Mechanical Properties of Amide Functionalized CNT/NBR at Different Temperatures: A Molecular Dynamics Study
Abstract
:1. Introduction
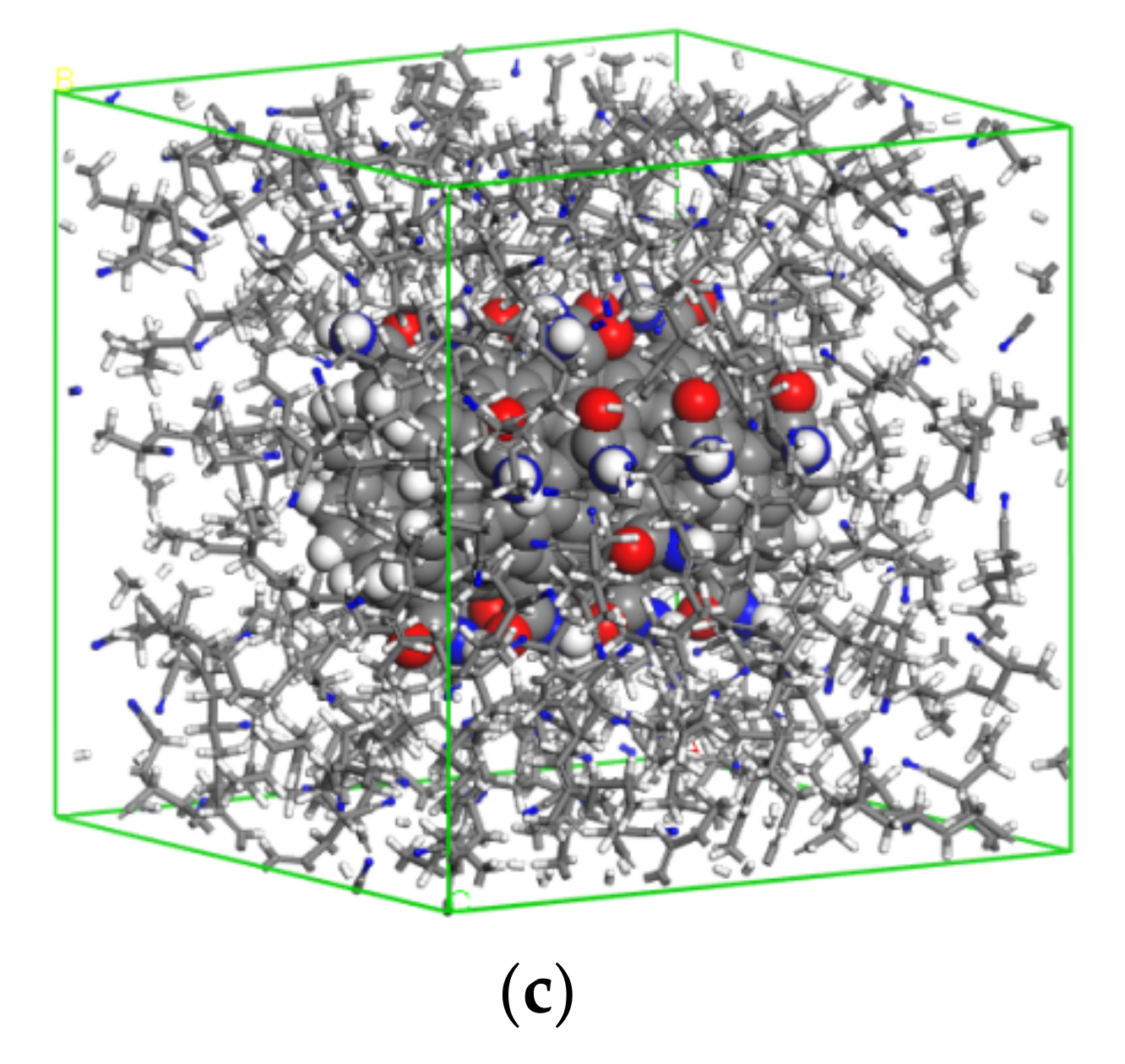
2. Modeling and Methods
3. Results and Discussions
3.1. Mechanical Properties of the Composites at Different Temperatures
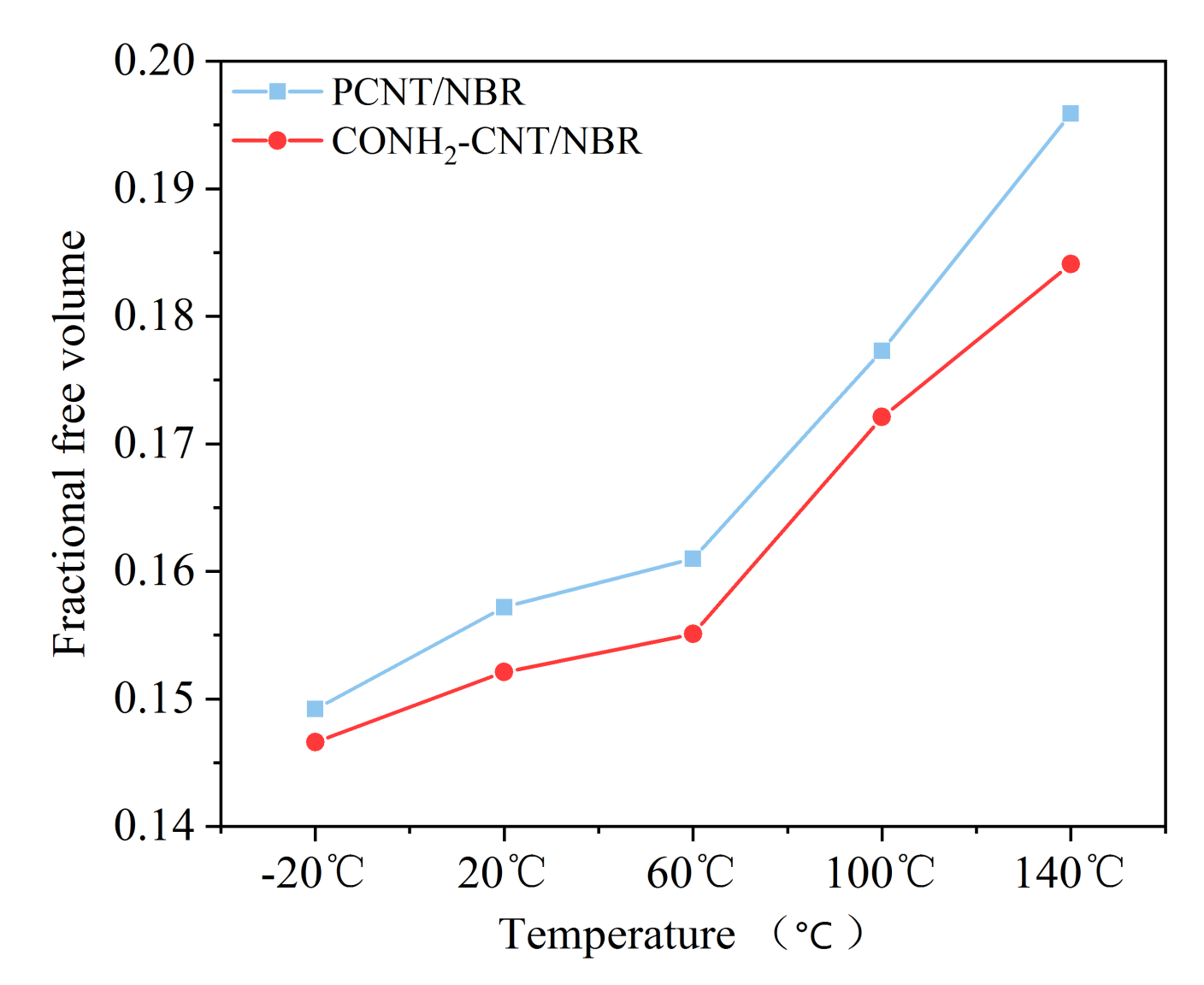
3.2. Fractional Free Volumes of the Composites at Different Temperatures
3.3. MSDs of the Composites at Different Temperatures
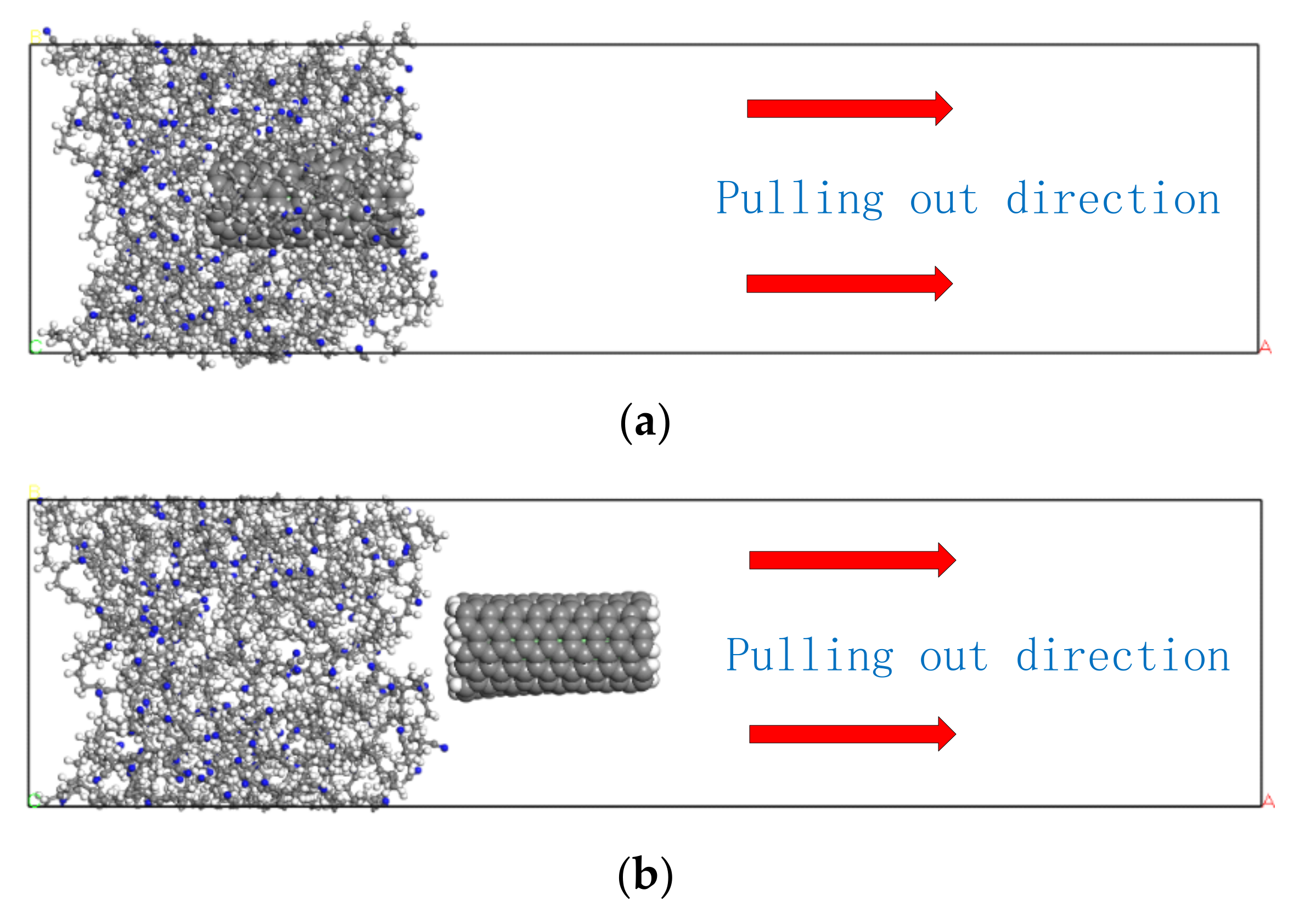
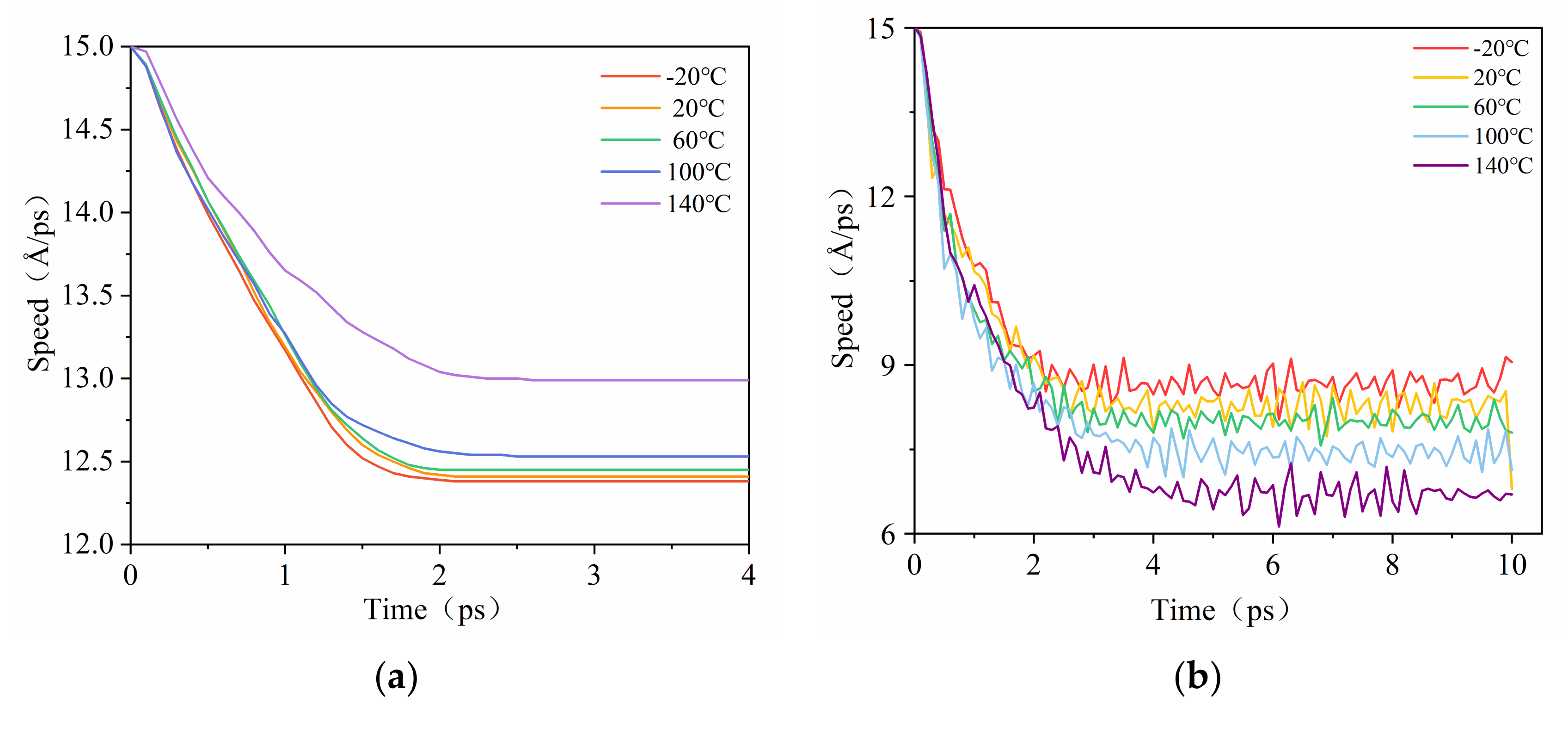
3.4. Simulation of the Pull-Out Behavior and Interfacial Binding Energy of the Composites in Different Temperatures
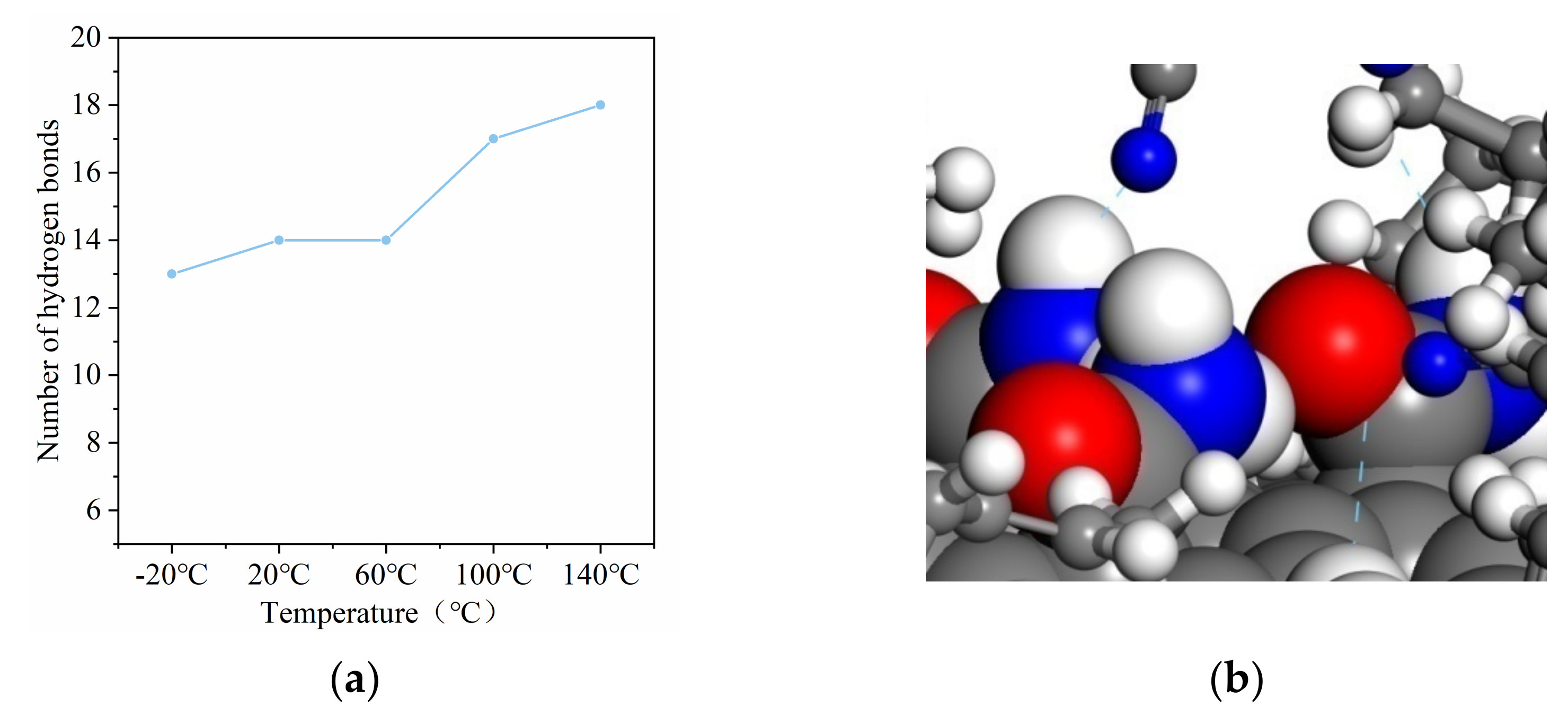
3.5. The Number of Hydrogen Bonds at Different Temperatures
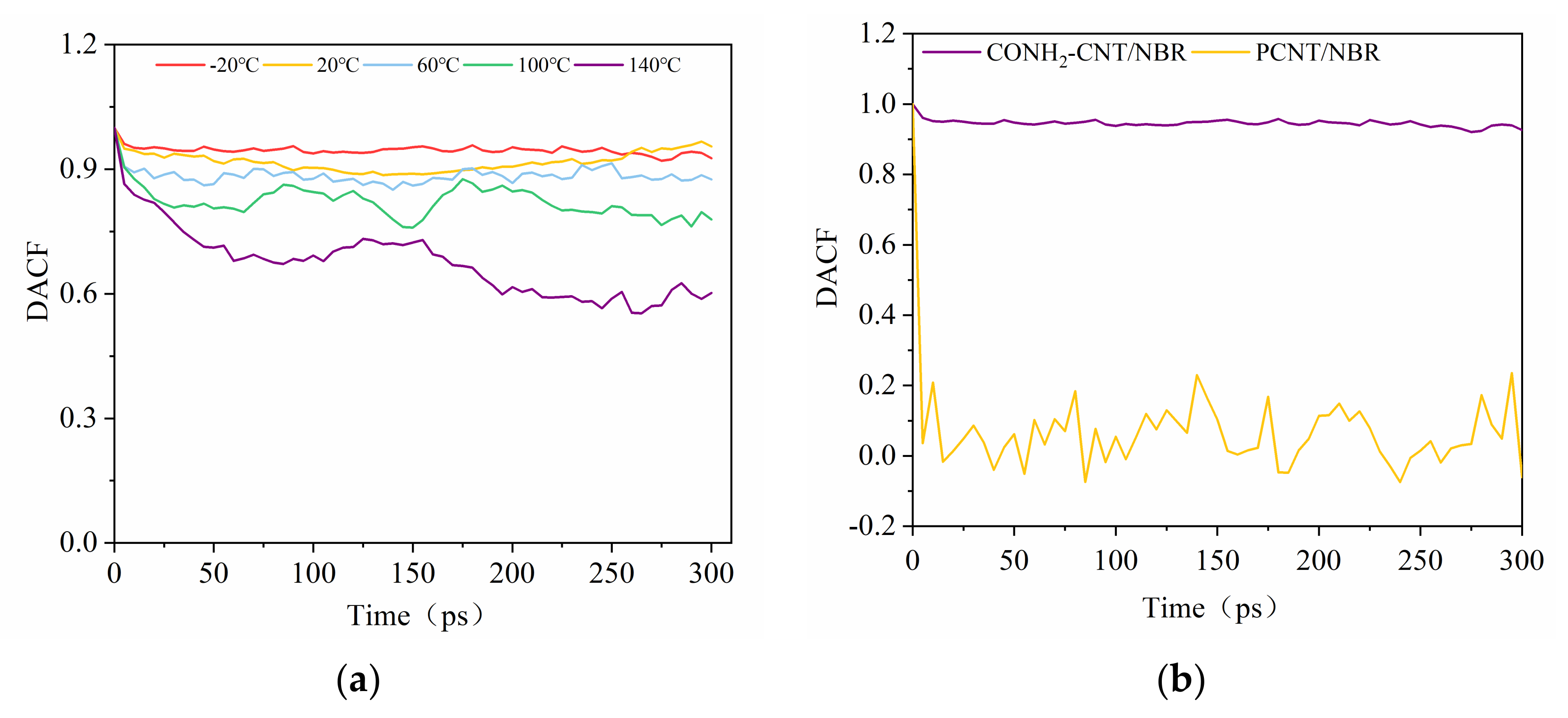
3.6. Dipole Autocorrelation Function of Composites at Different Temperatures
4. Conclusions
- (1)
- Five different temperatures and two different models, including pristine carbon nanotubes and functionalized carbon nanotubes with polar functional group, CONH2, were used to study the properties of the composite in different temperatures. Comparing the results of the mechanical properties of the PCNT/NBR composite and the CONH2–CNT/NBR material with different temperatures, the results show that the Young’s modulus, shear modulus, and bulk modulus of the two materials are reduced, but the CONH2–CNT/NBR composite performs better at each temperature with an improvement of the modulus by 5.02–25.93%.
- (2)
- The functionalization of CNT is beneficial to inhibit the influence of temperature on the FFV of the NBR matrix, it can further compress the free movement space of the NBR molecular chain in high temperatures. Therefore, more NBR molecular chains are adsorbed on the surface of functionalized CNT, the molecular motion is restricted. MSDs of the NBR composite are clearly decreased by the addition of the functionalized CNTs, thereby forming a more stable NBR matrix and further inhibiting the decrease of mechanical properties in high temperatures.
- (3)
- Interfacial binding energy is the fundamental reason for the enhancement of CNT on NBR. According to the comparison between the pull-out behaviors of two kinds of composite, electrostatic force played a more important role in the CONH2–CNT/NBR composite than in the PCNT/NBR composite, especially in high temperatures. This is attributed to the hydrogen bond interaction between CONH2–CNT and NBR which was increased as the temperature increased, having a bridging effect during the crack propagation process. Meanwhile, the value of DACF at each temperature remains consistent, making the functionalized NBR more stable in the matrix at both low and high temperatures, reducing agglomeration, and it has better dispersibility in the matrix than PCNT/NBR composite.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.J.; Kim, D.E. Molecular dynamics simulation of atomic-scale frictional behavior of corrugated nano-structured surfaces. Nanoscale 2012, 4, 3937–3944. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, D.L.; Zhong, W.S.; Zhang, L.; Fan, X.Q.; Cai, Z.B.; Zhu, M.H. Experimental and theoretical evaluations of the interfacial interaction between carbon nanotubes and carboxylated butadiene nitrile rubber: Mechanical and damping properties. Mater. Des. 2020, 186, 108318. [Google Scholar] [CrossRef]
- Li, Q.Y.; Dong, Y.L.; Perez, D.; Martini, A.; Carpick, R.W. Speed Dependence of Atomic Stick-Slip Friction in Optimally Matched Experiments and Molecular Dynamics Simulations. Phys. Rev. Lett. 2011, 106, 126101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palaty, S.; Joseph, R. Low Temperature Curing of NBR for Property Improvement. J. Elastomers Plast. 2006, 38, 199–209. [Google Scholar] [CrossRef]
- Yang, B.; Wang, S.J.; Li, Y.L.; Li, T.; En, H.; Song, S.Y. Molecular dynamics simulations of mechanical properties of swollen nitrile rubber composites by incorporating carbon nanotubes. Polym. Compos. 2020, 41, 3160–3169. [Google Scholar] [CrossRef]
- Chakraborty, S.K.M.K.K. Studies on Curing Characteristics of Natural Rubber-, Nitrile Rubber and Silicone Rubber-Based Gum Vulcanizates in the Presence of Boron Compounds. J. Elastomers Plast. 1993, 25, 358–380. [Google Scholar]
- Cui, J.Z.; Zhao, J.; Wang, S.J.; Wang, Y.; Li, Y.L. Effects of carbon nanotubes functionalization on mechanical and tribological properties of nitrile rubber nanocomposites: Molecular dynamics simulations. Comput. Mater. Sci. 2021, 196, 110556. [Google Scholar] [CrossRef]
- Luo, W.; Li, M.; Huang, Y.; Yin, B.; Hu, X. Effect of temperature on the tear fracture and fatigue life of carbon-black-filled rubber. Polymers 2019, 11, 768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieppati, J.; Schrittesser, B.; Wondracek, A.; Robin, S.; Holzner, A.; Pinter, G. Temperature impact on the mechanical and fatigue behavior of a non-crystallizing rubber. Int. J. Fatigue 2021, 144, 106050. [Google Scholar] [CrossRef]
- Ata, S.; Tomonoh, S.; Yamda, T.; Hata, K. Improvement in thermal durability of fluorinated rubber by the addition of single-walled carbon nanotubes as a thermally stable radical scavenger. Polymer 2017, 119, 112–117. [Google Scholar] [CrossRef]
- Zheng, G.B.; Sano, H.; Nakagoe, O.; Tanabe, S.J. A Molecular Dynamics Simulation of the Tensile Behavior of Y-B ranched-CNT/SiC Nanocomposite. Key Eng. Mater. Submitt. 2018, 804, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.; Yang, S.T.; Saafic, M. Molecular dynamics simulation of mechanical properties of intercalated GO/C-S-H nanocomposites. Comput. Mater. Sci. 2021, 18, 110012. [Google Scholar] [CrossRef]
- Abdul, R.E.S.; Muhd, J.N.B.; Abdul, H.Y.W. Reinforcement effect of nanocellulose on thermal stability of nitrile butadiene rubber (NBR) composites. J. Appl. Polym. Sci. 2018, 135, 46594. [Google Scholar] [CrossRef]
- Lev, Y.; Faye, A.; Volokh, K.Y. Experimental Study of the Effffect of Temperature on Strength and Extensibility of Rubberlike Materials. Exp. Mech. 2018, 58, 847–858. [Google Scholar] [CrossRef]
- Lee, J.K.; Park, J.; Kang, Y.C.; Koh, S.W. Spectroscopic and Mechanical Properties of Nano Silica Rubber Composite Material. J. Chosun Nat. Sci. 2016, 9, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Alian, A.R.; Meguid, S.A. Molecular dynamics simulations of the effect of waviness and agglomeration of CNTs on interface strength of thermoset nanocomposites. Phys. Chem. Chem. Phys. 2017, 19, 4426–4434. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Arash, B.; Wang, Q. A study on tribology of nitrile-butadiene rubber composites by incorporation of carbon nanotubes, Molecular dynamics simulations. Carbon 2016, 100, 145–150. [Google Scholar] [CrossRef]
- Varghese, T.V.; Kumar, H.A.; Anitha, S.; Rajeevc, R.S.; Rao, V.L. Reinforcement of acrylonitrile butadiene rubber using pristine few layer graphene and its hybrid fillers. Carbon 2013, 61, 476–486. [Google Scholar] [CrossRef]
- Yang, B.; Wang, S.; Song, Z.; Liu, L.F.; Li, H.L.; Li, Y.L. Molecular dynamics study on the reinforcing effect of incorporation of graphene/carbon nanotubes on the mechanical properties of swelling rubber. Polym. Test. 2021, 102, 107337. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Xu, L.; Zhang, P.Q. Investigation of aging behavior and mechanism of nitrile-butadiene rubber (NBR) in the accelerated thermal aging environment. Polym. Test. 2016, 54, 59–66. [Google Scholar] [CrossRef]




| Temperature | Uinter (kcal/mol) | Uelec | Portion of Uelec | UvdW | Portion of UvdW |
|---|---|---|---|---|---|
| −20 °C | −201.30 | −9.63 | 4.79% | −191.67 | 95.21% |
| 20 °C | −196.50 | −11.24 | 5.72% | −185.27 | 94.28% |
| 60 °C | −189.10 | −2.51 | 1.33% | −186.58 | 98.67% |
| 100 °C | −173.07 | −8.75 | 5.05% | −164.32 | 94.95% |
| 140 °C | −170.20 | −3.90 | 2.29% | −166.29 | 97.71% |
| Temperature | Uinter (kcal/mol) | Uelec | Portion of Uelec | UvdW | Portion of UvdW |
|---|---|---|---|---|---|
| −20 °C | −259.50 | −66.57 | 25.65% | −192.93 | 74.35% |
| 20 °C | −269.41 | −68.90 | 25.58% | −200.50 | 74.42% |
| 60 °C | −279.72 | −90.28 | 32.27% | −189.44 | 67.73% |
| 100 °C | −303.21 | −98.92 | 32.62% | −204.29 | 67.38% |
| 140 °C | −311.92 | −113.01 | 36.23% | −198.91 | 63.77% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, L.; Chen, L.; Lin, L.; Wang, S. Mechanical Properties of Amide Functionalized CNT/NBR at Different Temperatures: A Molecular Dynamics Study. Polymers 2022, 14, 1307. https://doi.org/10.3390/polym14071307
Ji L, Chen L, Lin L, Wang S. Mechanical Properties of Amide Functionalized CNT/NBR at Different Temperatures: A Molecular Dynamics Study. Polymers. 2022; 14(7):1307. https://doi.org/10.3390/polym14071307
Chicago/Turabian StyleJi, Longcheng, Lijia Chen, Li Lin, and Shijie Wang. 2022. "Mechanical Properties of Amide Functionalized CNT/NBR at Different Temperatures: A Molecular Dynamics Study" Polymers 14, no. 7: 1307. https://doi.org/10.3390/polym14071307








