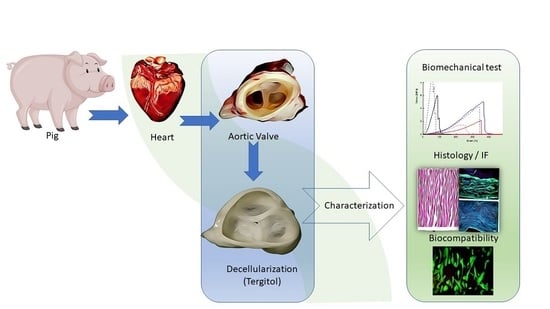
A New Decellularization Protocol of Porcine Aortic Valves Using Tergitol to Characterize the Scaffold with the Biocompatibility Profile Using Human Bone Marrow Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Procurement and Preservation
2.2. Decellularization Procedure
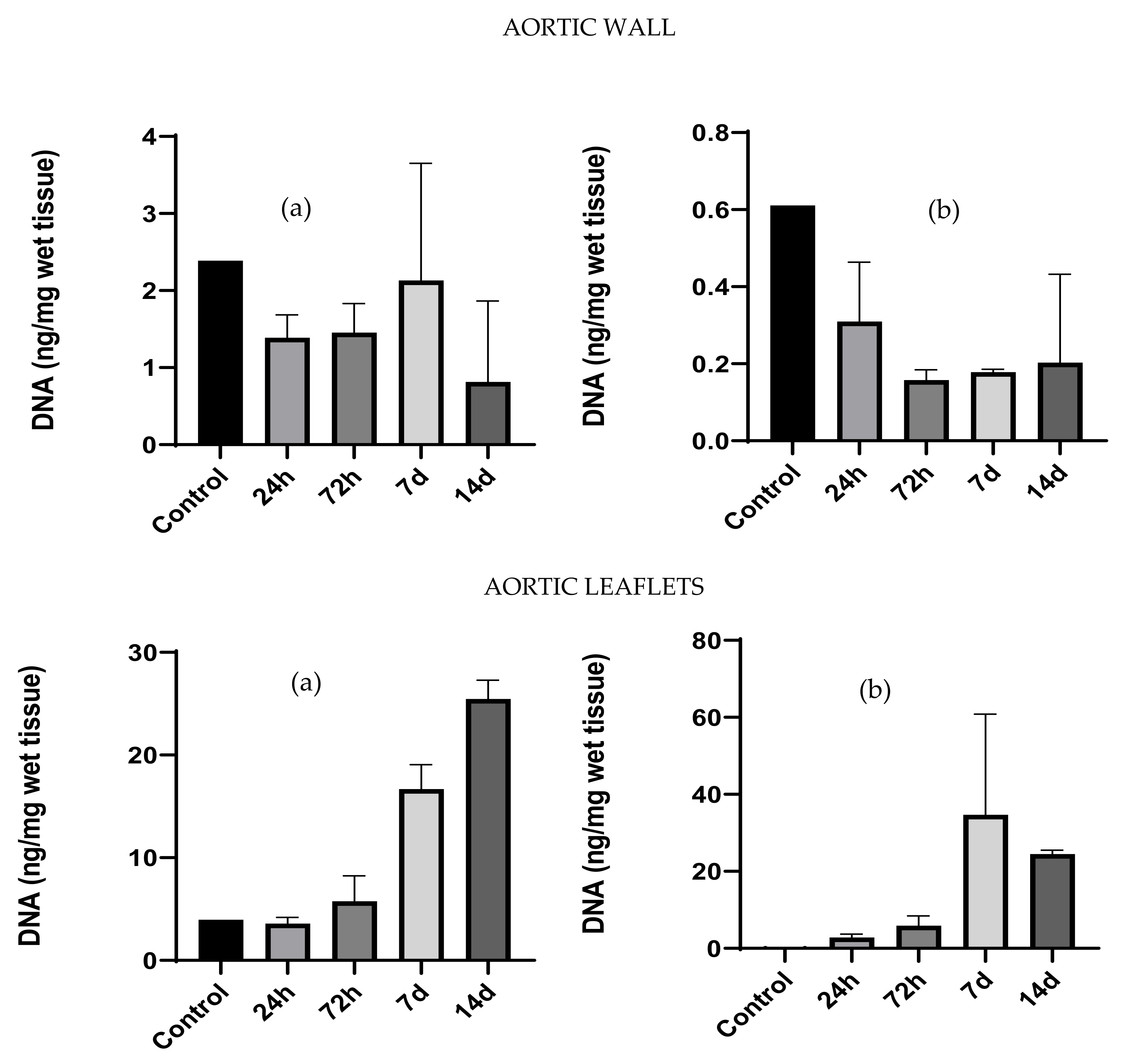
2.3. DNA Extraction and Quantification
2.4. Biochemical Assays
2.4.1. Elastin Quantification
2.4.2. Hydroxyproline Quantification
2.4.3. Glycosaminoglycans (GAGs) Quantification
2.5. Biomechanical Tests
2.6. Histology
2.7. Immunofluorescence
2.8. Determination of Alpha-Gal Epitope
2.9. Two Photon Microscopy
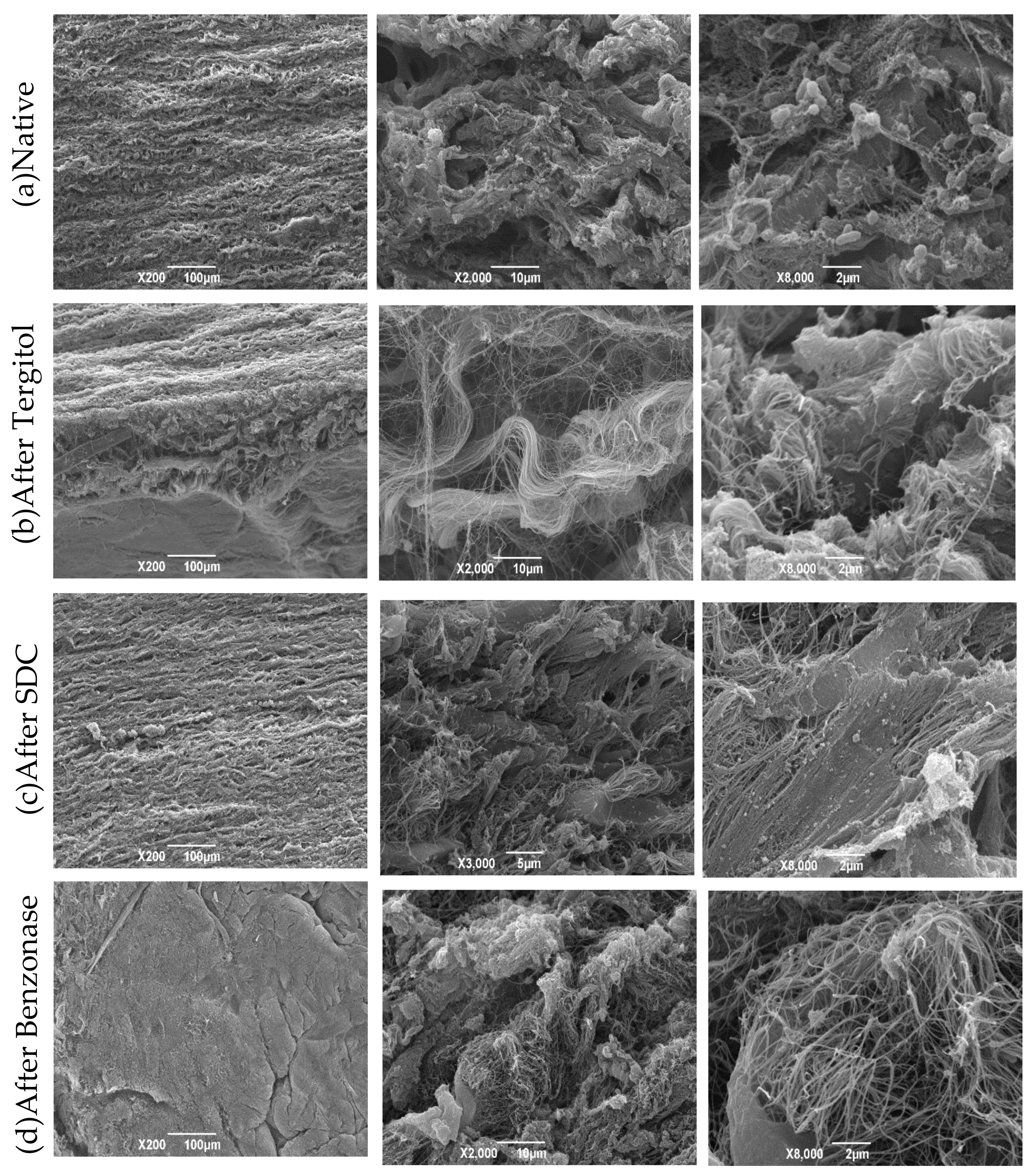
2.10. Scanning Electron Microscopy (SEM)
2.11. Sterility Assessment
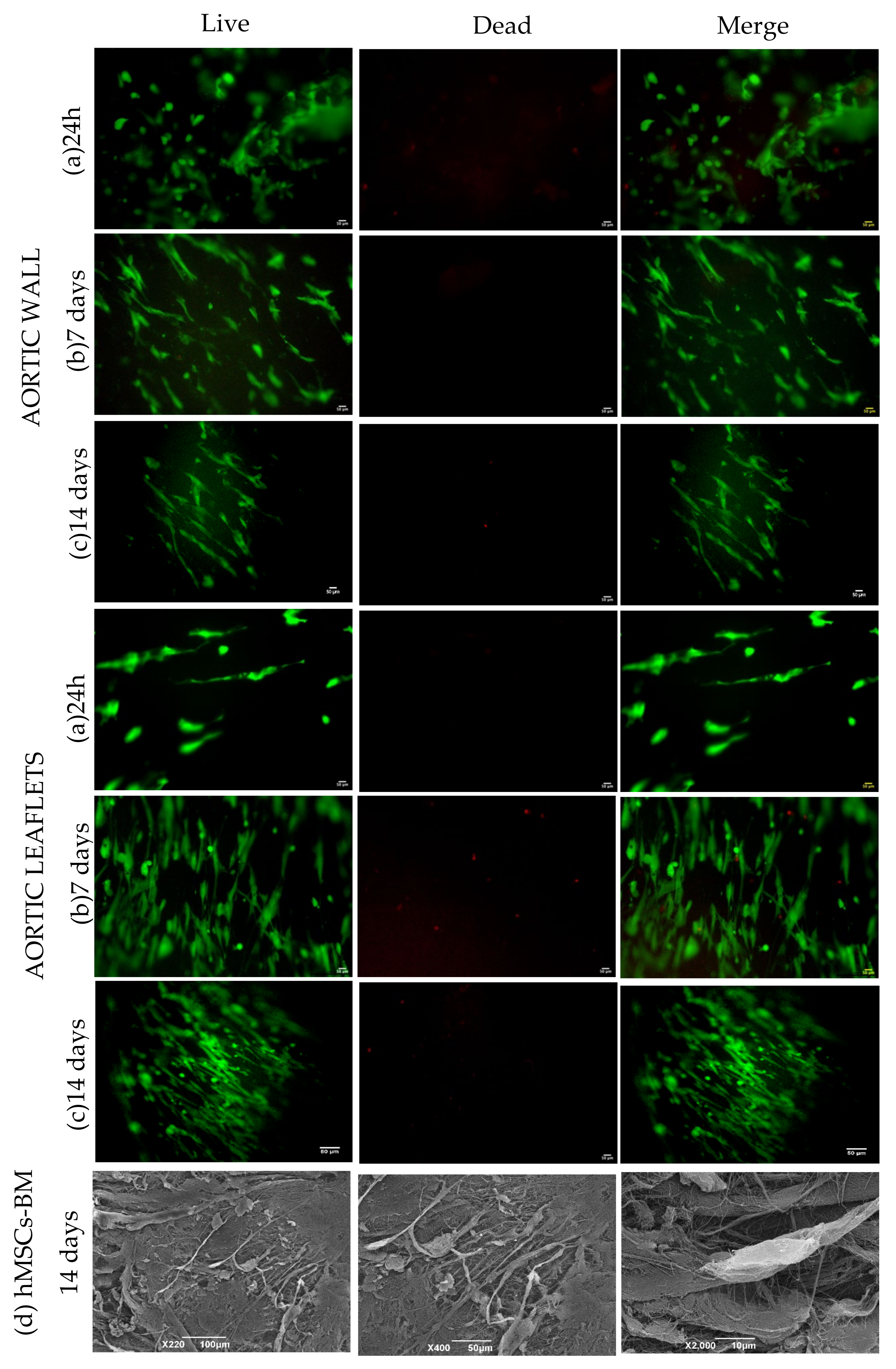
2.12. Biocompatibility Test
- (a)
- Live and Dead assay: Cell viability was evaluated using the Live/Dead viability/cytotoxicity kit (MP 03224, Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). Briefly, cells were stained with Calcein AM and Ethidium homodimer-1 with final concentrations of 2 and 4 µM, respectively, by incubation at 37 °C for 45 min. Images were obtained using Olympus IX71 microscope.
- (b)
- DNA extraction and quantification: Tissue patches were cultured with the hMSCs-BM and the amount of DNA was quantified to estimate cell proliferation. DNA extraction and quantification were performed as described in Section 2.3.
- (c)
- WST-1 assay: The cell Proliferation and Cytotoxicity Assay kit (AR1159, Boster, Pleasanton, CA, USA) was used to assess the viability/proliferation of cells cultured on decellularized patches at each time point. The assay was performed according to the suppliers’ instructions. Absorbance was measured at 450 nm using microplate reader (Spark 10M Tecan, Tecan). Cells plated on a multi-well plate served as a positive control.
- (d)
- Scanning electron microscopy (SEM): SEM was performed after culturing hMSCs-BM for 14 days on the decellularized and decontaminated aortic tissue patches (n = 3).
2.13. Data Analysis
3. Results
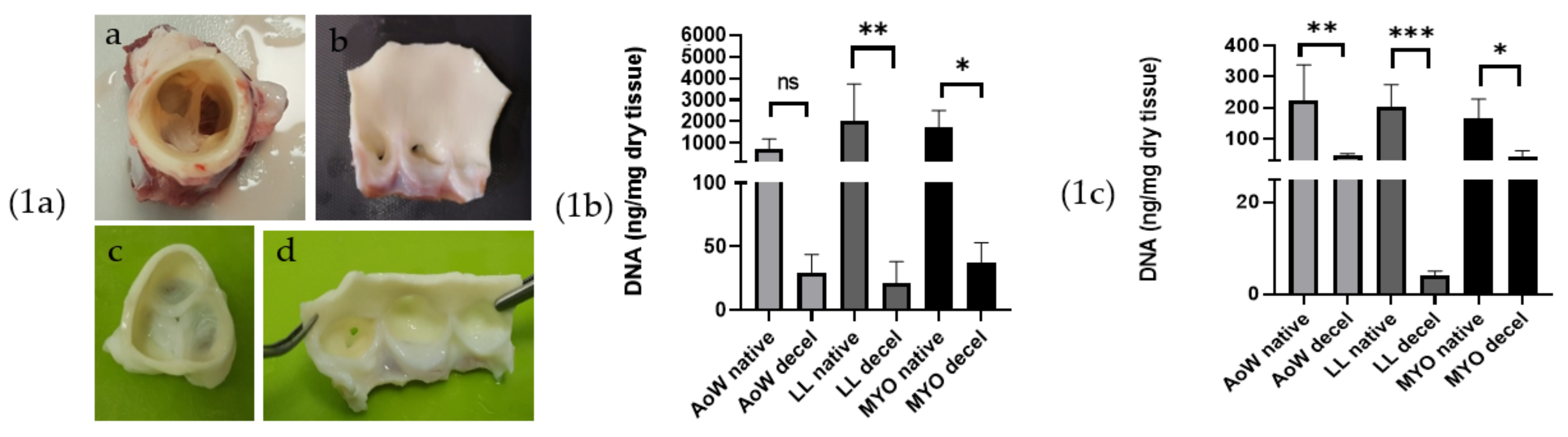
3.1. Visual Inspection
3.2. DNA Extraction and Quantification
3.3. Biochemical Assays
3.4. Biomechanical Tests
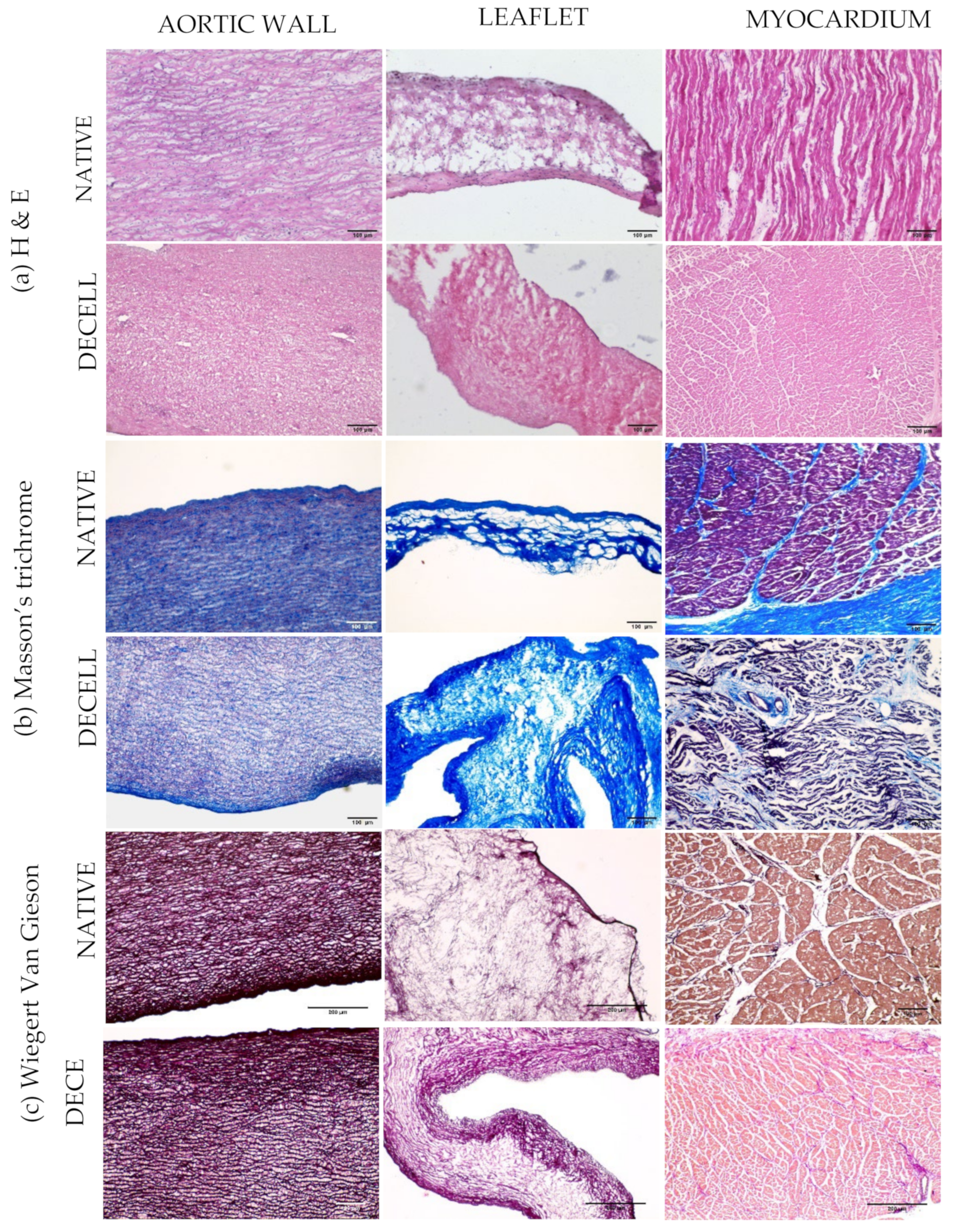
3.5. Histology
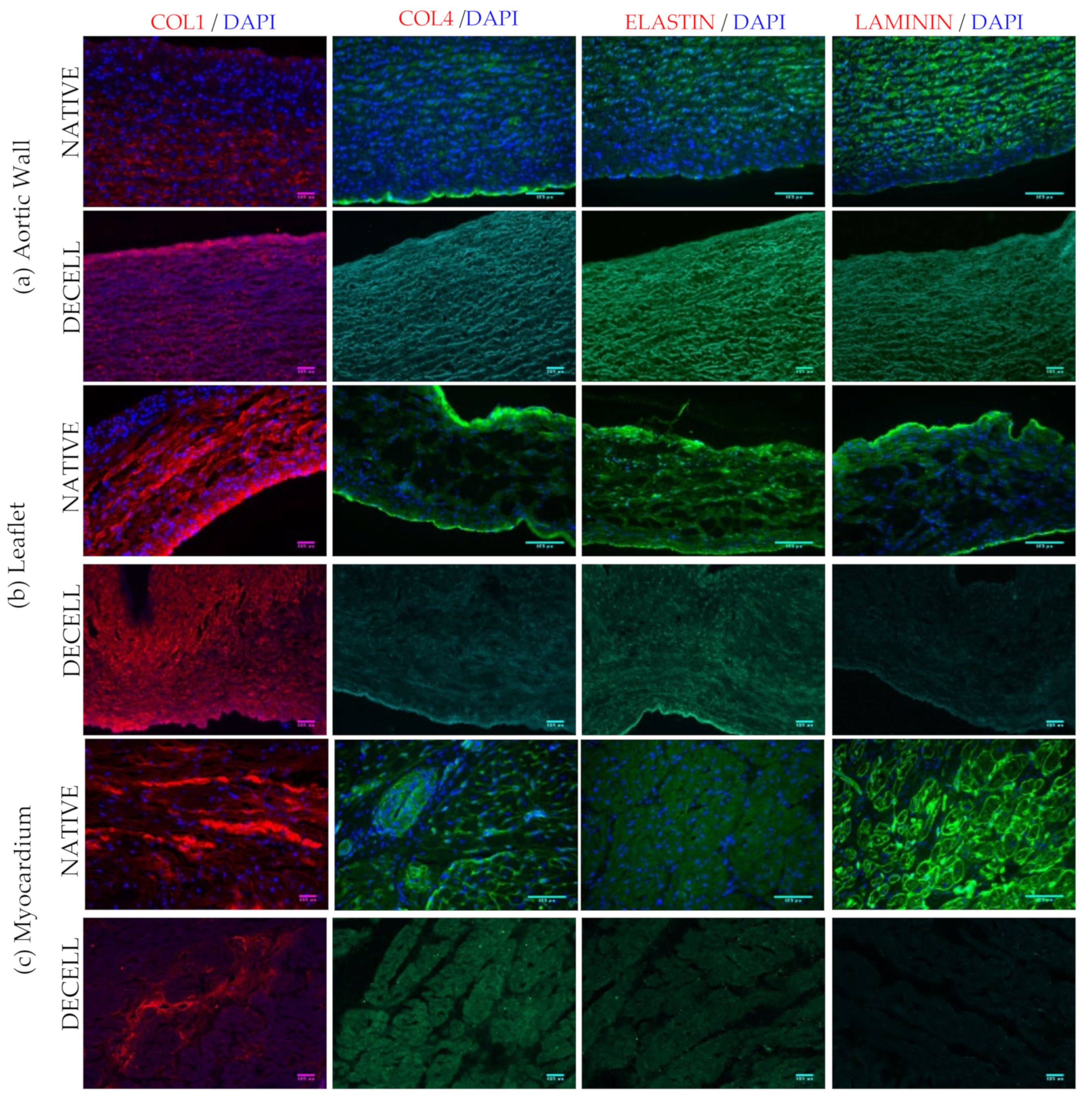
3.6. Immunofluorescence
3.7. Two Photon Microscopy
3.8. Scanning Electron Microscopy (SEM)
3.9. Sterility Assessment
3.10. Biocompatibility Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 2019, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Clavel, M.A.; Mathieu, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Gabella, T.; Voisine, P.; Puri, R.; Pibarot, P.; Rodés-Cabau, J. Aortic Bioprosthetic Valve Durability Incidence, Mechanisms, Predictors, and Managment of Surgical and Transcatheter Valve Degeneration. J. Am. Coll. Cardiol. 2017, 70, 1013–1028. [Google Scholar] [CrossRef]
- Brown, J.M.; O’Brien, S.M.; Wu, C.; Sikora, J.A.H.; Griffith, B.P.; Gammie, J.S. Isolated aortic valve replacement in North America comprising 108,678 patients i 10 years: Canges in risks, valve tyoes and outcomes in the Society of Thoracic Surgeons National Database. J. Thorac. Cardiovasc. Surg. 2008, 137, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilcox, H.E.; Korossis, S.A.; Booth, C.; Watterson, K.G.; Kearney, J.N.; Fisher, J.; Ingham, E. Biocompatibility and recellularization potential of an acellular porcine hearts valve matrix. J. Hear. Valve Dis. 2005, 14, 228. [Google Scholar]
- Fioretta, E.S.; von Boehmer, L.; Motta, S.E.; Lintas, V.; Hoerstrup, S.P.; Emmert, M.Y. Cardiovascular tissue engineering: From basic science to clinical application. Exp. Gerontol. 2019, 117, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sierad, L.N.; Shaw, E.L.; Bina, A.; Brazile, B.; Rierson, N.; Patnaik, S.S.; Kennamer, A.; Odum, R.; Cotoi, O.; Terezia, P.; et al. Functional heart valve scaffolds obtained by complete decellularization of porcine aortic roots in a novel differential pressure gradient perfusion system. Tissue Eng. Part C Methods 2015, 21, 1284–1296. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, P.L.; Fernández-Santos, M.E.; Costanza, S.; Climent, A.M.; Moscoso, I.; Gonzalez-Nicolas, M.A.; Sanz-Ruiz, R.; Rodríguez, H.; Kren, S.M.; Garrido, G.; et al. Acellular human heart matrix: A critical step toward whole hearts grafts. Biomaterials 2015, 61, 279–289. [Google Scholar] [CrossRef]
- Spina, M.; Ortolani, F.; Messlemani, A.E.; Gandaglia, A.; Bujan, J.; Garcia-Honduvilla, N.; Vesely, I.; Gerosa, G.; Casarotto, D.; Petrelli, L.; et al. Isolation of intact aortic valve scaffolds for heart-valve bioprostheses: Extracellular matrix structure, prevention from calcification, and cell repopulation features. J. Biomed. Mater. Res. Part A 2003, 67, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) 2017/999—Of 13 June 2017—Amending Annex XIV to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0999&from=EN (accessed on 17 February 2022).
- De la Parra-Guerra, A.; Olivero-Verbel, J. Toxicity of nonylphenol and nonylphenol ethoxylate on Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2020, 187, 109709. [Google Scholar] [CrossRef] [PubMed]
- Roderjan, J.G.; de Noronha, L.; Stimamiglio, M.A.; Correa, A.; Leitolis, A.; Bueno, R.R.L.; da Costa, F.D.A. Structural assessments in decellularized extracellular matrix of porcine semilunar heart valves: Evaluation of cell niches. Xenotransplantation 2019, 26, e12503. [Google Scholar] [CrossRef]
- Gallo, M.; Naso, F.; Poser, H.; Rossi, A.; Franci, P.; Bianco, R.; Micciolo, M.; Zanella, F.; Cucchini, U.; Aresu, L.; et al. Physiological Performance of a Detergent Decellularized Heart Valve Implanted for 15 Months in Vietnamese Pigs: Surgical Procedure, Follow-up, and Explant Inspection. Artif. Organs 2012, 36, E138–E150. [Google Scholar] [CrossRef] [PubMed]
- Winkelman, J. The Distribution of Tetraphenylporphinesulfonate in the Tumor-bearing Rat. Cancer Res. 1962, 22, 589–596. [Google Scholar] [PubMed]
- Neuman, R.E.; Logan, M.A. The determination of hydroxyproline. J. Biol. Chem. 1950, 184, 299–306. [Google Scholar] [CrossRef]
- Barbosa, I.; Garcia, S.; Barbier-Chassefière, V.; Caruelle, J.P.; Martelly, I.; Papy-García, D. Improved and simple micro assay for sulfated glycosaminoglycans quantification in biological extracts and its use in skin and muscle tissue studies. Glycobiology 2003, 13, 647–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Herrera, C.M.; Atienza, J.M.; Rojo, F.J.; Claes, E.; Guinea, G.V.; Celentano, D.J.; García-Montero, C.; Burgos, R.L. Mechanical behaviour and rupture of normal and pathological human ascending aortic wall. Med. Biol. Eng. Comput. 2012, 50, 559–566. [Google Scholar] [CrossRef]
- Filippi, A.; Dal Sasso, E.; Iop, L.; Armani, A.; Gintoli, M.; Sandri, M.; Gerosa, G.; Romanato, F.; Borile, G. Multimodal label-free ex vivo imaging using a dual-wavelength microscope with axial chromatic aberration compensation. J. Biomed. Opt. 2018, 23, 091403. [Google Scholar] [CrossRef]
- Council of Europe. 2.6.1. Sterility. Eur. Pharm. 2005, 5, 145–149. [Google Scholar]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horke, A.; Tudorache, I.; Laufer, G.; Andreas, M.; Pomar, J.L.; Pereda, D.; Quintana, E.; Sitges, M.; Meyns, B.; Rega, F.; et al. Early results from a prospective, single-arm European trial on decellularized allografts for aortic valve replacement: The ARISE study and ARISE registry data. Eur. J. Cardio-Thorac. Surg. 2020, 58, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Kasimir, M.T.; Seebacher, G.; Weigel, G.; Ullrich, R.; Salzer-Muhar, U.; Rieder, E.; Wolner, E. Early failure of the tissue engineered porcine heart valve SYNERGRAFTTM in pediatric patients. Eur. J. Cardio-Thorac. Surg. 2003, 23, 1002–1006. [Google Scholar] [CrossRef] [Green Version]
- Baraki, H.; Tudorache, I.; Braun, M.; Höffler, K.; Görler, A.; Lichtenberg, A.; Bara, C.; Calistru, A.; Brandes, G.; Hewicker-Trautwein, M.; et al. Orthotopic replacement of the aortic valve with decellularized allograft in a sheep model. Biomaterials 2009, 30, 6240–6246. [Google Scholar] [CrossRef] [PubMed]
- Tudorache, I.; Theodoridis, K.; Baraki, H.; Sarikouch, S.; Bara, C.; Meyer, T.; Höffler, K.; Hartung, D.; Hilfiker, A.; Haverich, A.; et al. Decellularized aortic allografts versus pulmonary autografts for aortic valve replacement in the growing sheep model: Haemodynamic and morphological results at 20 months after implantation. Eur. J. Cardio-Thorac. Surg. 2016, 49, 1228–1238. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, F.D.A.; Costa, A.C.B.A.; Prestes, R.; Domanski, A.C.; Balbi, E.M.; Ferreira, A.D.A.; Lopes, S.V. The early and midterm function of decellularized aortic valve allografts. Ann. Thorac. Surg. 2010, 90, 1854–1860. [Google Scholar] [CrossRef] [PubMed]




| Sample | Thickness [mm] |
|---|---|
| AoW Native | 1.8 ± 0.52 |
| AoW Decellularized | 1.7 ± 0.35 |
| LL Native | 0.45 ± 0.25 |
| LL Decellularized | 0.38 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faggioli, M.; Moro, A.; Butt, S.; Todesco, M.; Sandrin, D.; Borile, G.; Bagno, A.; Fabozzo, A.; Romanato, F.; Marchesan, M.; et al. A New Decellularization Protocol of Porcine Aortic Valves Using Tergitol to Characterize the Scaffold with the Biocompatibility Profile Using Human Bone Marrow Mesenchymal Stem Cells. Polymers 2022, 14, 1226. https://doi.org/10.3390/polym14061226
Faggioli M, Moro A, Butt S, Todesco M, Sandrin D, Borile G, Bagno A, Fabozzo A, Romanato F, Marchesan M, et al. A New Decellularization Protocol of Porcine Aortic Valves Using Tergitol to Characterize the Scaffold with the Biocompatibility Profile Using Human Bone Marrow Mesenchymal Stem Cells. Polymers. 2022; 14(6):1226. https://doi.org/10.3390/polym14061226
Chicago/Turabian StyleFaggioli, Marika, Arianna Moro, Salman Butt, Martina Todesco, Deborah Sandrin, Giulia Borile, Andrea Bagno, Assunta Fabozzo, Filippo Romanato, Massimo Marchesan, and et al. 2022. "A New Decellularization Protocol of Porcine Aortic Valves Using Tergitol to Characterize the Scaffold with the Biocompatibility Profile Using Human Bone Marrow Mesenchymal Stem Cells" Polymers 14, no. 6: 1226. https://doi.org/10.3390/polym14061226