Recent Advancements of Polysaccharides to Enhance Quality and Delay Ripening of Fresh Produce: A Review
Abstract
1. Introduction
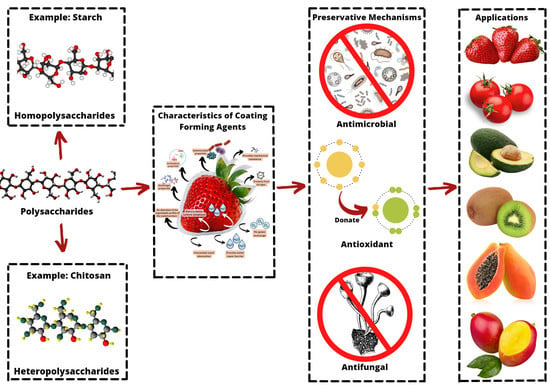
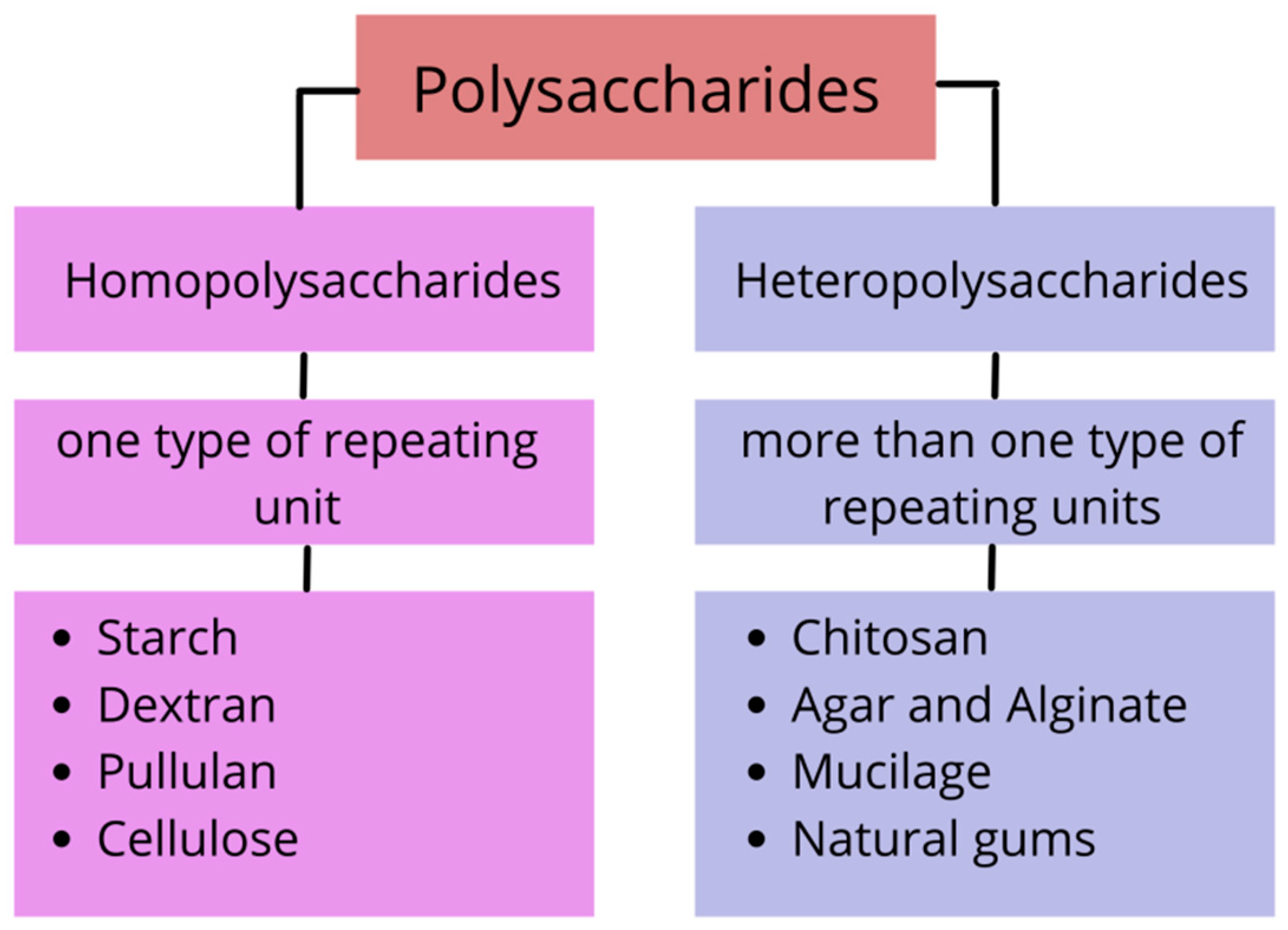
2. Types and Properties of Polysaccharides
2.1. Homopolysaccharide
2.1.1. Starch
2.1.2. Dextran
2.1.3. Pullulan
2.1.4. Cellulose
2.2. Heteropolysaccharide
2.2.1. Chitosan
2.2.2. Agar and Alginate
2.2.3. Mucilage
2.2.4. Natural Gums
3. The Coating-Forming Agents Designed to Preserve Fruits and Vegetables
4. Applications and Effects of Polysaccharides Coating on Fruits and Vegetable Postharvest
| Polysaccharides | Additives/Surfactants | Coated Fruits/Vegetables | Effects on Fruits and Vegetables | References |
|---|---|---|---|---|
| Gum arabic | Glycerol | Ponkan orange (Citrus poonensis) |
| [91] |
| Gum arabic, maize starch | Lemongrass oil, glycerol | Pomegranate (Punica granatum) |
| [92] |
| Gum arabic | Glycerol | Strawberry (Fragaria ananassa) |
| [73] |
| Gum arabic | - | Tomato (Solanum lycopersicum) |
| [104] |
| Gum arabic, cellulose | Moringa leaf extract | Avocado (Persea americana) |
| [105] |
| Gum arabic | Aloe vera gel, ethanolic | Mango (Mangifera indica) |
| [106] |
| Alginate | Citric acid, ascorbic acid | Apple (Malus pumila) |
| [93] |
| Alginate | Ascorbic acid | Pineapple (Ananas comosus) |
| [94] |
| Alginate | Salicylic acid, oxalic acid | Plum (Prunus salicina L. cv. ‘Black amber’) |
| [95] |
| Alginate | Orange essential oil, Tween 80 | Tomato |
| [96] |
| Chitosan, cellulose | Curcumin | Kiwi fruit (Actinidia deliciosa) |
| [97] |
| Chitosan, chitin, cellulose | - | Kiwi, avocado, strawberry, banana, nectarine, apricot |
| [98] |
| Chitosan, pullulan | Pomegranate peel extract | Green bell pepper (Capsicum annuum) |
| [28] |
| Chitosan | Acetic acid | Cucumber (Cucumis sativus) |
| [99] |
| Chitosan | Calcium chloride | Papaya (Carica papaya L.) |
| [100] |
| Pectin, pullulan | Grape seed extract (Vitis vinifera) | Peanut (Arachis hypogaea) |
| [101] |
| Xanthan gum | Citric acid, glycerol | Lotus root (Nelumbo nucifera) |
| [102] |
| Persian gum | Gelatin, shellac | ‘Valencia’ orange |
| [103] |
5. Preservation Mechanisms
| Polysaccharides Used | Fruits/Vegetables Used | Target | Preservative Mechanisms | Effect on Fresh Produce | References |
|---|---|---|---|---|---|
| Sodium alginate + Chitosan (SA/CS)3 | Strawberry (Fragaria ananassa) | Microbial cell membrane | Antimicrobial mechanisms of chitosan: Positively charged chitosan molecules interacting with negatively charged bacteria membranes inhibit microbial growth and toxin buildup. |
| [107] |
| Chitosan(CH) + cinnamon oil | Sweet cherry (Prunus avium L.) | Cell wall and cell membrane | Antimicrobial mechanisms of chitosan: The presence of chitosan micropores as a gas barrier and a carrier for cinnamon oil, along with: The presence of hydroxyl groups inhibits mycotoxin formation by creating hydrogen bonds with active enzymes. Antimicrobial mechanisms of cinnamon oil: Trans-cinnamaldehyde, a component in cinnamon, was shown to induce unevenness of hyphae cell walls of fungus, mechanical damage, and disrupted cell metabolism. |
| [108] |
| Aloe vera gel + Basil seed mucilage (AVG + BSM) | Apricot (Prunus armeanica cv. ‘Nouri’) | - | Antimicrobial mechanisms of AVG and BSM: TPC accumulated in response to AVG and BSM. Phenolic chemicals directly affect the defense process by inhibiting pathogen growth and reinforcing host tissues. |
| [69] |
| Aloe vera gel (AVG) | Grapes, Tomatoes, Peach, Sweet cherry, Litchi fruit, fresh-cut papaya, white button mushroom, fresh-cut guava, pomegranate arils | Phospholipid bilayer of fungal | Antifungal mechanisms of AVG: Bioactive compounds of aloe vera gel, Aloin, and Barbaloin disrupt the lipid/water interface in negatively charged phospholipids, causing fungal bilayer core rupture. Antioxidant mechanisms of AVG: Aloe-emodin, one of the bioactive components in AVG, prevents the degradation of flavonoid, FC and total phenolic content, TPC. |
| [111] |
| Chitosan (CH) + Alginate (AL) + Pomegranate peel extract (PPE) | Capsicum (Capsicum annuum L.) | Cell wall of the microorganisms | Antifungal mechanisms of pomegranate peel extract (PPE): PPE is high in phenolics, which are antioxidants and antimicrobial compounds. |
| [112] |
| Guar GUM (GG) + Ginseng extract (GSE) | Sweet cherry (Prunus avium L.) | - | Preservative mechanisms of GG-GSE: The formation of an extended network between GG and hydrophilic molecules, such as phenols via hydrogen bonding reduces respiration rate, water loss, and oxidation reaction. Enzyme related-defense mechanisms: High GSE bioactive component concentration reduces the oxygen supply needed for enzymatic oxidation of phenols. |
| [118] |
| Alginate + Chitosan + ZnO nanoparticles | Guava (Psidium guajava L.) | - | Antifungal activity of nanoZnO: ZnO nanoparticles prevent the development of Phyllosticta psidicola fungus when coated with alginate and chitosan. Antimicrobial activity of cationic starch and sodium alginate: Cationic groups are acquired via the production of cationic starch. Anionic groups were created using sodium alginate, a natural anionic polysaccharide. The antibacterial activity is measured by zone inhibition (disc diffusion method). |
| [109] |
| Citrus pectin + broken rice grain flour + cellulosic rice skin nanofiber | Avocado (Persea americana Milll.) | Apertures present in the dermal tissue of the fruit | Preservative mechanism of reducing respiratory rate: Coating essentially seals the dermal tissue’s apertures, severely reducing gas exchange or respiration. Coatings partially block natural fruit apertures, acting as a modified atmosphere package, slowing metabolism and enzyme activity, notably pectinases. |
| [119] |
| Chitosan (CH) + Locust Bean Gum (LBG) + Pomegranate Peel Extract (PPE) | Orange (Citrus sinensis) | - | Antifungal mechanisms of the coating: Inhibition halo requires both CH and LBG. CH has an inherent inhibitory action. The P. digitatum growth was considerably reduced by CH and LBG coatings supplemented with PPE. |
| [113] |
| Chitosan + carotenoproteins | Strawberries (Fragaria ananassa) | Fungal reproductive structure | Antifungal activity of the coating: For maximum bacterial suppression, use higher CS and CP concentrations in edible film creation. CS and CP improved fungicidal growth inhibition. CS’s potency was related to its ability to alter the morphology of fungal reproductive structures. |
| [114] |
| Sodium Alginate + Chitosan + Different Dietary Fibers (apple fiber, orange fiber, inulin, oligofructose) | Blueberries (Vaccinium sect. Cyanococcus) | Cell membrane | Antioxidant activity of oligofructose and orange fiber: The high amount of phytochemicals and phenols as well as an antioxidant capacity that triggers the phenylpropanoid metabolism allowed more significant reductions in yeast/mold counts than CH alone. Antifungal activity of CH–OL and CH–OF: OF and OL extract may include plant-derived bioactive substances with antifungal activity. |
| [115] |
| Chitosan + Lemongrass oil | Bell pepper (C. capsici) | Cell wall and cell membrane | Antifungal mechanism of CH and EO: The capacity of EO to permeate cell membranes damages biological processes, such as ion loss, coagulation of bacterial cytoplasm, and direct damage to proton pump and ATP generation sites. A reduction in membrane potential causes calcium ion leakage and other vital components. |
| [121] |
| Carboxymethylcellulose (CMC) | Plum fruits (Prunus domestica L.) | - | Antioxidant mechanism of CMC: The presence of carboxylic groups in CMC’s chemical structure results in hydrogen bonding within the coating matrix and between the coating and the fruit peel. Limiting O2 access and changing internal gas composition, lowering oxidative metabolism via increased peroxidase activity, and delaying textural changes in fruits. |
| [117] |
| Cactus Opuntia dillenii polysaccharide (ODP) | Fresh-cut potato | Bacterial cellular activity | Antimicrobial mechanism of ODP: ODP’s bacteriostatic effect slows bacterial growth by interfering with bacterial protein production, DNA replication, or other cellular metabolisms. |
| [110] |
| Fenugreek and flax polysaccharides coating | Apples (Malus domestica) | - | Preservative mechanism of reducing respiratory rate: Coating material alters the environment of fruit by forming a thick surface layer, which reduces the pace of respiration and the degradation process by preventing gas exchange. |
| [122] |
| Alginate + Chitosan + Carrageenan | Fresh-cut lettuce (Lactuca sativa L. var. ramosa Hort.) | - | Antioxidant mechanisms of the coating: Chitosan inhibits browning and increases antioxidant properties and controls the overproduction of ROS, and suppresses lipid peroxidation. Enzyme related-defense mechanisms: Inhibit the polyphenol oxidase (PPO) activity and postpone the time to reach its maximum level while triggering the formation of phenylalanine ammonia-lyase (PAL). Inhibit the phospholipase D, PLD and lipoxygenase, LOX activity while dramatically maintaining high activities of antioxidant enzyme (catalase, CAT; peroxidase, POD; superoxide dismutase, SOD; and ascorbate peroxidase, APX. |
| [116] |
| Polysaccharide isolated from Oudemansiella radicata | Shiitake mushrooms (Lentinus edodes) | - | Preservative mechanism of reducing respiratory rate: Capability to operate as a semi-permeable barrier to the flow of O2, CO2, moisture, and solutes, as well as to generate a modified environment to limit respiration rate. Coatings delay the loss of natural volatile components, allowing the original mushroom flavor to be preserved for a longer shelf life. |
| [120] |
6. Legislations and Safety Issues of Edible Films and Coatings
7. Benefits and Limitations of Natural Packaging
8. Conclusions and Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yahia, E.M.; García-Solís, P.; Celis, M.E. Contribution of Fruits and Vegetables to Human Nutrition and Health. In Post-Harvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead Publishing: Amsterdam, The Netherlands, 2019; pp. 19–45. [Google Scholar] [CrossRef]
- Salihoglu, G.; Salihoglu, N.K.; Ucaroglu, S.; Banar, M. Food loss and waste management in Turkey. Bioresour. Technol. 2018, 248, 88–99. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Verma, R.; Bhardwaj, P.; Sharma, S.; Kumar, D. Fruit and vegetable peels: Utilization of high value horticultural waste in novel industrial applications. Molecules 2020, 25, 2812. [Google Scholar] [CrossRef]
- Brasil, I.M.; Siddiqui, M.W. Postharvest Quality of Fruits and Vegetables: An Overview. Preharvest Modulation of Post-Harvest Fruit and Vegetable Quality; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–40. [Google Scholar] [CrossRef]
- Elik, A.; Yanik, D.K.; Istanbullu, Y.; Guzelsoy, N.A.; Yavuz, A.; Gogus, F. Strategies to reduce post-harvest losses for fruits and vegetables. Strategies 2019, 5, 29–39. [Google Scholar] [CrossRef]
- Parven, A.; Sarker, M.R.; Megharaj, M.; Meftaul, I.M. Prolonging the shelf life of Papaya (Carica papaya L.) using Aloe vera gel at ambient temperature. Sci. Hortic. 2020, 265, 109228. [Google Scholar] [CrossRef]
- Shah, S.; Hashmi, M.S. Chitosan–aloe vera gel coating delays post-harvest decay of mango fruit. Hortic. Environ. Biotechnol. 2020, 61, 279–289. [Google Scholar] [CrossRef]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface modification of polymers: Methods and applications. Adv. Mater. Interfaces. 2018, 5, 1801247. [Google Scholar] [CrossRef]
- Salehi, F. Edible coating of fruits and vegetables using natural gums: A review. Int. J. Fruit Sci. 2020, 20, S570–S589. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Mahunu, G.K.; Arslan, M.; Abdalhai, M.; Zhihua, L. Recent developments in gum edible coating applications for fruits and vegetables preservation: A review. Carbohydr. Polym. 2019, 224, 115141. [Google Scholar] [CrossRef] [PubMed]
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals from algae and cyanobacteria. In Algal Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 65–89. [Google Scholar] [CrossRef]
- Erna, K.H.; Felicia, W.X.L.; Rovina, K.; Vonnie, J.M.; Huda, N. Development of Curcumin/Rice Starch Films for Sensitive Detection of Hypoxanthine in Chicken and Fish Meat. Carbohydr. Polym. Technol. Appl. 2022, 3, 100189. [Google Scholar] [CrossRef]
- Oyom, W.; Xu, H.; Liu, Z.; Long, H.; Li, Y.; Zhang, Z.; Bi, Y.; Tahergorabi, R.; Prusky, D. Effects of modified sweet potato starch edible coating incorporated with cumin essential oil on storage quality of ‘early crisp’. LWT 2022, 153, 12475. [Google Scholar] [CrossRef]
- Trinh, B.M.; Smith, M.; Mekonnen, T.H. A nanomaterial-stabilized starch-beeswax Pickering emulsion coating to extend produce shelf-life. Chem. Eng. J. 2022, 431, 133905. [Google Scholar] [CrossRef]
- Rather, J.A.; Makroo, H.A.; Showkat, Q.A.; Majid, D.; Dar, B.N. Recovery of gelatin from poultry waste: Characteristics of the gelatin and lotus starch-based coating material and its application in shelf-life enhancement of fresh cherry tomato. Food Packag. Shelf Life 2022, 31, 100775. [Google Scholar] [CrossRef]
- Ghoshal, G.; Chopra, H. Impact of apricot oil incorporation in tamarind starch/gelatin based edible coating on shelf life of grape fruit. J. Food Meas. Charact. 2022, 16, 1274–1290. [Google Scholar] [CrossRef]
- Chen, M.; Yan, X.; Cheng, M.; Zhao, P.; Wang, Y.; Zhang, R.; Wang, X.; Wang, J.; Chen, M. Preparation, characterization and application of poly (lactic acid)/corn starch/eucalyptus leaf essential oil microencapsulated active bilayer degradable film. Int. J. Biol. Macromol. 2022, 195, 264–273. [Google Scholar] [CrossRef]
- Kawhena, T.G.; Opara, U.L.; Fawole, O.A. Effect of Gum Arabic and Starch-Based Coating and Different Polyliners on Postharvest Quality Attributes of Whole Pomegranate Fruit. Processes 2022, 10, 164. [Google Scholar] [CrossRef]
- Kusnadi, J.; Mahatmanto, T.; Marsheli, N.; Fawzia, N.; Rahmawani, D.E.; Alexander, K. Development of low-cost edible coatings based on polysaccharides with active lactic acid bacteria for the protection of fresh produce modeled using fresh cut apples. Food Sci. Technol. Int. 2022. [Google Scholar] [CrossRef] [PubMed]
- Lazić, V.; Vivod, V.; Peršin, Z.; Stoiljković, M.; Ratnayake, I.S.; Ahrenkiel, P.S.; Nedeljković, J.M.; Kokol, V. Dextran-coated silver nanoparticles for improved barrier and controlled antimicrobial properties of nanocellulose films used in food packaging. Food Packag. Shelf Life 2020, 26, 100575. [Google Scholar] [CrossRef]
- Davidović, S.; Miljković, M.; Tomić, M.; Gordić, M.; Nešić, A.; Dimitrijević, S. Response surface methodology for optimisation of edible coatings based on dextran from Leuconostoc mesenteroides T3. Carbohydr. Polym. 2018, 184, 207–213. [Google Scholar] [CrossRef]
- Davidović, S.; Miljković, M.; Gordic, M.; Cabrera-Barjas, G.; Nesic, A.; Dimitrijević-Branković, S. Dextran-Based Edible Coatings to Prolong the Shelf Life of Blueberries. Polymers 2021, 13, 4252. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Pobiega, K.; Gniewosz, M. Pullulan–biopolymer with potential for use as food packaging. Int. J. Food Eng. 2019, 15. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Ścibisz, I.; Gniewosz, M.; Mitek, M.; Pobiega, K.; Cendrowski, A. Effect of pullulan coating on postharvest quality and shelf-life of highbush blueberry (Vaccinium corymbosum L.). Materials 2017, 10, 965. [Google Scholar] [CrossRef]
- Ganduri, V.R. Evaluation of pullulan-based edible active coating methods on Rastali and Chakkarakeli bananas and their shelf-life extension parameters studies. J. Food Process. Preserv. 2020, 44, e14378. [Google Scholar] [CrossRef]
- Kumar, N.; Petkoska, A.T.; AL-Hilifi, S.A.; Fawole, O.A. Effect of Chitosan–Pullulan Composite Edible Coating Functionalized with Pomegranate Peel Extract on the Shelf Life of Mango (Mangifera indica). Coatings 2021, 11, 764. [Google Scholar] [CrossRef]
- Kumar, N.; Neeraj; Pratibha; Singla, M. Enhancement of storage life and quality maintenance of litchi (Litchi chinensis Sonn.) fruit using chitosan: Pullulan blend antimicrobial edible coating. Int. J. Fruit Sci. 2020, 20, S1662–S1680. [Google Scholar] [CrossRef]
- Kumar, N.; Ojha, A.; Upadhyay, A.; Singh, R.; Kumar, S. Effect of active chitosan-pullulan composite edible coating enrich with pomegranate peel extract on the storage quality of green bell pepper. LWT 2021, 138, 110435. [Google Scholar] [CrossRef]
- Kumar, N.; Neeraj; Pratibha; Trajkovska Petkoska, A. Improved shelf life and quality of tomato (Solanum lycopersicum L.) by using chitosan-pullulan composite edible coating enriched with pomegranate peel extract. ACS Food Sci. Technol. 2021, 1, 500–510. [Google Scholar] [CrossRef]
- Zhou, W.; He, Y.; Liu, F.; Liao, L.; Huang, X.; Li, R.; Li, J. Carboxymethyl chitosan-pullulan edible films enriched with galangal essential oil: Characterization and application in mango preservation. Carbohydr. Polym. 2021, 256, 117579. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, C.; Zhao, H. Application of pullulan and chitosan multilayer coatings in fresh papayas. Coatings 2019, 9, 745. [Google Scholar] [CrossRef]
- Pobiega, K.; Przybył, J.L.; Żubernik, J.; Gniewosz, M. Prolonging the shelf life of cherry tomatoes by pullulan coating with ethanol extract of propolis during refrigerated storage. Food Bioprocess Technol. 2020, 13, 1447–1461. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Zhao, G.; Du, J.; Chen, W.; Pan, M.; Chen, D. Preparation and thermostability of cellulose nanocrystals and nanofibrils from two sources of biomass: Rice straw and poplar wood. Cellulose 2019, 26, 8625–8643. [Google Scholar] [CrossRef]
- Khalil, H.A.; Tye, Y.Y.; Leh, C.P.; Saurabh, C.K.; Ariffin, F.; Fizree, H.M.; Suriani, A.B. Cellulose reinforced biodegradable polymer composite film for packaging applications. In Bionanocomposites for Packaging Applications; Springer: Berlin/Heidelberg, Germany, 2018; pp. 49–69. [Google Scholar] [CrossRef]
- Yu, K.; Zhou, L.; Xu, J.; Jiang, F.; Zhong, Z.; Zou, L.; Liu, W. Carboxymethyl cellulose-based water barrier coating regulated postharvest quality and ROS metabolism of pakchoi (Brassica chinensis L.). Postharvest Biol. Technol. 2022, 185, 111804. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Kordowska-Wiater, M.; Kałwa, K.; Skrzypek, T.; Sikora, M.; Łupina, K. Physiological, qualitative, and microbiological changes of minimally processed Brussels sprouts in response to coating with carboxymethyl cellulose/candelilla wax emulsion. J. Food Process. Preserv. 2019, 43, e14004. [Google Scholar] [CrossRef]
- Chen, C.; Peng, X.; Zeng, R.; Wan, C.; Chen, M.; Chen, J. Physiological and biochemical responses in cold-stored citrus fruits to carboxymethyl cellulose coating containing ethanol extract of Impatiens balsamina L. Stems. J. Food Process. Preserv. 2017, 41, e12999. [Google Scholar] [CrossRef]
- Zhang, J.; Ozturk, S.; Singh, R.K.; Kong, F. Effect of cellulose nanofiber-based coating with chitosan and trans-cinnamaldehyde on the microbiological safety and quality of cantaloupe rind and fresh-cut pulp. Part 1: Microbial safety. LWT 2020, 134, 109972. [Google Scholar] [CrossRef]
- Saba, M.K.; Amini, R. Nano-ZnO/carboxymethyl cellulose-based active coating impact on ready-to-use pomegranate during cold storage. Food Chem. 2017, 232, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Zhang, X.; Luo, X.; Wang, Y.; Li, Y.; Li, B.; Liu, S. Edible coating based on beeswax-in-water Pickering emulsion stabilized by cellulose nanofibrils and carboxymethyl chitosan. Food Chem. 2020, 331, 127108. [Google Scholar] [CrossRef]
- Liu, C.; Jin, T.; Liu, W.; Hao, W.; Yan, L.; Zheng, L. Effects of hydroxyethyl cellulose and sodium alginate edible coating containing asparagus waste extract on postharvest quality of strawberry fruit. LWT 2021, 148, 111770. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; Ni, Y. Chitosan as a preservative for fruits and vegetables: A review on chemistry and antimicrobial properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Shebis, Y.; Laskavy, A.; Molad-Filossof, A.; Arnon-Rips, H.; Natan-Warhaftig, M.; Jacobi, G.; Fallik, E.; Banin, E.; Poverenov, E. Non-radical synthesis of chitosan-quercetin polysaccharide: Properties, bioactivity and applications. Carbohydr. Polym. 2022, 284, 119206. [Google Scholar] [CrossRef]
- Liu, X.; Xue, F.; Li, C.; Adhikari, B. Physicochemical properties of films produced using nanoemulsions stabilized by carboxymethyl chitosan-peptide conjugates and application in blueberry preservation. Int. J. Biol. Macromol. 2022, 202, 26–36. [Google Scholar] [CrossRef]
- Guo, X.; Chu, L.; Gu, T.; Purohit, S.; Kou, L.; Zhang, B. Long-term quality retention and decay inhibition of chestnut using thymol loaded chitosan nanoparticle. Food Chem. 2022, 374, 131781. [Google Scholar] [CrossRef]
- Saki, M.; ValizadehKaji, B.; Abbasifar, A.; Shahrjerdi, I. Effect of chitosan coating combined with thymol essential oil on physicochemical and qualitative properties of fresh fig (Ficus carica L.) fruit during cold storage. J. Food Meas. Charact. 2019, 13, 1147–1158. [Google Scholar] [CrossRef]
- Qiao, G.; Xiao, Z.; Ding, W. Effect of chitosan/nano-titanium dioxide/thymol and tween films on ready-to-eat cantaloupe fruit quality. Coatings 2019, 9, 828. [Google Scholar] [CrossRef]
- Zheng, K.; Xiao, S.; Li, W.; Wang, W.; Chen, H.; Yang, F.; Qin, C. Chitosan-acorn starch-eugenol edible film: Physico-chemical, barrier, antimicrobial, antioxidant and structural properties. Int. J. Biol. Macromol. 2019, 135, 344–352. [Google Scholar] [CrossRef]
- La, D.D.; Nguyen-Tri, P.; Le, K.H.; Nguyen, P.T.; Nguyen, M.D.B.; Vo, A.T.; Nguyen, M.T.; Chang, S.W.; Tran, L.D.; Chung, W.J.; et al. Effects of antibacterial ZnO nanoparticles on the performance of a chitosan/gum arabic edible coating for post-harvest banana preservation. Prog. Org. Coat. 2021, 151, 106057. [Google Scholar] [CrossRef]
- Safari, Z.S.; Ding, P.; Nakasha, J.J.; Yusoff, S.F. Controlling Fusarium oxysporum Tomato Fruit Rot under Tropical Condition Using Both Chitosan and Vanillin. Coatings 2021, 11, 367. [Google Scholar] [CrossRef]
- Jaimun, R.; Sangsuwan, J. Efficacy of chitosan-coated paper incorporated with vanillin and ethylene adsorbents on the control of anthracnose and the quality of Nam Dok Mai mango fruit. Packag. Technol. Sci. 2019, 32, 383–394. [Google Scholar] [CrossRef]
- Bambace, M.F.; Moreira, M.D.R. Improving ready-to-eat apple cubes’ safety using chitosan-based active coatings. J. Food Saf. 2022, e12962. [Google Scholar] [CrossRef]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Akman, P.K.; Bozkurt, F.; Birech, K.; Tornuk, F. Development and characterization of sodium alginate based active edible films incorporated with essential oils of some medicinal plants. Int. J. Biol. Macromol. 2020, 145, 124–132. [Google Scholar] [CrossRef]
- Harnkarnsujarit, N.; Li, Y. Structure–property modification of microcrystalline cellulose film using agar and propylene glycol alginate. J. Appl. Polym. Sci. 2017, 134, 45533. [Google Scholar] [CrossRef]
- Hamzah, H.M.; Osman, A.; Tan, C.P.; Ghazali, F.M. Carrageenan as an alternative coating for papaya (Carica papaya L. cv. Eksotika). Postharvest Biol. Technol. 2013, 75, 142–146. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Quintero-Pimiento, C.; Díaz-Díaz, D.; Goyanes, S.; López-Córdoba, A. Improvement of andean blueberries postharvest preservation using carvacrol/alginate-edible coatings. Polymers 2020, 12, 2352. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Vishakha, K.; Banerjee, S.; Mondal, S.; Ganguli, A. Antibacterial and antibiofilm effectiveness of bioactive packaging materials from edible sodium alginate and vanillin: Assessment on lettuce. J. Food Process. Preserv. 2021, 45, e15668. [Google Scholar] [CrossRef]
- Sangsuwan, J.; Sutthasupa, S. Effect of chitosan and alginate beads incorporated with lavender, clove essential oils, and vanillin against Botrytis cinerea and their application in fresh table grapes packaging system. Packag. Technol. Sci. 2019, 32, 595–605. [Google Scholar] [CrossRef]
- Hu, W.; Jiang, A.; Xiu, Z.; Feng, K. Effect of thyme oil–alginate-based coating on quality and microbial safety of fresh-cut apples. J. Sci. Food Agric. 2018, 98, 2302–2311. [Google Scholar] [CrossRef]
- Bambace, M.F.; del Rosario Moreira, M.; Sánchez-Moreno, C.; De Ancos, B. Effects of combined application of high-pressure processing and active coatings on phenolic compounds and microbiological and physicochemical quality of apple cubes. J. Sci. Food Agric. 2021, 101, 4256–4265. [Google Scholar] [CrossRef] [PubMed]
- Shakerardekani, A.; Hashemi, M.; Shahedi, M.; Mirzaalian Dastjerdi, A. Enhancing the quality of fresh pistachio fruit using sodium alginate enriched with thyme essential oil. J. Agric. Sci. Technol. 2021, 23, 65–82. [Google Scholar]
- Glicerina, V.; Siroli, L.; Betoret, E.; Canali, G.; Dalla Rosa, M.; Lanciotti, R.; Romani, S. Characterization and evaluation of the influence of an alginate, cocoa and a bilayer alginate-cocoa coating on the quality of fresh-cut oranges during storage. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Xu, B.; Mi, H. Characterization of sodium alginate-based films incorporated with thymol for fresh-cut apple packaging. Food Control. 2021, 126, 108063. [Google Scholar] [CrossRef]
- Hu, W.; Feng, K.; Xiu, Z.; Jiang, A.; Lao, Y. Efficacy of thyme oil-alginate-based coating in reducing foodborne pathogens on fresh-cut apples. Int. J. Food Sci. Technol. 2019, 54, 3128–3137. [Google Scholar] [CrossRef]
- Beikzadeh, S.; Khezerlou, A.; Jafari, S.M.; Pilevar, Z.; Mortazavian, A.M. Seed mucilages as the functional ingredients for biodegradable films and edible coatings in the food industry. Adv. Colloid Interface Sci. 2020, 280, 102164. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Shahbazi, Y.; Shavisi, N.; Karami, N. Development of edible bioactive coating based on mucilages for increasing the shelf life of strawberries. J. Food Meas. Charact. 2021, 15, 394–405. [Google Scholar] [CrossRef]
- Nourozi, F.; Sayyari, M. Enrichment of Aloe vera gel with basil seed mucilage preserve bioactive compounds and postharvest quality of apricot fruits. Sci. Hortic. 2020, 262, 109041. [Google Scholar] [CrossRef]
- Mohammed, R.A.; Yousef, A.; Ali, A. Application of Biodegradable Aloe vera gel and Linseed mucilage for extending the shelf life of Plums. Res. J. Pharm. Technol. 2021, 14, 1579–1585. [Google Scholar] [CrossRef]
- Kozlu, A.; Elmacı, Y. Quince seed mucilage as edible coating for mandarin fruit; determination of the quality characteristics during storage. J. Food Process. Preserv. 2020, 44, e14854. [Google Scholar] [CrossRef]
- Liguori, G.; Gaglio, R.; Settanni, L.; Inglese, P.; D’Anna, F.; Miceli, A. Effect of Opuntia ficus-indica Mucilage Edible Coating in Combination with Ascorbic Acid, on Strawberry Fruit Quality during Cold Storage. J. Food Qual. 2021, 11, 1963. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Jiyong, S.; Mahunu, G.K.; Zhai, X.; Mariod, A.A. Quality and postharvest-shelf life of cold-stored strawberry fruit as affected by gum arabic (Acacia senegal) edible coating. J Food Biochem. 2018, 42, e12527. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D. Bioactive edible film based on Konjac glucomannan and probiotic Lactobacillus plantarum strains: Physicochemical properties and shelf life of fresh-cut kiwis. J. Food Sci. 2021, 86, 513–522. [Google Scholar] [CrossRef]
- Sati, F.; Qubbaj, T. Impact of gum Arabic and cactus mucilage as potential coating substances combined with calcium chloride treatment on tomato (Solanum lycopersicum L.) fruit quality attributes under ambient storage conditions. Can. J. Plant Sci. 2021. [Google Scholar] [CrossRef]
- Criado, P.; Fraschini, C.; Shankar, S.; Salmieri, S.; Lacroix, M. Influence of cellulose nanocrystals gellan gum-based coating on color and respiration rate of Agaricus bisporus mushrooms. J. Food Sci. 2021, 86, 420–425. [Google Scholar] [CrossRef]
- Ergin, S.Ö.; Yaman, H.; Dilek, M. The usage of edible films extracted from cherry and apricot tree gums for coating of strawberry (Fragaria ananassa) and loquat (Eriobotrya japonica) fruits. Turk. J. Agri. Food Sci. Technol. 2018, 6, 561–569. [Google Scholar] [CrossRef]
- Nasiri, M.; Barzegar, M.; Sahari, M.A.; Niakousari, M. Application of Tragacanth gum impregnated with Satureja khuzistanica essential oil as a natural coating for enhancement of postharvest quality and shelf life of button mushroom (Agaricus bisporus). Int. J. Biol. Macromol. 2018, 106, 218–226. [Google Scholar] [CrossRef]
- Sarpong, F.; Oteng-Darko, P.; Golly, M.K.; Amenorfe, L.P.; Rashid, M.T.; Zhou, C. Comparative study of enzymes inactivation and browning pigmentation of apple (Malus domestica) slices by selected gums during low temperature storage. J. Food Biochem. 2018, 42, e12681. [Google Scholar] [CrossRef]
- Saleem, M.S.; Ejaz, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Hussain, S.; Ali, S.; Canan, İ. Postharvest application of gum arabic edible coating delays ripening and maintains quality of persimmon fruits during storage. J. Food Process. Preserv. 2020, 44, e14583. [Google Scholar] [CrossRef]
- Palou, L.; Valencia-Chamorro, S.A.; Pérez-Gago, M.B. Antifungal edible coatings for fresh citrus fruit: A review. Coatings 2015, 5, 962–986. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable Poly (Butylene Adipate-Co-Terephthalate) and Thermoplastic Starch-Blended TiO2 Nanocomposite Blown Films as Functional Active Packaging of Fresh Fruit. Polymers 2021, 13, 4192. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Rozilah, A.; Jaafar, C.N.; Sapuan, S.M.; Zainol, I.; Ilyas, R.A. The effects of silver nanoparticles compositions on the mechanical, physiochemical, antibacterial, and morphology properties of sugar palm starch biocomposites for antibacterial coating. Polymers 2020, 12, 2605. [Google Scholar] [CrossRef]
- Sapper, M.; Chiralt, A. Starch-based coatings for preservation of fruits and vegetables. Coatings 2018, 8, 152. [Google Scholar] [CrossRef]
- Kumar, R.; Ghoshal, G.; Goyal, M. Biodegradable composite films/coatings of modified corn starch/gelatin for shelf life improvement of cucumber. J. Food Sci. Technol. 2021, 58, 1227–1237. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Nanoemulsions as edible coatings. Curr. Opin. Food Sci. 2017, 15, 43–49. [Google Scholar] [CrossRef]
- Azam, M.; Ejaz, S.; Rehman, R.N.; Khan, M.; Qadri, R. Postharvest Quality Management of Strawberries. Strawberry-Pre-and Post-Harvest Management Techniques for Higher Fruit Quality; BoD: London, UK, 2019. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M. An extensive review of natural polymers used as coatings for post-harvest shelf-life extension: Trends and challenges. Polymers 2021, 3, 3271. [Google Scholar] [CrossRef]
- Morales-de la Peña, M.; Welti-Chanes, J.; Martín-Belloso, O. Novel technologies to improve food safety and quality. Curr. Opin. Food Sci. 2019, 30, 1–7. [Google Scholar] [CrossRef]
- Huang, Q.; Wan, C.; Zhang, Y.; Chen, C.; Chen, J. Gum Arabic Edible Coating Reduces Postharvest Decay and Alleviates Nutritional Quality Deterioration of Ponkan Fruit during Cold Storage. Front. Nutr. 2021, 8, 717596. [Google Scholar] [CrossRef]
- Kawhena, T.G.; Opara, U.L.; Fawole, O.A. Optimization of gum arabic and starch-based edible coatings with lemongrass oil using response surface methodology for improving post-harvest quality of whole “Wonderful” pomegranate fruit. Coatings 2021, 11, 442. [Google Scholar] [CrossRef]
- Najafi Marghmaleki, S.; Mortazavi, S.M.; Saei, H.; Mostaan, A. The Effect of Alginate-Based Edible Coating Enriched with Citric Acid and Ascorbic Acid on Texture, Appearance and Eating Quality of Apple Fresh-Cut. Int. J. Fruit Sci. 2021, 21, 40–51. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Aldana-Usme, A. Edible coatings based on sodium alginate and ascorbic acid for application on fresh-cut pineapple (Ananas comosus (L.) Merr). Agron. Colomb. 2019, 37, 317–322. [Google Scholar] [CrossRef]
- Bal, E. Effects of alginate edible coating enriched with salicylic and oxalic acid on preserving plum fruit (Prunus salicina L. cv. ‘Black amber’) quality during post-harvest storage. Acta Sci. Pol. Hortorum Cultus 2019, 18, 35–46. [Google Scholar] [CrossRef]
- Das, S.; Vishakha, K.; Banerjee, S.; Mondal, S.; Ganguli, A. Sodium alginate-based edible coating containing nanoemulsion of Citrus sinensis essential oil eradicates planktonic and sessile cells of food-borne pathogens and increased quality attributes of tomatoes. Int. J. Biol. Macromol. 2020, 162, 1770–1779. [Google Scholar] [CrossRef]
- Ghosh, T.; Nakano, K.; Katiyar, V. Curcumin doped functionalized cellulose nanofibers based edible chitosan coating on kiwifruits. Int. J. Biol. Macromol. 2021, 184, 936–945. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Q.; Picha, D.H.; Ferguson, M.H.; Ndukwe, I.E.; Azadi, P. Comparative performance of bio-based coatings formulated with cellulose, chitin, and chitosan nanomaterials suitable for fruit preservation. Carbohydr. Polym. 2021, 259, 117764. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Park, J.J.; Lee, J.J.; Lee, W.Y. Combined effect of chitosan coating and modified atmosphere packaging on fresh-cut cucumber. Food Sci. Nutr. 2019, 7, 1043–1052. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Baños, S.B.; Sivakumar, D. Shelf life extension of fresh fruit and vegetables by chitosan treatment. Crit. Rev. Food 2017, 57, 579–601. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Riahi, Z.; Rhim, J.W. Antioxidant pectin/pullulan edible coating incorporated with Vitis vinifera grape seed extract for extending the shelf life of peanuts. Postharvest Biol. Technol. 2022, 183, 111740. [Google Scholar] [CrossRef]
- Lara, G.; Yakoubi, S.; Villacorta, C.M.; Uemura, K.; Kobayashi, I.; Takahashi, C.; Nakajima, M.; Neves, M.A. Spray technology applications of xanthan gum-based edible coatings for fresh-cut lotus root (Nelumbo nucifera). Food Res. Int. 2020, 137, 109723. [Google Scholar] [CrossRef]
- Khorram, F.; Ramezanian, A.; Hosseini, S.M.H. Shellac, gelatin and Persian gum as alternative coating for orange fruit. Sci. Hortic. 2017, 225, 22–28. [Google Scholar] [CrossRef]
- Adiamo, O.Q.; Eltoum, Y.A.; Babiker, E.E. Effects of gum arabic edible coatings and sun-drying on the storage life and quality of raw and blanched tomato slices. J. Culin. Sci. Technol. 2019, 17, 45–58. [Google Scholar] [CrossRef]
- Kubheka, S.F.; Tesfay, S.Z.; Mditshwa, A.; Magwaza, L.S. Evaluating the Efficacy of Edible Coatings Incorporated with Moringa Leaf Extract on Postharvest of ‘Maluma’ Avocado Fruit Quality and Its Biofungicidal Effect. HortScience 2020, 55, 410–415. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Rastegar, S. Preservation of mango fruit with guar-based edible coatings enriched with Spirulina platensis and Aloe vera extract during storage at ambient temperature. Sci. Hortic. 2020, 265, 109258. [Google Scholar] [CrossRef]
- Du, Y.; Yang, F.; Yu, H.; Cheng, Y.; Guo, Y.; Yao, W.; Xie, Y. Fabrication of novel self-healing edible coating for fruits preservation and its performance maintenance mechanism. Food Chem. 2021, 351, 129284. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, Q.; Yang, S.X.; Chen, C.; Tang, Y.; Sun, S.; Li, X. Preservation mechanism of chitosan-based coating with cinnamon oil for fruits storage based on sensor data. J. Sens. 2016, 16, 1111. [Google Scholar] [CrossRef]
- Arroyo, B.J.; Bezerra, A.C.; Oliveira, L.L.; Arroyo, S.J.; de Melo, E.A.; Santos, A.M.P. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.). Food Chem. 2020, 309, 125566. [Google Scholar] [CrossRef]
- Wu, S. Extending shelf-life of fresh-cut potato with cactus Opuntia dillenii polysaccharide-based edible coatings. Int. J. Biol. Macromol. 2019, 130, 640–644. [Google Scholar] [CrossRef]
- Nicolau-Lapena, I.; Colas-Meda, P.; Alegre, I.; Aguilo-Aguayo, I.; Muranyi, P.; Vinas, I. Aloe vera gel: An update on its use as a functional edible coating to preserve fruits and vegetables. Prog. Org. Coat. 2021, 151, 106007. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Characterization and antifungal activity of pomegranate peel extract and its use in polysaccharide-based edible coatings to extend the shelf-life of capsicum (Capsicum annuum L.). Food Bioprocess Technol. 2018, 11, 1317–1327. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Parafati, L.; Licciardello, F.; Muratore, G.; Hamdi, M.; Cirvilleri, G.; Restuccia, C. Edible coatings incorporating pomegranate peel extract and biocontrol yeast to reduce Penicillium digitatum post-harvest decay of oranges. Food Microbiol. 2018, 74, 107–112. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Affes, S.; Boufi, S.; Nasri, M. Optimization of the formulation of chitosan edible coatings supplemented with carotenoproteins and their use for extending strawberries post-harvest life. Food Hydrocoll. 2018, 83, 375–392. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ponce, A.G.; Moreira, M.R. Influence of polysaccharide-based edible coatings as carriers of prebiotic fibers on quality attributes of ready-to-eat fresh blueberries. J. Sci. Food Agric. 2018, 98, 2587–2597. [Google Scholar] [CrossRef]
- Li, L.; Yi, P.; Li, C.; Xin, M.; Sun, J.; He, X.; Tang, Y. Influence of polysaccharide-based edible coatings on enzymatic browning and oxidative senescence of fresh-cut lettuce. Food Sci. Nutr. 2021, 9, 888–899. [Google Scholar] [CrossRef]
- Panahirad, S.; Naghshiband-Hassani, R.; Ghanbarzadeh, B.; Zaare-Nahandi, F.; Mahna, N. Shelf life quality of plum fruits (Prunus domestica L.) improves with carboxymethylcellulose-based edible coating. HortScience 2019, 54, 505–510. [Google Scholar] [CrossRef]
- Dong, F.; Wang, X. Guar gum and ginseng extract coatings maintain the quality of sweet cherry. LWT 2018, 89, 117–122. [Google Scholar] [CrossRef]
- Careli-Gondim, Í.; Mesquita, T.C.; Boas, E.V.D.B.V.; Caliari, M.; Júnior, M.S.S. The effect of active coating and refrigerated storage on the quality of avocado cultivar, Quintal. J. Food Sci. Technol. 2020, 57, 143–151. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, X.; Song, Z.; Kong, W.; Kang, Y.; Kong, W.; Ng, T.B. Coating shiitake mushrooms (Lentinus edodes) with a polysaccharide from Oudemansiella radicata improves product quality and flavor during post-harvest storage. Food Chem. 2021, 352, 12935. [Google Scholar] [CrossRef]
- Ali, A.; Noh, N.M.; Mustafa, M.A. Antimicrobial activity of chitosan enriched with lemongrass oil against anthracnose of bell pepper. Food Packag. 2015, 3, 56–61. [Google Scholar] [CrossRef]
- Rashid, F.; Ahmed, Z.; Hussain, S.; Kausar, T.; Nadeem, M.; Ainee, A.; Mehmood, T. Optimization of fenugreek and flax polysaccharides-based edible coating formulation to preserve the quality and storability of apple after harvesting. J. Food Process. Preserv. 2020, 44, e14812. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive Review of Polysaccharide-Based Materials in Edible Packaging: A Sustainable Approach. Foods 2021, 10, 1845. [Google Scholar] [CrossRef]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel materials in the preparation of edible films and coatings—A review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Yang, W.; Huang, H. Preparation, deproteinization and comparison of bioactive polysaccharides. Trends Food Sci. Technol. 2021, 109, 564–568. [Google Scholar] [CrossRef]
- Pitkänen, L.; Heinonen, M.; Mikkonen, K.S. Safety considerations of plant polysaccharides for food use: A case study on phenolic-rich softwood galactoglucomannan extract. Food Funct. 2019, 9, 1931–1943. [Google Scholar] [CrossRef]
- Gultekin, F.; Doguc, D.K. Allergic and immunologic reactions to food additives. Clin. Rev. Allergy Immunol. 2013, 45, 6–29. [Google Scholar] [CrossRef] [PubMed]
- Skypala, I.J. Food-induced anaphylaxis: Role of hidden allergens and cofactors. Front. Immunol. 2019, 10, 673. [Google Scholar] [CrossRef] [PubMed]
- Milani, J.M.; Tirgarian, B. An overview of edible protein-based packaging: Main sources, advantages, drawbacks, recent progressions and food applications. J. Package Technol. Res. 2020, 4, 103–115. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felicia, W.X.L.; Rovina, K.; Nur’Aqilah, M.N.; Vonnie, J.M.; Erna, K.H.; Misson, M.; Halid, N.F.A. Recent Advancements of Polysaccharides to Enhance Quality and Delay Ripening of Fresh Produce: A Review. Polymers 2022, 14, 1341. https://doi.org/10.3390/polym14071341
Felicia WXL, Rovina K, Nur’Aqilah MN, Vonnie JM, Erna KH, Misson M, Halid NFA. Recent Advancements of Polysaccharides to Enhance Quality and Delay Ripening of Fresh Produce: A Review. Polymers. 2022; 14(7):1341. https://doi.org/10.3390/polym14071341
Chicago/Turabian StyleFelicia, Wen Xia Ling, Kobun Rovina, Md Nasir Nur’Aqilah, Joseph Merillyn Vonnie, Kana Husna Erna, Mailin Misson, and Nur Fatihah Abdul Halid. 2022. "Recent Advancements of Polysaccharides to Enhance Quality and Delay Ripening of Fresh Produce: A Review" Polymers 14, no. 7: 1341. https://doi.org/10.3390/polym14071341
APA StyleFelicia, W. X. L., Rovina, K., Nur’Aqilah, M. N., Vonnie, J. M., Erna, K. H., Misson, M., & Halid, N. F. A. (2022). Recent Advancements of Polysaccharides to Enhance Quality and Delay Ripening of Fresh Produce: A Review. Polymers, 14(7), 1341. https://doi.org/10.3390/polym14071341