Synthesis of Alginate Nanogels with Polyvalent 3D Transition Metal Cations: Applications in Urease Immobilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Alginate Nanogels and Encapsulation of Urease Enzyme
2.2. Characterization
2.2.1. Size Determination and Surface Charge Studies
2.2.2. SEM-EDX Measurement
2.2.3. FT-IR Spectral Study
2.3. Enzyme Assay
2.4. Steady-State Kinetics
2.5. Storage and Stability Studies of Urease in Nanogels
3. Results and discussion
3.1. Alginate Nanogels by Emulsification Method
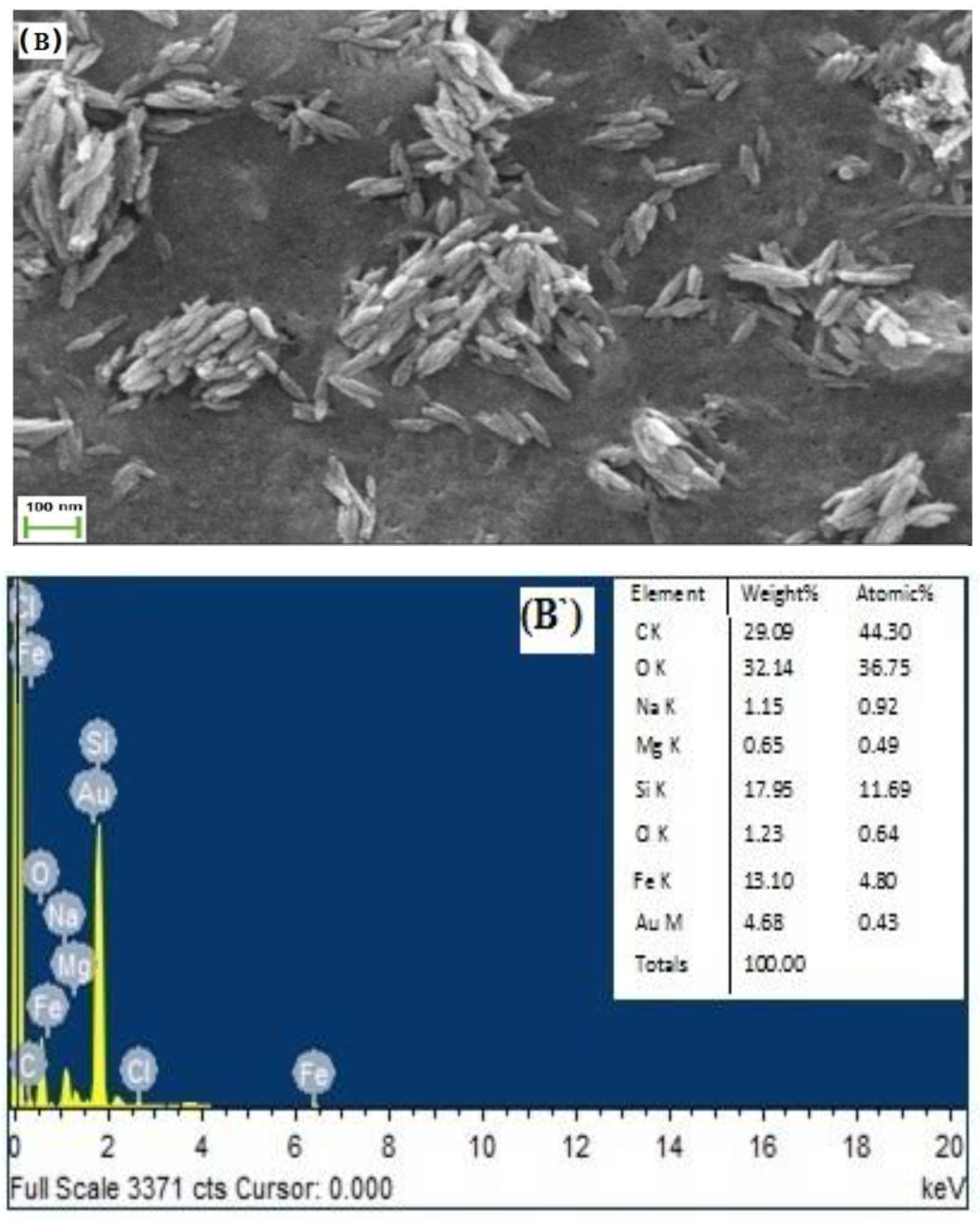
3.2. Morphology Analysis by SEM& EDX Studies
3.3. Characterization of Alginate Nanogels Cross-Linked with Polyvalent Cations (Mn, Fe, or Co) by FT-IR Studies
3.4. Surface Charge and Stability
3.5. Enzyme Assay, Using Urease Enzyme Encapsulated in Alginate Nanogels
3.5.1. pH Based Stability Studies
3.5.2. Steady-State Kinetics
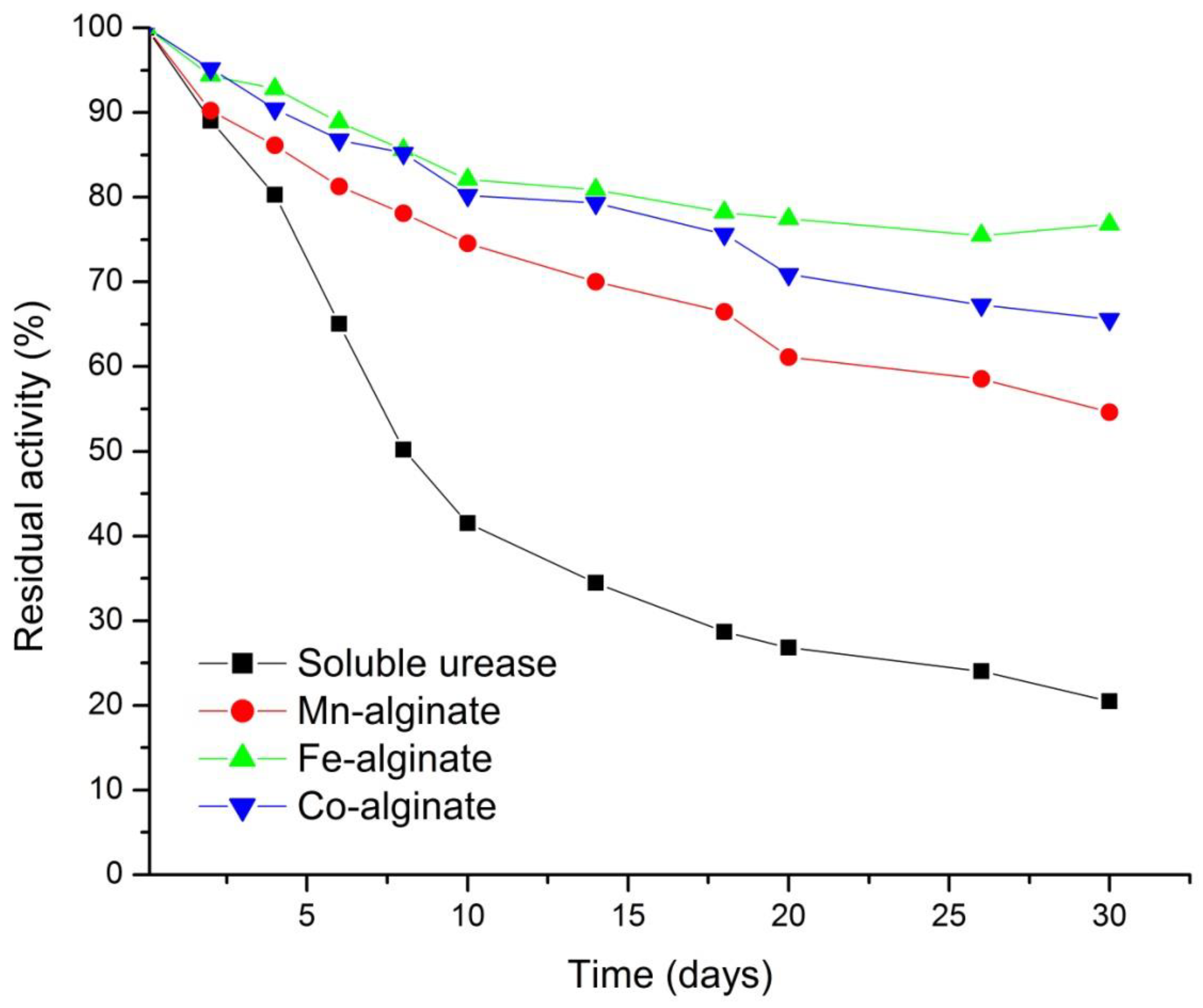
3.5.3. Storage Stability of Urease in Nanogel
3.6. Analytical Application of the Fe–Alginate Nanogels
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinsen, A.; Skjåk-Braek, G.; Smidsrød, O. Alginate as immobilization material: I. Correlation between chemical and physical properties of alginate gel beads. Biotechnol. Bioeng. 1989, 33, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lacík, I.; Brissová, M.; Anilkumar, A.V.; Prokop, A.; Hunkeler, D.; Green, R.; Shahrokhi, K.; Powers, A.C. An encapsulation system for the immunoisolation of pancreatic islets. Nat. Biotechnol. 1997, 15, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Lee, J.-H.; Huh, Y.-S.; Takayama, S. Alginate Microencapsulation for Three-dimensional in vitro Cell Culture. ACS Biomater. Sci. Eng. 2021, 7, 2864–2879. [Google Scholar] [CrossRef]
- Piras, C.C.; Smith, D.K. Multicomponent polysaccharide alginate-based bioinks. J. Mater. Chem. B 2020, 8, 8171–8188. [Google Scholar] [CrossRef] [PubMed]
- Mizrahy, S.; Peer, D. Polysaccharides as building blocks for nanotherapeutics. Chem. Soc. Rev. 2012, 41, 2623–2640. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rodrigues, J.; Tomás, J. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef]
- Kupikowska-Stobba, B.; Lewińska, D. Polymer microcapsules and microbeads as cell carriers for in vivo biomedical applications. Biomater. Sci. 2020, 8, 1536–1574. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorptionbybrownalgae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Hong, H.-J.; Park, I.-S.; Ryu, T.; Jeong, H.S.; Ryu, J. Demonstration of Seawater Strontium (Sr(II)) Extraction and Enrichment by a Biosorption Technique through Continuous Column Operation. Ind. Eng. Chem. Res. 2018, 57, 38. [Google Scholar] [CrossRef]
- Cabodi, M.; Choi, N.W.; Gleghorn, J.P.; Lee, C.S.D.; Bonassar, L.J.; Stroock, A.D. A Microfluidic Biomaterial. J. Am. Chem. Soc. 2005, 127, 13788–13789. [Google Scholar] [CrossRef]
- Navrátil, M.; Gemeiner, P.; Klein, P.; Šturdík, E.; Malovíková, A.; Nahálka, J.; Vikartovská, A.; Dömény, Z.; Šmogrovičová, D. Properties of hydrogel materials used for entrapment of microbial cells in production of fermented beverages. Artif. Cells Blood Substit. Immobil. Biotechnol. 2009, 30, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, S.; Dong, Y.; Zhi, H.; Zong, W. Tea polyphenols incorporated into alginate-based edible coating for quality maintenance of Chinese winter jujube under ambienttemperature. LWT 2016, 70, 155–161. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Paques, J.P.; Linden, E.; Rijn, C.J.M.; Sagis, L.M.C. Preparation methods of alginate nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Jeoh, T.; Wong, D.E.; Strobel, S.A.; Hudnall, K.; Pereira, N.R.; Williams, K.A.; Arbaugh, B.M.; Cunniffe, J.C.; Scher, H.B. How alginate properties influence in situ internal gelation in crosslinked alginate microcapsules (CLAMs) formed by spray drying. PLoS ONE 2021, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brus, J.; Urbanova, M.; Czernek, J.; Pavelkova, M.; Kubova, K.; Vyslouzil, J.; Abbrent, S.; Konefal, R.; Horský, J.; Vetchy, D.; et al. Structure and Dynamics of Alginate Gels Cross-Linked by Polyvalent Ions Probed via Solid State NMR Spectroscopy. Biomacromolecules 2017, 18, 2478–2488. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Smidsrød, O. Selectivity of some anionic polymers for divalent metal ions. Acta Chem. Scand. 1970, 24, 843–854. [Google Scholar] [CrossRef]
- Haug, A. The affinity of some divalent metals for different types of alginates. Acta Chem. Scand. 1961, 15, 1794–1795. [Google Scholar] [CrossRef]
- Mørch, Y.A.; Donati, I.; Strand, B.L.; Skjåk-Bræk, G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef]
- Mørch, Y.A.; Sandvig, I.; Olsenc, Ø.; Donati, I.; Thuen, M.; Skjåk-Bræk, G.; Haraldseth, O.; Brekken, C. Mn-alginate gels as a novel system for controlled release of Mn2+ in manganeseenhanced MRI. Contrast Media Mol. Imaging 2011, 7, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Hecht, H.; Srebnik, S. Structural characterization of sodium alginate and calcium alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Agulhon, P.; Markova, V.; Robitzer, M.; Quignard, F.; Mineva, T. Structure of alginate gels: Interaction of diuronate units with divalent cations from density functional calculations. Biomacromolecules 2012, 13, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Poncet-Legrand, C.; Somers, S.; Slade, A.; Yip, C.; Duft, A.M.; Winnik, F.M.; Chang, P.L. Properties of a Novel Magnetized Alginate for Magnetic Resonance Imaging. Biotechnol. Bioeng. 2003, 83, 282–292. [Google Scholar] [CrossRef]
- Winkleman, A.; Bracher, P.J.; Gitlin, I.; Whitesides, G.M. Fabrication and Manipulation of Ionotropic Hydrogels Cross-Linked by Paramagnetic Ion. Chem. Mater. 2007, 19, 1362–1368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Machida-Sano, I.; Ogawa, S.; Ueda, H.; Kimura, Y.; Satoh, N.; Namiki, H. Effects of Composition of Iron-Cross-Linked Alginate Hydrogels for Cultivation of Human Dermal Fibroblasts. Int. J. Biomater. 2012, 2012, 820513. [Google Scholar] [CrossRef]
- Kang, I.-K.; Moon, J.-K.; Jeon, H.M.; Meng, W.; Kim, Y.I.; Hwang, Y.J.; Kim, S. Morphology and metabolism of Ba-alginate encapsulated hepatocytes with galactosylated poly(allyl amine) and poly(vinyl alcohol) as extracellular matrices. J. Mater. Sci. Mater. Med. 2005, 16, 533–539. [Google Scholar] [CrossRef]
- Widerøe, H.; Danielsen, S. Evaluation of the use of Sr2+ in alginate immobilization of cells. Naturwissenschaften 2001, 88, 224–228. [Google Scholar]
- Larsen, B.E.; Bjørnstad, J.; Pettersen, E.O.; Tønnesen, H.H.; Melvik, J.E. Rheological characterization of an injectable alginate gel system. BMC Biotechnol. 2015, 15, 29. [Google Scholar] [CrossRef]
- Giammanco, G.E.; Sosnofsky, C.T.; Ostrowski, A.D. Light-Responsive Iron(III)−Polysaccharide Coordination Hydrogels for Controlled Delivery. ACS Appl. Mater. Interfaces 2015, 7, 3068–3076. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Coradin, T.; Fie’vet-Vincent, F.; Livage, J.; Fie’vet, F. Algal polysaccharide capsule-templated growth of magnetic Nanoparticles. New J. Chem. 2005, 29, 681–685. [Google Scholar] [CrossRef]
- Tafjord, J.; Rytter, E.; Holmen, A.; Myrstad, R.; Svenum, I.-H.; Christensen, B.E.; Yang, J. Transition-metal nanoparticle catalysts anchored on carbon supports via short-chain alginate linkers. ACS Appl. Nano Mater. 2021, 4, 3900–3910. [Google Scholar] [CrossRef]
- Ma, H.-L.; Qi, X.-R.; Maitani, Y.; Nagai, T. Preparation and characterization of superparamagnetic iron oxide nanoparticles stabilized by alginate. Int. J. Pharm. 2007, 333, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Horniblow, R.; Dowle, M.; Iqbal, T.H.; Latunde-Dada, G.O.; Palmer, R.E.; Pikramenou, Z.; Tselepis, C. Alginate-Iron Speciation and Its Effect on In Vitro Cellular Iron Metabolism. PLoS ONE 2015, 10, e0138240. [Google Scholar] [CrossRef]
- Johnson, A.S.; O’Sullivan, E.; D’Aoust, L.N.; Omer, A.; Bonner-Weir, S.; Fisher, R.J.; Weir, G.C.; Colton, C.K. Quantitative assessment of islets of Langerhans encapsulated in alginate. Tissue Eng. Part C Methods 2011, 17, 435–449. [Google Scholar] [CrossRef]
- Focaroli, S.; Teti, G.; Salvatore, V.; Orienti, I.; Falconi, M. Calcium/Cobalt Alginate Beads as Functional Scaffolds for Cartilage Tissue Engineering. Stem Cells Int. 2016, 2016, 2030478. [Google Scholar] [CrossRef]
- Chuang, J.-J.; Huang, Y.-Y.; Lo, S.-H.; Hsu, T.-F.; Huang, W.-Y.; Huang, S.-H.; Lin, Y.-S. Effects of pH on the Shape of Alginate Particles and Its Release Behavior. Int. J. Polym. Sci. 2017, 2017, 3902704. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-induced gelation of alginate: Mechanism and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Kerman, K.; Saito, M.; Tamiya, E.; Yamamura, S.; Takamura, Y. Nanomaterial based electrochemical biosensors for medical applications. TrAC Trends Anal. Chem. 2008, 27, 585–592. [Google Scholar] [CrossRef]
- Niemeyer, C.M. Nanoparticles, Proteins, and Nucleic Acids: Biotechnology Meets Materials Science. Angew. Chem. Int. Ed. 2001, 40, 4128–4158. [Google Scholar] [CrossRef]
- De, S.; Robinson, D. Polymer relationships during preparation of chitosan-alginate and poly-L-lysine-alginate nanospheres. J. Control Release 2003, 89, 101–112. [Google Scholar] [CrossRef]
- Palivan, C.G.; Onaca, O.F.; Delcea, M.; Itel, F.; Meier, W. Protein–polymer nanoreactors for medical applications. Chem. Soc. Rev. 2012, 41, 2800–2823. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Wei, H.; Zhang, Q.; Zhang, X.Z.; Cheng, S.X.; Zhuo, R.X. Effect of Ions on the Aggregation Behavior of Natural Polymer Alginate. J. Phy. Chem. B 2009, 113, 14839–14843. [Google Scholar] [CrossRef] [PubMed]
- Lertsutthiwong, P.; Noomun, K.; Jongaroonngamsang, N.; Rojsitthisak, P.; Nimmannit, U. Preparation of alginate nanocapsules containing turmeric oil. Carbohydr. Polym. 2008, 74, 209–214. [Google Scholar] [CrossRef]
- Azevedo, M.A.; Bourbon, A.I.; Vicente, A.A.; Cerqueira, M.A. Alginate/chitosan nanoparticles for encapsulation and controlled release of vitamin B2. Int. J. Biol. Macromol. 2014, 71, 141–146. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Jia, L.-H.; Yin, B.-C.; Zhang, X.-H.; Cheng, S.-X.; Zhuo, R.-X. Fabrication of Nanospheres and Vesicles as Drug Carriers by Self-Assembly of Alginate. J. Phys. Chem. C 2008, 112, 16774–16778. [Google Scholar] [CrossRef]
- You, J.-O.; Peng, C.-A. Calcium-Alginate Nanoparticles Formed by Reverse Microemulsion as Gene Carriers. Macromol. Symp. 2005, 219, 147–153. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and characterization of alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Katuwavila, N.P.; Perera, A.D.L.C.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A.J.; Karunaratne, D.N. Alginate nanoparticles protect ferrous from oxidation: Potential iron delivery system. Int. J. Pharm. 2016, 513, 404–409. [Google Scholar] [CrossRef]
- Xue, Y.; Xia, X.; Yu, B.; Luo, X.; Cai, N.; Long, S.; Yu, F. A green and facile method for the preparation of a pH-responsive alginate nanogel for subcellular delivery of doxorubicin. RSC Adv. 2015, 5, 73416–73423. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.W.; Han, E.J.; Ahn, G. Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem. Eng. Sci. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Bazban-Shotorbani, S.; Dashtimoghadam, E.; Karkhaneh, A.; Hasani-Sadrabadi, M.M.; Jacob, K.I. Microfluidic Directed Synthesis of Alginate Nanogels with Tunable Pore Size for Efficient Protein Delivery. Langmuir 2016, 32, 4996–5003. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.P.; Munnier, E.; Souce1, M.; Perse, X.; David, S.; Bonnier, F.; Vial, F.; Yvergnaux, F.; Perrier, T.; Cohen-Jonathan, S.; et al. Novel alginate-based nanocarriers as a strategy to include high concentrations of hydrophobic compounds in hydrogels for topical application. Nanotechnology 2015, 26, 255101. [Google Scholar] [CrossRef] [PubMed]
- Choukaife, H.; Doolaanea, A.A.; Alfatama, M. Alginate Nanoformulation: Influence of Process and Selected Variables. Pharmaceuticals 2020, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Bhattacharya, A.; Kumar, S.; Epstein, I.R.; Sahney, R. Biopolymer matrix for nano-encapsulation of urease- a model protein and its application in urea detection. J. Colloid Interface Sci. 2017, 490, 452–461. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; He, C.; Liu, X.; Zhao, W.; Sun, S.; Zhao, C. Safe and Effective Removal of Urea by Urease-Immobilized, Carboxyl-Functionalized PES Beads with Good Reusability and Storage Stability. ACS Omega 2019, 4, 2853–2862. [Google Scholar] [CrossRef]
- Arqué, X.; Romero-Rivera, A.; Feixas, F.; Patiño, T.; Osuna, S.; Sánchez, S. Intrinsic enzymatic properties modulate the self-propulsion of micromotors. Nat. Commun. 2019, 10, 2826. [Google Scholar] [CrossRef]
- Panja, S.; Adams, D.J. Urea-Urease Reaction in Controlling Properties of Supramolecular Hydrogels: Pros and Cons. Chem. Eur. J. 2021, 27, 8928–8939. [Google Scholar] [CrossRef] [PubMed]
- Andreescu, S.; Njagi, J.; Ispas, C. Nanostructured materials for enzyme immobilization and biosensors—Chapter 7. In The New Frontiers of Organic and Composite Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 355–394. [Google Scholar]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Corbett, J.C.W.; McNeil-Watson, F.; Jack, R.O.; Howarth, M. Measuring surfacezeta potential using phase analysis light scattering in a simple dip cell arrangement. Colloids Surf. A Physicochem. Eng. Asp. 2012, 396, 169–176. [Google Scholar] [CrossRef]
- Kumar, S.; Dwevedi, A.; Kayastha, A.M. Immobilization of soybean (Glycine max) urease on alginate and chitosan beads showing improved stability: Analytical applications. J. Mol. Catal. B Enzym. 2009, 58, 138–145. [Google Scholar] [CrossRef]
- Pignolet, L.H.; Waldman, A.D.; Schechinger, L.; Govindarajoo, G.; Nowick, J.S. The alginate demonstration: Polymers, food science, and ion exchange. J. Chem. Educ. 1998, 75, 1430. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hulteen, J.C.; Martin, C.R. A general template-based method for the preparation of nanomaterials. J. Mater. Chem. 1997, 7, 1075–1087. [Google Scholar] [CrossRef]
- Liu, Y.; Goebl, J.; Yin, Y. Templated synthesis of nanostructured materials. Chem. Soc. Rev. 2013, 42, 2610. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kocaefe, D.; Chen, C.; Kocaefe, Y. Review of Research on Template Methods in Preparation of Nanomaterials. J. Nanomater. 2016, 2016, 2302595. [Google Scholar] [CrossRef]
- Correa, N.M.; Silber, J.J.; Riter, R.E.; Levinger, N.E. Nonaqueous Polar Solvents in Reverse Micelle Systems. Chem. Rev. 2012, 112, 8–4569. [Google Scholar] [CrossRef]
- Hoar, T.P.; Schulman, J.H. Transparent water-in-oil dispersions: The oleopathic hydro-micelle. Nature 1943, 152, 102–103. [Google Scholar] [CrossRef]
- Langevin, D. Microemulsions—Interfacial aspects. Adv. Colloid Interface Sci. 1991, 34, 583–595. [Google Scholar] [CrossRef]
- Pileni, M.P. Reverse micelles: A microreactor. J. Phys. Chem. 1993, 97, 9661–9668. [Google Scholar] [CrossRef]
- Pileni, M.P. Nanosized particles made in colloidal assemblies. Langmuir 1997, 13, 3266–3276. [Google Scholar] [CrossRef]
- Sanchez-Dominguez, M.; Pemartin, K.; Boutonnet, M. Preparation of inorganic nanoparticles in oil-in-water microemulsions: A soft and versatile approach. Curr. Opin. Colloid Interface Sci. 2012, 17, 297–305. [Google Scholar] [CrossRef]
- Peltonen, L.; Yliruusi, J. Surface Pressure, Hysteresis, Interfacial Tension, and CMC of Four Sorbitan Monoesters at Water–Air, Water–Hexane, and Hexane–Air Interfaces. J. Colloid Interface Sci. 2000, 227, 1–6. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J.; Yliruusi, J. The Behavior of Sorbitan Surfactants at the Water–Oil Interface:Straight-Chained Hydrocarbons from Pentane to Dodecane as an Oil Phase. J. Colloid Interface Sci. 2001, 240, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Opawale, F.O.; Burgess, D.J. Influence of Interfacial Properties of Lipophilic Surfactants on Water-in-Oil Emulsion Stability. J. Colloid Interface Sci. 1998, 197, 142–150. [Google Scholar] [CrossRef]
- Coradin, T.; Nassif, N.; Livage, J. Silica-alginate composites for microencapsulation. Appl. Microbiol. Biotechnol. 2003, 61, 429–434. [Google Scholar] [CrossRef]
- Kurayama, F.; Suzuki, S.; Bahadur, N.M.; Furusawa, T.; Ota, H.; Sato, M.; Suzuki, N.N. Preparation of aminosilane-alginate hybrid microcapsules and their use for enzyme encapsulation. J. Mater. Chem. 2012, 22, 15405–15411. [Google Scholar] [CrossRef]
- Johnston, S.T.; Faria, M.; Crampin, E.J. 2018 An analytical approach for quantifying the influence of nanoparticle polydispersity on cellular delivered dose. J. R. Soc. Interface 2018, 15, 20180364. [Google Scholar] [CrossRef]
- Abou-Nemeh, I.; Bart, H.J. Microstructures in the System Water/D2EHPA/Span-80/n-Dodecane. Langmuir 1998, 14, 4451–4459. [Google Scholar] [CrossRef]
- Siladitya, B.; Chatterjee, M.; Ganguli, D. Role of organic solvents and surface active agents in the sol emulsion gel synthesis of spherical alumina powders. J. Mat. Res. 2000, 15, 176–185. [Google Scholar]
- Wedlock, D.J. Controlled Particle, Droplet and Bubble Formation; Elsevier Publication: Amsterdam, The Netherlands; Butterworth-Heinemann: Oxford, UK, 1994; pp. 91–216. ISBN 9780750614948. [Google Scholar]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Mackie, W.; Perez, S.; Rizzo, R.; Taravel, F.; Vignon, M. Aspects of the conformation of polyguluronate in the solid state and in solution. Int. J. Biol. Macromol. 1983, 5, 329–341. [Google Scholar] [CrossRef]
- Perić, L.; Pereira, C.S.; Perez, S.; Hünenberger, P.H. Conformation, dynamics and ion-binding properties of single-chain polyuronates: A molecular dynamics study. Mol. Simul. 2008, 34, 421–446. [Google Scholar] [CrossRef]
- Draget, K.I.; Skjåk-Bræk, G.; Stokke, B.T. Similarities and differences between alginic acid gels and ionically crosslinked alginate gels. Food Hydrocoll. 2006, 20, 170–175. [Google Scholar] [CrossRef]
- Agulhon, P.; Robitzer, M.; David, L.; Quignard, F. Structural Regime Identification in Ionotropic Alginate Gels: Influence of the Cation Nature and Alginate Structure. Biomacromolecules 2012, 13, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Furst, E.M. Fabrication of Unusual Asymmetric Colloids at an Oil−Water Interface. Langmuir 2010, 26, 10406–10410. [Google Scholar] [CrossRef]
- Ungphaiboon, S.; Attia, D.; d’Ayala, G.G.; Sansongsak, P.; Cellesi, F.; Tirelli, N. Materials for microencapsulation: What toroidal particles (“doughnuts”) can do better than spherical beads. Soft Matter 2010, 6, 4070–4083. [Google Scholar] [CrossRef]
- Choi, C.-H.; Lee, J.; Yoon, K.; Tripathi, A.; Stone, H.A.; Weitz, D.A.; Lee, C.-S. Surface tension induced synthesis of complex particles using confined polymeric fluids. Angew. Int. Ed. 2010, 122, 7914–7918. [Google Scholar] [CrossRef]
- Pileni, M.-P. The role of soft colloidal templates in controlling the size and shape of inorganic nanocrystals. Nat. Matter 2003, 2, 145–150. [Google Scholar] [CrossRef]
- Yoo, J.-W.; Mitragotri, S. Polymer particles that switch shape in response to a stimulus. Proc. Natl. Acad. Sci. USA 2010, 107, 11205–11210. [Google Scholar] [CrossRef]
- Kiosseoglou, V.D.; Sherman, P. The influence of egg yolk lipoproteins on the rheology and stability of O/W emulsions and mayonnaise. Colloid Polym. Sci. 1983, 261, 502–507. [Google Scholar] [CrossRef]
- Jeon, C.; Nah, I.W.; Hwang, K.-Y. Adsorption of heavy metals using magnetically modified alginic acid. Hydrometallurgy 2007, 86, 140–146. [Google Scholar] [CrossRef]
- Pathak, T.S.; Yun, J.-H.; Lee, J.; Paeng, K.-J. Effect of calcium ion (cross-linker) concentration on porosity, surface morphology and thermal behavior of calcium alginates prepared from algae (Undaria pinnatifida). Carbohydr. Polym. 2010, 81, 633–639. [Google Scholar] [CrossRef]
- Liang, H.-F.; Hong, M.-H.; Ho, R.-M.; Chung, C.-K.; Lin, Y.-H.; Chen, C.-H.; Sung, H.-W. Novel Method Using a Temperature-Sensitive Polymer (Methylcellulose) to Thermally Gel Aqueous Alginate as a pH-Sensitive Hydrogel. Biomacromolecules 2004, 5, 1917–1925. [Google Scholar] [CrossRef]
- Zhu, H.; Srivastava, R.; Brown, J.Q.; McShane, M.J. Combined Physical and Chemical Immobilization of Glucose Oxidase in Alginate Microspheres Improves Stability of Encapsulation and Activity. Bioconjugate Chem. 2005, 16, 1451–1458. [Google Scholar] [CrossRef]
- Smith, B.C. Infrared Spectral Interpretation—A Systematic Approach; CRC Press: Boca Raton, FL, USA, 1998; p. 130. [Google Scholar]
- Cantor, C.R.; Schimmel, P.R. Techniques for the Study of Biological Structure and Function. Biophysical Chemistry: Part II; WH Freeman and Co.: Oxford, UK, 1980; p. 503. [Google Scholar]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.O.; Williams, P.A. Handbook of Hydrocolloids, 2nd ed.; Woodhead Publishing: Duxford, UK, 2009; p. 948. ISBN -9781845694142. [Google Scholar]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Quong, D.; Neufeld, R.J.; Skjåk-Bræk, G.; Poncelet, D. External versus internal source of calcium during the gelation of alginate beads for DNA encapsulation. Biotechnol. Bioeng. 1998, 57, 438–446. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, S.; Dhiman, A. Acacia gum polysaccharide based hydrogel wound dressings: Synthesis, characterization, drug delivery and biomedical properties. Carbohydr. Polym. 2017, 165, 294–303. [Google Scholar] [CrossRef]
- Galante, R.; Ghisleni, D.; Paradiso, P.; Alves, V.D.; Pinto, T.J.A.; Colaço, R.; Serro, A.P. Sterilization of silicone-based hydrogels for biomedical application using ozone gas: Comparison with conventional techniques. Mater. Sci. Eng. C 2017, 78, 389–397. [Google Scholar] [CrossRef]
- Wu, J.; Eisenberg, A. Proton diffusion across membranes of vesicles of poly(styrene-b-acrylic acid) diblock copolymers. J. Am. Chem. Soc. 2006, 128, 2880–2884. [Google Scholar] [CrossRef]
- Cao, L.; Schmid, R.D. Carrier-Bound Immobilized Enzymes: Principles, Applications and Design; Wiley-VCH Publications: Hoboken, NJ, USA, 2005; p. 578. ISBN 978-3-527-31232-0. [Google Scholar]
- Hayashi, T.; Ikada, Y. Protease immobilization onto polyacrolein microspheres. Biotechnol. Bioeng. 1990, 35, 518–524. [Google Scholar] [CrossRef]
- Miyata, T.; Jikihara, A.; Nakamae, K. Permeation decomposition of urea through asymmetrically urease-immobilized ethylene-vinyl alcohol copolymer membranes. J Appl. Polym. Sci. 1997, 63, 1579–1588. [Google Scholar] [CrossRef]
- Chen, J.-P.; Chiu, S.-H. A poly(N-isopropylacrylamide-co-N-acryloxysuccinimide-co-2-hydroxyethyl methacrylate) composite hydrogel membrane for urease immobilization to enhance urea hydrolysis rate by temperature swing. Enzyme Microb. Technol. 2000, 26, 359–364. [Google Scholar] [CrossRef]
- Illanes, A. Enzyme Biocatalysis-Principles and application; Springer: Berlin/Heidelberg, Germany, 2008; pp. 162–172. ISBN 978-1-4020-8360-0. [Google Scholar]
- Howell, S.F.; Sumner, J.B. The specific effects of buffers upon urease activity. J. Biol. Chem. 1934, 104, 619–626. [Google Scholar] [CrossRef]
- Fahmy, A.S.; Bagos, V.B.; Mohammed, T.M. Immobilization of Citrullus vulgaris urease on cyanuric chloride deae-cellulose ether: Preparation and properties. Bioresour. Technol. 1998, 64, 121–129. [Google Scholar] [CrossRef]
- Fidaleo, M.; Lavecchia, R. Kinetic study of enzymatic urea hydrolysis in the pH range 4–9. Chem. Biochem. Eng. Q. 2003, 17, 311–318. [Google Scholar]
- Hoare, J.P.; Laidler, K.J. The molecular kinetics of the urea-urease system. II. The inhibition by products. J. Am. Chem. Soc. 1950, 72, 2487–2489. [Google Scholar] [CrossRef]
- Cao, S.; Xu, P.; Ma, Y.; Yao, X.; Yao, Y.; Zong, M.; Li, X.; Lou, W. Recent advances in immobilized enzymes on nanocarriers. Chin. J. Catal. 2016, 37, 1814–1823. [Google Scholar] [CrossRef]
- Yeon, K.-H.; Lueptow, R.M. Urease immobilization on an ion-exchange textile for urea hydrolysis. J. Chem. Technol. Biotechnol. 2006, 81, 940–950. [Google Scholar] [CrossRef]
- Laboratory Reference Values. Urea nitrogen (BUN). Rochester, Minn.: Mayo Foundation for Medical Education and Research. 2010. Available online: http://www.arborassays.com/wp-content/files_mf/1344286592K024H1.pdf (accessed on 3 November 2021).
- Waiker, S.S.; Bonventre, J.V. Biomarkers for the diagnosis of acute kidney injury. Nephron Clin. Pract. 2008, 109, c192–c197. [Google Scholar] [CrossRef]
- Donatan, S.; Yashchenok, A.; Khan, N.; Parakhonskiy, B.; Cocquyt, M.; Pinchasik, B.-L.; Khalenkow, D.; Möhwald, H.; Konrad, M.; Skirtach, A. Loading Capacity versus Enzyme Activity in Anisotropic and Spherical Calcium Carbonate Microparticles. ACS Appl. Mater. Interfaces 2016, 8, 14284–14292. [Google Scholar] [CrossRef]
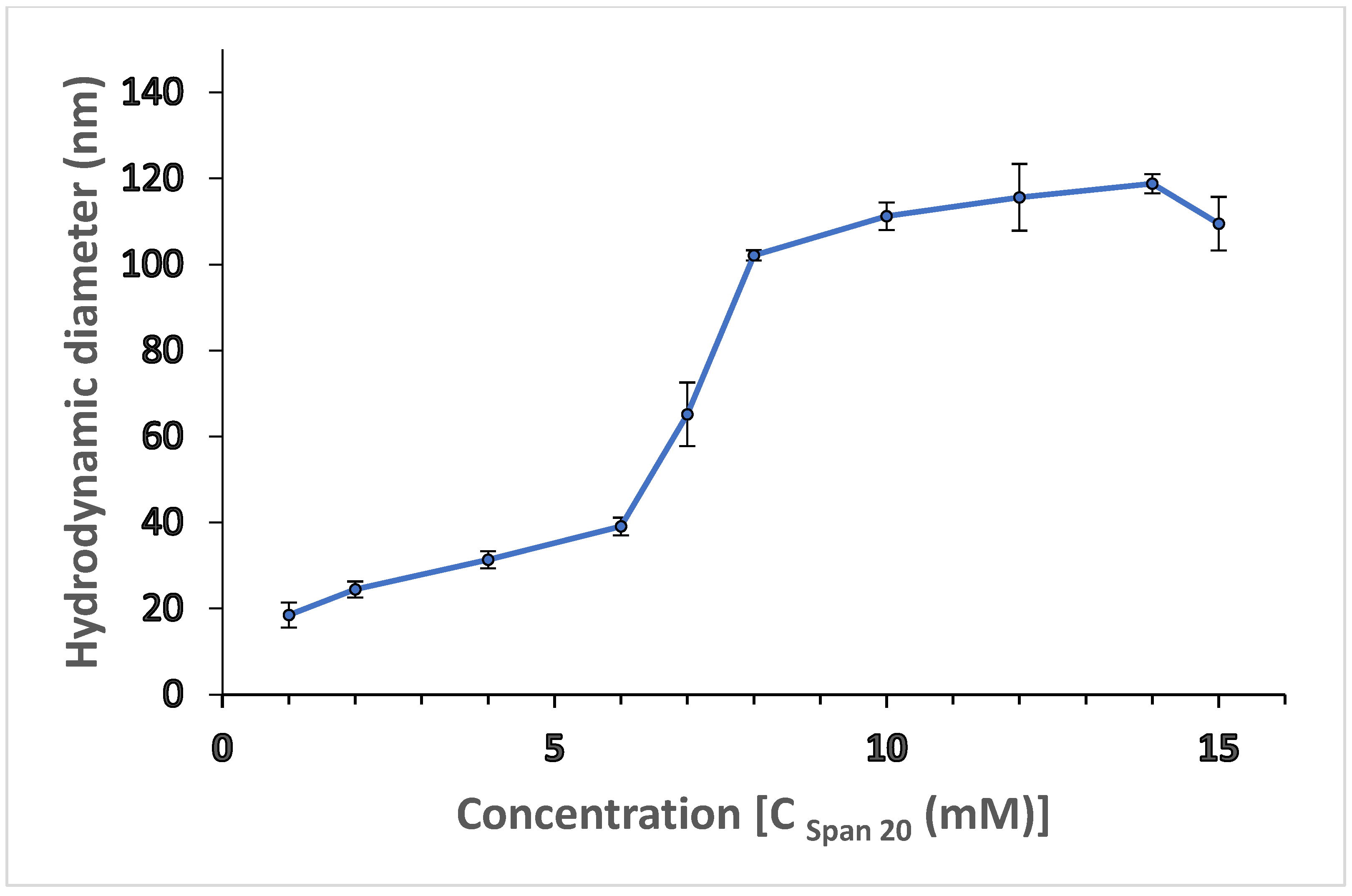
 ), circles (
), circles ( ) and triangles (
) and triangles ( ) correspond to the microemulsion regions where the micelle size varies from 110 nm to 231 nm, 431 nm to 586 nm, and 886 nm to 1163 nm, respectively.
) correspond to the microemulsion regions where the micelle size varies from 110 nm to 231 nm, 431 nm to 586 nm, and 886 nm to 1163 nm, respectively.
 ), circles (
), circles ( ) and triangles (
) and triangles ( ) correspond to the microemulsion regions where the micelle size varies from 110 nm to 231 nm, 431 nm to 586 nm, and 886 nm to 1163 nm, respectively.
) correspond to the microemulsion regions where the micelle size varies from 110 nm to 231 nm, 431 nm to 586 nm, and 886 nm to 1163 nm, respectively.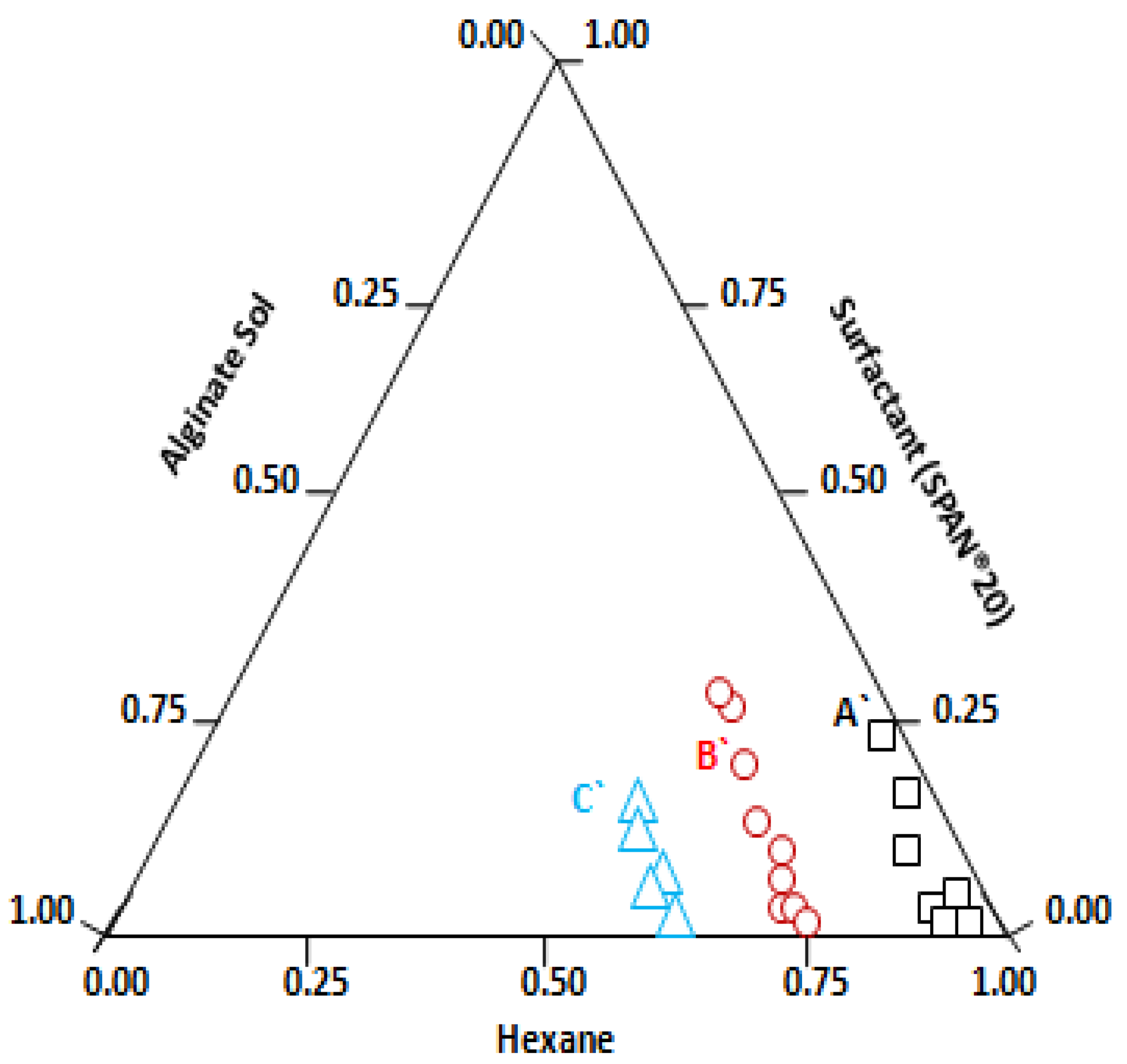




| S. No. | Gelling Solution (0.1 M–2 mL) | Droplet Size of Microemulsion without Aqueous Phase (nm) before Gelation | Recovered Nanogels with Cations (nm) after Gelation | Shape | Cationic Size (Å) | Number of Cross-Linking Ions (ICPMS) (PPM) |
|---|---|---|---|---|---|---|
| 1. | MnCl2 | 110 ± 1.42 | 275 ± 3.12 | Spherical | 0.67 | 197 |
| 2. | FeCl3 | 110 ± 1.42 | 120 ± 5.77 | Spherical | 0.61 | 248 |
| 3. | CoCl2 | 110 ± 1.42 | 7.6 ± 3.38 | Spherical | 0.65 | 186 |
| (A) | ||||
| Spectral Band | Na–Alginate Salt (Powder) | Mn–Alginate Lyophilized Nanogel | Fe–Alginate Lyophilized Nanogel | Co–Alginate Lyophilized Nanogel |
| ν(O–H) (1) | 3496 | 3472 | 3482 | 3512 |
| ν(C = O) (2) | 1646 | 1644 | 1648 | 1672 |
| ν(C–OH) (3) | 1474 | 1447 | 1464 | 1464 |
| ν(OC–OH) (4) | 1110 | 1116 | 1118 | 1134 |
| (B) | ||||
| Spectral Band | Urease (Free) | Urease-Encapsulated Mn–Alginate | Urease-Encapsulated Fe–Alginate | Urease-Encapsulated Co–Alginate |
| ν(O–H) (1) | 3412 | 3420 | 3382 | 3448 |
| νsym(COO−) (2) | 1610 | 1640 | 1599 | 1652 |
| νasym(COO−) (3) | --- | 1439 | 1404 | 1452 |
| ν(OC–OH) (4) | 1458 | 1110 | 1074 | 1116 |
| S. No. | Type of Alginates | Zeta Potential (mV) |
|---|---|---|
| 1. | Sodium alginate (microemulsion droplet) | −57.13 ± 0.33 |
| 2. | Mn–alginate nanogels | −2.63 ± 0.02 |
| 3. | Fe–alginate nanogels | −8.78 ± 0.02 |
| 4. | Co–alginate nanogels | −4.83 ± 0.02 |
| S. No. | Type of Alginate Nanogel Containing Urease | Concentration of Sodium Alginate % (w/v) | Protein/mL of Alginate Sol (mg) | % Immobilization | Enzyme Loading Efficiency (%) | Enzyme Loading Capacity (10−3) |
|---|---|---|---|---|---|---|
| 1. | Urease | 0.0 | - | - | 100 | - |
| 2. | Mn–alg | 0.2 | 1 | 52.7 | 68.4 | 0.6793 |
| 0.2 | 5 | 58.0 | 52.5 | 0.5214 | ||
| 0.2 | 7 | 35.14 | 70.16 | 0.6968 | ||
| 3. | Fe–alg | 0.2 | 1 | 47.72 | 63 | 0.6257 |
| 0.2 | 5 | 75.3 | 76.9 | 0.7637 | ||
| 0.2 | 7 | 38.41 | 81.25 | 0.8069 | ||
| 4. | Co–alg | 0.2 | 1 | 48.32 | 67.2 | 0.6674 |
| 0.2 | 5 | 59.7 | 66.4 | 0.6594 | ||
| 0.2 | 7 | 35.17 | 80.8 | 0.8025 |
| S. No. | Type of Alginate Nanogel Containing Urease | Vmax (mmol/min) | Km (mM) | Linear Range of Calibration Curve (mM) | Turn Over No. |
|---|---|---|---|---|---|
| 1. | Urease | -- | 2.4 | -- | -- |
| 2. | Mn–alg | 1.16 | 2.597 | 5.0–15.0 | 49.42 |
| 3. | Fe–alg | 1.62 | 0.31 | 5.0–25.0 | 80.52 |
| 4. | Co–alg | 1.33 | 0.51 | 1.0–5.0 | 61.29 |
| S. No. | Urea Concentration (mg/dL) (Clinical Method) | Urea Concentration (mg/dL) (Present Method) | Relative Error |
|---|---|---|---|
| 1. | 35.8 | 36.4 ± 2.5 | 1.675 |
| 2. | 50.4 | 52.4 ± 4.5 | 3.968 |
| 3. | 82.5 | 85.1 ± 2.2 | 3.151 |
| 4. | 116.4 | 119.6 ± 2.6 | 2.749 |
| 5. | 129.8 | 135.4 ± 2.4 | 4.314 |
| 6. | 138.4 | 145.1 ± 2.5 | 4.841 |
| 7 | 143 | 150.2 ± 3 | 5.034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saxena, A.; Sharda, S.; Kumar, S.; Kumar, B.; Shirodkar, S.; Dahiya, P.; Sahney, R. Synthesis of Alginate Nanogels with Polyvalent 3D Transition Metal Cations: Applications in Urease Immobilization. Polymers 2022, 14, 1277. https://doi.org/10.3390/polym14071277
Saxena A, Sharda S, Kumar S, Kumar B, Shirodkar S, Dahiya P, Sahney R. Synthesis of Alginate Nanogels with Polyvalent 3D Transition Metal Cations: Applications in Urease Immobilization. Polymers. 2022; 14(7):1277. https://doi.org/10.3390/polym14071277
Chicago/Turabian StyleSaxena, Abhishek, Shivani Sharda, Sumit Kumar, Benu Kumar, Sheetal Shirodkar, Praveen Dahiya, and Rachana Sahney. 2022. "Synthesis of Alginate Nanogels with Polyvalent 3D Transition Metal Cations: Applications in Urease Immobilization" Polymers 14, no. 7: 1277. https://doi.org/10.3390/polym14071277
APA StyleSaxena, A., Sharda, S., Kumar, S., Kumar, B., Shirodkar, S., Dahiya, P., & Sahney, R. (2022). Synthesis of Alginate Nanogels with Polyvalent 3D Transition Metal Cations: Applications in Urease Immobilization. Polymers, 14(7), 1277. https://doi.org/10.3390/polym14071277






