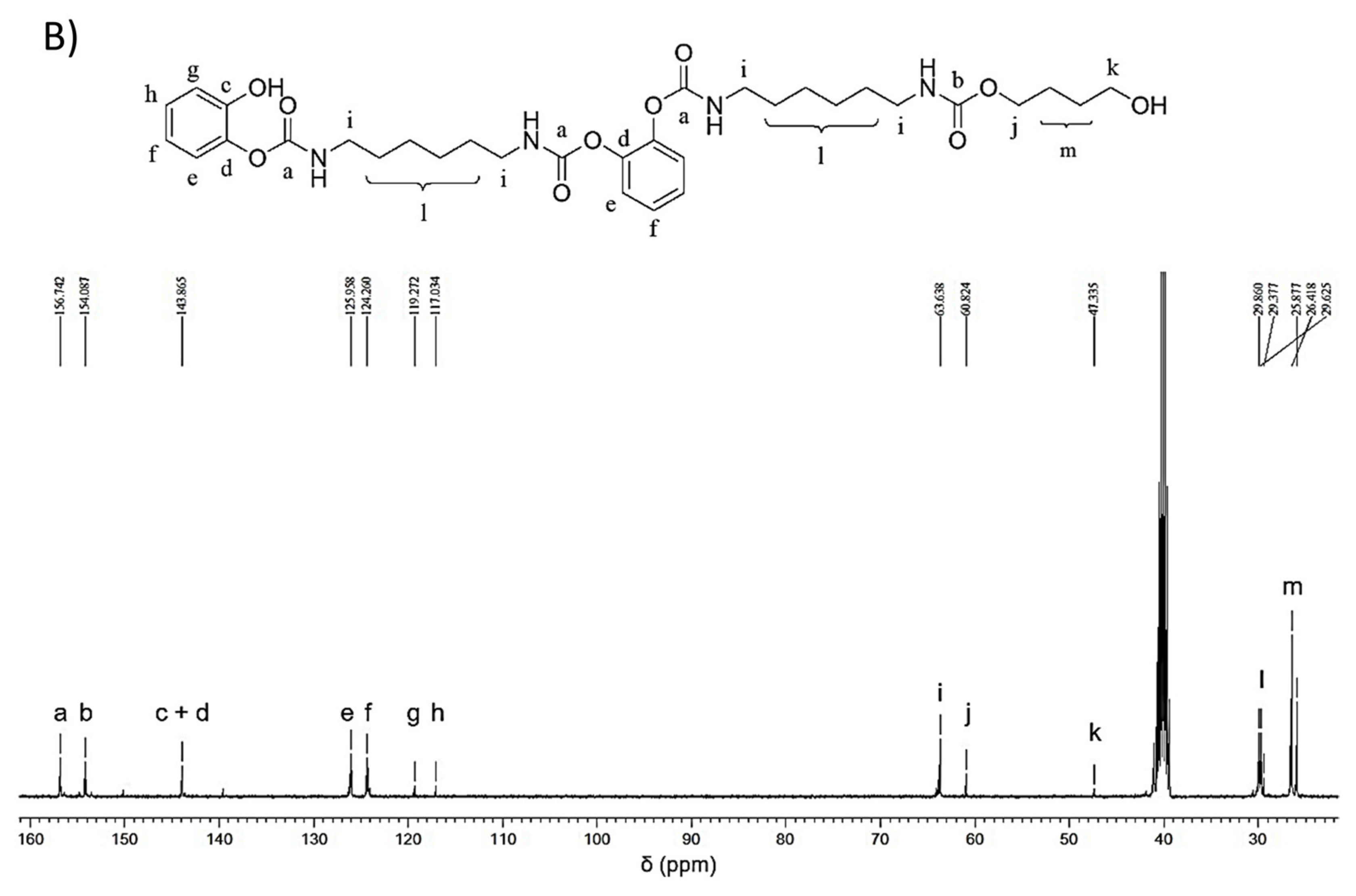
2.5. Model Reactions
2.5.1. Model Reaction for the Synthesis of PU
2.5.2. Model Reaction for Studying the Effect of G and G-OH on the Formation of Urethane Bond
3. Results and Discussion
3.1. Functionalization of Carbon Allotropes
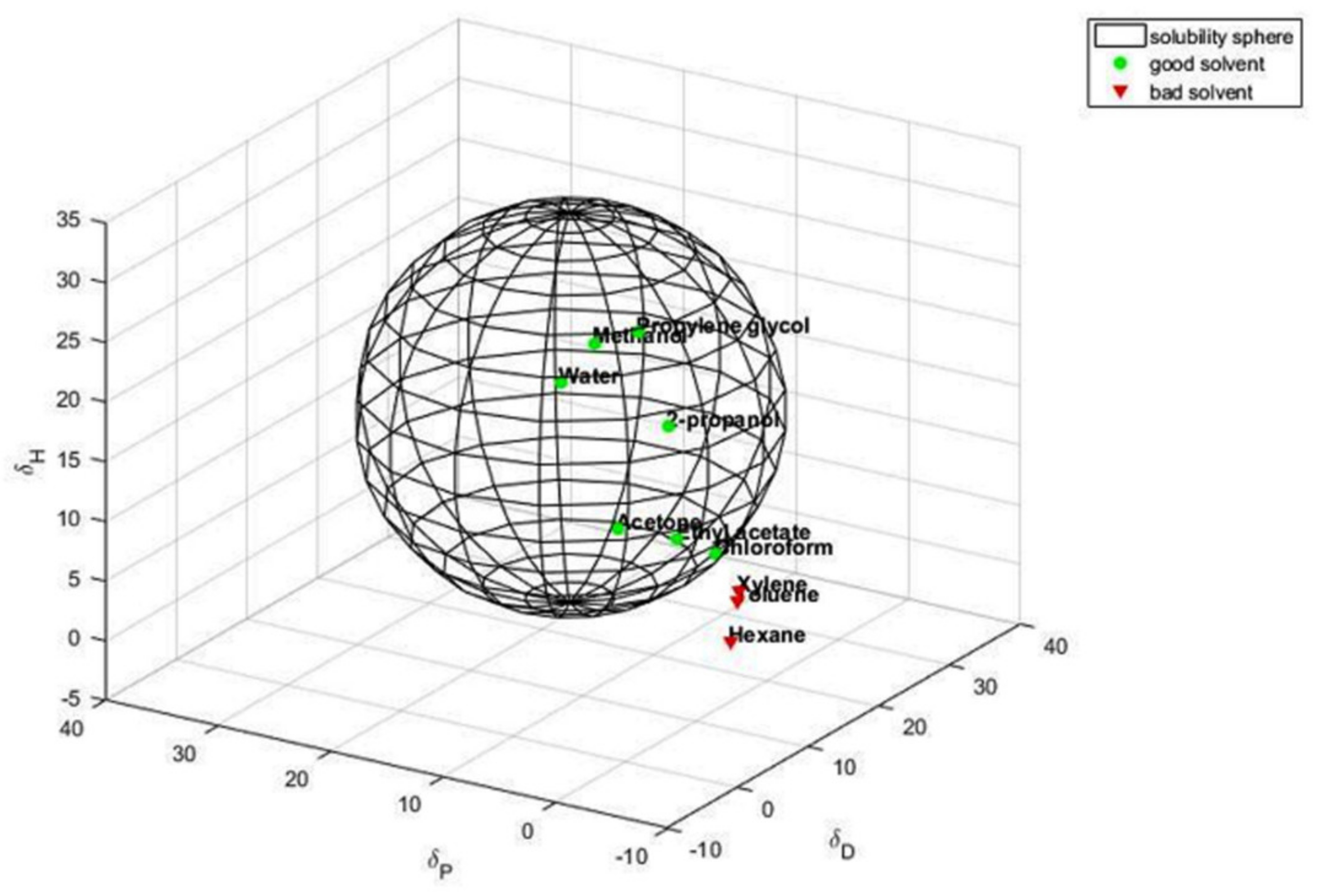
3.2. Calculation of the Hansen Solubility Sphere and Hansen Solubility Parameters
3.3. Dispersion of HSAG and G-OH in Polyol
3.4. Reactivity of Polyhydroxylated Carbon Allotropes with Isocyanates: Model Reaction
3.5. Polyurethane Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, M.J.; Gray, C.A.; Reznek, S.A.; Mahmud, K.; Kutsovsky, Y. Kirk-Othmer Encyclopedia of Chemical Technology; Carbon Black, Inc.: Waltham, MA, USA, 2003. [Google Scholar]
- Voet, A.; Morawski, J.C.; Donnet, J.B. Reinforcement of elastomers by silica. Rubber Chem. Technol. 1977, 50, 342. [Google Scholar]
- Wudl, F. Fullerene materials. J. Mater. Chem. 2002, 12, 1959–1963. [Google Scholar] [CrossRef]
- Diederich, F.; Gómez-López, M. Supramolecular fullerene chemistry. Chem. Soc. Rev. 1999, 28, 263–277. [Google Scholar] [CrossRef]
- Maiti, M.; Bhattacharya, M.; Bhowmick, A.K. Elastomer nanocomposites. Rubber Chem. Technol. 2008, 81, 384. [Google Scholar]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187. [Google Scholar] [CrossRef] [Green Version]
- Galimberti, M.; Cipolletti, V.; Musto, S.; Cioppa, S.; Peli, G.; Mauro, M.; Guerra, G.; Agnelli, S.; Riccò, T.; Kumar, V. Recent advancements in rubber nanocomposites. Rubber Chem. Technol. 2014, 87, 417–442. [Google Scholar] [CrossRef]
- Galimberti, M.; Cipolletti, V.; Coombs, M. Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Bokobza, L. Multiwall carbon nanotube elastomeric composites: A review. Polymer 2007, 48, 4907. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, M.; Maiti, M.; Bhowmick, A.K. Tailoring properties of styrene butadiene rubber nanocomposite by various nanofillers and their dispersion. Polym. Eng. Sci. 2009, 49, 81. [Google Scholar]
- Bhowmick, A.K.; Bhattacharya, M.; Mitra, S. Exfoliation of nanolayer assemblies for improved natural rubber properties: Methods and theory. J. Elastomers Plast. 2010, 42, 517. [Google Scholar] [CrossRef]
- Al-Solamya, F.R.; Al-Ghamdib, A.A.; Mahmou, W.E. Piezoresistive behavior of graphite nanoplatelets based rubber nanocomposites. Polym. Adv. Technol. 2012, 23, 478. [Google Scholar] [CrossRef]
- Galimberti, M.; Kumar, V.; Coombs, M.; Cipolletti, V.; Agnelli, S.; Pandini, S.; Conzatti, L. Filler networking of a nanographite with a high shape anisotropy and synergism with carbon black in poly (1, 4-cis-isoprene)–based nanocomposites. Rubber Chem. Technol. 2014, 87, 197. [Google Scholar] [CrossRef]
- Sardon, H.; Pascual, A.; Mecerreyes, D.; Taton, D.; Cramail, H.; Hedrick, J.L. Synthesis of polyurethanes using organocatalysis: A perspective. Macromolecules 2015, 48, 3153–3165. [Google Scholar] [CrossRef]
- Szycher, M. (Ed.) Szycher’s Handbook of Polyurethanes; Taylor & Francis: Boca Raton, FL, USA, 1999. [Google Scholar]
- Sharmin, E.; Zafar, F. Polyurethane: An introduction (Chapter 1). In Polyurethane; IntechOpen: London, UK, 2012; pp. 3–16. [Google Scholar]
- Sonnenschein, M.F. Polyurethanes: Science, Technology, Markets, and Trends; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Chattopadhyay, D.K.; Raju KV, S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Grand View Research. Polyurethane Market Size, Share. Global PU Industry Report 2019–2025. 2020. Available online: https://www.grandviewresearch.com/industry-analysis/polyurethane-pu-market (accessed on 5 May 2021).
- Engels, H.-W.; Pirkl, H.-G.; Albers, R.; Albach, R.W.; Krause, J.; Hoffmann, A.; Casselmann, H.; Dormish, J. Polyurethanes: Versatile materials and sustainable problem solvers for today’s challenges. Angew. Chem. Int. Ed. 2013, 52, 9422–9441. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Jadhav, S.; Mulge, S.; Thomas, S. Applications of polyurethane based composites and nanocomposites. In Polyurethane Polymers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 559–573. [Google Scholar]
- Souri, H.; Nam, I.W.; Lee, H.K. Electrical properties and piezoresistive evaluation of polyurethane-based composites with carbon nano-materials. Compos. Sci. Technol. 2015, 121, 41–48. [Google Scholar] [CrossRef]
- Sattar, R.; Kausar, A.; Siddiq, M. Advances in thermoplastic polyurethane composites reinforced with carbon nanotubes and carbon nanofibers: A review. J. Plast. Film. Sheeting 2015, 31, 186–224. [Google Scholar] [CrossRef]
- Vaithylingam, R.; Ansari MN, M.; Shanks, R.A. Recent advances in polyurethane-based nanocomposites: A review. Polym.-Plast. Technol. Eng. 2017, 56, 1528–1541. [Google Scholar] [CrossRef]
- Badamshina, E.; Estrin, Y.; Gafurova, M. Nanocomposites based on polyurethanes and carbon nanoparticles: Preparation, properties and application. J. Mater. Chem. A 2013, 1, 6509–6529. [Google Scholar] [CrossRef]
- Ciecierska, E.; Jurczyk-Kowalska, M.; Bazarnik, P.; Gloc, M.; Kulesza, M.; Kowalski, M.; Lewandowska, M. Flammability, mechanical properties and structure of rigid polyurethane foams with different types of carbon reinforcing materials. Compos. Struct. 2016, 140, 67–76. [Google Scholar] [CrossRef]
- Kim, J.H.; Dao, T.D.; Jeong, H.M. Aluminum hydroxide–CNT hybrid material for synergizing the thermal conductivity of alumina sphere/thermoplastic polyurethane composite with minimal increase of electrical conductivity. J. Ind. Eng. Chem. 2016, 33, 150–155. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, J.; Son, Y. Effect of a Monomer Composition on the Mechanical Properties and Glass Transition Temperature of a Waterborne Polyurethane/Graphene Oxide and Waterborne Polyurethane/MWCNT Nanocomposite. Polymers 2020, 12, 2013. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Verma, P.; Dhawan, S.K.; Choudhary, V. Tailored graphene based polyurethane composites for efficient electrostatic dissipation and electromagnetic interference shielding applications. RSC Adv. 2015, 5, 97349–97358. [Google Scholar] [CrossRef]
- Akram, N.; Saeed, M.; Usman, M.; Mansha, A.; Anjum, F.; Zia, K.; Mahmood, I.; Mumtaz, N.; Khan, W.G. Influence of graphene oxide contents on mechanical behavior of polyurethane composites fabricated with different diisocyanates. Polymers 2021, 13, 444. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, Z.E.; Solouk, A.; Shafieian, M.; Nazarpak, M.H. Electrospun polyurethane/carbon nanotube composites with different amounts of carbon nanotubes and almost the same fiber diameter for biomedical applications. Mater. Sci. Eng. C 2021, 118, 111403. [Google Scholar] [CrossRef]
- Mai, V.D.; Nguyen DC, T.; Vu, V.P.; Lee, S.H. Fast healing conductive polymer composite based on carbon black and polyurethane containing disulfide bonds. J. Vinyl Addit. Technol. 2021, 28, 115–124. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Zhou, Y.; Hu, L. The study of mechanical behavior and flame retardancy of castor oil phosphate-based rigid polyurethane foam composites containing expanded graphite and triethyl phosphate. Polym. Degrad. Stab. 2013, 98, 2784–2794. [Google Scholar] [CrossRef]
- Gama, N.; Costa, L.C.; Amaral, V.; Ferreira, A.; Barros-Timmons, A. Insights into the physical properties of biobased polyurethane/expanded graphite composite foams. Compos. Sci. Technol. 2017, 138, 24–31. [Google Scholar] [CrossRef]
- Gama, N.V.; Silva, R.; Mohseni, F.; Davarpanah, A.; Amaral, V.S.; Ferreira, A.; Barros-Timmons, A. Enhancement of physical and reaction to fire properties of crude glycerol polyurethane foams filled with expanded graphite. Polym. Test. 2018, 69, 199–207. [Google Scholar] [CrossRef]
- Vasquez, L.; Campagnolo, L.; Athanassiou, A.; Fragouli, D. Expanded graphite-polyurethane foams for water–oil filtration. ACS Appl. Mater. Interfaces 2019, 11, 30207–30217. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Liu, H.; Huang, W.; Yang, X.; Dai, K.; Zheng, G.; Liu, C.; Shen, C.; Guo, J.; Guo, Z. Organic vapor sensing behaviors of conductive thermoplastic polyurethane–graphene nanocomposites. J. Mater. Chem. C 2016, 4, 4459–4469. [Google Scholar] [CrossRef]
- Liu, H.; Dong, M.; Huang, W.; Gao, J.; Dai, K.; Guo, J.; Guo, Z. Lightweight conductive graphene/thermoplastic polyurethane foams with ultrahigh compressibility for piezoresistive sensing. J. Mater. Chem. C 2017, 5, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Huang, W.; Gao, J.; Dai, K.; Zheng, G.; Liu, C.; Shen, C.; Yan, X.; Guo, J.; Guo, Z. Piezoresistive behavior of porous carbon nanotube-thermoplastic polyurethane conductive nanocomposites with ultrahigh compressibility. Appl. Phys. Lett. 2016, 108, 011904. [Google Scholar]
- Tran, M.T.; Tung, T.T.; Sachan, A.; Losic, D.; Castro, M.; Feller, J.F. 3D sprayed polyurethane functionalized graphene/carbon nanotubes hybrid architectures to enhance the piezo-resistive response of quantum resistive pressure sensors. Carbon 2020, 168, 564–579. [Google Scholar] [CrossRef]
- Chen, K.; Liu, H.; Zhou, J.; Sun, Y.; Yu, K. Polyurethane Blended with Silica-Nanoparticle-Modified Graphene as a Flexible and Superhydrophobic Conductive Coating with a Self-Healing Ability for Sensing Applications. ACS Appl. Nano Mater. 2022, 5, 615–625. [Google Scholar] [CrossRef]
- Kausar, A. Shape memory polymer/graphene nanocomposites: State-of-the-art. e-Polymers 2022, 22, 165–181. [Google Scholar] [CrossRef]
- Gao, Y.; Lv, J.; Liu, L.; Yu, Y. Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer. e-Polymers 2020, 20, 469–481. [Google Scholar] [CrossRef]
- Mauro, M.; Cipolletti, V.; Galimberti, M.; Longo, P.; Guerra, G. Chemically reduced graphite oxide with improved shape anisotropy. J. Phys. Chem. C 2012, 116, 24809–24813. [Google Scholar] [CrossRef]
- Barbera, V.; Porta, A.; Brambilla, L.; Guerra, S.; Serafini, A.; Valerio, A.M.; Galimberti, M. Polyhydroxylated few layer graphene for the preparation of flexible conductive carbon paper. RSC Adv. 2016, 6, 87767–87777. [Google Scholar] [CrossRef]
- Zhou, J.; Sui, Z.; Zhu, J.; Li, P.; Chen, D.; Dai, Y.; Yuan, W. Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FTIR. Carbon 2007, 45, 785–796. [Google Scholar] [CrossRef]
- Barbera, V.; Brambilla, L.; Porta, A.; Bongiovanni, R.M.; Vitale, A.; Torrisi, G.; Galimberti, M. Selective edge functionalization of graphene layers with oxygenated groups by means of Reimer-Tiemann and domino Reimer-Tiemann/Cannizzaro reactions. J. Mater. Chem. A 2018, 6, 7749–7761. [Google Scholar] [CrossRef]
- Barbera, V.; Bernardi, A.; Palazzolo, A.; Rosengart, A.; Brambilla, L.; Galimberti, M. Facile and sustainable functionalization of graphene layers with pyrrole compounds. Pure Appl. Chem. 2018, 90, 253–270. [Google Scholar] [CrossRef] [Green Version]
- Barbera, V.; Brambilla, L.; Milani, A.; Palazzolo, A.; Castiglioni, C.; Vitale, A.; Bongiovanni, R.; Galimberti, M. Domino Reaction for the Sustainable Functionalization of Few-Layer Graphene. Nanomaterials 2019, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vittore, A.; Acocella, M.R.; Guerra, G. Edge-oxidation of graphites by hydrogen peroxide. Langmuir 2019, 35, 2244–2250. [Google Scholar] [CrossRef]
- Barbera, V.; Bernardi, A.; Torrisi, G.; Porta, A.; Galimberti, M. Controlled functionalization of sp2 carbon allotropes for the reinforcement of diene elastomers. Elastomery 2017, 21, 235–251. [Google Scholar]
- Hildebrand, J.H.; Scott, R.L. The Solubility of Nonelectrolytes, 3rd ed.; Dover Publications: New York, NY, USA, 1964. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Geim, A.K. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Reich, S.; Thomsen, C. Raman spectroscopy of graphite. Philos. Trans. R. Soc. A 2004, 362, 2271–2288. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cancado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef]
- Castiglioni, C.; Tommasini, M.; Zerbi, G. Raman spectroscopy of polyconjugated molecules and materials: Confinement effect in one and two dimensions. Philos. Trans. R. Soc. A 2004, 362, 2425–2459. [Google Scholar] [CrossRef]
- Tommasini, M.; Di Donato, E.; Castiglioni, C.; Zerbi, G.; Severin, N.; Bohme, T.; Rabe, J.P. Resonant Raman spectroscopy of nanostructured carbon-based materials: The molecular approach. AIP Conf. Proc. 2004, 723, 334. [Google Scholar]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially resolved Raman spectroscopy of single-and few-layer graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Casiraghi, C.; Hartschuh, A.; Qian, H.; Piscanec, S.; Georgi, C.; Fasoli, A.; Ferrari, A.C. Raman spectroscopy of graphene edges. Nano Lett. 2009, 9, 1433–1441. [Google Scholar] [CrossRef] [Green Version]
- Radovic, L.R.; Bockrath, B. On the chemical nature of graphene edges: Origin of stability and potential for magnetism in carbon materials. J. Am. Chem. Soc. 2005, 127, 5917–5927. [Google Scholar] [CrossRef]
- Barbera, V.; Guerra, S.; Brambilla, L.; Maggio, M.; Serafini, A.; Conzatti, L.; Galimberti, M. Carbon papers and aerogels based on graphene layers and chitosan: Direct preparation from high surface area graphite. Biomacromolecules 2017, 18, 3978–3991. [Google Scholar] [CrossRef]
- Casiraghi, C.; Pisana, S.; Novoselov, K.S.; Geim, A.K.; Ferrari, A.C. Raman fingerprint of charged impurities in graphene. Appl. Phys. Lett. 2007, 91, 233108. [Google Scholar] [CrossRef] [Green Version]
- Bergin, S.D.; Sun, Z.; Rickard, D.; Streich, P.V.; Hamilton, J.P.; Coleman, J.N. Multicomponent solubility parameters for single-walled carbon nanotube−solvent mixtures. ACS Nano 2009, 3, 2340. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Lotya, M.; Rickard, D.; Bergin, S.D.; Coleman, J.N. Measurement of multicomponent solubility parameters for graphene facilitates solvent discovery. Langmuir 2010, 26, 3208. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, M.; Barbera, V.; Guerra, S.; Bernardi, A. Facile functionalization of sp2 carbon allotropes with a biobased Janus molecule. Rubber Chem. Technol. 2017, 90, 285–307. [Google Scholar] [CrossRef] [Green Version]
- Galimberti, M.; Barbera, V.; Guerra, S.; Conzatti, L.; Castiglioni, C.; Brambilla, L.; Serafini, A. Biobased Janus molecule for the facile preparation of water solutions of few layer graphene sheets. RSC Adv. 2015, 5, 81142–81152. [Google Scholar] [CrossRef]
- Tounici, A.; Martín-Martínez, J.M. Addition of graphene oxide in different stages of the synthesis of waterborne polyurethane-urea adhesives and its influence on their structure, thermal, viscoelastic and adhesion properties. Materials 2020, 13, 2899. [Google Scholar] [CrossRef]
- Tounici, A.; Martín-Martínez, J.M. Addition of small amounts of graphene oxide in the polyol during the synthesis of waterborne polyurethane urea adhesives for improving their adhesion properties. Int. J. Adhes. Adhes. 2021, 104, 102725. [Google Scholar] [CrossRef]
- Tounici, A.; Martín-Martínez, J.M. Influence of the Surface Chemistry of Graphene Oxide on the Structure–Property Relationship of Waterborne Poly(urethane urea) Adhesive. Materials 2021, 14, 4377. [Google Scholar] [CrossRef]
- Tounici, A.; Martín-Martínez, J.M. Structure and adhesion properties of waterborne poly (urethane urea) s containing small amounts of different graphene derivatives. J. Adhes. Sci. Technol. 2021, 35, 2758–2789. [Google Scholar] [CrossRef]
- Patel, K.P.; Gayakwad, E.M.; Shankarling, G.S. Graphene Oxide as a Metal-free Carbocatalyst for Direct Amide Synthesis from Carboxylic Acid and Amine Under Solvent-Free Reaction Condition. ChemistrySelect 2020, 5, 8295–8300. [Google Scholar] [CrossRef]
- Mauro, M.; Acocella, M.R.; Corcione, C.E.; Maffezzoli, A.; Guerra, G. Catalytic activity of graphite-based nanofillers on cure reaction of epoxy resins. Polymer 2014, 55, 5612–5615. [Google Scholar] [CrossRef]
- Acocella, M.R.; Mauro, M.; Falivene, L.; Cavallo, L.; Guerra, G. Inverting the diastereoselectivity of the mukaiyama–michael addition with graphite-based catalysts. ACS Catal. 2014, 4, 492–496. [Google Scholar] [CrossRef]

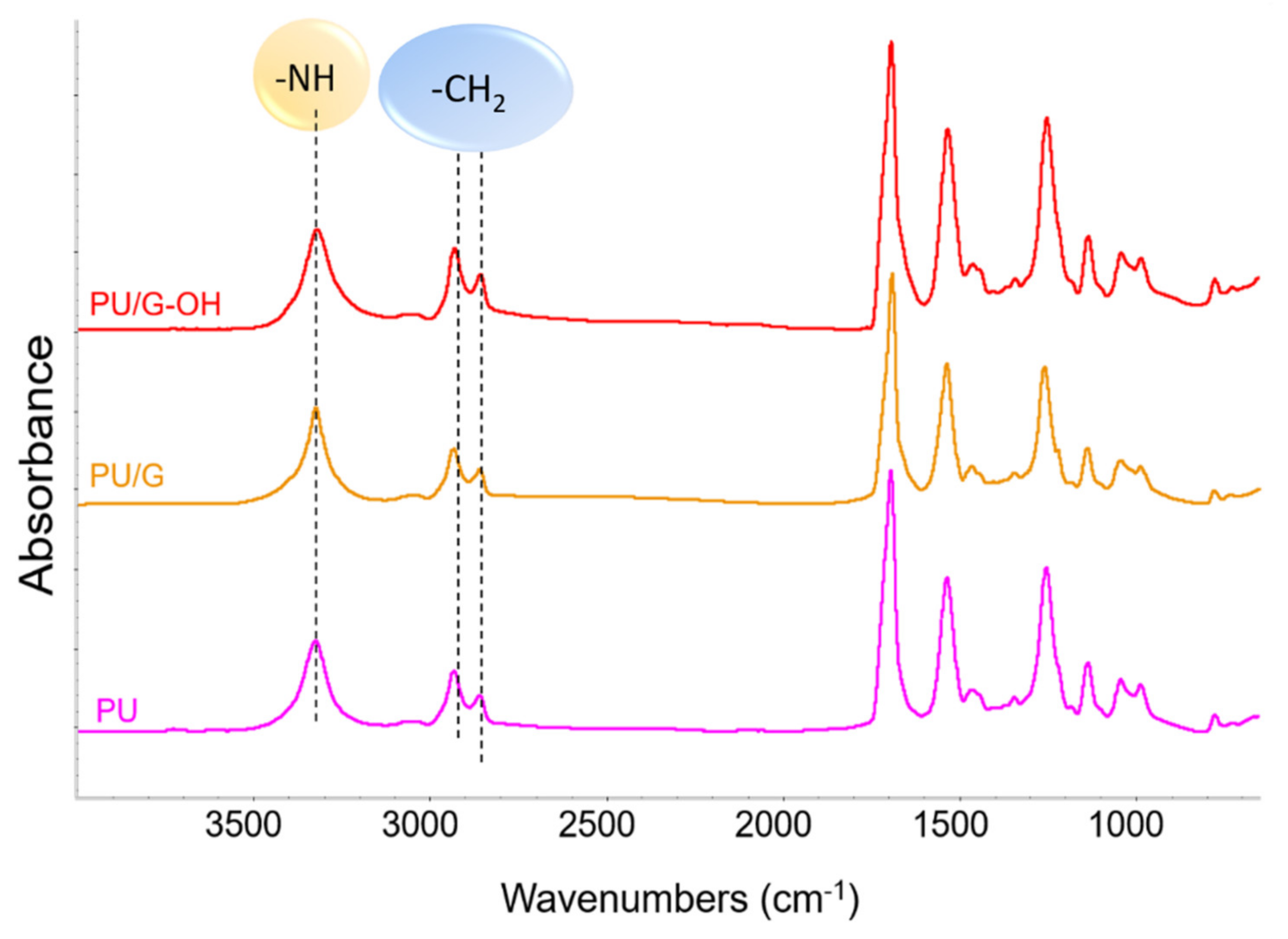
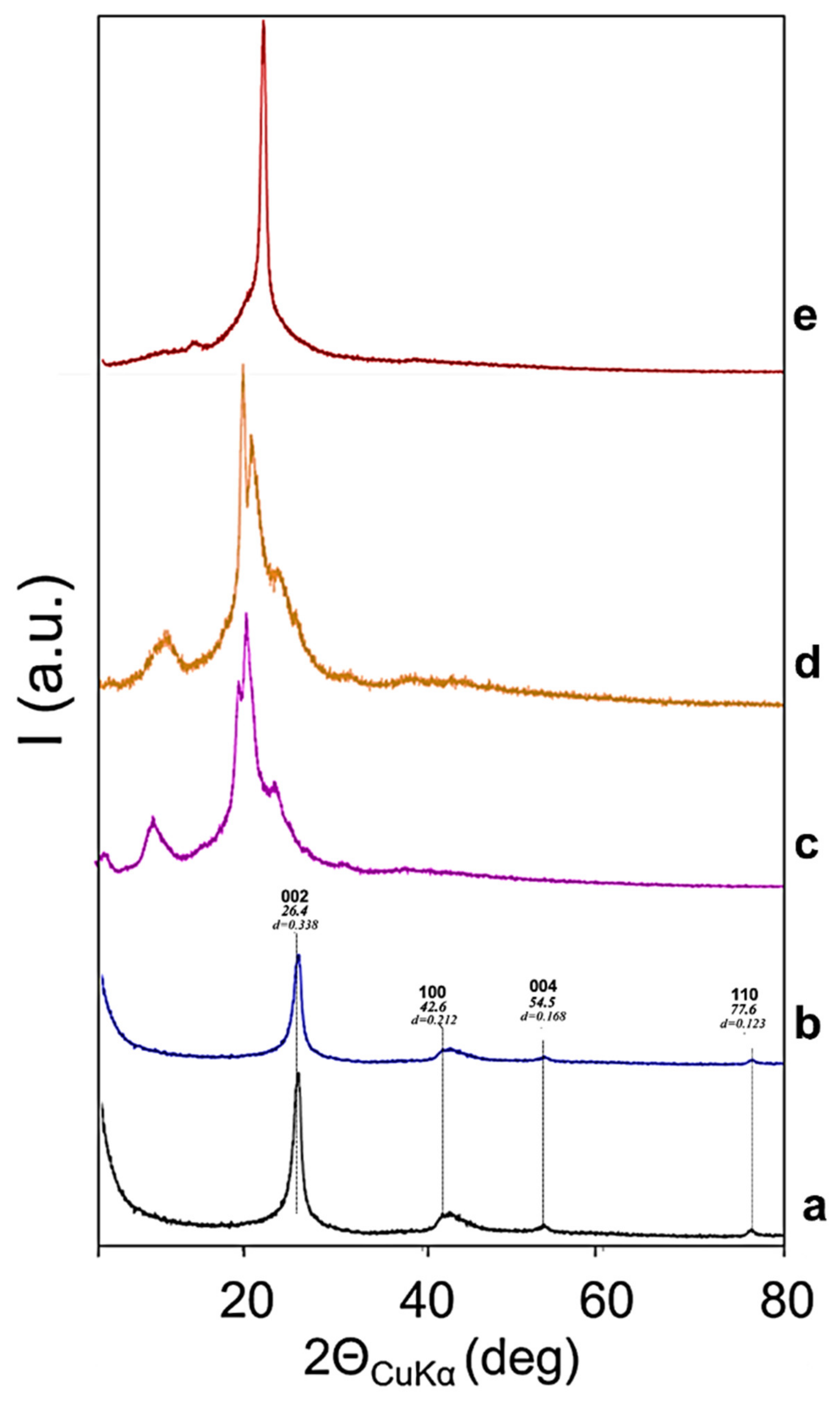
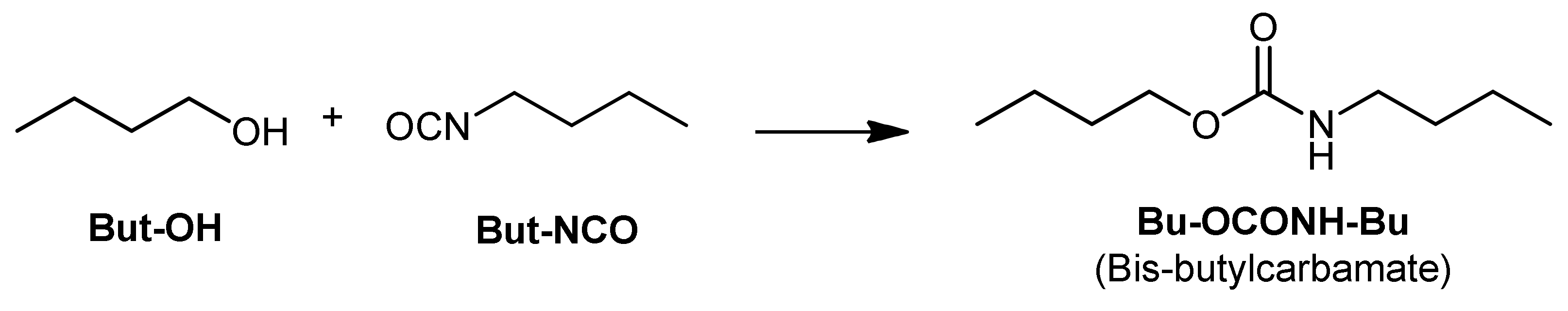
| Run | PU Sample | Diol a | Isocyanate b | Catalyst c | Filler |
|---|---|---|---|---|---|
| [g] | [g] | [mg] | (3 %w) | ||
| 1 | PU | 10 | 19 | 2.54 | n.u. d |
| 2 | PU/G | 10 | 19 | 2.54 | HSAG |
| 3 | PU/G-OH | 9.42 e | 19 | 2.54 | G-OH |
| Sample | δD | δP | δH | Radius | δTb | Ref |
|---|---|---|---|---|---|---|
| G | 18 | 2 | 4 | 1 | 22 | 49 |
| G-OH | 16 | 15 | 16 | 16 | 27 | This work |
| Sample | Tg (°C) | Tc (°C) | ΔHc (J/g) | Tm (°C) | ΔHm (J/g) |
|---|---|---|---|---|---|
| PU | 12 | 88 | 34 | 121 | 34 |
| PU/G | 10 | 87 | 28 | 126 | 28 |
| PU/G-OH | 8 | 99 | 34 | 130 | 37 |
| Run | Catalyst (3 %w) a | Time (h) | Yield (%) b |
|---|---|---|---|
| 1 | = | 24 | 84 |
| 2 | HSAG | 2 | 93 |
| 3 | G-OH | 2 | 88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubino, L.; Torrisi, G.; Brambilla, L.; Rubino, L.; Ortenzi, M.A.; Galimberti, M.; Barbera, V. Polyhydroxylated Nanosized Graphite as Multifunctional Building Block for Polyurethanes. Polymers 2022, 14, 1159. https://doi.org/10.3390/polym14061159
Rubino L, Torrisi G, Brambilla L, Rubino L, Ortenzi MA, Galimberti M, Barbera V. Polyhydroxylated Nanosized Graphite as Multifunctional Building Block for Polyurethanes. Polymers. 2022; 14(6):1159. https://doi.org/10.3390/polym14061159
Chicago/Turabian StyleRubino, Lucia, Giulio Torrisi, Luigi Brambilla, Luca Rubino, Marco Aldo Ortenzi, Maurizio Galimberti, and Vincenzina Barbera. 2022. "Polyhydroxylated Nanosized Graphite as Multifunctional Building Block for Polyurethanes" Polymers 14, no. 6: 1159. https://doi.org/10.3390/polym14061159
APA StyleRubino, L., Torrisi, G., Brambilla, L., Rubino, L., Ortenzi, M. A., Galimberti, M., & Barbera, V. (2022). Polyhydroxylated Nanosized Graphite as Multifunctional Building Block for Polyurethanes. Polymers, 14(6), 1159. https://doi.org/10.3390/polym14061159









