Modulating Role of Co-Solutes in Complexation between Bovine Serum Albumin and Sodium Polystyrene Sulfonate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Buffer, Protein and Polyelectrolyte Stock Solutions
2.3. Turbidimetric Titrations
2.4. Fluorimetry
2.5. Circular Dichroism (CD)
3. Theoretical Methods
4. Results and Discussion
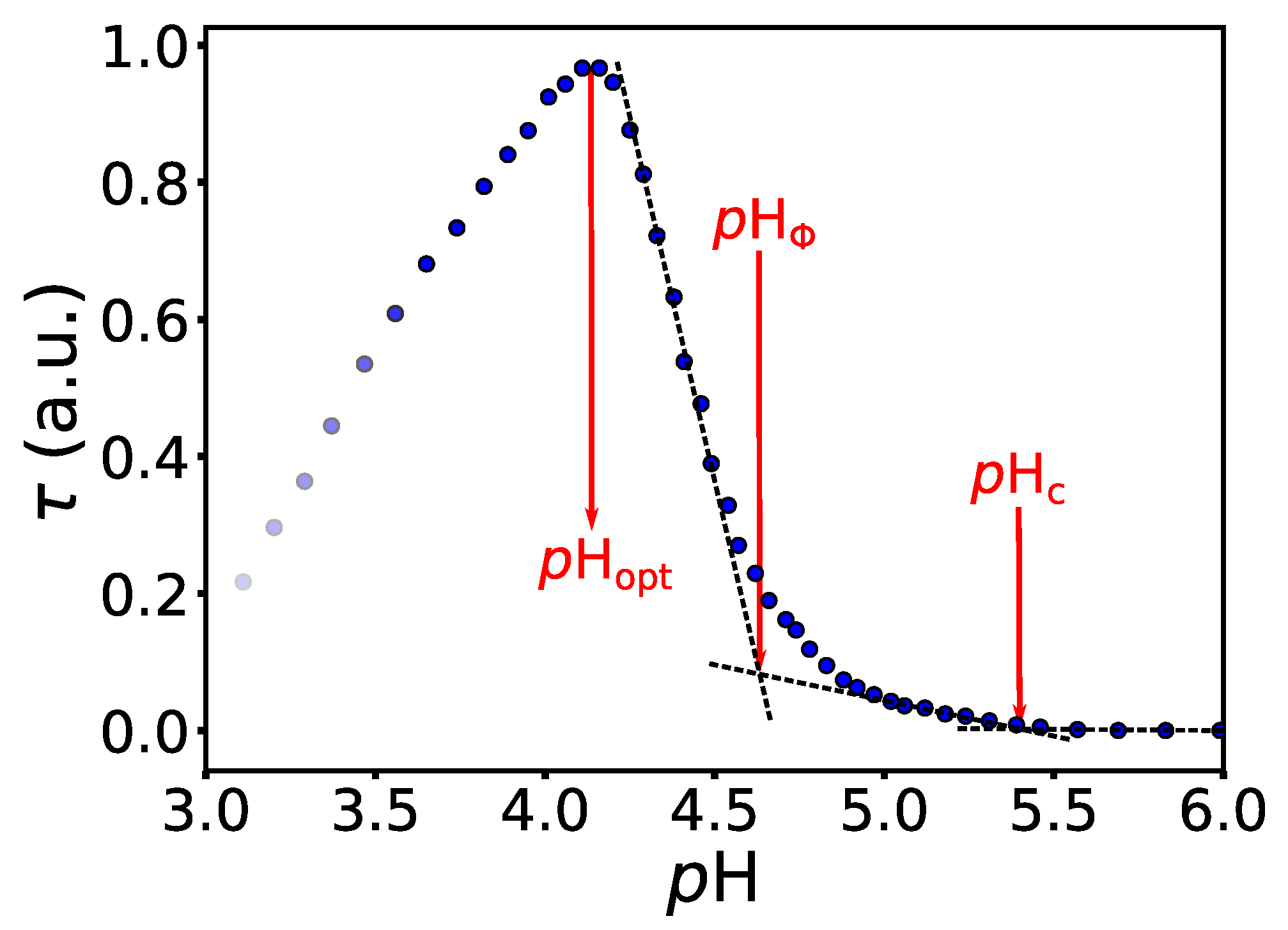
4.1. The BSA/NaPSS Solutions without Co-Solute
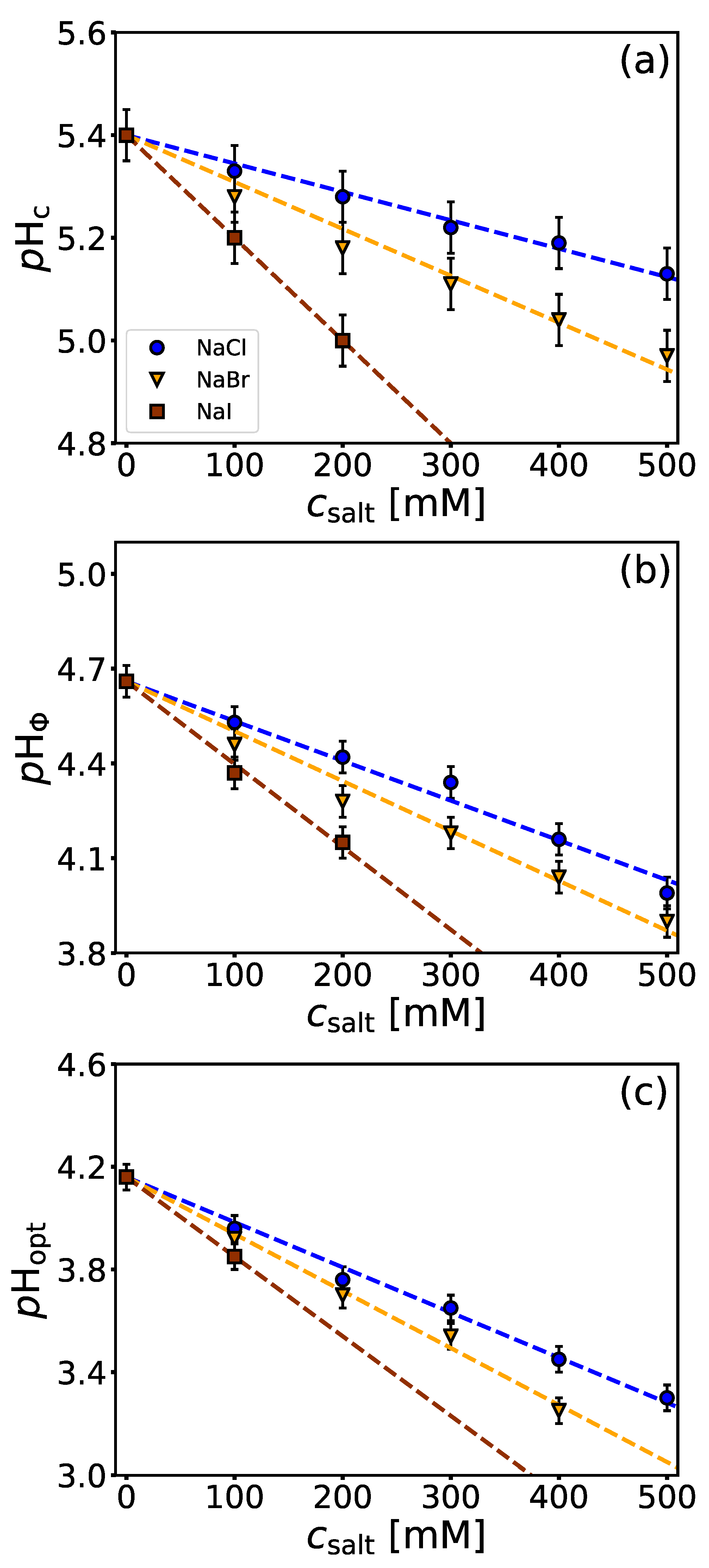
4.2. Salt-Specific Influence on BSA/NaPSS Complexation
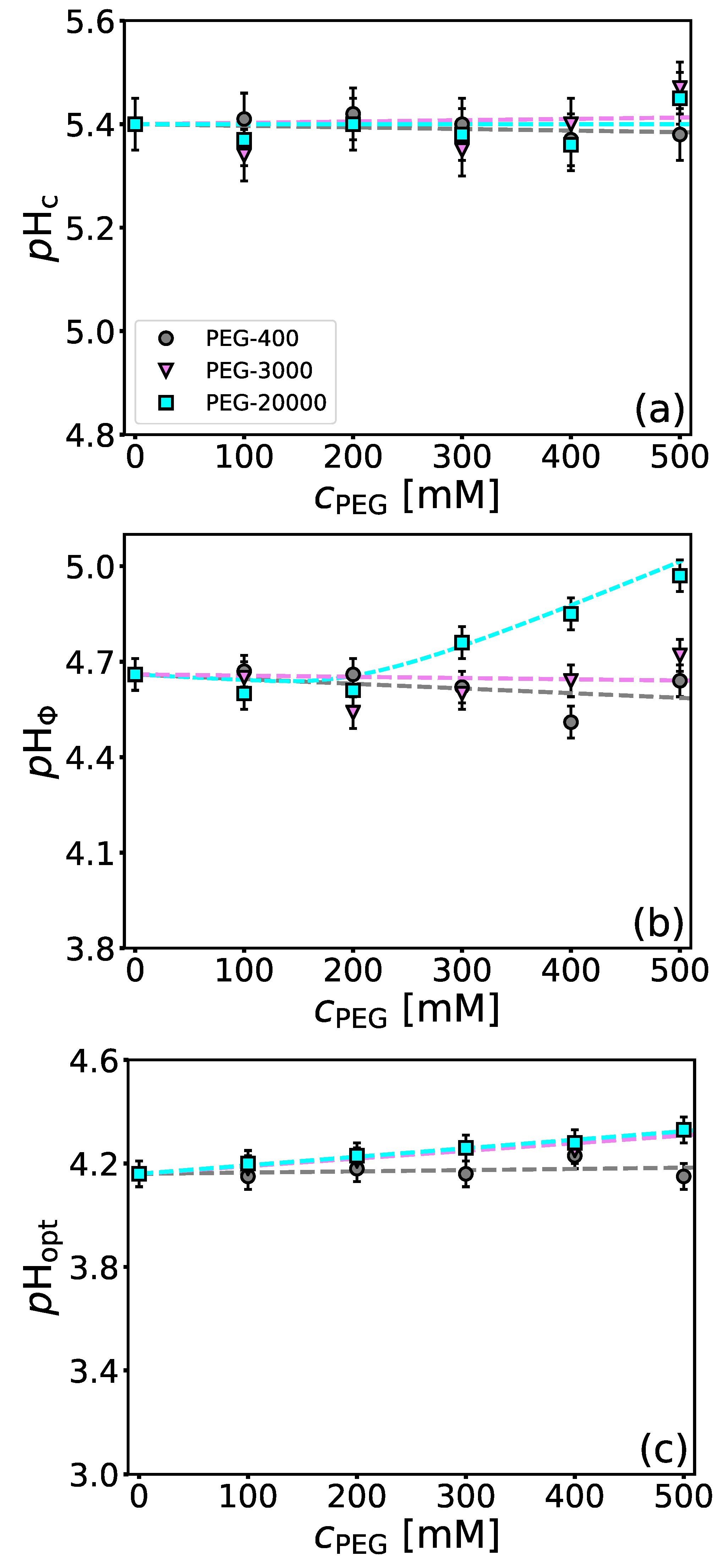
4.3. Influence of Molecular Crowders on BSA/NaPSS Complexation
4.4. Influence of Sugars on BSA/NaPSS Complexation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PE | polyelectrolyte |
| BSA | bovine serum albumin |
| NaPSS | sodium polystyrene sulfonate |
| PEG | polyethylene glycol |
References
- Comert, F.; Dubin, P.L. Liquid-liquid and liquid-solid phase separation in protein-polyelectrolyte systems. Adv. Colloid Interface Sci. 2017, 239, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Patel, H.M.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. Polyelectrolyte complexes: Mechanisms, critical experimental aspects, and applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1615–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Mazzawi, M.; Chen, K.; Sun, L.; Dubin, P.L. Protein purification by polyelectrolyte coacervation: Influence of protein charge anisotropy on selectivity. Biomacromolecules 2011, 12, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Chen, Y.; Wang, X.; Zhao, X.; Qian, W.; Xu, Y. Selective protein separation based on charge anisotropy by spherical polyelectrolyte brushes. Langmuir 2020, 36, 10528–10536. [Google Scholar] [CrossRef] [PubMed]
- Van Lente, J.J.; Claessens, M.M.; Lindhoud, S. Charge-based separation of proteins using polyelectrolyte complexes as models for membraneless organelles. Biomacromolecules 2019, 20, 3696–3703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Lente, J.J.; Lindhoud, S. Extraction of Lysozyme from Chicken Albumen Using Polyelectrolyte Complexes. Small 2021, 18, 2105147. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, K.; Kurinomaru, T.; Tomita, S. Wrap-and-Strip Technology of Protein–Polyelectrolyte Complex for Biomedical Application. Curr. Med. Chem. 2016, 23, 276–289. [Google Scholar] [CrossRef] [Green Version]
- Batrakova, E.V.; Li, S.; Reynolds, A.D.; Mosley, R.L.; Bronich, T.K.; Kabanov, A.V.; Gendelman, H.E. A Macrophage-Nanozyme delivery system for Parkinson’s disease. Bioconjug. Chem. 2007, 18, 1498–1506. [Google Scholar] [CrossRef] [Green Version]
- Kwon, T.K.; Kim, J.C. Complex coacervation-controlled release from monoolein cubic phase containing silk fibroin and alginate. Biomacromolecules 2011, 12, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Skwarczynski, M.; Toth, I. Polyelectrolyte-based platforms for the delivery of peptides and proteins. ACS Biomater. Sci. Eng. 2019, 5, 4937–4950. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Niwa, O.; Kurita, R. Artificial modification of an enzyme for construction of cross-reactive polyion complexes to fingerprint signatures of proteins and mammalian cells. Anal. Chem. 2016, 88, 9079–9086. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Liu, W.; Zhu, G.; Zhou, R.; Niu, Y. A review of the preparation and application of flavour and essential oils microcapsules based on complex coacervation technology. J. Sci. Food Agric. 2014, 94, 1482–1494. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef]
- Korolev, N.; Allahverdi, A.; Lyubartsev, A.P.; Nordenskiöld, L. The polyelectrolyte properties of chromatin. Soft Matter 2012, 8, 9322–9333. [Google Scholar] [CrossRef]
- Pathak, J.; Priyadarshini, E.; Rawat, K.; Bohidar, H. Complex coacervation in charge complementary biopolymers: Electrostatic versus surface patch binding. Adv. Colloid Interface Sci. 2017, 250, 40–53. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef]
- Meka, V.S.; Sing, M.K.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Dubin, P.; Kayitmazer, A.; Turksen, S. Polyelectrolyte–protein complexes. Curr. Opin. Colloid Interface Sci. 2005, 10, 52–78. [Google Scholar] [CrossRef]
- Gao, S.; Holkar, A.; Srivastava, S. Protein–polyelectrolyte complexes and micellar assemblies. Polymers 2019, 11, 1097. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, K.; Si, Y.; Guo, X.; Xu, Y. Protein–polyelectrolyte interaction: Thermodynamic analysis based on the titration method. Polymers 2019, 11, 82. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, C.; Turgeon, S.L. Protein/polysaccharide complexes and coacervates in food systems. Adv. Colloid Interface Sci. 2011, 167, 63–70. [Google Scholar] [CrossRef]
- Ou, Z.; Muthukumar, M. Entropy and enthalpy of polyelectrolyte complexation: Langevin dynamics simulations. J. Chem. Phys. 2006, 124, 154902. [Google Scholar] [CrossRef] [PubMed]
- Yigit, C.; Heyda, J.; Ballauff, M.; Dzubiella, J. Like-charged protein-polyelectrolyte complexation driven by charge patches. J. Chem. Phys. 2015, 143, 064905. [Google Scholar] [CrossRef]
- Turgeon, S.; Schmitt, C.; Sanchez, C. Protein–polysaccharide complexes and coacervates. Curr. Opin. Colloid Interface Sci. 2007, 12, 166–178. [Google Scholar] [CrossRef]
- Sadman, K.; Wang, Q.; Chen, Y.; Keshavarz, B.; Jiang, Z.; Shull, K.R. Influence of hydrophobicity on polyelectrolyte complexation. Macromolecules 2017, 50, 9417–9426. [Google Scholar] [CrossRef]
- Park, J.M.; Muhoberac, B.B.; Dubin, P.L.; Xia, J. Effects of protein charge heterogeneity in protein-polyelectrolyte complexation. Macromolecules 1992, 25, 290–295. [Google Scholar] [CrossRef]
- Mattison, K.W.; Dubin, P.L.; Brittain, I.J. Complex formation between bovine serum albumin and strong polyelectrolytes: Effect of polymer charge density. J. Phys. Chem. B 1998, 102, 3830–3836. [Google Scholar] [CrossRef] [Green Version]
- Kaibara, K.; Okazaki, T.; Bohidar, H.; Dubin, P. pH-induced coacervation in complexes of bovine serum albumin and cationic polyelectrolytes. Biomacromolecules 2000, 1, 100–107. [Google Scholar] [CrossRef]
- Antonov, M.; Mazzawi, M.; Dubin, P.L. Entering and exiting the protein- polyelectrolyte coacervate phase via nonmonotonic salt dependence of critical conditions. Biomacromolecules 2010, 11, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Asimakopoulos, T.; Staikos, G. Complexation of bovine serum albumin with cationic polyelectrolytes at pH 7.40—Formation of soluble complexes. Eur. Polym. J. 2015, 71, 567–574. [Google Scholar] [CrossRef]
- Boeris, V.; Farruggia, B.; Picó, G. Chitosan–bovine serum albumin complex formation: A model to design an enzyme isolation method by polyelectrolyte precipitation. J. Chromatogr. B 2010, 878, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Seyrek, E.; Dubin, P.L.; Tribet, C.; Gamble, E.A. Ionic strength dependence of protein-polyelectrolyte interactions. Biomacromolecules 2003, 4, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Jiang, F. Complexation of bovine serum albumin and sugar beet pectin: Stabilising oil-in-water emulsions. J. Colloid Interface Sci. 2012, 388, 103–111. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Stoleru, E.; Diaconu, A.; Tudorachi, N. Tailorable polyelectrolyte protein complex based on poly(aspartic acid) and bovine serum albumin. Des. Monomers Polym. 2016, 19, 596–606. [Google Scholar] [CrossRef] [Green Version]
- Chodankar, S.; Aswal, V.; Kohlbrecher, J.; Vavrin, R.; Wagh, A. Structural study of coacervation in protein-polyelectrolyte complexes. Phys. Rev. E 2008, 78, 031913. [Google Scholar] [CrossRef] [Green Version]
- Milyaeva, O.Y.; Lin, S.Y.; Noskov, B. Influence of sodium polystyrene sulfonate on dynamic surface properties of bovine serum albumin solutions. Colloid J. 2014, 76, 459–464. [Google Scholar] [CrossRef]
- Dautzenberg, H. Polyelectrolyte Complex Formation in Highly Aggregating Systems. 1. Effect of Salt: Polyelectrolyte Complex Formation in the Presence of NaCl. Macromolecules 1997, 30, 7810–7815. [Google Scholar] [CrossRef]
- Spruijt, E. Strength, Structure and Stability of Polyelectrolyte Complex Coacervates; Wageningen University: Wageningen, The Netherlands, 2012. [Google Scholar]
- Atha, D.H.; Ingham, K.C. Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume. J. Biol. Chem. 1981, 256, 12108–12117. [Google Scholar] [CrossRef]
- Kamerzell, T.J.; Esfandiary, R.; Joshi, S.B.; Middaugh, C.R.; Volkin, D.B. Protein–excipient interactions: Mechanisms and biophysical characterization applied to protein formulation development. Adv. Drug Deliv. Rev. 2011, 63, 1118–1159. [Google Scholar] [CrossRef]
- Lai, J.j.; Yan, H.y.; Liu, Y.; Huang, Y. Effects of PEG molecular weight on its interaction with albumin. Chin. J. Polym. Sci. 2015, 33, 1373–1379. [Google Scholar] [CrossRef]
- Rawat, S.; Suri, C.R.; Sahoo, D.K. Molecular mechanism of polyethylene glycol mediated stabilization of protein. Biochem. Biophys. Res. Commun. 2010, 392, 561–566. [Google Scholar] [CrossRef]
- Imberti, S.; McLain, S.E.; Rhys, N.H.; Bruni, F.; Ricci, M.A. Role of Water in Sucrose, Lactose, and Sucralose Taste: The Sweeter, The Wetter? ACS Omega 2019, 4, 22392–22398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowe, J.H.; Crowe, L.M.; Oliver, A.E.; Tsvetkova, N.; Wolkers, W.; Tablin, F. The trehalose myth revisited: Introduction to a symposium on stabilization of cells in the dry state. Cryobiology 2001, 43, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Mimura, M.; Tsumura, K.; Matsuda, A.; Akatsuka, N.; Shiraki, K. Effect of additives on liquid droplet of protein–polyelectrolyte complex for high-concentration formulations. J. Chem. Phys. 2019, 150, 064903. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Annunziata, O. Comparison between Protein-Polyethylene Glycol (PEG) Interactions and the Effect of PEG on Protein-Protein Interactions Using the Liquid-Liquid Phase Transition. J. Phys. Chem. B 2007, 111, 1222–1230. [Google Scholar] [CrossRef] [Green Version]
- Aune, K.C.; Tanford, C. Thermodynamics of the denaturation of lysozyme by guanidine hydrochloride. I. Dependence on pH at 25 °C. Biochemistry 1969, 8, 4579–4585. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, J.; Matsuoka, H.; Kitano, H.; Hasegawa, M.; Ise, N. Revisit to the intrinsic viscosity-molecular weight relationship of ionic polymers. 2. Viscosity behavior of salt-free aqueous solutions of sodium poly(styrenesulfonates). J. Am. Chem. Soc. 1990, 112, 587–592. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Berendsen, H.J.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Schmid, N.; Eichenberger, A.P.; Choutko, A.; Riniker, S.; Winger, M.; Mark, A.E.; van Gunsteren, W.F. Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur. Biophys. J. 2011, 40, 843–856. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Simončič, M.; Lukšič, M. Mechanistic differences in the effects of sucrose and sucralose on the phase stability of lysozyme solutions. J. Mol. Liq. 2021, 326, 115245. [Google Scholar] [CrossRef] [PubMed]
- Bujacz, A. Structures of bovine, equine and leporine serum albumin. Acta Crystallogr. D 2012, 68, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.H.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent treatment of internal and surface residues in empirical pKa predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Salis, A.; Boström, M.; Medda, L.; Cugia, F.; Barse, B.; Parsons, D.F.; Ninham, B.W.; Monduzzi, M. Measurements and theoretical interpretation of points of zero charge/potential of BSA protein. Langmuir 2011, 27, 11597–11604. [Google Scholar] [CrossRef]
- Weinbreck, F.; De Vries, R.; Schrooyen, P.; De Kruif, C. Complex coacervation of whey proteins and gum arabic. Biomacromolecules 2003, 4, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Low, N.H.; Nickerson, M.T. Effect of pH, salt, and biopolymer ratio on the formation of pea protein isolate-gum arabic complexes. J. Agric. Food Chem. 2009, 57, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Klemmer, K.; Waldner, L.; Stone, A.; Low, N.; Nickerson, M. Complex coacervation of pea protein isolate and alginate polysaccharides. Food Chem. 2012, 130, 710–715. [Google Scholar] [CrossRef]
- Gummel, J.; Boué, F.; Clemens, D.; Cousin, F. Finite size and inner structure controlled by electrostatic screening in globular complexes of proteins and polyelectrolytes. Soft Matter 2008, 4, 1653–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhao, Q.; Huang, Q. Understanding complex coacervation in serum albumin and pectin mixtures using a combination of the Boltzmann equation and Monte Carlo simulation. Carbohydr. Polym. 2014, 101, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Srivastava, S.; Andreev, M.; Marciel, A.B.; de Pablo, J.J.; Tirrell, M.V. Phase behavior and salt partitioning in polyelectrolyte complex coacervates. Macromolecules 2018, 51, 2988–2995. [Google Scholar] [CrossRef]
- Fu, J.; Schlenoff, J.B. Driving forces for oppositely charged polyion association in aqueous solutions: Enthalpic, entropic, but not electrostatic. J. Am. Chem. Soc. 2016, 138, 980–990. [Google Scholar] [CrossRef]
- Janc, T.; Vlachy, V.; Lukšič, M. Calorimetric studies of interactions between low molecular weight salts and bovine serum albumin in water at pH values below and above the isoionic point. J. Mol. Liq. 2018, 270, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Džudžević-Čančar, H.; Vivod, M.B.; Vlachy, V.; Lukšič, M. Phase stability of aqueous mixtures of bovine serum albumin with low molecular mass salts in presence of polyethylene glycol. J. Mol. Liq. 2022, 349, 118477. [Google Scholar] [CrossRef]
- Feldötö, Z.; Varga, I.; Blomberg, E. Influence of salt and rinsing protocol on the structure of PAH/PSS polyelectrolyte multilayers. Langmuir 2010, 26, 17048–17057. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.E.; Zastrow, H.; Jaeger, W.; von Klitzing, R. Specific ion versus electrostatic effects on the construction of polyelectrolyte multilayers. Langmuir 2009, 25, 14061–14070. [Google Scholar] [CrossRef]
- Salomäki, M.; Tervasmäki, P.; Areva, S.; Kankare, J. The Hofmeister anion effect and the growth of polyelectrolyte multilayers. Langmuir 2004, 20, 3679–3683. [Google Scholar] [CrossRef]
- Collins, K.D. Charge density-dependent strength of hydration and biological structure. Biophys. J. 1997, 72, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Collins, K.D. The behavior of ions in water is controlled by their water affinity. Q. Rev. Biophys. 2019, 52, e11. [Google Scholar] [CrossRef] [PubMed]
- Brini, E.; Fennell, C.J.; Fernandez-Serra, M.; Hribar-Lee, B.; Lukšič, M.; Dill, K.A. How water’s properties are encoded in its molecular structure and energies. Chem. Rev. 2017, 117, 12385–12414. [Google Scholar] [CrossRef] [Green Version]
- Schwierz, N.; Horinek, D.; Sivan, U.; Netz, R. Reversed Hofmeister series—The rule rather than the exception. Curr. Opin. Colloid Interface Sci. 2016, 23, 10–18. [Google Scholar] [CrossRef]
- Asakura, S.; Oosawa, F. On Interaction between Two Bodies Immersed in a Solution of Macromolecules. J. Chem. Phys. 1954, 22, 1255–1257. [Google Scholar] [CrossRef]
- Chen, W.Y.; Hsu, M.Y.; Tsai, C.W.; Chang, Y.; Ruaan, R.C.; Kao, W.H.; Huang, E.W.; Chung Chuan, H.Y.T. Kosmotrope-like hydration behavior of polyethylene glycol from microcalorimetry and binding isotherm measurements. Langmuir 2013, 29, 4259–4265. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Barnes, R.; Lin, Y.; Jeon, B.j.; Najafi, S.; Delaney, K.T.; Fredrickson, G.H.; Shea, J.E.; Hwang, D.S.; Han, S. Dehydration entropy drives liquid-liquid phase separation by molecular crowding. Commun. Chem. 2020, 3, 83. [Google Scholar] [CrossRef]
- Marianelli, A.M.; Miller, B.M.; Keating, C.D. Impact of macromolecular crowding on RNA/spermine complex coacervation and oligonucleotide compartmentalization. Soft Matter 2018, 14, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.M.; Chatterjee, A.P.; Schweizer, K.S.; Zukoski, C.F. Effects of polyethylene glycol on protein interactions. J. Chem. Phys. 2000, 113, 9863–9873. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, C.; Lin, W.; Hu, R.; Wang, Q.; Chen, H.; Li, L.; Chen, S.; Zheng, J. Binding characteristics between polyethylene glycol (PEG) and proteins in aqueous solution. J. Mater. Chem. B 2014, 2, 2983–2992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, S.; Wang, Y.; Fei, Z.; Cao, J. Binding mechanism of five typical sweeteners with bovine serum albumin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 205, 40–47. [Google Scholar] [CrossRef] [PubMed]


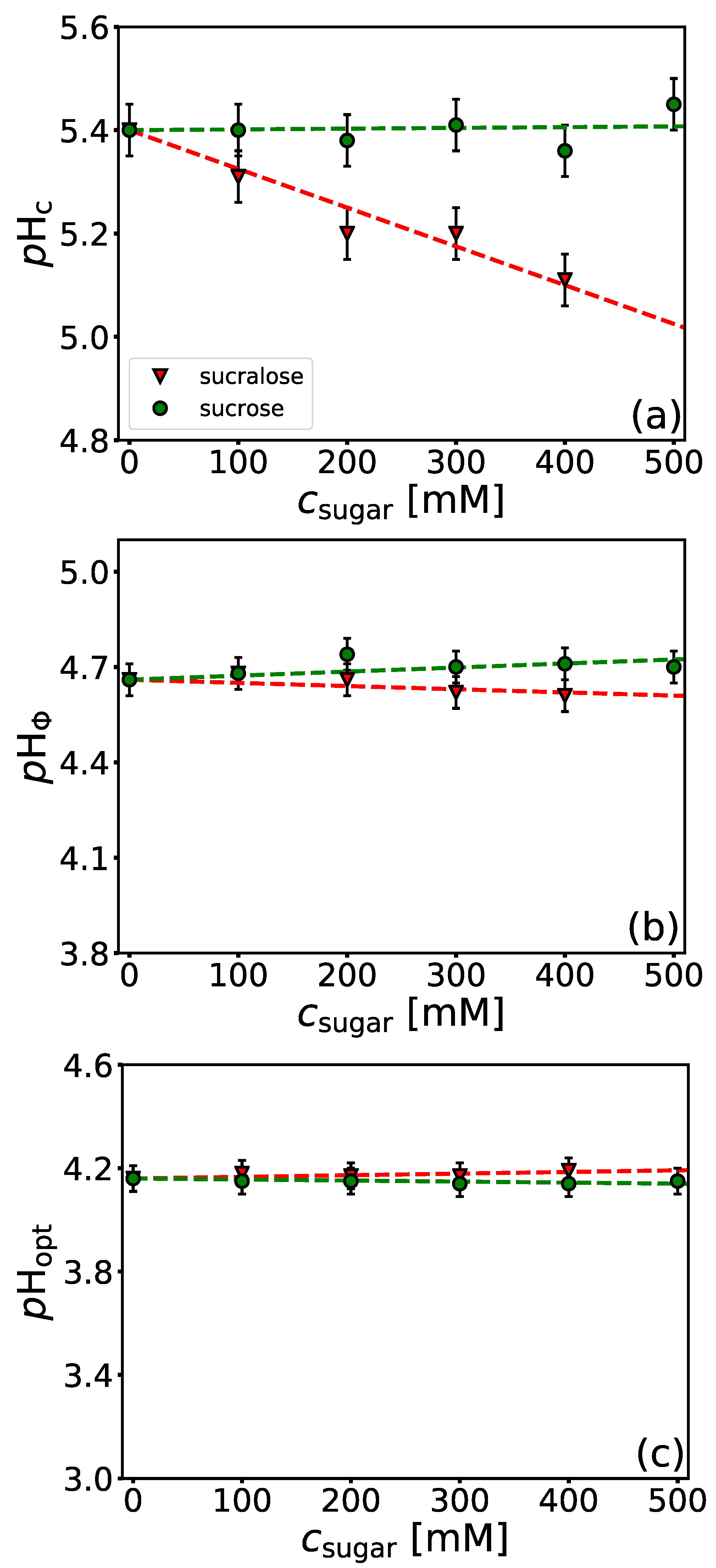
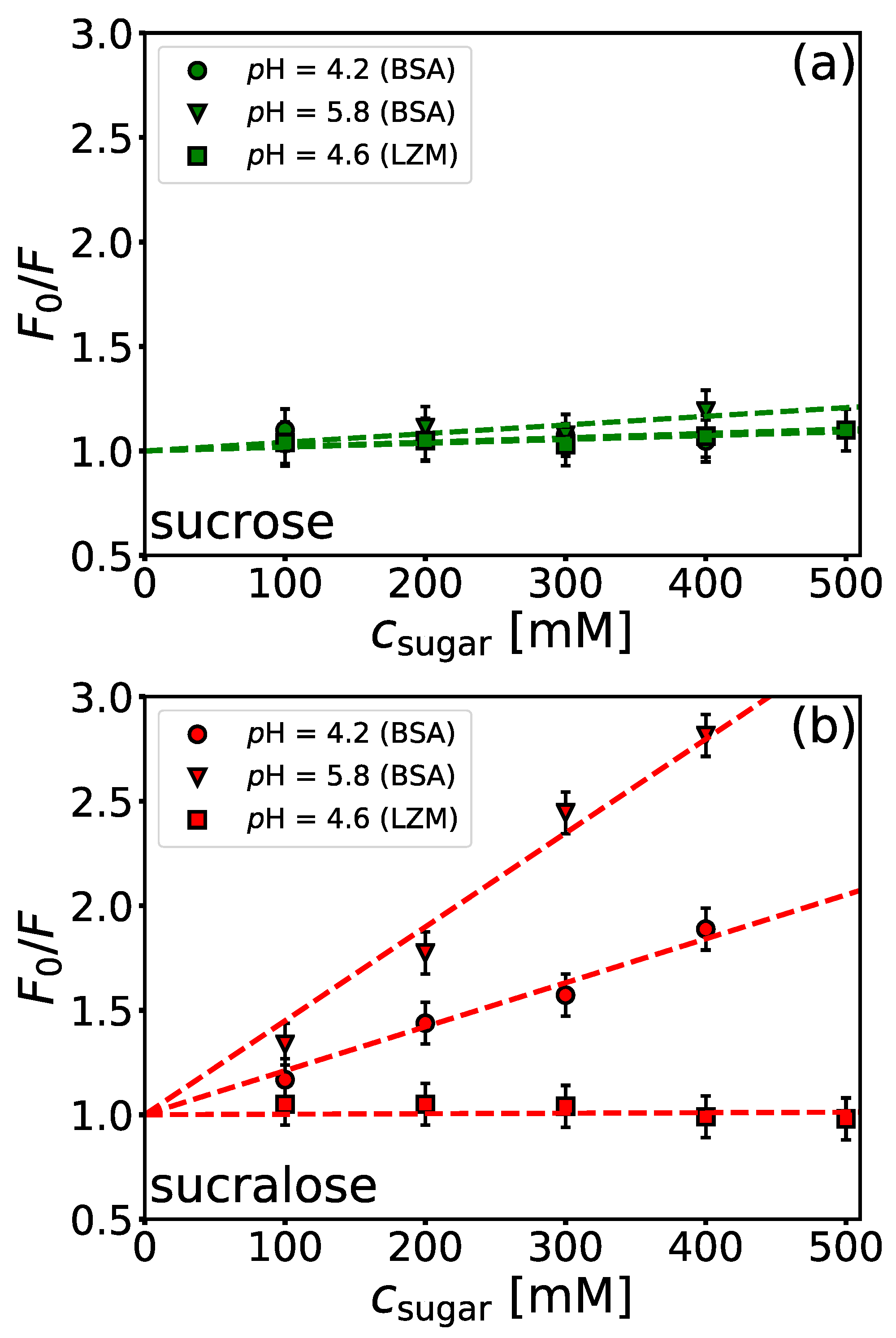
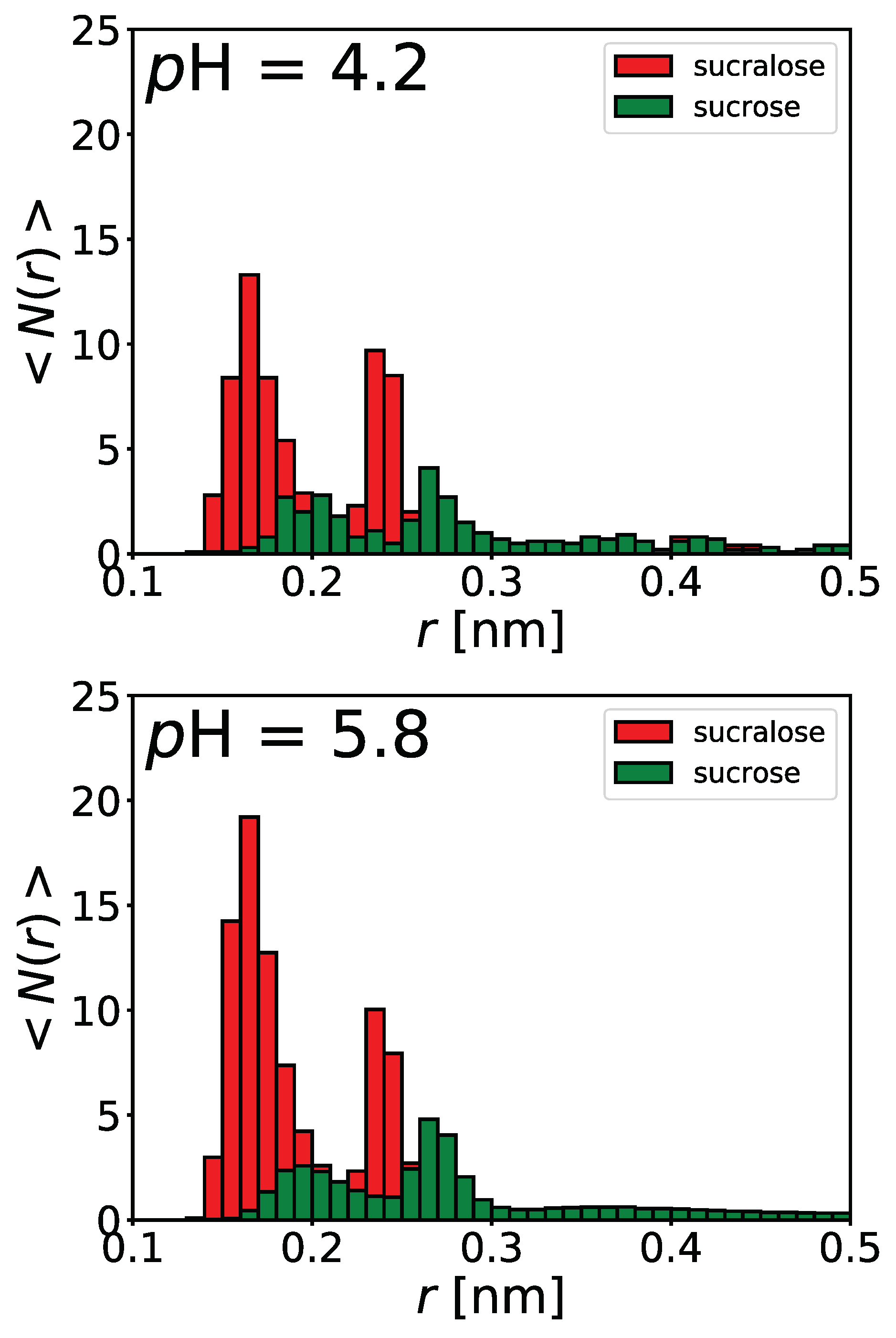
| Co-Solute | pH = 4.2 | pH = 5.8 |
|---|---|---|
| PEG-3000 | 0.15 ± 0.08 | 0.15 ± 0.08 |
| PEG-20000 | 0.22 ± 0.06 | 0.17 ± 0.03 |
| BSA | BSA | LZM | |
|---|---|---|---|
| Co-Solute | H = 4.2 | H = 5.8 | H = 4.6 |
| sucrose | 0.21 ± 0.09 | 0.41 ± 0.07 | 0.18 ± 0.02 |
| sucralose | 2.10 ± 0.09 | 4.5 ± 0.2 | 0.02 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simončič, M.; Lukšič, M. Modulating Role of Co-Solutes in Complexation between Bovine Serum Albumin and Sodium Polystyrene Sulfonate. Polymers 2022, 14, 1245. https://doi.org/10.3390/polym14061245
Simončič M, Lukšič M. Modulating Role of Co-Solutes in Complexation between Bovine Serum Albumin and Sodium Polystyrene Sulfonate. Polymers. 2022; 14(6):1245. https://doi.org/10.3390/polym14061245
Chicago/Turabian StyleSimončič, Matjaž, and Miha Lukšič. 2022. "Modulating Role of Co-Solutes in Complexation between Bovine Serum Albumin and Sodium Polystyrene Sulfonate" Polymers 14, no. 6: 1245. https://doi.org/10.3390/polym14061245
APA StyleSimončič, M., & Lukšič, M. (2022). Modulating Role of Co-Solutes in Complexation between Bovine Serum Albumin and Sodium Polystyrene Sulfonate. Polymers, 14(6), 1245. https://doi.org/10.3390/polym14061245